Abstract
Good health and normal sexual function in males require functioning testicles, a fact known since antiquity. It is only more recently that the source of that effect was attributed to testosterone. Several centuries ago, Arnould Berthold castrated a rooster as part of an experiment, which resulted in loss of the physical signs of masculinization. Berthold realized that something manufactured by the testes was responsible for maintenance of virilization; returning the testicles to the intra-abdominal cavity of the rooster led to a restoration of its phenotype. Because Berthold had severed the neurovascular connection of the testis to the body, he correctly surmised that the testicles produced a secretion that must act through the circulatory system. It was Charles Brown-Sequard who confirmed the testes as the source of the secretion of this “hormone.” After he extracted fluid from the testis of dogs and injected himself with this fluid, he experienced improvements in strength, appetite, and mentation and published the results. The exact chemical messenger remained unidentified until 1935, when David et al. published the structure of the steroidal chemical and named the compound “testosterone.” Shortly thereafter, Butenandt and Ruzicka published near simultaneous manuscripts describing the methods for testosterone synthesis, which resulted in them subsequently sharing the Nobel Prize.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Erectile Function
- Premature Ejaculation
- Serum Testosterone Level
- Testosterone Replacement
- Testosterone Replacement Therapy
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Good health and normal sexual function in males require functioning testicles, a fact known since antiquity. It is only more recently that the source of that effect was attributed to testosterone [1]. Several centuries ago, Arnould Berthold castrated a rooster as part of an experiment, which resulted in loss of the physical signs of masculinization. Berthold realized that something manufactured by the testes was responsible for maintenance of virilization; returning the testicles to the intra-abdominal cavity of the rooster led to a restoration of its phenotype. Because Berthold had severed the neurovascular connection of the testis to the body, he correctly surmised that the testicles produced a secretion that must act through the circulatory system. It was Charles Brown-Sequard who confirmed the testes as the source of the secretion of this “hormone.” After he extracted fluid from the testis of dogs and injected himself with this fluid, he experienced improvements in strength, appetite, and mentation and published the results. The exact chemical messenger remained unidentified until 1935, when David et al. published the structure of the steroidal chemical and named the compound “Testosterone.” Shortly thereafter, Butenandt and Ruzicka published near simultaneous manuscripts describing the methods for testosterone synthesis, which resulted in them subsequently sharing the Nobel Prize.
Men with low or absent testosterone production suffer significant and recognizable physical consequences. Classic male hypogonadism, a relatively rare condition, may be due to central (hypogonadotropic hypogonadism) or peripheral (hypergonadotropic hypogonadism) loss of testosterone production. German, Swiss, and Dutch chemists successfully synthesized synthetic testosterone in the 1930s, and an oral form of testosterone for replacement therapy became available in the 1940s. Testosterone replacement therapy (TRT), which maintains serum testosterone levels in the normal range, rapidly became the standard of care for hypogonadal men due to the availability of synthetic testosterone. The initial indication for TRT was somewhat limited. It included men suffering from genetic abnormalities like Kleinfelter’s syndrome, true endocrinopathies with near castrate levels of testosterone, and the most severe form of hypogonadism. The results of TRT in these men were dramatic, and the most notable was the restoration of erectile function. As a consequence, the use of testosterone as therapy for erectile dysfunction became commonplace, primarily due to the absence of any other effective therapy at the time. This “off label” use of testosterone for the treatment of erectile dysfunction was marginally effective at best. Testosterone was first approved in 1972 by the Food and Drug Administration (FDA) as a treatment for the signs and symptoms of male hypogonadism. The use of TRT in men with prostate cancer has been contra-indicated since its original approval. The contraindication for the use of TRT in hypogonadal men with a history of prostate cancer remains controversial, despite the absence of evidence of significant risk.
Medicalization of Aging
All living organisms have a finite life cycle, and all forms of life ultimately expire at the end of that cycle. Disease represents conditions of nature and environment that may shorten the life cycle of any individual. Good health allows the individual to live out the full lifespan of the species. Organized medicine represents efforts to identify and modify disease by scientific means, and to reverse the effects of disease on the life cycle. Scientists, physicians, and other health care providers study the accumulated scientific and experiential body of knowledge, and bring the results of these efforts to the benefit of the individual patient and the health of society.
Aging in men leads to decline in multiple physiologic and psychological parameters. The decline is progressive and is in part due to decreasing androgen production. The reduction of serum levels of total, free, and bioavailable testosterone throughout aging has been well documented. The symptoms attendant to this drop in androgens, like depression, loss of energy, and sexual dysfunction, are so common that some would say that they are not symptoms at all but the normal consequence of age itself. The physical effects of aging broadly recognized include weight gain, loss of muscle mass, and skin changes. Menopause is a universal event for aging women; however, the treatment remains controversial. “Male menopause” or “andropause” are terms associated with a male syndrome similar to menopause, but sufficiently different. This causes some to question the wisdom of androgen replacement in men suffering a significant decrease in quality of life. Efforts to reverse the effects of aging are themselves timeless (e.g., the search for the “fountain of youth”). The role of the testes in rejuvenation was first appreciated by Eugen Steinbach who, together with urologist Robert Lichtenstern, developed a surgical procedure a century ago (vasectomy) that purportedly led to the improvement in the symptoms of aging [2].
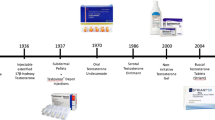
Initial testosterone preparations were administered by oral or parenteral routes; while effective in raising serum testosterone levels, adverse effects in some men accompanied each. The development of transdermal testosterone delivery by skin patch, approved by the FDA in 1995, led to an increase in disease awareness and testosterone use. Concurrently, the population of patients and the indications for testosterone replacement were broadening. Since the introduction of transdermal testosterone gel in 2000, the market for TRT has increased dramatically, for men with age related decline of testosterone. It was also promoted as a reversal for the effects of aging in men. In 2002, this led the National Institute of Aging and the National Cancer Institute to request that the Institute of Medicine (IOM) develop “an independent assessment of clinical research on testosterone therapy and make recommendations for future direction for this field.” The resulting white paper was published in 2004 and suggested that small efficacy trials be performed in seven clinical areas prior to undertaking a large and long-term study of safety in testosterone replacement in the aging male [3]. The IOM report recommended studies in older men with low testosterone (recommendation 1); with specific requests for short-term randomized double-blind, placebo-controlled efficacy trials (recommendation 2). The IOM panel felt that long-term risks and benefits should be determined by further clinical trial only if substantial evidence of clinically significant benefit was witnessed in the short-term studies (recommendation 3).
To gauge how successful the IOM recommendations have been to date, an OVID search was performed on the seven keywords (strength/frailty/disability, cognitive function, sexual function, and vitality/well-being/quality of life) and cross-referenced with the keyword “testosterone.” All publications published between 2003 and 2011 sharing testosterone and one of the keywords from 2004 to 2012 were identified, characterized (clinical trial, series, review, and other), and tabulated (Table 1.1). The list is not exclusive and a publication with more than one keyword identified may appear more than once. As with many clinical conditions, when confirmatory evidence of treatment efficacy is lacking, the number of review articles often exceeds the number of clinical trial manuscripts. When http://www.clinicaltrials.gov web site was searched for testosterone clinical trials, a total of 598 clinical trials were listed. After elimination of trials of testosterone in women, prostate cancer, nongeriatric related male trials, and pharmacokinetic trials, the remaining trials were plotted based on the date the trial was first received (Fig. 1.1). An initial increase in recorded trials occurred in the first few years after the IOM publication, which has now receded somewhat.
Number of testosterone clinical trials by year of first registration 2005–2011 (based on data from http://www.ClinicalTrials.Gov)
Sexual Dysfunction
Testosterone is closely tied with sexual function, so the majority of publications in the immediate period after the publication of the IOM report dealt with the intersection of testosterone and sexual function. Several manuscripts investigated the validity of new patient reported survey instruments in aging men to see if they were truly reflective of the association between aging and androgens. Others continued to investigate the “aging versus disease state” conundrum: whether sexual dysfunction is a result of abnormally low testosterone levels or just another physical aspect of aging. Finally, a large meta-analysis of 17 studies published between 1979 and 2003/2004 outlined the positive and negative effects of TRT on sexual function.
In a study of two commonly used patient reported outcome (PRO) instruments, Basar et al. investigated the relationship between testosterone levels and symptoms using the Aging Male Symptoms (AMS) score and International Index of Erectile Function (IIEF) [4]. The AMS and IIEF questionnaires were administered to 348 men (21–67 years; mean 49.6 years); serum sex hormone levels (total testosterone (TT), free testosterone (FT), estradiol (E2), and dehydroepiandrosterone-sulfate (DHEA-S)) were drawn. Serum DHEA-S and FT levels and age correlated significantly with the IIEF scores; although serum total testosterone, FT, and DHEA-S levels correlated significantly with the andrologic domain of AMS, the only correlation of the total AMS score was with age.
The Androgen Deficiency of Aging Men (ADAM) questionnaire, another popular PRO, was also evaluated for efficacy in diagnosing hypogonadism. Blumel et al. administered the ADAM questionnaire and drew serum sex hormone profiles on 96 men aged 40 years and older in a cross-sectional study in Chile [5]. Total testosterone, sex hormone binding globulin (SHBG), and albumin were measured, and bioavailable testosterone was calculated. Standard scoring for the ADAM questionnaire was used to evaluate for androgen deficiency (if items 1 or 7 or any three other questions of the ADAM questionnaire were positive). A total of 78 men (81.3%) were identified as androgen deficient by the ADAM questionnaire; however, available testosterone confirmed the diagnosis in only 27 cases (28.1%). Low libido was a better predictor of hypogonadism by itself, rather than the ADAM questionnaire (63.3% sensitivity and 66.7% specificity). For now we do not have a statistically valid, rigorous PRO for androgen deficiency in aging men, and the need remains, a topic discussed in a later chapter of this text.
While efforts to find a good PRO continue, others tried to determine whether there exists a threshold at which patients become symptomatic, or whether each symptom may have an individual serum testosterone threshold at which it emerges. In such an attempt, Lackner et al. evaluated 675 healthy men using the Aging Male Symptoms (AMS) scale, the Beck Depression Index (BDI) and the International Index of Erectile Function (IIEF), and attempted to terrelate scores with AM serum testosterone levels [6]. The patients were divided into two groups: those with symptoms and those without. Patients suffering from lack of concentration, decreased libido, listlessness, and both somatic/psychological symptoms by AMS demonstrated testosterone levels that were different from those in men without these symptoms. However, this association was lost when multivariate analysis was applied; loss of libido, lack of vigor, and sexual dysfunction were associated with age rather than with testosterone. Thus, levels of serum testosterone at which specific symptoms emerged could not be identified in this study.
Two manuscripts examined the relationship of testosterone levels with erection and ejaculation. Gades et al. evaluated the association between sex hormone serum levels, erectile function, and sexual drive in a population-based sample of men (the Olmsted County Study of Urinary Symptoms and Health Status) [7]. In a random sample of men residing in Olmsted County, MN, 414 men completed the Brief Male Sexual Function Inventory (BMSFI), underwent physical examination, and had serum hormone measurements performed. Of these men, 294 had a regular sexual partner and androgen measurements after 14 years of follow-up. At 14 years, total testosterone and erectile function (but not libido) demonstrated significant correlation, despite adjustment for age. Bioavailable testosterone was significantly correlated with both erectile function and libido.
Corona et al. evaluated the role of testosterone and hypogonadism in the control of the ejaculatory reflex, comparing subjects with premature ejaculation (PE) or delayed ejaculation (DE) to those without ejaculatory dysfunction [8]. Serum hormonal and biochemical parameters were studied in 2,437 men with sexual dysfunction. Premature ejaculation was reported by 714 (25.9%) and delayed ejaculation by 121 (4.4%). Testosterone levels significantly correlated with the type of ejaculation. The youngest men (25–40 years) with PE had higher TT and FT levels; the oldest (55–70 years), with DE, had lower TT and FT levels. As would be expected from these results, hypogonadism was more common in men with DE (26%) and least common in men suffering PE (12%). Adjustment for age and libido did not change the results.
Positive correlations between threshold testosterone levels and specific sexual symptoms were found in a study of TRT reported by Seftel et al. [9]. Four hundred and six aging symptomatic hypogonadal men (mean age 58 years) were randomized to transdermal testosterone gel (50 and 100 mg/day), transdermal testosterone patch, or placebo. Patients were evaluated at 30 and 90 days after initiation of treatment for significant change in the frequency of intercourse, nighttime erections, and libido. At day 30, a significant increase was seen for all three primary outcome measures for those on 100 mg/day T gel compared to the others; the results at 90 days for sexual desire and nighttime erections vs. placebo were similar. The authors defined a “threshold average daily serum T level for sexual response” which was the testosterone level at which (1) while the testosterone level might be within the normal range, the sexual response was no different from that of the group of subjects with the lowest serum T level (0–300 ng/dL); and (2) there was a significant change when treated with testosterone compared with that of the group of subjects with the lowest serum level. This threshold f was 400 ng/dL for nighttime erections; at or below this serum level, the frequency of nighttime erections was no different than that for hypogonadal men. For frequency of sexual intercourse, the “threshold average daily serum testosterone level was 500 ng/dL, and it was 600 ng/dL for sexual desire. While the authors felt these data demonstrated a clear relationship between restoring serum T concentrations and improvement in certain parameters of sexual function, they do state that the study was not designed to explicitly measure a threshold serum level.
Isidori et al. performed a systematic review and meta-analysis of placebo-controlled studies published in the past 30 year period (1975–2004) [10]. MEDLINE, the Cochrane Library, EMBASE, and Current Contents were reviewed, of which 17 randomized placebo-controlled trials were found to be eligible. The total number of evaluable patients over the 17 studies was 656, of which 284 were randomized to testosterone and 284 to placebo (P), and 88 were treated in crossover. The majority of the patients received parenteral testosterone, though a few men received transdermal or buccal testosterone. The average length of therapy across the studies was 3 months (range 1–36 months). Results of the meta-analysis revealed that in hypogonadal men with an initial testosterone level below 12 ng/dL, TRT led to an improvement in multiple parameters: nocturnal erections, sexual thoughts and motivation, number of successful intercourses, scores for erectile function, and overall sexual satisfaction. Testosterone replacement had no effect on sexual function in eugonadal men and was equivalent to placebo. The effects of T on erectile function, but not libido, were inversely related to the mean baseline T concentration. Results of this meta-analysis must be viewed critically as the majority of the studies were performed during a period when sexual function, and in particular erectile function, was the most common outcome used to judge clinical response to testosterone replacement, and the physiology of erection and pathophysiology of erectile dysfunction were poorly understood. Conclusions from studies performed during this period are not necessarily valid given our understanding of androgens and erectile function today.
Well-Being/Quality of Life/Cognitive Function
As men get older, they experience many conditions, often together, that eventually result in the inability to perform many activities of daily living. Some men may experience an increased propensity to fall and decreased independence. Elderly men also have increased incidence of anemia, higher rates of metabolic syndrome, decreased sexual function, and memory impairment. These conditions likely have multiple causes, but one likely contributing cause is a low serum testosterone concentration. When young hypogonadal men are treated with testosterone, they experience improvements in sexual function, muscle mass and strength, bone mineral density (BMD), and sense of well-being. In aging men, the benefits of testosterone therapy are controversial, particularly those related to psychosocial improvement. A limited number of studies have examined these issues since the IOM report, the following are the most important.
The degree to which androgen deficiency leads to a decline in the quality of life in elderly is unknown. To examine this question, Finas et al. investigated 24 hypogonadal men aged 50 years or older (defined as free testosterone <200 ng/dL) and compared them to 24 eugonadal age-matched controls. All of the men were under treatment for benign prostatic hyperplasia. The Short Form (SF)-12 Health Survey (physical health and mental health index) assessed health-related “Quality of Life.” The investigators modified the questionnaire to broaden it, importing “vitality” and “psychological well-being” scales from SF-36. The SF-12 physical health index was reduced in the hypogonadal group, but the mental health index was not. Patients with low FT demonstrated lower scores on vitality than eugonadal men, but no differences were detected in psychological well-being between hypogonadal and eugonadal men [11].
T’Sjoen et al. used the AMS rating scale to assess the relationship of male aging symptoms and androgen levels in asymptomatic elderly men in generally good health [12]. After the AMS questionnaire was completed, serum sex steroids levels, sex hormone binding globulin, gonadotropins, and physiologic responses were measured in 161 ambulatory, elderly men, aged 74–89. Mild psychological and mild to moderate somato-vegetative symptoms were associated with diminished serum testosterone levels as assessed by AMS. However, none of the three AMS domain scale scores significantly correlated with testosterone, free testosterone or bioavailable testosterone. In healthy elderly men, the AMS was not useful in predicting androgen levels because no consistent relationship was demonstrated.
Androgens may play a role in maintenance of cognitive function in men. Patients suffering from derangements of cognitive function such as Alzheimer’s disease may benefit from TRT. Previously, Hogervorst et al. found lower levels of testosterone in men with Alzheimer’s disease compared with controls [13]. To determine whether abnormal pituitary regulation of androgens was responsible for the testosterone deficiency, the investigators compared sex hormones (follicle stimulating hormone (FSH) and luteinizing hormone (LH), and sex hormone binding globulin (SHBG)) and testosterone in 45 men with Alzheimer’s, 15 men with other types of dementia, and 133 elderly controls. There were no observed differences in LH, FSH, or SHBG levels between Alzheimer’s disease patients and controls. However, testosterone levels were significantly lower in the men with Alzheimer’s.
The association of depression and low testosterone in men is recognized; what is not as well known is whether there is a causal relationship between low testosterone and depression. In a large cross-sectional study, Almeida et al. sought to determine whether the association between serum testosterone concentration and mood in older men is independent of physical comorbidity [14]. The authors investigated 3,987 men aged 71–89 years from a community-based setting. Patients were evaluated using the Geriatric Depression Scale (GDS-15) to assess mood; physical health was assessed with Physical Component Summary score of the 36-Item Short Form Health Survey. Two hundred and three men (5.1%) had depression. These men had significantly lower total and free testosterone concentrations than nondepressed men. Depressed men also had higher obesity levels and more physical limitations. After adjusting for these factors and for age, the association between depression and low total and free testosterone concentrations did not change.
Giltay et al. examined the reversibility of hypogonadal induced depression secondary to testosterone deficiency in a randomized, placebo-controlled, double-blind, phase III trial (ClinicalTrials.gov identifier: NCT00696748) [15]. In this trial, 184 men with metabolic syndrome and low testosterone were randomized 2:1 to receive testosterone or placebo injections. Mood, well-being, and sexual function were assessed at three time periods (baseline, 18 and 30 weeks) using the Beck Depression Inventory (BDI-IA), Aging Males’ Symptoms (AMS) scale, and International Index of Erectile Function 5-item (IIEF-5) scale. Restoration to a eugonadal state led to significant improvements in all three parameters (BDI-IA, AMS, and IIEF); the greatest improvement occurred in men with a baseline total testosterone level <7.7 ng/dL.
Disability/Frailty/Vitality
The majority of studies published since the IOM report have dealt with the impact of androgen deficiency and replacement on frailty in the aging, and the disability and loss of vitality that accompany frailty. These reports are comprehensive and come from large populations worldwide. The outcomes are tangible and more easily measured (i.e., bone mineral density, muscle strength, etc.). There is also more certainty that therapeutic interventions are causal to the observed improvements (Table 1.2).
Schaap et al. reported on a cross-sectional population-based study, based on data from the Longitudinal Ageing Study Amsterdam (LASA). Sex hormones were measured, and physical performance, functional limitations, and muscle strength were assessed. Analysis of falls was performed prospectively for 3 years [16]. Men in the highest quartile of the estradiol/SHBG ratio had significantly higher physical performance scores than men in the lowest quartile. Serum levels of total testosterone were positively associated with muscle strength. Calculated bioavailable testosterone levels were positively associated with physical performance and muscle strength. Low levels of sex hormones were associated with impaired mobility and low muscle strength in men.
In an observational study of 1,705 men in Australia, Travison et al. used longitudinal measurements to assess sex hormones and their relationship to the prevalence and progression of frailty in older men [17]. Measurements were obtained at baseline (2005–2007) and at 2-year follow-up (2007–2009). Frailty syndrome was measured according to the Cardiovascular Health Study (CHS) and Study of Osteoporotic Fractures (SOF) indices. Significant age-adjusted associations were seen between serum androgens and estrogens and concurrent frailty. Subjects in the lowest T quintile had 2.2-fold odds of exhibiting greater CHS frailty as compared with the highest T quintile (P < 0.001). A 2-year decrease of one standard deviation in T, calculated free T, or LH was associated with a 1.2- to 1.3-fold increase in the odds of progression (increase in severity) of frailty. Control for comorbid medical conditions did not affect the age-related changes. The author concluded that Serum androgens and estrogens may contribute to the development or progression of frailty in men.
Cawthon et al. reported a large multicentered cross-sectional/longitudinal study of sex hormones and frailty based in the United States [18]. The Osteoporotic Fractures in Men (MrOS) study involved 1,469 men at least 65 year old; 1,245 men had frailty status reassessed after 4 years. Frailty was assessed by using a modification of the CHS, with patients exhibiting three or more symptoms (shrinking/sarcopenia, weakness, slowness, low activity level, and exhaustion). Bioavailable testosterone was the only serum sex hormone associated with frailty. Men in the lowest quarter of bioavailable testosterone had 1.39-fold increase in odds of greater frailty status compared to men in the highest quartile, and a 1.51-fold increase in odds of greater frailty status 4 years later. Comorbid conditions had little effect. A second US based study used a modification of the Cardiovascular Health System to assess sex hormones and frailty in older men [19]. The Massachusetts Male Aging Study Examined 646 men aged 50+ with frailty defined as the presence of three or more of the following: weight loss, exhaustion, low physical activity, slowness, and weakness. TT, free T, and SHBG levels were investigated for association with frailty and with degree of frailty. Total and free T were not associated with frailty in these aging men; however, SHBG did show a significant association with frailty. Grip strength and physical activity (but not exhaustion, slow walking, or weight loss) were associated with total T levels, whereas SHBG was related to weight loss, exhaustion, and physical activity.
Tajar et al. [20] reported the results of the European Male Aging Study. Three thousand two hundred and nineteen men had frailty assessed as an index (FI) according to the number (out of 43 possible) of health deficits (symptoms, signs, and functional impairments), and relationships between FI and hormone levels (as outcomes). Results were explored using regression models. Mean FI was highest in the oldest group and higher levels of FI were significantly associated with lower levels of total T, free T, and DHEA-S and higher levels of gonadotropins and SHBG. In the “Health In Men” study of frailty and androgens, Hyde et al. examined 3,616 elderly men living in Australia [21]. Sex hormones (testosterone SHBG, LH, and calculated free testosterone) were measured. The authors used a “FRAIL scale”; patients were scored on five physical tasks: fatigue, difficulty climbing a flight of stairs, difficulty walking more than 100 m, more than five illnesses present, or weight loss greater than 5%. Men with three or more positive symptoms were deemed frail. Frailty was assessed at baseline; of the 3,616, men 548 (15.2%) were judged frail (at least three deficits). At following reassessment in 2008–2009, frailty increased to 23.0% (364/1,586). Of the sex hormones evaluated, only free testosterone predicted frailty after statistical adjustment for age and other comorbid factors.
Four clinical trials were published in the period after the IOM report, which document the beneficial effects of androgen replacement in frail men. Though much smaller in number, the aging patients demonstrated measurable improvements in objectively measurable physiologic parameters. Three different routes of administration were used to achieve normal serum testosterone levels (oral, parenteral, and transdermal). In a single center randomized study, Kenny et al. evaluated the effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels [22]. This study was designed after publication of the IOM report and incorporated the IOM recommendations for small efficacy studies: focus on health outcomes for which preliminary evidence of efficacy and limited alternative therapies exist. One hundred and thirty-one aging men (mean age 77 years) with low testosterone, history of fracture, or osteoporosis (T-score less than −2.0) were included in the study. Frailty was assessed based on Fried and colleagues’ parameters (frail = 3–5 characteristics, intermediate frail = 1–2 characteristics, nonfrail (n = 0 characteristics). Patients received 5 mg/day of testosterone or placebo for 12–24 months. Additionally, all men received calcium (1,500 mg/day diet and supplement) and cholecalciferol (1,000 IU/day). Multiple outcome measures were assessed, including BMD (hip, lumbar spine, and mid-radius), body composition, sex hormones, calcium-regulating hormones, bone turnover markers, strength, physical performance, and safety parameters. Ninety-nine men (75.6%) completed 12 months. Sixty-two (47.3%) completed end of therapy (mean 23 months). BMD on testosterone increased 1.4% at the femoral neck and 3.2% at the lumbar spine. There was an increase in lean mass and a decrease in fat mass in the testosterone group but no differences in strength or physical performance. There were no differences in safety parameters.
Transdermal testosterone gel was also used by Srinivas-Shankar et al. to investigate testosterone replacement on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men [23]. In a single-center study, 274 elderly men at least 65 years of age with hypogonadism (total T ≤ 12 nmol/L or free T at or below 250 pmol/L) were randomized to transdermal T (50 mg/day) or placebo gel for 6 months. Outcome measures included muscle strength, lean and fat mass, physical function, and self-reported quality of life. At the end of the study, muscle strength improved in the T group (vs. placebo at 6 months), while lean body mass increased and fat mass decreased significantly. Somatic and sexual symptom scores decreased with T treatment.
In Thailand, Permpongkosol et al. conducted a retrospective review of 161 men with symptomatic late onset hypogonadism who had received treatment with parenteral testosterone undecanoate. The effects of the therapy on body composition, lipids, and psychosexual complaints were noted [24]. Late onset hypogonadism was defined as pretreatment T < 300 ng/day. The mean duration of treatment was 90.6 weeks. One hundred men had used parenteral TU for >12 months. Body mass index (BMI), waist circumference, percentage body fat, total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, prostate-specific antigen (PSA), and hematocrit were measured. Further, the AMS scale and the IIEF (IIEF-5 and 15) were scored. Testosterone replacement led to a significant decline in waist circumference and percentage body fat, but no change in BMI. The psychological, somato-vegetative, and sexual factor scores of subscales of AMS decreased. Erectile function domain, orgasmic function domain, sexual desire domain, intercourse satisfaction domain, and overall satisfaction domain improved. No clinically significant adverse effects occurred in these men. Oral testosterone supplementation was used in an attempt to prevent muscle loss in eugonadal frail men over a 12-month period [25]. Seventy-six men, aged 60 years or older, received testosterone undecanoate (80 mg twice daily) or placebo for 1 year. Measurements of body composition, muscle strength, hormones, and safety parameters were obtained at 0, 6, and 12 months. Lean body mass increased and fat mass decreased in the testosterone treated group. There were no significant effects on muscle strength. Again, no clinically significant adverse effects were seen.
Over 10,000 aging men from four continents were included in the studies listed above (Table 1.3). All of the studies related increasing frailty status to decreasing androgens. However, there was disagreement between studies as to which androgen measurement was predictive of frailty. Each study attempts to objectively assess frailty status. Many use commonly accepted scales (Cardiovascular Health System, and Study of Osteoporotic Fractures), but modifications were made locally making comparisons and measurements sometimes difficult to interpret. Methodologies, though similar, do not utilize the IOM recommended trial design. The prospective treatment studies were composed of different testosterone preparations and duration and measured different outcomes. All of these factors reduce the ability to generalize the conclusions of individual studies.
One study, published in the period since the IOM report, has received significant notoriety. The Testosterone In Older Men With Mobility Limitations (TOM) trial was a single center, placebo-controlled, randomized clinical trial designed to comprehensively determine the effects of testosterone administration on muscle strength and physical function in older men with mobility limitations [26]. Community-dwelling men, 65 years of age or older, with limitations in mobility and a total serum testosterone level of 100–350 ng/dL or a free serum testosterone level <50 pg/mL, underwent randomization to placebo gel or testosterone gel for 6 months. Adverse events were categorized with the use of the Medical Dictionary for Regulatory Activities (MedRA). At midterm review, the data and safety monitoring board recommended that the trial be discontinued early. A significantly higher rate of adverse cardiovascular events was found in the testosterone group compared to the placebo group. A total of 209 men (mean age, 74 years) were enrolled at study termination. Of the 209 men, a total of 23 subjects in the testosterone group, as compared to 5 in the placebo group, had cardiovascular-related adverse events. The relative risk of a cardiovascular-related adverse events remained constant throughout the 6-month treatment period. The authors did admit in the manuscript that the small size of the trial and the unique population prevented broader inferences from being made about the safety of testosterone therapy (ClinicalTrials.gov number, NCT00240981).
The TOM trial raised concerns about the safety of testosterone therapy in aging men, but was justifiably criticized for several shortcomings. The initial dose of testosterone used in the study was greater than the recommended starting dose in the product label. Patients were also titrated to doses above the maximal approved dose. Several of the adverse effects experienced in the treatment group were known effects of testosterone replacement (peripheral edema) and clinically insignificant in nature. Yet, appropriate concern was used when the Data Safety Monitoring committee made the decision to terminate the study early. Definitive demonstration of an acceptable risk/benefit ratio, as defined in the IOM report, remains unfulfilled.
The Testosterone Trial in Older Men
Officially known as “Randomized, Placebo-controlled, Double-blind Study of Five Coordinated Testosterone Treatment Trials in Older Men,” this multicentered, randomized, placebo-controlled, double-blind treatment efficacy and safety study meets the challenge for clinical trial design as recommended by the IOM 2004 report [27]. The testosterone “Trial” will determine if 1 year of active testosterone replacement will lead to improvement in five primary outcome measures: walking speed, sexual activity, vitality scale, verbal memory test, and correction of anemia (ClinicalTrials.gov Identifier: NCT00799617). The trial is sponsored and funded by the University of Pennsylvania with the National Institute for Aging, the National Institute of Neurological Disorders and Stroke, the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Heart Lung & Blood Institutes serving as collaborators, with additional assistance from Abbott Corporation. Eight hundred men, aged 65 or older, with related androgen deficiency will enroll at 1 of the 12 participating medical centers beginning in November 2009 and ending in June 2015. The laboratory definition of low testosterone in this study is a total morning (drawn between 7 and 10 a.m.) serum concentration of <275 and <300 ng/dL at each of two screening visits. Within the “Testosterone in Men” trial, two sub-trials will be performed prospectively. Two additional trials have been incorporated into the T Trial. One, a cardiovascular trial, examines the effects of testosterone treatment on cardiovascular risk factors compared to placebo. In the second, the bone trial attempts to document an increase in volumetric trabecular BMD of the lumbar spine.
The testosterone trial is the result of multiple investigators’ efforts to incorporate the IOM recommendations into a large safety and efficacy trial. There are commonly agreed upon evaluative tools, PRO measures, primary and secondary physical outcomes, and adverse event reports. The large size of the trial requires support of both government and industry. We will await the results of the testosterone trial with great interest.
References
Freeman ER, Bloom DA, McGuire EJ. A brief history of testosterone. J Urol. 2001;165(2):371–3.
Schultheiss D, Denil J, Jonas U. Rejuvenation in the early 20th century. Andrologia. 1997;29(6):351–5.
Liverman CT, Blazer DG, editors. Testosterone and aging: clinical research directions. Washington, DC: National Academies Press; 2004.
Basar MM, Aydin G, Mert HC, et al. Relationship between serum sex steroids and Aging Male Symptoms score and International Index of Erectile Function. Urology. 2005;66(3):597–601.
Blumel JE, Chedraui P, Gili SA, Navarro A, Valenzuela K, Vallejo S. Is the Androgen Deficiency of Aging Men (ADAM) questionnaire useful for the screening of partial androgenic deficiency of aging men? Maturitas. 2009;63(4):365–8.
Lackner JE, Rucklinger E, Schatzl G, Lunglmayr G, Kratzik CW. Are there symptom-specific testosterone thresholds in aging men? BJU Int. 2011;108(8):1310–5.
Gades NM, Jacobson DJ, McGree ME, et al. The associations between serum sex hormones, erectile function, and sex drive: the Olmsted County Study of Urinary Symptoms and Health Status among men. J Sex Med. 2008;5(9):2209–20.
Corona G, Jannini EA, Mannucci E, et al. Different testosterone levels are associated with ejaculatory dysfunction. J Sex Med. 2008;5(8):1991–8.
Seftel AD, Mack RJ, Secrest AR, Smith TM. Restorative increases in serum testosterone levels are significantly correlated to improvements in sexual functioning. J Androl. 2004;25(6):963–72.
Isidori AM, Giannetta E, Gianfrilli D, et al. Effects of testosterone on sexual function in men: results of a meta-analysis. Clin Endocrinol. 2005;63(4):381–94.
Finas D, Bals-Pratsch M, Sandmann J, et al. Quality of life in elderly men with androgen deficiency. Andrologia. 2006;38(2):48–53.
T’Sjoen G, Goemaere S, De Meyere M, Kaufman JM. Perception of males’ aging symptoms, health and well-being in elderly community-dwelling men is not related to circulating androgen levels. Psychoneuroendocrinology. 2004;29(2):201–14.
Hogervorst E, Combrinck M, Smith AD. Testosterone and gonadotropin levels in men with dementia. Neuro Endocrinol Lett. 2003;24(3–4):203–8.
Almeida OP, Yeap BB, Hankey GJ, Jamrozik K, Flicker L. Low free testosterone concentration as a potentially treatable cause of depressive symptoms in older men. Arch Gen Psychiatry. 2008;65(3):283–9.
Giltay EJ, Tishova YA, Mskhalaya GJ, Gooren LJG, Saad F, Kalinchenko SY. Effects of testosterone supplementation on depressive symptoms and sexual dysfunction in hypogonadal men with the metabolic syndrome. J Sex Med. 2010;7(7):2572–82.
Schaap LA, Pluijm SMF, Smit JH, et al. The association of sex hormone levels with poor mobility, low muscle strength and incidence of falls among older men and women. Clin Endocrinol. 2005;63(2):152–60.
Travison TG, Nguyen A-H, Naganathan V, et al. Changes in reproductive hormone concentrations predict the prevalence and progression of the frailty syndrome in older men: the concord health and ageing in men project. J Clin Endocrinol Metabol. 2011;96(8):2464–74.
Cawthon PM, Ensrud KE, Laughlin GA, et al. Sex hormones and frailty in older men: the osteoporotic fractures in men (MrOS) study. J Clin Endocrinol Metabol. 2009;94(10):3806–15.
Mohr BA, Bhasin S, Kupelian V, Araujo AB, O’Donnell AB, McKinlay JB. Testosterone, sex hormone-binding globulin, and frailty in older men. J Am Geriatr Soc. 2007;55(4):548–55.
Tajar A, O’Connell MDL, Mitnitski AB, et al. Frailty in relation to variations in hormone levels of the hypothalamic-pituitary-testicular axis in older men: results from the European male aging study. J Am Geriatr Soc. 2011;59(5):814–21.
Hyde Z, Flicker L, Almeida OP, et al. Low free testosterone predicts frailty in older men: the health in men study. J Clin Endocrinol Metabol. 2010;95(7):3165–72.
Kenny AM, Kleppinger A, Annis K, et al. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels, low bone mass, and physical frailty. J Am Geriatr Soc. 2010;58(6):1134–43.
Srinivas-Shankar U, Roberts SA, Connolly MJ, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metabol. 2010;95(2):639–50.
Permpongkosol S, Tantirangsee N, Ratana-olarn K. Treatment of 161 men with symptomatic late onset hypogonadism with long-acting parenteral testosterone undecanoate: effects on body composition, lipids, and psychosexual complaints. J Sex Med. 2010;7(11):3765–74.
Wittert GA, Chapman IM, Haren MT, Mackintosh S, Coates P, Morley JE. Oral testosterone supplementation increases muscle and decreases fat mass in healthy elderly males with low-normal gonadal status. J Gerontol A Biol Sci Med Sci. 2003;58(7):618–25.
Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363(2):109–22.
Cook NL, Romashkan S. Why do we need a trial on the effects of testosterone therapy in older men? Clin Pharmacol Ther. 2011;89(1):29–31.
Disclaimer The opinions expressed by the author are his alone and do not reflect those of Eli Lilly & Company.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Donatucci, C.F. (2013). The Institute of Medicine White Paper on Testosterone: Current Perspective. In: Hellstrom, W. (eds) Androgen Deficiency and Testosterone Replacement. Current Clinical Urology. Humana Press, Totowa, NJ. https://doi.org/10.1007/978-1-62703-179-0_1
Download citation
DOI: https://doi.org/10.1007/978-1-62703-179-0_1
Published:
Publisher Name: Humana Press, Totowa, NJ
Print ISBN: 978-1-62703-178-3
Online ISBN: 978-1-62703-179-0
eBook Packages: MedicineMedicine (R0)