Abstract
Extirpation and transplantation of endocrine glands are among the earliest tools in experimental endocrinology. The testes, with their exposed position, are vulnerable and easily accessible to external manipulation, including trauma and forceful removal. Thus, the effects of testosterone and its absence as a consequence of loss of the testes became known quite early in the history of mankind. Castration, leading to a loss of virility and fertility, has been used over centuries for punishment of criminal acts and high treason, for the creation of obedient slaves and servants and for preserving the prepubertal soprano voices for musical entertainment. Although the testes were correctly identified as the source of androgenicity, attempts to use them in organotherapy can only have led to placebo effects. Testosterone itself was isolated only in 1935 and, since then, has been available as a powerful medication for the treatment of hypogonadism. Only recently has it become possible to produce injectable or transdermal testosterone preparations generating serum levels in the physiological range.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Testosterone
- Testosterone pellets
- Testosterone suppositories
- Testosterone tablets
- Testosterone propionate
- Testosterone enanthate
- Testosterone buciclate
- Testosterone undecanoate
- Transdermal testosterone
- Mesterolone
- 17α-methyl testosterone
- Castration
- Transplantation
- Castrati singers
- Organotherapy
- Eunuchs
“The Oldest Key to the Endocrine Treasure Trove : The Testicles”
With that phrase Victor C. Medvei [37], the master historian of endocrinology, highlights the role played by the testes in unravelling mankind’s knowledge of endocrine functions. The testes, in their exposed position, are vulnerable and easily accessible to manipulation including both accidental trauma and forceful removal. Loss of virility and fertility are easily recognizable, not only by physicians but also by laymen, so that the results of lost testicular function have been known since antiquity and long before the discovery of sperm and their function in the seventeenth and eighteenth century, and long before testosterone, as the active agent, was isolated and synthesized in the twentieth century.
This chapter describes how knowledge about androgenic functions of the testes evolved, that detours and blind alleys that were taken toward the actual discovery of testosterone and finally, how testosterone preparations have been developed for clinical use. An earlier historical report can aid as a supplement to this chapter [45].
Effects of Testis Removal
In Greek mythology, Chronos (Saturn) castrated his father Uranos (Zeus) because he did not allow him to procreate with his mother Gea, where upon Chronos’ testes fell into the sea, causing a gigantic foaming, from which Aphrodite (Venus) was born, already indicating the magic powers attributed to the testes in ancient times (depicted masterly by Giorgio Varsari [1511–1574] in a fresco in the Palazzo Vecchio, Florence). In hellenistic times (fourth to first century B.C.) the power attributed to the testes is also reflected in the cult devoted to Diana Ephesina. Seeking favors, good luck, health, and fertility, worshipers affixed the testes of sacrificed bulls to wooden statutes of the goddess. In some places, effigies of the so-decorated goddess were made of marble and colored. Some of these statutes have survived to modern times as exhibited in the Archeological Museum of Naples (Italy) (Fig. 1.1). It was clarified quite recently that the bull testes were originally mistaken for supernumerary breasts as signs of the goddess of fertility .
At the Chinese imperial court, documented since the Ming dynasty (1368–1644), eunuchs were not only custodians of the harem, but they obtained high-ranking political positions as exemplified by Admiral Zhèng Hé (1371–1435), leader of seven large expeditions into countries around the Indian and Pacific oceans, or Lin Yin (1451–1510), who is still considered among the richest people in history. The last imperial eunuch, Sun Yaoting, died in 1996 at the age of 94. Castration had previously been used to produce obedient slaves who are loyal to their masters and rulers. Over the centuries in Islamic societies castrated slaves, who were predominantly imported from Subsahara Africa as well as from Europe and Asia, were the work force and constituted elite troops who were deployed in wars of conquest.
Castration has also been used as lawful punishment. In medieval Scandinavia high treason was not subject to capital punishment but rather to castration combined with blinding. This was adopted by the Normans who introduced this custom wherever they ruled [70]. After the invasion of England in 1066, William the Conqueror largely abolished the Anglo-Saxon death penalty and replaced it with castration and blinding: “I also forbid that anyone shall be slain or hanged for any fault, but let his eyes be put out and let him be castrated”.
For other, more benign purposes, castration was carried out prior to puberty in young boys to maintain the high pitch so that soprano and alto voices resulted with the acoustic volume an adult male. They were featured in operas of the seventeenth and eighteenth centuries, such as those composed by Georg Friedrich Händel (1685–1779) or Nicola Porporra (1686–1768). Such voices could be heard until the twentieth century in the Vatican choirs. Some of those castrated became famous soloists, such as Carlo Farinelli (1705–1782) or Domenico Annibaldi (1705–1779). The last castrato, Alessandro Moreschi, born in 1858, died in 1922 and left behind the only recordings of the castrato voice as a collection of arias he sang in the Vatican .
Surgeons in the Italian cities of Norcia and neighbouring Preci, secluded in the Sibellini Mountains in Umbria, specialized on delicate operations including castration of young boys. Going back to the thirteenth century, 30 family dynasties formed the Scuola Chirurgica di Preci [17] and monopolized the trade there, guaranteing utmost secrecy concerning this operation which had been forbidden by church law under Pope Benedict IV (1675–1758) and reinforced by Pope Clement XIV (1705–1774), although the Vatican itself was one of the foremost employers of castrated singers. The operation, carried out without anesthesia and under deplorable hygienic conditions, in all probability cost the lives of hundreds of boys, however—if the procedure was successful—chances for a lucrative career compensated for the risks. Because of their high-pitched voices, but with a significantly larger volume than in women, castrati were in high demand for opera performances.
The skills of the surgeons in Preci and Norcia have been adopted by local butchers working on animal “models”, advertising for “agnello castrato” as their speciality (Fig. 1.2). The Scuola Chirurgica di Preci was also famous for other surgical procedures such as cataract extraction, and the surgeons were in demand throughout Europe. Cesare Scacchi (born 1555) was called to the English Court to operate on Queen Elizabeth I in 1588, and successfully removed her opaque lenses. Modest museums in the town hall of Preci and the San Eutizio Monastery nearby give a flavor of this fascinating piece of medical history and exhibit some of the tools used by the surgeons [21].
Those early castrates served in what can be considered a posthumous clinical trial to test the hypothesis that testosterone shortens male life expectancy: a comparison of the lifespan of 50 sixteenth to nineteenth-century castrates with 50 contemporary intact singers demonstrated not only the stressful lives both these groups of artists had to endure, but also revealed no difference in their life expectancy [51]. In contrast, the lives of eunuchs at the imperial court of the Korean Chosun Dynasty (1392–1910) showed a longer lifespan for the eunuchs than for normal men at the time [39]. However, the Korean eunuchs were castrated as adults and spent their life in a well-protected environment shielded from the hostile outside world. In addition, the time point of the loss of the testes in the castrati singers and the Korean eunuchs may also account for the difference .
Lessons from Experimental Testis Transplantation
While removal of endocrine glands is one basic concept of experimental endocrinology, replacing the glands is the other. As a surgeon in the Seven Years’ War (1756–1763), John Hunter (1728–1793) saw the need for transplantation of organs and limbs. This stimulated his experiments of transplanting testes from cocks to hens, thereby demonstrating the “vital principle” of living organs. Far from any endocrine belief, his goal was to demonstrate the survival of the transplant resulting from nerve growth, with the intention of replacing limbs and organs in wounded soldiers. Thus Hunter, among many other achievements, can be considered as one of the fathers of modern transplant surgery.
At the University of Göttingen Arnold Adolph Berthold (1803–1861) (Fig. 1.3) also used chickens as an experimental model. In 1849 he observed and published that testes transplanted from roosters to capons restored androgenic functions: “[The transplanted capons] crowed quite considerably, often fought among themselves and with other young roosters and showed a normal inclination to hens.” He concluded that these effects “must be affected through the productive relationship of the testes, that is to say, through their action on the blood, and then through the suitable ensuing action of the blood on the organism as a whole” [10]. He was thus the first to postulate a humoral effect of the testes (and of an endocrine gland in general) on distant organs. At the same time, Franz Leydig (1821–1908), at the University of Würzburg, described the interstitial Leydig cells in the testes in many species, without, however, knowing their real function and importance [34].
It would take another 50 years until an endocrine function was clearly attributed to the Leydig cells, when Ancel and Bouin [1] summarized their conclusions from extensive experimentation as follows: “In numerous previous studies we have assembled a group of morphological, physiological and chemical facts that, taken together, allow us to formulate the following hypothesis: that the general action of the testes on the organism, ascribed in the past to the testes as a whole, is actually due to the interstitial gland” (translated by [14]). They did not (yet) use the terms “hormone” or hormone action which would have been appropriate for their findings, as this term was coined and first published only a year later by Ernest Starling [65] in London.
Berthold’s experiments were initially not accepted by the scientific community—including his own department director Rudolf Wagner (1805–1864)— until Moritz Nussbaum at the University of Bonn in 1908 [54] and A. Pézard in Paris in 1910 [56] repeated and confirmed Berthold’s results using frogs and chickens, respectively.
Erroneous Conclusions from Berthold’s Experiments
Possibly prompted by these findings, surgeons turned to testes transplantation as a means to treat hypogonadism and bring about rejuvenation and therapy for all types of disorders. George Frank Lydston (1858–1923) at Cook County Hospital in Chicago was one of the first to perform human testicular transplantation from accident victims to recipients [36]. Also in Chicago, Victor D. Lespinase (1878–1946) published his experience with transplanting human testes from donors to patients for rejuvenation [33] and Leo Stanley (1886–1976), at the California State Prison San Quentin, reported 20 cases of transplantation of testes from executed prisoners to other inmates who reported signs of revitalization. Stanley later turned to rams as sources for his testicular grafts and reported satisfaction on the part of the patients, which included 13 physicians [63, 64].
John Romulus Brinkly (1885–1942), a half-educated medic, turned goat testis transplantation in his clinic in Milford, Kansas, into a booming business between 1918 and 1930. However, in 1939 he was found guilty by a Texas judge of acting as a charlatan and quack, thus unleashing a series of lawsuits demanding millions of dollars as compensation. Brinkly declared bankruptcy and died of a heart attack soon thereafter.
In Vienna, Eugen Steinach (1861–1944) performed vasoligation for rejuvenation [66]. One of his followers, Serge Voronoff (1866–1951) turned to xenotransplantation and used monkey testes to be transplanted for rejuvenation [73]. He first offered the surgery in Paris, but after several scandals, continued his questionable operations in Algiers, where he was visited by patients from all over the world. Voronoff’s followers xenotransplanted animal testes and pieces thereof to patients demanding rejuvenation in many countries of the world. When unrest among the medical profession grew regarding this quackery, the Royal Society of Medicine (London) sent an international committee to Voronoff in Algiers in 1927. The committee concluded their investigations by declaring Voronoff’s claims as unsubstantiated.
Those scandals and the hope that steroid biochemistry would ultimately lead to the discovery and synthesis of the male sex hormone, following that of female sex hormones, finally terminated the questionable business of testes transplantation. However, before the chapter of modern testosterone biochemistry and pharmacology can be opened, another century-long medical misapprehension must be discussed.
Testes for Organotherapy
Knowledge of the powerful function of the testes in the normal male organism induced patients and healers to turn to the ingestion of these organs in various modalities over the centuries. In Rome, Gaius Plinius Secundus (23–79) prescribed the consumption of animal testes for the treatment of symptoms of hypogonadism and impotence. For the same purpose in Baghdad, the Arabic physician Mesue the Elder (777–837) recommended testis extracts. Additionally, in Chinese medicine—at least since 1132—Hsue Shu-Wei prescribed raw and desiccated animal testes. The “Universal Doctor” and founder of the University of Cologne, Albertus Magnus (1192–1280), concerned with the taste of his prescription, recommended powdered hog testes in wine as an alternate method of consumption [37].
Charles-Edouard Brown-Séquard (1847–1894), the well-known scientist and member of several high-ranking learned societies, gave organotherapy a new dimension when, at the age of 72, he published the results of his dubious self-experimentation in the Lancet [11]. He self-injected a mixture of testicular vein blood, semen and juice extracted from dog or guinea-pig testes and observed signs of rejuvenation, which, at best, must have been placebo effects, because the testes synthesize testosterone, but do not store it (as e.g. the thyroid does with its hormones), and the amount administered was minute [18]. However, “extracts of animal organs by the Brown-Séquard method ” were immediately sold worldwide and factories sprang up in Europe as well as in the United States. This elixir soon became not only the source of high revenues, but also the object of ridicule in comics and songs as demonstrated by “The greatest comic song of the day” (words and music by J. Winchell Forbes, 1889)
“Undertakers, wigmakers, and gravediggers swear,
Till the air with their curses is blue.
At the man who invented Elixir Séquard,
And left them with nothing to do.
And even the doctors are rattled at last,
For when their best patients are sick, sir,
They just step around to the corner drug store,
And “shake” for a dose of “Elixir”.
The elixirs were consumed by everyday patients as well as celebrities and were even used for doping in sports, as exemplified by Pud Galvin (1856–1902). The American National Association baseball pitcher was the first Major League 300-game-winner (elected to the Baseball Hall of Fame in 1965) who attributed his final successes to the Brown-Séquard elixir.
The craze for these products caused concern about the overall image of the relatively new field of endocrinology. Harvey W. Cushing (1869–1939), a famous neurosurgeon, went so far as to discuss “endocriminology” in the context of this organotherapy . Nevertheless, many companies worldwide continued to manufacture extracts and pills, well into the period of time when genuine testosterone was already long on the market. It was not until 1961 that Ciba (Switzerland) withdrew Androstin® (= “biologically titrated full extract from male gonads” for oral and parenteral use) from the market, after three decades of successful sales for the treatment of “male gonadal insufficiency, impotence, infantilism, premature aging and endocrine obesity ” [58].
Isolation and Synthesis of Testosterone
The pharmaceutical industry, finally reacting to the hype generated by testicular transplantation and organotherapy , started cooperating with academic research to rehabilitate endocrinology and replace organotherapy with proper hormone substitution. In 1935, the era of testosterone emerging as a biochemical and marketable entity, Ciba (Switzerland) and Schering (Germany), pharmaceutical companies both active in the field, began a cooperative effort to inform each other about progress and forced their academic protagonists Leopold Ruzicka (1887–1976) at the Technical University of Zürich and Adolf Butenandt (1903–1995) at the University of Göttingen, to exchange their respective advances, to which the former rivals reluctantly agreed. In 1937 the Ciba-Schering cooperation was extended to include Boehringer (Germany), Chimio Roussel (France), and Organon (The Netherlands) to form a syndicate to share knowledge, to stake their market claims worldwide, and to agree on product pricing [58].
Important steps in the isolation of testosterone were the development of biological tests for androgen activity. Loewe and Voss [35] described androgenic activity in urine and developed the Loewe-Voss-Test for measuring androgenic activity. Moore et al. [40] refined and standardized the capon comb test as the unity of androgenicity. That biological test helped to resolve the question of whether only one or several androgenic steroids existed and, if more than one—which might be the more potent.
In 1931 Adolf Butenandt isolated the androgenic steroid androsterone (androstan-3α-ol-17-one) from 15,000 l of urine provided by young policemen from Berlin and then processed by Schering to obtain 15 mg of this first androgen [12]. In 1935 Ernst Laqueur (1866–1947) and his group at Organon and the University of Amsterdam extracted and isolated 10 mg of testosterone (17β-hydroxy-4-androstene-3one) from 100 kg of bull testes and found to be more active than androsterone in biological tests [19]. They called the hormone “testosterone”. In the same year Butenandt and Hanisch [13] in Göttingen, (Fig. 1.4) as well as Ruzicka and Wettstein [60] in Zurich/Basel (Fig. 1.5), released the chemical synthesis of testosterone, marking the beginning of modern clinical pharmacology of testosterone and male reproductive physiology.
The synthesis of testosterone from dehydroandrosterone as described and shown by Butenandt and Hanisch in their original paper in 1935 [13]
Degradative synthesis of testosterone (43) and 17α-methyltestosterone (45) as described by Ruzicka and Wettstein in 1935. Interestingly, in his autobiography Ruzicka [59] considered the synthesis of 17α-methyltestosterone as “a greater intellectual achievement” than that of testosterone
The close cooperation of the researchers reinforced by the two pharmaceutical companies may explain why these discoveries were released at approximately the same time. Marius Tausk, one of the former heads of research at Organon who knew the competing protagonists personally, very vividly describes the race to testosterone isolation and synthesis and how the key respective papers were submitted for publication in short sequence in 1935 [68]. In 1939, Butenandt and Laqueur jointly received the Nobel Prize for chemistry, Butenandt “for his work on sex hormones” (estrogen, progesterone, and androsterone) and Ruzicka “for his work on polymethylenes and higher terpens”, guiding him to the androgens. Why Laqueur’s contribution was not recognized remains unclear. The Nazi regime prevented Butenandt from accepting the Nobel Prize and he received it only after the end of World War II.
The research work was financially rewarding beyond scientific recognition, as Ruzicka wrote in autobiographical notes: “The patents for the degradative synthesis of testosterone and methyltestosterone earned me during subsequent years an enormous (compared with my professorial standard) amount of money as royalties from Ciba in Basel and Ciba in the USA.” [59]. During 1939 alone, Ciba transferred 56,744 Swiss Francs to Ruzicka as royalties [58]. Part of this money was invested in a collection of seventeenth century Flemish and Dutch paintings that Ruzicka donated to the Kunsthalle Zürich in 1947 [59].
However, while this pioneering work was well recognized in science and by pharmaceutical companies, clinicians were skeptical about whether the early preparations of testosterone would contain enough of the hormone to produce any biological and clinical effects, as documented in a textbook of endocrinology at the time: “The number of testis hormone preparations is still very low. Comprehensive clinical investigations are not yet available, determining the doses for human therapy. It is therefore unknown whether the available preparations can be administered in sufficient concentrations” [31]. Their skepticism was soon to be defeated by the chemists’ continued efforts and skills.
Evolution of Testosterone Preparations for Clinical Use
Soon after its synthesis it became clear that in reasonable doses, testosterone was not effective orally or—as we know today—would require extremely high doses that were simply not available or were too expensive. We currently know that the lack of oral effectiveness is a result of the inactivation of testosterone by the first-pass effects in the liver (Fig. 1.6) [48, 50]. Three approaches were used to overcome this problem:
Serum testosterone levels in normal volunteers after ingestion of either 65 mg crystalline testosterone (lower lines) or 100 mg testosterone undecanoate capsules (equivalent testosterone doses) (adapted from [48])
-
1.
Chemical modification of the steroid molecule,
-
2.
Parenteral application, and
-
3.
Esterification in position 17β of the testosterone molecule.
For a complete description of the many testosterone preparations and routes of administration, the reader is referred to reviews by Nieschlag and Behre [43] and Behre and Nieschlag [5] and to respective chapters in this text.
As early as 1935, the year when testosterone was first isolated and synthesized, Ruzicka et al. [61] also synthesised 17α-methyl-testosterone (Fig. 1.5). Its oral effectiveness was demonstrated and it was soon licensed for clinical use and was well accepted because of the ease of oral application [22]. However, liver toxicity because of the 17α-structure of this molecule soon became evident, particularly with long-term use and at higher doses [79]. It further became clear that the toxic effect would be shared by all 17α-substituted androgens [46], thus giving testosterone in general a bad name among physicians. In the 1980s, however, 17α-methyl-testosterone became obsolete for clinical use—at least in Europe, when another orally effective preparation free of toxic side effects became available (see below).
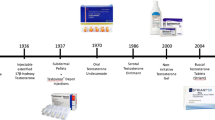
As testosterone proved to be orally ineffective, parenteral routes were explored. Subdermal testosterone pellet implants were the first to be investigated [20] and pellets are still in use today [5]. They were manufactured by Organon (The Netherlands) until 2007, when the company was taken over by Schering Plough (USA) and then until 2009 when Schering Plough was bought by Merck, Sharp & Dohme/MSD (USA). Their application requires a small surgical procedure and harbors the risk of infection and extrusion. However, if enough pellets are implanted they may provide substitution for upto six months. The pellets are predominantly currently used in Australia.
Other parenteral routes were tested in the course of the steroid’s existence during which testosterone suppositories were marketed by Ferring [23], but yielded rather unpredictable serum levels [49] and are no longer commercially available. The most recent development in this area is bioadhesive buccal testosterone tablets , placed on the gingiva and resulting in effective serum levels if applied twice daily [76]. However, because of low patient compliance they have never penetrated the market.
When injected, testosterone has an extremely short half-life of only 10 min and as such is not suitable for substitution. Therefore, the third possibility for making testosterone clinically effective is esterification at the 17β-hydroxy-group of the molecule, making it suitable for intramuscular injection. Testosterone propionate was the first of these esters marketed by Ciba as Perandren® and by Schering as Proviron® in 1936. However, this ester has a short half-life and effective serum levels are reached for only 1–2 days. When mesterolone (1α-methyl-5α-androstan-17βol-one) became available, Schering used the then well-established name Proviron® for this new oral preparation [24, 41] and continued to market testosterone proprionate as Testoviron® . Because mesterolone is not aromatizable it has little effect on gonadotropins, bones, and other estrogen-dependent functions and could not be used for full substitution of hypogonadism, but was mainly utilized for male infertility treatment—without evidence-based proof of its effectiveness for that indication [28].
Following the propionate ester, testosterone enanthate was synthesised by Junkmann [26, 27] at Schering and marketed as intramuscular Testoviron® Depot injection in 250 mg doses, providing substitution for 2–3 weeks [49]. However, the pharmacokinetics are characterized by transient supraphysiological peaks for a few days, followed by slow decline to levels below the lower limit of normal. Although patients do not appreciate the up and down mood swings and libido between injections, this remained the major testosterone preparation for substitution of hypogonadism for half a century.
In the late 1950s and 1960s, instead of improving modes of application, the pharmaceutical industry became more interested in the chemical modification of the testosterone molecule in order to disentangle its various effects and produced predominantly erythropoietic or anabolic androgenic steroids (AAS ). Although hundreds of androgens were synthesised, it proved impossible to produce androgens with only one desired effect out of the wide spectrum of testosterone activities. Nevertheless, while some AAS were applied clinically, they disappeared again in the wake of evidence-based medicine. They did, however, retain a shadow existence for doping in sports and bodybuilding, potentially causing considerable undesired effects [46, 47]. Regrettably, at that time the pharmaceutical industry ignored the needs of hypogonadal patients, as pharmacokinetic studies had revealed that the existing testosterone preparations resulted in unphysiologically high or low serum levels, not desirable for substitution purposes so that the World Health Organization (WHO) made an appeal for more physiologic modalities of substitution [80].
Unfortunately, the pharmaceutical industry also turned a blind eye on another potentially huge application of testosterone: hormonal male contraception. In the 1970s the WHO Human Reproduction Program and the Population Council of the Rockefeller Foundation had identified male contraception as an unmet need for family planning and as a means against global overpopulation. Hormonal male contraception, a combination of testosterone and a progestin, was at that time—and remains so to date—the most likely candidate for general use. However, the existing testosterone preparations required too frequent applications (for review of clinical trials see [42]). To overcome this deficiency both organizations started programs in search of long-acting testosterone preparations. Under the auspices of the WHO, testosterone buciclate was synthesised [16] and identified as a long-acting preparation, well suited for male contraception—and by the same token, also for substitution [7]. However, no pharmaceutical company could be inspired to further develop this promising preparation [74], so in its ensuing clinical trials for male contraception, the WHO switched to intramuscular testosterone undecanoate as described below [8].
Meanwhile, the Population Council had turned to 7α-methyl-19-nortestosterone ( MENT ) as its preferred androgen for male contraception. This androgen might have the advantage of lacking conversion to DHT (dihydrotestosterone) and thereby have little effect on the prostate. Because MENT has a short half-life, it was administered in subdermal silastic implants, delivering the active substance for a year—or perhaps even longer—thus being well suited for contraception in addition to substitution ([53, 67, 72]). However, the company, although interested in further clinical research with this androgen, dropped its plans in the wake of being taken over by another company not interested in continuing the research.
In the late 1970s the orally effective testosterone undecanoate , absorbed from the gut via the lymph to avoid the first-pass effect in the liver (Fig. 1.6) [15, 25], had been added to the spectrum of testosterone preparations available for replacement therapy. Peter Kicovic, in charge of clinical development at Organon at the time, approached the senior author for first clinical trials with the new substance as he had developed a radioimmunoassay for testosterone and was able to measure testosterone levels in small serum volumes lending themselves to pharmacokinetic studies [44]. Because the assay and the testosterone-antiserum produced for the assay [69] were widely used and quoted, both papers became citation classics in 1982. While initial clinical testing revealed that oral testosterone undecanoate was best absorbed with food, the testosterone peaks were short-lived so that 3–4 capsules had to be taken during the day [48, 62]. Oral testosterone undecanoate was introduced to the market worldwide (except in the USA) in the late 1970s under the brand name Andriol®. More recently, an oral self-emulsifying delivery system for testosterone undecanoate has shown better pharmacokinetics than testosterone undecanote in arachis oil [81] and as such, may become acceptable to the FDA.
In the mid-1990s, transdermal testosterone films applied to the scrotal skin became the first transdermal testosterone preparation in clinical use. Invented by Virgil Place (1924–2012) at ALZA in Palo Alto, California and first tested in clinical trials in Münster [3, 4, 57], they showed excellent pharmacokinetic and clinical results and, for the first time, physiological testosterone serum levels could be achieved (Fig. 1.7). Patients were satisfied with this physiological pharmacokinetic profile, as long-term substitution revealed [2, 9]. However, physicians were reluctant to prescribe a medication to be applied to the scrotum, preferring a subsequently developed non-scrotal testosterone system , Androderm® [38]. That, however, caused unpleasant skin reactions because it required an enhancer to drive testosterone through the skin. For that reason the advent of the first transdermal testosterone gel in 2000 was welcomed for substitution [75]. Of the various gels available currently, the one with the highest testosterone concentration (2.5 % Testotop® ) can be washed off the skin shortly after application, thereby reducing the danger of contaminating children or women. It has also been tested for scrotal application. Because of the high absorptive capacity of scrotal skin, only 20 % of the gel needed for non-scrotal application is required, making this form of application economically and ecologically desirable [32].
First clinical trial of transdermal testosterone: serum testosterone values in seven hypogonadal men following the scrotal application of a transdermal testosterone film (adapted from [3])
Finally, in 2004, the intramuscular testosterone undecanoate preparation entered the market and soon achieved great popularity as a real depot preparation. Testosterone undecanoate, originally used in oral capsules, as mentioned previously, had been turned into an injectable preparation by Chinese investigators using tea seed oil as a vehicle [77]. When the authors came across it at a meeting in Bejing in 1993 (Fig. 1.8), samples were brought to Germany, injected into monkeys and showed a surprisingly long half-life [55]. In a further study in monkeys, the kinetics and biological effects of intramuscular testosterone undecanoate were compared with those of testosterone enanthate and with testosterone buciclate , a preparation duly synthesized under WHO auspices primarily for male contraceptive purposes as discussed above, further demonstrating the superior properties of intramuscular testosterone undecanoate [78]. The long half-life and serum levels, consistently in the physiological range, could be confirmed in volunteering hypogonadal men who all showed serum levels in the normal range for several weeks [6]. Several pharmaceutical companies were contacted for further development of the preparation, however, although all were convinced of the excellent pharmacokinetic properties, none were interested in taking it on board, probably disregarding its potential because male hypogonadism was too small an indication for a financial commitment, and the aging male had not yet been “discovered”. When Jenapharm became interested in this fascinating preparation, tea seed oil was replaced by castor oil as a vehicle and the injection intervals could be extended to 12 weeks of physiological serum testosterone levels [6, 9, 52, 71].
Meanwhile, the new testosterone preparation had also been tested in trials for hormonal male contraception and had proved to be effective in combination with norethisterone enanthate [29, 30]. Our 10-year experience with intramuscular testosterone undecanoate has been summarized [82] and has demonstrated that the CAG repeat polymorphism in exon 1 of the androgen receptor influences the pharmacokinetic and biological effects of testosterone and may thus provide a clue to a personalized administration of testosterone.
In 2004, intramuscular testosterone undecanoate was first licensed in Germany under the trade name of Nebido® and licensing in over 100 countries followed either under this brand name or as Reandron® . The latest approval came from the FDA in 2014, licensing ampules of 750 mg testosterone undecanoate under the brand name Aveed® for the treatment of hypogonadism in the USA.
References
Ancel P, Bouin P. Recherches sur la signification physiologique de la glande interstitielle du testicule des mammifères. II. Role de la glande interstitielle chez l’embryon, les sujets jeunes et agés; ses variations fonctionelle. J Physiol Pathol Gén. 1904;6:1039–50.
Atkinson LE, Chang YL, Snyder PJ. Long-term experience with testosterone replacement through scrotal skin. In: Nieschlag E, Behre HM, editors. Testosterone: action, deficiency, substitution. 2nd ed. Heidelberg: Springer; 1998. p. 365–88.
Bals-Pratsch M, Knuth UA, Yoon YD, Nieschlag E. Transdermal testosterone substitution therapy for male hypogonadism. Lancet. 1986;2:943–6.
Bals-Pratsch M, Langer K, Place VA, Nieschlag E. Substitution therapy of hypogonadal men with transdermal testosterone over one year. Acta Endocrinol (Copenh). 1988;118:7–13.
Behre HM, Nieschlag E. Testosterone preparations for clinical use in males. In: Nieschlag E, Behre HM, Nieschlag S, editors. Testosterone: action, deficiency, substitution. 4th ed. Cambridge: Cambridge University Press; 2012. p. 309–35.
Behre HM, Abshagen K, Oettel M, Hubler D, Nieschlag E. Intramuscular injection of testosterone undecanoate for the treatment of male hypogonadism: phase I studies. Eur J Endocrinol. 1999;140:414–9.
Behre HM, Baus S, Kliesch S, Keck C, Simoni M, Nieschlag E. Potential of testosterone buciclate for male contraception: endocrine differences between responders and nonresponders. J Clin Endocrinol Metab. 1995;80:2394–403.
Behre HM, Zitzmann M, Anderson RA, Handelsman DJ, Lestari SW, McLachlan RI, Meriggiola MC, Miso MM, Noe G, Wu FCW, Festin MPR, Habib NA, Vogelsong KM, Callahan MM, Linton KA, Colvard DS. Efficacy and safety of an injectable combination hormonal contraceptive for men. J Clin Endocrinol Metab. 2016 PIMD 27788052.
Behre HM, von Eckardstein S, Kliesch S, Nieschlag E. Long-term substitution therapy of hypogonadal men with transscrotal testosterone over 7-10 years. Clin Endocrinol (Oxf). 1999;50:629–35.
Berthold AA. Über die transplantation der Hoden. Arch Anat Physiol Wiss Med. 1849;16:42–6.
Brown-Séquard E. The effects produced on man by subcutaneous injections of a liquid obtained from the testicles of animals. Lancet. 1889;20:105–7.
Butenandt A. Über die chemische Untersuchung des Sexualhormons. Z Angew Chem. 1931;44:905–8.
Butenandt A, Hanisch G. Über Testosteron. Umwandlung des Dehydroandrosterons in Androstendiol und Testosteron; ein Weg zur Darstellung des Testosterons aus Cholesterin. Hoppe-Seyler Z Physiol Chem. 1935;237:89–98.
Christensen AK. A history of studies on testicular Leydig cells: the first century. In: Payne AH, Hardy MP, Russell LD, editors. The Leydig cell. Vienna, IL: Cache River Press; 1996.
Coert A, Geelen J, de Visser J, van der Vies J. The pharmacology and metabolism of testosterone undecanoate (TU), a new orally active androgen. Acta Endocrinol (Copenh). 1975;79:789–800.
Crabbé P, Archer S, Benagiano G, Diczfalusy E, Djerassi C, Fried J, Higuchi T. Long-acting contraceptive agents: design of the WHO Chemical Synthesis Programme. Steroids. 1983;41:243–53.
Cruciani GF. Cerusici e Fisici. Preciani et Nursini dal XIV al XVIII secolo. Storia e Antologia, Norcia. Edizioni THYRUS, Grafiche Millefiorini; 1999.
Cussons AJ, Bhagat CI, Fletcher SJ, Walsh JP. Brown-Séquard revisited: a lesson from history on the placebo effect of androgen treatment. Med J Aust. 2002;177:678–9.
David K, Dingemanse E, Freud J, Laquer E. Über krystallinisches männliches Hormon aus Hoden (Testosteron), wirksamer als aus Harn oder aus Cholesterin bereitetes Androsteron. Hoppe-Seyler Z Physiol Chem. 1935;233:281–2.
Deansley R, Parkes AS. Factors influencing effectiveness of administered hormones. Proc R Soc London. 1937;124:279–98.
Fabbi A. La scuola chirurgica di Preci. Spoleto: Panetto and Petrelli; 1974.
Foss GL. The oral application of methyl testosterone and its simplification of androgen therapy. Br Med J. 1939;2:11–2.
Hamburger C. Testosterone treatment and 17-ketosteroid excretion. Acta Endocrinol. 1958;28:529–36.
Hornstein O. Contribution to the long-term treatment of severe androgen deficiency with 1-α –methyl-5-α -androstan-17-β -ol-3-one (mesterolone). Arzneimittelforschung. 1966;16:466–8.
Horst HJ, Höltje WJ, Dennis M, Coert A, Geelen J, Voigt KD. Lymphatic absorption and metabolism of orally administered testosterone undecanoate in man. Klin Wochenschr. 1976;54:875–9.
Junkmann K. Über protrahiert wirksame Androgene. Arch Pathol Pharmacol. 1952;215:85–92.
Junkmann K. Long-acting steroids in reproduction. Recent Prog Horm Res. 1957;13:389–419.
Kamischke A, Nieschlag E. Analysis of medical treatment of male infertility. Hum Reprod. 1999;14 suppl 1:1–23.
Kamischke A, Venherm S, Plöger D, von Eckardstein S, Nieschlag E. Intramuscular testosterone undecanoate and norethisterone enanthate in a clinical trial for male contraception. J Clin Endocrinol Metab. 2001;86:303–9.
Kamischke A, Heuermann T, Krüger K, von Eckardstein S, Schellschmidt I, Rübig A, Nieschlag E. An effective hormonal male contraceptive using testosterone undecanoate with oral or injectable norethisterone preparations. J Clin Endocrinol Metab. 2002;87:530–9.
Kemp T, Okkels J. Lehrbuch der Endokrinologie. Leipzig: Barth; 1936.
Kühnert B, Byrne M, Simoni M, Köpcke W, Gerss J, Lemmnitz G, Nieschlag E. Testosterone substitution with a new transdermal, hydroalcoholic gel applied to scrotal or non-scrotal skin: a multicentre trial. Eur J Endocrinol. 2005;153:317–26.
Lespinase VD. Transplantation of the testicle. J Am Med Assoc. 1913;18:251–3.
Leydig F. Zur Anatomie der männlichen Geschlechtsorgane und Analdrüsen der Säugethiere. Z Wiss Zool. 1850;2:1–57.
Loewe S, Voss HE. Der Stand der Erfassung des männlichen Sexualhormons (Androkinins). Klin Wschr. 1930;9:481–7.
Lydston GF. Sex gland implantation. N Y Med J. 1915;51:601–8.
Medvei VC. The history of clinical endocrinology. MTP Press, Laucaster, 1982.
Meikle AW, Arver S, Dobs AS, Sanders SW, Rajaram L, Mazer NA. Pharmacokinetics and metabolism of a permeation-enhanced testosterone transdermal system in hypogonadal men: influence of application site - a clinical research center study. J Clin Endocrinol Metab. 1996;81:1832–40.
Min KJ, Lee CK, Park HN. The lifespan of Korean eunuchs. Curr Biol. 2012;22:R792.
Moore CR, Gallagher TF, Koch FC. The effects of extracts of testis in correcting the castrated condition in the fowl and in the mammal. Endocrinology. 1929;13:367–74.
Neumann F, Wiechert R, Kramer M, Raspé G. Experimental animal studies with a new androgen – mesterolone (1-α –methyl-5-α -androstan-17-β -ol-3-one). Arzneimittelforschung. 1966;16:455–8.
Nieschlag E. Clinical trials in male hormonal contraception. Contraception. 2010;82:457–70.
Nieschlag E, Behre HM. Clinical uses of testosterone in hypogonadism and other conditions. In: Nieschlag E, Behre HM, Nieschlag S, editors. Testosterone: action, deficiency, substitution. 4th ed. Cambridge: Cambridge University Press; 2012. p. 292–308.
Nieschlag E, Loriaux DL. Radioimmunoassy for plasma testosterone. Z Klin Chem Klin Biochem. 1972;10:164.
Nieschlag E, Nieschlag S. Testosterone deficiency: a historical perspective. Asian J Androl. 2014;16(2):161–8.
Nieschlag E, Vorona E. Medical consequences of doping with anabolic androgenic steroids (AAS): effects on reproductive functions. Eur J Endocrinol. 2015;173:R47–58. doi:10.1530/EJE-15-0080.
Nieschlag E, Vorona E. Doping with anabolic androgenic steroids (AAS): adverse effects on non-reproductive organs and functions. Rev Endocr Metab Disord. 2015;16:199.
Nieschlag E, Mauss J, Coert A, Kicovic P. Plasma androgen levels in men after oral administration of testosterone or testosterone undecanoate. Acta Endocrinol. 1975;79:366–74.
Nieschlag E, Cüppers HJ, Wiegelmann W, Wickings EJ. Bioavailability and LH-suppressing effect of different testosterone preparations in normal and hypogonadal men. Horm Res. 1976;7:138–45.
Nieschlag E, Cüppers EJ, Wickings EJ. Influence of sex, testicular development and liver function on the bioavailability of oral testosterone. Eur J Clin Invest. 1977;7:145–7.
Nieschlag E, Nieschlag S, Behre HM. Life expectancy and testosterone. Nature. 1993;366:215.
Nieschlag E, Büchter D, von Eckardstein S, Abshagen K, Simoni M, Behre HM. Repeated intramuscular injections of testosterone undecanoate for substitution therapy in hypogonadal men. Clin Endocrinol (Oxf). 1999;51:757–63.
Nieschlag E, Kumar N, Sitruk-Ware R. 7α-methyl-19-nortestosterone (MENTR): the population council’s contribution to research on male contraception and treatment of hypogonadism. Contraception. 2013;87:288–95.
Nussbaum M. Hoden und Brunstorgane des braunen Landfrosches (Rana fusca). Pflügers Arch Eur J Physiol. 1909;126:519–77.
Partsch CJ, Weinbauer GF, Fang R, Nieschlag E. Injectable testosterone undecanoate has more favourable pharmacokinetics and pharmacodynamics than testosterone enanthate. Eur J Endocrinol. 1995;132:514–9.
Pézard A. Sur la détermination des caractères sexuels secondaires chez les gallinacés. Cpt Rend Scienc. 1911;153:1027.
Place VA, Atkinson L, Prather DA, Trunnell N, Yates FE. Transdermal testosterone replacement through genital skin. In: Nieschlag E, Behre HM, editors. Testosterone: action, deficiency, substitution. Berlin: Springer; 1990. p. 165–81.
Ratmoko C. Damit die Chemie stimmt. Die Anfänge der industriellen Herstellung von weiblichen und männlichen Sexualhormonen 1914-1938. Zürich: Chronos; 2010.
Ruzicka L. In the borderland between bioorganic chemistry and biochemistry. Ann Rev Biochem. 1973;42:1–20.
Ruzicka L, Wettstein A. Synthetische Darstellung des Testikelhormons Testosteron (Androsten 3-on-17-ol). Helv Chim Acta. 1935;18:1264–75.
Ruzicka L, Goldberg MW, Rosenberg HR. Herstellung des 17alpha-Methyl- testosterons und anderer Androsten- und Androstanderivate. Zusammenhänge zwischen chemischer Konstitution und männlicher Hormonwirkung. Helv Chim Acta. 1935;18:1487–98.
Schürmeyer T, Wickings EJ, Freischem CW, Nieschlag E. Saliva and serum testosterone following oral testosterone undecanoate administration in normal and hypogonadal men. Acta Endocrinol (Copenh). 1983;102:456–62.
Stanley LL. Experiences in testicle transplantation. Cal State J Med. 1920;18:251–3.
Stanley LL. An analysis of one thousand testicular substance implantations. Endocrinology. 1923;7:787–94.
Starling EH. The chemical correlation of the functions of the body I. Lancet. 1905;2:339–41.
Steinach E. Verjüngung durch experimentelle Neubelebung der alternden Pubertätsdrüse. Berlin: J Springer Verlag; 1920.
Sundaram K, Kumar N, Bardin CW. 7-alpha-Methyl-nortesosterone (MENT): the optimal androgen for male contraception. Ann Med. 1993;25:199–205.
Tausk M. A brief endocrine history of the German-speaking peoples. In: Kracht J, von zur Mühlen A, Scriba PC, editors. Endocrinology guide: Federal Republic of Germany. Giessen: Brühlsche Universitäts-Druckerei; 1976.
Vaitukaitis J, Robbins JB, Nieschlag E, Ross GT. A method for producing specific antisera with small doses of immunogen. J Clin Endocrinol Metab. 1971;33:988–91.
Van Eickels K. Gendered violence: castration and blinding as punishment for treason in Normandy and Anglo-Norman England. Gend Hist. 2004;16:588–602.
von Eckardstein S, Nieschlag E. Treatment of hypogonadism with testosterone undecanoate injected at extended intervals of 12 weeks: phase II study. J Androl. 2002;23:419–25.
von Eckardstein S, Noe G, Brache V, Nieschlag E, Croxatto H, Alvarez F, Moo-Young A, Sivin I, Kumar N, Small M, Sundaram K, International Committee for Contraception Research, The Population Council. A clinical trial of 7 alpha-methyl-19-nortestosterone implants for possible use as a long-acting contraceptive for men. J Clin Endocrinol Metab. 2003;88(11):5232–9.
Voronoff S. Testicular grafting from ape to man. London: Brentanos Ltd.; 1920.
Waites GM. Development of methods of male contraception: impact of the World Health Organization Task Force. Fertil Steril. 2003;80(1):1–15.
Wang C, Berman N, Longstreth JA, Chuapoco B, Hull L, Steiner B, Faulkner S, Dudley RE, Swerdloff RS. Pharmacokinetics of transdermal testosterone gel in hypogonadal men: application of gel at one site versus four sites: a general clinical research center study. J Clin Endocrinol Metab. 2000;85:964–9.
Wang C, Swerdloff R, Kipnes M, Matsumoto AM, Dobs AS, Cunningham G, Katznelson L, Weber TJ, Friedman TC, Snyder P, Levine HL. New testosterone buccal system (Striant) delivers physiological testosterone levels: pharmacokinetics study in hypogonadal men. J Clin Endocrinol Metab. 2004;89:3821–9.
Wang L, Shi DC, Lu SY, Fang RY. The therapeutic effect of domestically produced testosterone undecanoate in Klinefelter syndrome. New Drug Mark. 1991;8:28–32.
Weinbauer GF, Partsch CJ, Zitzmann M, Schlatt S, Nieschlag E. Pharmacokinetics and degree of aromatization rather than total dose of different preparations determine the effects of testosterone: a non-human primate study. J Androl. 2003;24:765–74.
Werner SC, Hamger FM, Kritzler RA. Jaundice during methyltestosterone therapy. Am J Med. 1950;8:325–31.
World Health Organization, Nieschlag E, Wang C, Handelsman DJ, Swerdloff RS, Wu F, Einer-Jensen N, Waites G. Guidelines for the use of androgens. Geneva: WHO; 1992.
Yin AY, Htun M, Swerdloff RS, Diaz-Arjonilla M, Dudley RE, Faulkner S, Bross R, Leung A, Baravarian S, Hull L, Longstreth JA, Kulback S, Flippo G, Wang C. Reexamination of pharmacokinetics of oral testosterone undecanoate in hypogonadal men with a new self-emulsifying formulation. J Androl. 2012;33:190–201.
Zitzmann M, Nieschlag E. Androgen receptor gene CAG repeat length and body mass index modulate the safety of long-term intramuscular testosterone undecanoate therapy in hypogonadal men. J Clin Endocrinol Metab. 2007;92:3844–53.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Nieschlag, E., Nieschlag, S. (2017). The History of Testosterone and The Testes: From Antiquity to Modern Times. In: Hohl, A. (eds) Testosterone. Springer, Cham. https://doi.org/10.1007/978-3-319-46086-4_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-46086-4_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-46084-0
Online ISBN: 978-3-319-46086-4
eBook Packages: MedicineMedicine (R0)