Keypoints
-
1.
Tinnitus pathophysiology should explain both tinnitus distress and tinnitus intensity.
-
2.
Distress in tinnitus is most likely generated by an aspecific distress network consisting of the amygdala–anterior cingulate and anterior insula.
-
3.
Tinnitus intensity might be encoded by gamma band activity in the contralateral auditory cortex.
-
4.
This gamma band activity might result from thalamocortical dysrhythmia.
-
5.
Tinnitus distress can be seen as phase-synchronized co-activation of the auditory cortex activity and the aspecific distress network.
-
6.
For tinnitus to be perceived consciously, it requires the auditory cortex activity be embedded in a larger network.
-
7.
This larger network could be the global workspace, the self-perception network.
-
8.
The tinnitus network changes in time, hypothetically via an allostatic mechanism.
-
9.
In chronic tinnitus, the parahippocampus, insula, and dorsolateral prefrontal cortex networks are critical.
-
10.
The parahippocampus is involved via its auditory sensory gating mechanism, suppressing redundant auditory information.
*With a critical review by the members of the TRI neurostimulation workgroup: Nathan Weisz, Berthold Langguth, Marco Congedo, Winnie Schlee, Arnaud Norena. Other reviewers include Ana Belen Elgoyhen, Elsa van der Loo, Sven Vanneste, Mark Plazier, Thomas Elbert, Paul van de Heyning, and Aage Møller. Not every idea presented in this heuristic model has full support of all the reviewers.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Tinnitus
- Gamma
- Theta
- Thalamocortical dysrhythmia
- Distress
- Deafferentation
- Plasticity
- Reorganization
- Networks
Introduction
If rational treatments for tinnitus are to be developed, its pathophysiology needs to be understood. However, current knowledge of auditory system physiology is largely insufficient for this purpose. Available data on auditory physiology and the neural correlate of tinnitus can be supplemented by translating physiological data from other systems studied more extensively, such as the visual and somatosensory systems, and by extrapolating from pathophysiological mechanisms known in potentially analogous symptoms such as pain. Being aware of the limitations and the potential risks of such an approach, the proposed model has to be considered as a heuristic approach that results in the generation of testable hypotheses and needs to be corrected and improved accordingly.
Pathophysiology of Tinnitus
The pathophysiological working model of tinnitus has to include the mechanisms involved in the generation of the auditory percept and the intensity of a phantom sound as well as the mechanisms causing the tinnitus-related distress.
Tinnitus Intensity
The auditory system consists of two main parallel pathways supplying auditory information to the cerebral cortex; the same two ascending systems also have a descending counterpart, the tonotopically organized parvalbumin staining lemniscal system and the non-tonotopic calbindin staining extralemniscal system [1–4]. The lemniscal pathways use the ventral part of the medial geniculate body, the neurons of which project to the primary auditory cortex, whereas the extralemniscal pathways use the dorsal part of the medial geniculate body that projects to the secondary auditory cortex and association cortices, thus bypassing the primary cortex [5], Table 21.1. While neurons in the lemniscal pathways only respond to auditory stimulation, many neurons in the extralemniscal pathway are multimodal. Neurons in the ventral thalamus fire in a tonic or semi-tonic mode while neurons in the dorsal thalamus fire in bursts [6, 7]. Burst firing consists of dense packets of action potentials followed by periods of quiescence [8]. Information theory suggests that, in general, both tonic and burst firing efficiently transmit information about the stimulus. Burst and tonic firing might therefore be parallel computations in the auditory and other sensory systems [8, 9] (Table 21.1).
Based on the differences between the two parallel auditory pathways – the lemniscal being tonotopic and the extralemniscal being less tonotopic – it has been hypothesized that white-noise tinnitus may be caused by synchronous hyperactivity of burst firing in the non-tonotopic extralemniscal system, whereas pure-tone tinnitus may be the result of increased synchronous tonic firing in the tonotopic (lemniscal) system [43]. Narrow band tinnitus could be the result of a co-activation of the lemniscal and extralemniscal pathways.
Tinnitus Distress Matrix
The same subjectively reported tinnitus intensity can be related with severe distress in some people but may well be tolerated in others. The emotional component involved in tinnitus is most likely generated in the emotional circuit imbedded in our brain. Components of the emotional system are the amygdala, the subgenual and dorsal anterior cingulate cortex (ACC), the anterior insula, the ventromedial prefrontal cortex (VMPFC), and the orbitofrontal cortex [44–47]. Some of these areas such as the amygdala [48], the ACC [49], and the orbitofrontal cortex [50] are also involved in the reward system, together with the ventral tegmental area, nucleus accumbens, and mediodorsal nucleus of the thalamus [51].
The brain resolves perceptual ambiguity by anticipating the forthcoming sensory environment, generating a template against which to match observed sensory evidence. The ventromedial prefrontal cortex has been implicated as the source of this template [52]. Positive feedback results when sensory evidence is indeed as predicted and raises hemodynamic activity in the ventral striatum (nucleus accumbens) and the posterior cingulated cortex, related to a reward and storing the received information, respectively; negative feedback activates the dACC and the anterior insula, mediated via the habenula [53]. Thus, when the brain has not obtained the information, it needs to guide subsequent behavior, it activates the dACC–insula network to get more information.
Whenever new information is presented, the brain cannot compare this to a template, and therefore activity levels of the dorsal ACC (dACC) might also reflect the salience of the new information for predicting future outcomes [54, 55], guiding optimal decision making in an uncertain world [56].
Functional connectivity studies reveal that the dACC is functionally connected to the anterior insula [57] as well as the thalamus and brainstem [58]. The combined dACC–anterior insula activity possibly subserves intrinsic alertness [58], as the dACC and anterior insula are co-activated during states of arousal [55, 59, 60] and anticipatory arousal [61]. It has been shown that the amount of baseline activity in the dACC and insula predicts how intense a subsequent pain stimulus is being perceived [62]. The combined anterior insula and dACC activation has been suggested to act as a switch from the interoceptive default state to an exteroceptive executive brain state [63].
The human dACC has developed a parallel specialization for motivational drive via a thalamocortical pathway relaying in the mediodorsal thalamus [49]. The direct activation of both the interoceptive cortex and the dACC by the distinct homeostatic modalities corresponds with the simultaneous generation of both a sensation and a motivation [49, 64]. Thus, the function of the dACC might be to integrate motivationally important information with appropriate autonomic and motor responses [61] related to the survival needs of the body [64]. This might be based on the reward learning system, which uses dopamine as one of its major neurotransmitters. Dopamine neurons emit an alerting message about the surprising presence or absence of rewards [65, 66]. Dopamine neurons in the ventral tegmental area (VTA) are activated by rewarding events that are better than predicted, remain uninfluenced by events that are as good as predicted, and are depressed by events that are worse than predicted.
The right anterior insula has been implicated in interoceptive awareness [64, 67] related to the autonomic nervous system, the amygdala could be a relevance detector [68], and the ventromedial prefrontal cortex could be a major link between the autonomic nervous system, regulation of emotion, and stress reactivity [69]. Imaging studies on distress in posttraumatic stress disorder (PTSD) demonstrate activation of the amygdala, insula, medial prefrontal cortex, and anterior cingulate cortex [70], which overlaps with the distress network noted in pain and tinnitus. In anxiety disorders (such as social phobia, specific phobia, or PTSD) during emotional processing, the amygdalae and insulae are hyperactive; in PTSD specifically, the dACC and medial prefrontal cortex are hypoactive [71]. This could hypothetically reflect the brain’s suppression of the salience (dACC [54]) of the traumatic template (VMPFC [52]). Thus, even though the same network is active, its composing structures might be differentially activated depending on the task and pathology involved.
In tinnitus, using whole head magnetoencephalography (MEG) phase synchronization analysis has shown that functional connectivity between ACC and the right frontal lobe and ACC and right parietal lobe is correlated to tinnitus intrusiveness, a measure of tinnitus distress. The phase synchronization between ACC and right frontal lobe was inversely correlated with tinnitus intrusiveness, whereas the phase synchronization between ACC and right parietal lobe was positively correlated with tinnitus intrusiveness [72]. Even though no specific studies have looked at the tinnitus distress, Positron Emission Tomography (PET) studies have demonstrated activation of this distress network as well. Tinnitus distress, as measured by the Tinnitus Questionnaire (TQ) [73], is correlated with anterior cingulate activity [74], and the anterior insula is activated in tinnitus [75].
It has been suggested that there is a lateralization of the two components of the autonomic system, with the right insula controlling the sympathetic system and the left insula the parasympathetic system [59, 76, 77]. The same lateralization has been found in the ventromedial prefrontal cortex [78, 79], consistent with earlier data on hemispheric lateralization of parasympathetic and sympathetic control [80]. This could explain why the difference between severe but compensating and severe but decompensating tinnitus distress is related to activation of the right anterior insula (Vanneste submitted), confirmed by heart rate variability data correlated to anterior insula spontaneous activity (van der Loo, unpublished data). Both studies are based on Low Resolution brain Electric Tomography (LORETA) EEGs [81] (Fig. 21.1).
Based on the clinical analogies between tinnitus distress and pain distress and based on neuroimaging data, it is tempting to speculate that the tinnitus distress network and the pain matrix are identical [82]: unpleasantness of pain activates the anterior cingulate [83] and orbitofrontal cortices, amygdala, hypothalamus, posterior insula, primary motor cortex, and frontal pole [84]. One may further speculate that the perception of tinnitus and pain intensity could be related to auditory and somatosensory cortex activation, respectively, but that the distress associated with its perception might be related to activation of a common general non-specific “distress network.” This notion is supported by a recent study that demonstrates activation of this distress network during unpleasant symptoms in a somatoform disorder, even in the absence of a real physical stimulus [85].
Furthermore, the emotional network involved in pain and dyspnea [86] is similar, suggesting that the distress network might be a non-specific system that can be activated by many different kinds of external and internal stimuli.
The conscious perception of tinnitus distress and pain distress could be due to a co-activation of the thalamocortical auditory and somatosensory activity and distress network activity, possibly through synchronization of neuronal activity [72]. This heuristic model can also explain the clinical observation that tinnitus distress is frequently related to the development of tinnitus in stressful periods. Thus, a person in which the distress network is already sensitized, for whatever reason (divorce, work-related problems, etc.), would be more vulnerable to develop distressing tinnitus by increased activation of the auditory system. Once established, the co-activation between the auditory pathways and the distress network might stabilize and become self-sustaining.
Developmental and Adult Plasticity
Plasticity refers to the capacity of the nervous system to modify its organization [87]. The response of the nervous system to environmental changes can involve functional and structural changes. These changes can be induced not only by normal sensory input but also by abnormal sensory input, adaptation to damage of the nervous system, or sensory deprivation [87]. There seems to be a greater potential for plastic changes during development than during adulthood, even though similar mechanisms seem to govern both developmental and adulthood plasticity.
Any alteration of auditory input during the development of the tonotopy will result in reorganization of the tonotopic map according to the altered pattern of incoming neural activity. Thus, the Lamarckian and Darwinian (pangenesis) principle of “use it or lose it” guides both development and subsequent changes in the tonotopy. The auditory system develops in two stages [88, 89]. A first stage of synapse formation or auditory tract formation is genetically determined [90] and requires the release of a chemotropic factor [89, 91]. This is followed by fine-tuning of the synapses, leading to the formation of a tonotopic structure [92]. The development of tonotopy requires electrical activity resulting from auditory input during a critical period [93, 94]. It is the result of self-organization [95] via apoptotic resorption of surplus synapses and neurons [91, 96].
The mature auditory system still demonstrates an important capacity for reorganization, adjusting itself to any change in the auditory environment [97, 98]. The tonotopic maps are not rigid and may alter or reorganize under influence of normal physiological stimuli, as in learning, adjusting the tonotopic map to relevant environmental stimuli [97, 99, 100]. However, the plastic changes also occur in pathological situations such as sound overexposure [101], partial unilateral hearing loss [93, 102], or tinnitus [103].
In addition, the tonotopic map can also reorganize via direct cortical stimulation, as demonstrated in the big brown bat. Electrical auditory cortex stimulation can change the tonotopic map at a cortical [104], thalamic [105], or inferior colliculus level [97, 105], suggesting that the corticofugal pathway is involved in this tonotopical reorganization [98]. This corticofugal system acts as a positive feedback system, which in combination with lateral inhibition sharpens and adjusts tuning of neurons in the thalamus and inferior colliculus [98, 106]. In other words, the corticofugal system acts as a mechanism for reorganization of the thalamus and the inferior colliculus [105], adjusting the tonotopy to auditory experience [97].
Focal electrical stimulation of the cortex activates this corticofugal system resulting in reorganization of the thalamus and inferior colliculus [107], all the way to the cochlea [108], as well as the auditory cortex itself [104]. It induces tonotopic changes by decreasing best frequencies slightly higher than those electrically stimulated, and increasing best frequencies slightly lower than those electrically stimulated [104].
Auditory cortex plasticity is under the influence of the major neuromodulatory systems, such as the cholinergic nucleus basalis [99, 109], the dopaminergic ventral tegmental area [110], the serotoninergic dorsal raphe [111], and the noradrenergic locus coeruleus. The effects on the auditory cortex are understandably not identical for all these neuromodulatory systems. For example, the effect of the nucleus basalis [109] and the VTA [110] can be summarized as follows:
Stimulation of | NB | VTA |
|---|---|---|
Size of functional auditory cortex | Increased | Increased |
Size of functional AI | No change | Increased |
Stimulus frequency representation | Increased | Increased |
Adjacent frequency representation | Decreased | Increased |
Spectral selectivity | Increased | No change |
Non-monotonic responses | Increased | No change |
Frequency specificity of the effects | Sharper | Broader |
Tuning of secondary auditory cortex | Yes | No |
Temporal asymmetry of the effects | Yes | No |
Modulation of stimulus-following rate | Undetermined | Yes |
Cross-area synchronization | Yes | Does not apply |
The differential effects of these neuromodulatory systems on auditory cortex plasticity might benefit future tinnitus treatments.
Plasticity and Reorganization in Tinnitus
After noise trauma, tonotopic organization in the cortex is changed such that cortical neurons with characteristic frequencies in the frequency region of the hearing loss no longer respond according to their place in the tonotopic map but reflect instead the frequency tuning of their less affected neighbors [112, 113]. Providing an acoustically enriched environment, spectrally matching the hearing loss prevents this reorganization [114]. Neurons in the reorganized region also demonstrate spontaneous hyperactivity and increased neural synchrony [115–117], which can also be abolished by providing a spectrally matched and enriched acoustic environment. Magnetic source imaging studies [103] confirm this reorganization in humans: the auditory cortex is reorganized such that the frequency area corresponding to the tinnitus pitch is represented adjacent to where magnetic activity is expected on the tonotopic axis. Furthermore, in this study, the amount of reorganization was correlated with the perceived strength of the tinnitus, similarly to what is found in phantom pain [118]. In tinnitus patients, this reorganization is not correlated with the amount of hearing loss [103], which is the primary activator of changes in tonotopic maps [119]. This suggests that reorganization of the cortical tonotopic map, changes in neuron response properties, and tinnitus are correlated.
Deafferentation, Tinnitus, and Synchronized Auditory Hyperactivity
In tinnitus, firing rate and synchrony of firing are increased both in the extralemniscal and in the lemniscal systems. In the extralemniscal system, increased firing is observed [120–122] in the dorsal and external inferior colliculus [120], the thalamus [123], and the secondary auditory cortex [121, 122]. Furthermore, quinine, known to generate tinnitus, induces an increased regularity in burst firing, at the level of the auditory cortex, inferior colliculus, and frontal cortex [124]. This fits with the fact that in tinnitus an increased synchrony is found in the cochlear nerve [125–127] and auditory cortex [128, 129]. In tinnitus, an increased tonic firing rate is present in the lemniscal system as demonstrated in the lemniscal dorsal cochlear nucleus [130–135], inferior colliculus [136–139], and primary auditory cortex [140]. Interestingly, in the primary auditory cortex, not only tonic firing is increased, generating the phantom sound, but also the burst firing [129] at a regular basis.
Repetitive stimulus presentation results in decreased neuronal response to that stimulus, known as auditory habituation at the single cell level [141], also known as auditory-mismatch negativity at multiple cell level [141, 142]. Tinnitus is usually constantly present, i.e., there is no auditory habituation to this specific activation at this specific frequency. This corresponds, to some extent, to habituation deficits described in chronic pain.
The Neural Correlate of Tinnitus: Gamma Band Thalamocortical Firing
The EEG power spectrum (of the oscillation rate) and the level of consciousness are correlated [143]. Slow delta frequencies (0.5–4 Hz) are recorded in patients under deep sleep, anaesthesia, and coma. Somewhat higher frequencies, called theta waves (4–7 Hz), are noted in light sleep, and alpha waves (8–13 Hz) are recorded from all sensory areas in a resting state. Frontal beta waves (13–30 Hz) are recorded predominantly when people pay attention to external or internal stimuli. Synchronization of separate gamma band activities (30–80 Hz), present in different thalamocortical columns [144], is proposed to bind [145, 146] distributed neural gamma activity into one coherent auditory percept [147–152]. In general, coherent gamma band activity is present only in locally restricted areas of the cortex for short periods of time [152–156]. Thus, persisting gamma activity localized in one brain area can be considered pathological.
Recent data from the visual system suggest stimuli that reach consciousness and those that do not reach consciousness are characterized by a similar increase of local gamma oscillations in the EEG [157, 158]. Thus, gamma band activity, per se, is not related to conscious perception. Data from the olfactory bulb, as homologue for the thalamus, indicate that percept of odor could be related to amplitude modulation of the gamma band, suggesting that the gamma band is no more than a carrier wave [159, 160]. This idea is based on the fact that a signal (information) must sometimes be attached or superimposed on other voltages at frequencies that move easier in the transmission medium. Attaching signals to other carrier signals is called modulation. Carrier waves are known frequencies that can be readily detected (using a narrow bandwidth receiver tuned to transmitted signal). Retrieving the tinnitus-related information from the gamma carrier wave might therefore be attempted by different methods: by amplitude modulation analysis, frequency modulation analysis, pulse modulation analysis, or by completely different methods such as principal or independent component analysis (ICA) of the spectrally filtered gamma band or raw EEG.
In clinical practice, source analysis of the gamma band activity in tinnitus patients can be performed with LORETA EEGs [81]. If gamma band activity is localized in the auditory cortex, an ICA of the raw EEG filtered for gamma band activity can be performed, and the independent component that co-localizes with the gamma band activity could be considered to contain the tinnitus-related information. Intracranial recordings (iEEG) give a unique way to measure brain activity directly at the site of the electrode, bypassing skin and skull resistance. Comparing these intracranial recordings to simultaneously recorded scalp EEG activity, validation of the independent components measured at scalp level has been given at the site of the intracranial electrode [161]. According to our data, the ICA of scalp EEG could indeed serve as a tool to detect the neural correlate of tinnitus, similarly to what has been suggested for contralateral auditory cortex gamma band activity [162, 163]. Incorporating this concept into the thalamocortical dysrythmia model of Llinas (see below for further information), 40 Hz is a carrier wave, carrying the tinnitus-related information, which could potentially be represented by a co-localized gamma band filtered independent component (Fig. 21.2).
Independent component analysis performed on a 19-channel EEG recording in a patient with right-sided pure-tone tinnitus. The 16th independent component co-localizes with 40 Hz activity. Note that this component is not based on gamma band filtered EEG, which would be essential for if looking for the tinnitus information carried on the gamma wave
Thalamocortical Dysrhythmia
Tinnitus correlates with gamma band activity, and Llinas has developed this hypothesis further in this thalamocortical dysrhythmia model [163]. This model can be summarized as follows: the thalamus and cortex are interconnected and act in a coherent way. In the sleeping state, the thalamus fires at 4–7 Hz (1–3 Hz during slow wave sleep); in the resting awake state the thalamus fires around 10 Hz, driving the cortex to fire at the same rate [164]. When auditory stimuli are presented, the thalamocortical rhythm becomes activated and increases its firing rate to gamma band activity (>30 Hz). However, in a deafferented state, the thalamocortical columns fire in a burst mode with a frequency of 4–7 Hz. This leads to a decrease of lateral inhibition in the adjacent areas and results in a halo of gamma band activity, called the edge effect. It is hypothesized that this spontaneous and constant gamma band hyperactivity causes tinnitus [156].
Tinnitus is usually constantly present, which suggests that tinnitus-related gamma activity is continuously present, in contrast to normal physiological gamma activity, which waxes and wanes [152–156]. Therefore, it should be possible to retrieve this gamma band activity from the auditory cortex by analyzing short-term recordings of spontaneous electrical activity from the brain. Magnetoencephalography studies demonstrate that indeed gamma band activity is increased in the auditory cortex contralaterally to the side of tinnitus perception [162]. Whether the gamma band activity in the auditory cortex is related to the percept per se or is just an intensity coding mechanism is not clear. The first LORETA EEG data suggest that the spontaneous gamma band activity might be encoding tinnitus intensity [165].
Using data from implanted electrodes overlying the secondary auditory cortex, power versus frequency plots can be made of spontaneous electrical activity. The normal power versus frequency plots demonstrate the typical individual alpha peak of the sensory cortices. In thalamocortical dysrhythmia tinnitus, a theta peak can sometimes be found on iEEG recordings (De Ridder, submitted) similarly to what has been described for MEG. When recording during a period of residual inhibition, after electrical stimulation at the area of the theta peak when no more tinnitus is present, the theta peak disappears, suggesting that the theta peak is causally related to the tinnitus, either the theta itself or, hypothetically, via the decrease of nested gamma [166]. This seems to confirm Llinas’ model, at least at a cortical level.
When analyzing four implanted patients, in whom stimulation results in a decrease of tinnitus intensity, iEEG recordings can be performed with tinnitus at two different tinnitus intensities: one performed while the tinnitus is at rest and another performed during a period of residual inhibition. Theta band activity is higher on all poles of the electrodes when tinnitus intensity is high in comparison with low (Z = −1.826, p = 0.068), a nearly significant result with only four patients.
Using co-registration of the preoperative functional Magnetic Resonance Imaging (fMRI) and the postoperative CT, it can be shown that gamma band activity is highest at the area of Blood Oxygen-Dependent Level (BOLD) activation in all patients. These data give some support at a group level for the idea of thalamocortical dysrhythmia.
Tinnitus is usually constantly present, indicating that no habituation occurs for the tinnitus-related neuronal activity. Using EEG-mismatch negativity, abnormalities have been demonstrated in tinnitus sufferers who are specific to frequencies located at the audiometrically normal lesion edge as compared to normal hearing controls [167], which is compatible with Llinas’ thalamocortical dysrhythmia model [163].
Thalamocortical Dysrhythmia and Reorganization Go Hand in Hand
Increased “synchrony” in theta and gamma band firing in thalamocortical dysrhythmic tinnitus may induce cortical reorganization by simple Hebbian plasticity mechanisms [168]: cells that fire together, wire together. This model would predict that over time the tinnitus-related neuronal changes become more and more stabilized and the tinnitus more difficult to treat. Hebbian learning in the adult requires that the event is behaviorally relevant, i.e., input from nucleus basalis (NB) and VTA in addition to the firing of cortical cells or thalamocortical circuits in parallel. Therefore, the model would emphasize appraisal of the tinnitus, only predicting long-term changes when the tinnitus is given significant attention. The central nucleus of the amygdala and midbrain–striatal dopamine systems are critically involved in the alteration of attentional and emotional processing of initially neutral stimuli by associative learning [169–171], via its influence on the VTA [169]and nucleus basalis [170]. The insula and anterior cingulate receive the most pronounced innervations from the VTA [172]. It has been demonstrated that 10–50 Hz stimulation at the VTA (in contrast to the MD nucleus of the thalamus) activates the anterior cingulate via a dopaminergic pathway in a frequency-dependent manner [173].
Thus, co-activation of the dorsal ACC with the anterior insulae could result in attaching salience [54, 174, 175] to the tinnitus sound, resulting in reward-based Hebbian long-term plasticity as a (clinically negative) consequence. The dACC exerts a top–down influence on secondary auditory cortex (BA22) gamma band responses [176]. Cortical gamma band activity with associated attentive behavior is under control of the dopaminergic VTA [177]. Stimulating the VTA together with an auditory stimulus of a particular tone increases the cortical area and selectivity of the neural responses to that sound stimulus in AI and via coherent activity in A2 as well [110].
The anterior insula is not only involved in sound detection and in the entry of the sound into awareness but also in allocating auditory attention and in processing of novel versus familiar auditory stimuli [178]. Lesions in the anterior insula lead to contralateral auditory agnosia [179–181].
Under physiological situations, the hippocampus detects new information, which is not already stored in its long-term memory as it arrives. The resulting novelty signal is conveyed through the subiculum, accumbens, and ventral pallidum to the VTA where it contributes (along with salience and goal information) to the novelty-dependent firing of these cells. This results in dopamine release within the hippocampus producing an enhancement of Long-Term Potentiation (LTP) and learning [182]. In the auditory system, the auditory input enters the hippocampus via the parahippocampus [183, 184]. Complex novel sounds in humans activate the left and right superior temporal gyrus and the left inferior and middle frontal gyrus as well as the left parahippocampal gyrus [185]. In a similar fashion, the left superior temporal and left parahippocampal gyrus, along with left inferior frontal regions, are associated with listening to meaningful sounds [186]. The parahippocampal area is involved in sensory gating of irrelevant or redundant auditory information after both 100 ms and 400 ms [183]. This area is activated with the dACC, which peaks at 120 ms and after 240 ms [187]. It is of interest that onset of auditory hallucinations is related to activation of the left anterior insula and right middle temporal gyrus [188, 189], associated with deactivation of the parahippocampal area and anterior cingulate [188].
Thus, in summary, the amygdala might perceive a sound as salient or not [190], which activates the VTA [169] to mobilize the dACC and insulae [173], switching the default state to an executive brain state [63]. The dACC exerts a top–down influence on A2 [191], from where the left parahippocampal area is also activated if the sound is novel [185] or meaningful [186]. The VTA and the (tinnitus) sound result in plastic changes in the primary auditory cortex and from there in the secondary auditory cortex [110]. The posterior parahippocampus is the main node of entry for auditory information from A2 to the medial temporal lobe memory system, where salient information is encoded into long-term memory [184]. The parahippocampus also has an auditory gating function, suppressing irrelevant or redundant auditory information [183], as the dACC does somewhat earlier [187, 192]. Thus, when the dACC and parahippocampus are deactivated, as in the onset of complex auditory phantom percepts (hallucinations), the irrelevant and redundant information is not suppressed anymore, and the activation of the anterior insula and temporal cortex permits the internally generated auditory information to be perceived consciously and attended to [178]. Thus, it can be hypothesized that tinnitus onset could be characterized by deactivation of the dACC and parahippocampus, with activation of the insula and superior temporal gyrus.
Extending Thalamocortical Dysrhythmia to Darwinian Plasticity: Reverse Thalamocortical Dysrhythmia
Thalamocortical dysrhythmia predicts that the hyperactive symptoms related to gamma band activity are expressed at the lesion edge, thus adjacent to the missing sensory input. However, both in the auditory system [193] and in the somatosensory system [194], phantom perceptions are those coming from the missing input and not from the edge. This could be explained by including Darwinian plasticity to the thalamocortical dysrhythmia model. Sensory deafferentation results in expansion of the adjacent non-deafferented region into the vacated area, both in the somatosensory and in the auditory cortex. It has been suggested that a reverse form of plasticity could also exist: deafferented sensory cortex neurons seek information elsewhere in an attempt to survive (hence the name Darwinian plasticity). Neurophysiological and neuroanatomical data, functional imaging, clinical and human electrical brain stimulation data suggest a Darwinian model of brain plasticity. This model is capable of explaining deafferentation-induced symptomatology, which was not well explained by classical plasticity [195]. Whereas the lemniscal thalamocortical dysrhythmia model predicts a reduction of the oscillation frequency in deafferentiated thalamocortical columns, the proposed reverse thalamocortical dysrhythmia model can explain that the deafferented thalamocortical units also oscillate at gamma frequencies and thus can generate phantom percepts that fit the clinical data. Due to increased lateral inhibition related to gamma activity, a halo of low-frequency activity will develop at the lesion edge. This could be called reverse thalamocortical dysrhythmia, which explains that the perceived tinnitus pitch matches the deafferented frequencies (Fig. 21.3).
Cortical reorganization in tinnitus can be visualized using MSI (Magnetic Source Imaging, a fusion of MEG and MRI; Muhlnickel, Elbert et al. [103]). However, MEG is an expensive technique, restricted to a very limited amount of research centers. Therefore, using fMRI as a means of visualizing tinnitus would be advantageous in routine clinical practice, as this technique is available at many clinics and can provide images at high resolution.
fMRI measures a relative difference in oxygen consumption between a resting state and activated state. BOLD contrast takes advantage of the fact that the magnetic properties of haemoglobin depend on its oxygenation. The blood oxygenation in turn reflects changes in neuronal activity. As such, BOLD contrast can be used to provide in vivo real-time maps of blood oxygenation in the brain under normal physiological conditions [196]. Thus, a focal area of increased oxygen consumption can be depicted by subtraction of two MRI images, one at rest and one with increased oxygen consumption due to a specific task. As increased oxygen consumption is correlated to increasing metabolic demands, the BOLD effect is related to event-related synchronization of gamma band activity [197], and BOLD is highly coupled to gamma local field potentials (EEG) in the auditory cortex [198, 199]. This strongly suggests that fMRI can visualize the gamma band-synchronized activity associated with tinnitus.
A scanning paradigm, using music as a stimulus, adequately visualizes the auditory pathways in tinnitus patients [200]. fMRI activation is symmetrical in patients with bilateral tinnitus at all investigated areas of the auditory pathways (auditory cortex, thalamus, and inferior colliculus). fMRI activation is significantly decreased in patients with right-sided tinnitus in the left primary auditory cortex (AC) and in the left inferior colliculli (IC). In patients with left-sided tinnitus, fMRI activation is significantly decreased in the right medial geniculate body (MGB). In summary, the contralateral auditory pathways seem to be involved in patients with unilateral tinnitus. fMRI activation always represents a difference in neural activity instead of absolute neural activity. An increase of spontaneous neural activity, such as postulated in tinnitus patients, would mean that the affected brain area during the rest condition is more active than the unaffected side, and that the active condition (sound presentation) will only give rise to a limited increase in activity due to a ceiling effect (known as the saturation model) in comparison to the non-affected side. This can explain the fact that constant pathological neuronal hyperactivity can be correlated to hypoactivation in fMRI [200].
A similar study for tinnitus using tinnitus pitch and character-specific stimuli is currently being conducted. In this study, we compare BOLD activation for tinnitus-specific sound presentation to non-tinnitus sounds presented in the scanner. Only tinnitus-specific sounds induce a significant BOLD change, as demonstrated by a lateralization effect, in contrast to non-tinnitus sounds, which generate a bilateral symmetrical BOLD activation.
Even for tinnitus-specific frequencies, the exact representation might be important. For patients suffering from pure-tone tinnitus, auditory presentation of a pure tone generates a marked asymmetrical BOLD activation, whereas presentation of a narrow band noise creates less BOLD activation, and a white noise generates almost no asymmetry (Kovacs, unpublished data) (Fig. 21.4). However, as mentioned before, auditory tract activation is insufficient to objectively diagnose tinnitus solely based on functional imaging.
Tinnitus frequency-specific BOLD changes on fMRI in a patient with left-sided pure-tone tinnitus. Auditory presentation of non-tinnitus sound (OF other frequency) generates a bilateral BOLD activity on white noise (BPW band pass wide), narrow band noise (BPS band pass small), and pure tones. In contrast, for the tinnitus frequency (TF), a pure tone generates a marked asymmetrical BOLD activation, a narrow band noise less so, and a white noise creates almost no asymmetry
A disadvantage of fMRI studies is that a contrast is needed, e.g. by presenting a sound and comparing this to a resting state or other conditions (e.g. other sound). The active condition may include different unspecific components, e.g., different arousal and differences in a patient’s understanding of verbal instruction. Therefore, fMRI studies might suffer from various confounds. The fact that the fMRI-related activation changes are specific for the perceived phantom sound (Fig. 21.4) does, however, suggest that fMRI can indeed be used to study tinnitus.
Isolated Thalamocortical Dysrhythmia and the Global Workspace Model (Electrophysiologically Explored)
As tinnitus is a persistent conscious auditory percept, it is important to understand the neural correlates of auditory consciousness, defined as the minimal neuronal mechanisms jointly sufficient for any auditory conscious percept [201]. This understanding is an essential requirement for defining neurostimulation targets to suppress this auditory phenomenon. It has been suggested by Crick and Koch that in the visual system V1 activation is necessary but insufficient for visual awareness [202, 203]. Thus, isolated thalamocortical dysrhythmia in the primary auditory cortex is most likely not enough to generate the conscious percept of tinnitus. Studies in patients in persistent vegetative state (PVS), who are awake but without awareness – without conscious percepts [204] – demonstrate that these patients have a decreased metabolism in a network of areas consisting of midline areas, such as the anterior cingulate (ACC), which extends into the ventromedial prefrontal cortex (VMPFC), and the posterior cingulate (PCC), which extends into the precuneus. However, the lateral cortical regions also have less metabolic activity, more specifically the parietal and dorsolateral prefrontal cortex areas [205]. Not only is metabolism decreased in these patients, but functional connectivity is also decreased between the intralaminar nuclei of the thalamus and ACC/VMPFC and PCC/precuneus regions, and between ACC/VMPFC and PCC/precuneus [204, 205]. Recovery from PVS is associated with normalization of metabolism and connectivity, suggesting this decreased metabolism and loss of connectivity is critically involved and causally related to the neural correlate of consciousness [206, 207]. Extending these studies to auditory processing of patients in PVS, it was shown that the activation associated with auditory stimuli was restricted to the primary auditory cortex bilaterally in patients in a PVS without functional connectivity between the secondary auditory cortex and temporal and prefrontal association cortices [208], similarly to what has been shown for pain processing [209]. Based on these data, it can be proposed that activity restricted to the primary auditory cortex does not lead to auditory conscious perception, similarly to the somatosensory and visual system, but that this auditory activity becomes conscious when functionally connected to the ACC/VMPFC and prefrontal cortex (BA10) [206].
Baars has proposed the global workspace theory [210], which was extended and electrophysiologically refined for the visual system by Dehaene [211, 212]. The global workspace model, as perfected by Dehaene, can be translated to the auditory system as follows [213]: in (unconscious) preconscious processing, auditory stimulus processing is blocked at the level of the global neuronal workspace, i.e., it remains limited to the primary auditory cortex, while the global workspace is temporarily occupied by another task or is non-active, such as in PVS. A preconscious auditory stimulus may be temporarily buffered within the primary auditory cortex (discussed below) and later accessed by the frontoparietal system, once it is released by its present distracting task. In this case, information switches from unconscious to conscious. Conscious processing occurs when the accumulated stimulus-evoked activation exceeds a threshold and evokes a dynamic state of global reverberation [214] (“ignition”) across multiple high-level cortical areas forming a “global neuronal workspace,” particularly involving prefrontal, cingulate, and parietal cortices, the same areas that are decreased in metabolism and functional connectivity in PVS. These areas can maintain the information on-line and broadcast it to a variety of other processors, thus serving as a central hub for global access to information – a key property of conscious states.
Subliminal processing corresponds to a data-limited situation where the auditory stimulus reaches only specialized cerebral sensory networks (i.e., secondary and auditory association areas), without reaching a threshold for global ignition and, thus, without global reportability. The orientation and depth of subliminal processing may nevertheless depend on the top–down state of attention.
So, when an auditory stimulus is presented, it will activate the primary auditory cortex after about 17 to 30 ms [215, 216] and the primary auditory cortex (A1) remains activated up to 300 ms generating a Pa (P50), Nb, Na, P1 en N100 ERP [217]. This persistent A1 activation is characterized by an early (85 ms) posterior and a late (115 ms) anterior N1 component [218, 219]. In other words, the primary auditory cortex neurons synchronize multiple times to generate positive and negative ERP peaks. At 50 ms, the information is not only processed in the primary, secondary, and association auditory cortex [220] but also in the frontal cortex [183, 221], more specifically, in Brodmann’s areas 6 and 24 [192, 221, 222]. There might be a parallel signal transmission to the ACC and auditory cortex analogous to what has been shown for the somatosensory system. Somatosensory stimuli arrive at the ACC and somatosensory cortex simultaneously, as evidenced by intracranial recordings of evoked potentials [223]. This might reflect simultaneous processing of sensory and affective components of the stimulus. Auditory information processing is dependent on sensory gating, a mechanism of suppression of irrelevant auditory input or auditory habituation. Sensory gating seems to depend both on frontal and on auditory cortex activity [192, 221, 222, 224], and predominantly on gamma band activity [225]. At 100 ms, the auditory information also arrives in the posterior parahippocampus [183] and is still present frontally at the dorsal anterior cingulate extending into the insula, electrophysiologically recorded as N100 [226–228]. At the same time, information is processed in the PCC [227]. After 200 ms, the information will still be processed in the auditory cortices, ACC in VMPFC, extending to frontopolar cortex (BA10), and posteriorly in the precuneus (coming from PCC), altogether generating a N200 ERP [220, 229]. The anterior circuit, which is activated earlier, is most likely related to attentive processes, whereas the posterior activity is more related to sensory memory updating. After 300 ms, the information also extends into the temporoparietal junction and inferior and superior parietal area [230, 231], which is required for conscious perception (in the visual system). It has to be mentioned that a P300 is different from a P600 in its neural generators (P600 has generator in basal ganglia) [232], suggesting that any positive peak between 250 and 900 ms should not be called a P300 as is commonly done [233]. After 400 ms, the signal (if semantic) reaches the parahippocampus again [183], mediating sensory gating (presenting repetitious stimuli and measuring the degree of neural inhibition that occurs) [234] of irrelevant or redundant auditory input [183].
The Functional Networks of the Brain
The brain is organized into multiple systems that have distinct and potentially competing functional roles [235]; at least four functional systems have been described by functional connectivity analysis:
-
1.
The dorsal attention system, which is associated with externally directed cognition, includes regions in the frontal eye fields, ventral premotor cortex, superior parietal lobule, intraparietal sulcus, and motion-sensitive middle temporal area [236–238].
-
2.
The hippocampal-cortical memory system, a network of regions that are active during passive mental states linked to internally directed cognition (the default network) [239, 240], includes regions in ventral medial prefrontal cortex, posterior inferior parietal lobule, retrosplenial cortex, posterior cingulate, and the lateral temporal lobe [235, 236, 239, 241, 242].
-
3.
The frontoparietal control system is an executive control system guiding decision making by integrating information from the external environment with stored internal representations [243]. It includes many regions identified as supporting cognitive control and decision-making processes including lateral prefrontal cortex, anterior cingulate cortex, and inferior parietal lobule [235].
-
4.
The emotional system is a network based on functional connectivity with the amygdala and includes subgenual and dorsal anterior cingulate, orbitofrontal, insular, and dorsolateral prefrontal cortex, as well as strong interactions between amygdala and parahippocampal gyrus [244].
The global workspace has not been delineated anatomically. It can be hypothesized that the areas involved in the global workspace overlap with regions of these four networks.
However, that is still more than the minimal requirement for conscious perception [201].
Sleep studies have shown that the inferior and midfrontal gyrus, inferior parietal area, and medial parietal area are less active in Rapid Eye Movement (REM) sleep in comparison to wakefulness [245], suggesting that these areas are important for wakefulness and processing of external input but less important for awareness. The superior frontal and superior parietal areas with the intraparietal sulcus are equally active during wakefulness and REM sleep, as well as the VMPFC [245], suggesting that these areas are important for awareness/consciousness and could potentially be the minimal network required for awareness. It is striking that the dorsal attentional network, which selects and links stimuli and responses and hereby influences subsequent processing of stimuli in sensory cortex, is located in exactly the same areas: intraparietal sulcus (IPS) and superior parietal lobule (SPL), and dorsal frontal cortex along the precentral sulcus [237, 246], except for the VMPFC. The ventral attentional network, which interrupts and resets ongoing activity, consists of the temporoparietal junction (including the STS and gyrus), the inferior parietal lobule, and the mid and inferior frontal gyrus as well as the frontal operculum, and anterior insula [237, 246]. Thus, the inferior parietal and mid- and inferior frontal area, which are less active in REM compared to wakefulness [245] and are part of a resetting network [246], might be critically involved in updating current conscious information processing with novel external input.
Thus, based on both PVS and sleep studies, it can be proposed that the network consisting of the superior frontal–superior parietal–VMPFC–intralaminar nuclei has to be functionally connected for internally or externally generated auditory stimuli to be consciously perceived. These areas are activated after 200–300 ms and are involved in the generation of the P300, which is one of the requirements for stimuli to be perceived consciously (in the visual system).
Subliminal stimuli can be deeply processed and activate similar brain areas as consciously perceived stimuli [158]. Both perceived and non-perceived visual stimuli cause a similar increase of local (gamma) oscillations in the EEG, but only perceived words induce a transient long-distance synchronization of gamma oscillations across widely separated regions of the brain [157, 158], compatible with the global workspace model. Furthermore, only visual stimuli that are consciously perceived induce enhanced theta oscillations over frontal regions and demonstrate an increase of the P300 component of the event-related potential and an increase in power and phase synchrony of gamma oscillations [158].
As previously mentioned, the neural generators of the auditory P300 are the inferior parietal lobe/temporoparietal junction (TPJ), the supplementary motor cortex (SMA), the dorsal anterior cingulate cortex (dACC), the superior temporal gyrus (STG), the insula, and the dorsolateral prefrontal cortex [231] (in other words, the ventral attentional network plus dorsolateral prefrontal cortex). Thus, the P300 seems to interrupt and reset ongoing activity to what is being processed in the DLPFC, or in working memory [247]. This is very similar to the frontoparietal control system [235].
It has been suggested that the P300 is the electrophysiological correlate of global workspace activation, implying that the global workspace consists of the dorsolateral prefrontal cortex, dACC, SMA, and inferior parietal area extending into the STS [248].
If auditory cortex activation is essential but not sufficient for auditory conscious perception, where is the percept being transformed into a conscious percept? Data from monkey studies in the somatosensory system suggest it could be the prefrontal cortex [249]. Activity of primary somatosensory cortex neurons co-varies with the stimulus strength but not with the animal’s perceptual reports. This is similar in tinnitus: tinnitus intensity correlates with gamma band activity in the contralateral auditory cortex [165]. In contrast, the activity of the medial premotor cortex (MPC) neurons does not co-vary with the stimulus strength but does so with the animal’s perceptual reports [249]. In further agreement with the global workspace model, it has been demonstrated in the somatosensory system that the neural correlate of subjective sensory experience gradually builds up across cortical areas starting at the somatosensory cortex and ending in the premotor areas of the frontal lobe [250], which might have a hidden sensory function [251]. This idea of premotor cortex activity related to conscious sensory perception fits with the sensorimotor contingency philosophy of consciousness [252] described in the book Action in Perception [253], which suggests that seeing is a way of acting, a way of exploring the environment. This intentionality driven sensation dates back to Aristotle and Thomas Aquinas [254] and has been proposed to be a working mechanism in olfaction as well [255]. Thus, neural activity alone is not sufficient to produce vision, but neural activity contributes to experience only as enabling mastery and exercise of laws of sensorimotor contingency [252].
It is of interest that it was shown that N1, P2, and P3 are attenuated in chronic tinnitus patients [256, 257]. However, no source analysis was performed, and N1 attenuation is not found all the time [258]. One explanation can be that N1 is only attenuated in patients with low distress [259]. Another study found a difference in N1-P2 in unilateral tinnitus sufferers on the basis of N1-P2 intensity dependence and N1-P2 amplitude. A bilateral tinnitus group differed from controls by greater intensity dependence of the N1-P2 component and shorter N1 latency [260]. Using MEG, it was also shown that amplitude ratio M200/M100 represents a clear-cut criterion to distinguish between tinnitus patients and individuals without tinnitus [261], and the abnormal M200/M100 normalized when the tinnitus disappeared [262].
However, this M200/M100 abnormality in tinnitus patients could not be confirmed by another study [263].
Based on the above-mentioned heuristic model, it can be hypothesized that the ERPs should be performed with tinnitus-matched sound and non-tinnitus–matched sound. Obtaining a LORETA ICA of N100 should correlate to two aspects of tinnitus: one component relating to tinnitus distress (the ACC component) and one component to tinnitus intensity (auditory cortex component). In a similar way, the P/N 200 should be analyzed by ICA to make the distinction between distress and intensity. Similarly, the P300 should be analyzed for the presence of tinnitus, with P3a gamma band activity examined for the presence of distress and P3b for the presence of the sound.
It can be further hypothesized that the P50 (and N400) might be abnormal in tinnitus, as there is no sensory gating involved for the tinnitus-matched sound, whereas the P50 and N400 could be normal for non-tinnitus–matched sound.
PET studies have shown which areas of the brain are involved in the tinnitus global workspace network (Fig. 21.5): primary [75, 264–267] and secondary auditory cortex, extending into the temporoparietal junction (the auditory association area) [265, 268], (para)hippocampus [75], medial geniculate body, [75], anterior [74] and posterior cingulate cortex [269, 270], and precuneus and inferior lateral parietal cortex [271]. Voxel-based morphometry adds the subgenual ACC extending into the nucleus accumbens area [272], the hippocampus, and the inferior colliculus [273], which is confirmed by fMRI [274, 275]. Magnetoencephalography also finds abnormal spontaneous activity as well in the prefrontal cortex (BA10) [276]. Most of the tinnitus network overlaps with an aversive sound-processing network consisting of the primary and secondary auditory cortex, parahippocampus, amygdala, and right superior, middle, and inferior dorsolateral prefrontal cortex [277]. Later studies extended the aversive sound network to the auditory association, nucleus accumbens, and insula area [278].
The Tinnitus Network Changes in Time
Clinical data suggest that the longer tinnitus lasts the more difficult it becomes to treat. This has been shown for microvascular decompressions [279–285] and transcranial magnetic stimulations [269, 286–288]. Even though it is most likely a gradual continuous change, tinnitus duration of 4 years might be a practical point for clinicians to differentiate acute from chronic tinnitus (De Ridder, in press, Neurosurgery). This was first noted in microvascular decompressions by Møller, later by others performing the same surgery [279–285], and most recently was extended to rTMS investigations [286, 287]. A MEG study looking at phase-locked connectivity in the tinnitus network found that in patients with a tinnitus history of less than 4 years, the left temporal cortex was predominant in the gamma network, whereas in patients with tinnitus duration of more than 4 years, the gamma network was more widely distributed including more frontal and parietal regions [289]. Thus, even though the areas involved might still be the same, the functional connectivity and weight of the hubs between the involved areas might change.
In a recent EEG study, these network changes were also analyzed spectrally. Results indicate that the generators involved in tinnitus of recent onset (<4 years) seem to change in time with increased synchronized activity contralaterally in the auditory cortex, DLPFC/premotor cortex, dACC, and inusla. This is associated with an increase in gamma band connectivity between the parahippocampal cortex, auditory cortex, and the insula ipsilaterally to the tinnitus side and DLPFC contralaterally to the tinnitus side. All other connections seem to decrease in time (vanneste, submitted).
It is interesting to note that in chronic tinnitus, the degree of response to auditory cortex rTMS on TQ distress was correlated with tinnitus-associated activation of the anterior cingulate cortex [74].
Recently, the idea of allostasis, defined as the adaptive process for actively maintaining stability (homeostasis) through change [290], has been introduced in medicine [290]. It has been shown that allostasis is controlled by the brain [291, 292]. Homeostasis relates to the mechanisms that maintain stability within the physiological systems and hold all the parameters of the organisms internal milieu within limits that allow an organism to survive [290, 293, 294]. Allostasis, on the other hand, relates to the maintenance of stability outside of the normal homeostatic range, where an organism must vary all the parameters of its physiological systems to match them appropriately to chronic demands, for example, by resetting the system parameters at a new set point [290, 295, 296]. An allostatic state has been defined as a state of chronic deviation of the regulatory systems from their normal state of operation with establishment of a new set point [296]. It has been especially investigated with regard to the Darwinian [297] adaptive nature of stress and its possible maladaptive consequences, called allostatic load. The allostatic load then leads to pathology [291, 292, 298]. Drug addiction is hypothesized to involve a change in drug reward set point and reflects an allostatic, rather than a homeostatic, adaptation (i.e., outside the normal set point) [295, 296].
The brain areas controlling allostasis in stress are suggested to be the amygdala and the prefrontal cortex [291, 292, 297], as well as the ACC and insula [175]. Based on parallels between addiction and pain, it has been suggested that in chronic pain the concomitant tolerance (adaptive decreases of the drug’s efficacy) and hyperalgesia might be the result of the development of a new allostatic equilibrium [299]. Conceptually, in chronic tinnitus, a new allostatic equilibrium could develop, resulting in hyperacusis and persistence of the phantom sound. The dorsal ACC is involved in adaptive decision making and value evaluation [300] by adapting its activity when a new piece of information is witnessed, reflecting its salience for predicting future outcomes [54] by utilizing dopamine reward prediction error signals, but only when something can be learned [301]. Thus, the dorsal ACC might be involved in resetting this equilibrium. Metaphorically speaking, the dorsal ACC attributes salience to the phantom sound and resets its equilibrium allostaticly, so that the sound remains consciously perceived via resetting the parahippocampal auditory gating.
The allostatic equilibrium resetting can be located in the dACC and parahippocampus, as both regions are involved in auditory sensory gating [183, 192], i.e. suppression of irrelevant or redundant auditory information. Thus, if there is an allostatic reset of what auditory information is important or not, the dACC will be important as well as the parahippocampal area.
The parahippocampus is functionally connected to the inferior lateral parietal cortex regions along the midline including posterior cingulate and retrosplenial cortex extending into the precuneus, and subgenual ACC extending into the ventral medial prefrontal cortex [241].
The posterior parahippocampus is the main node of entry for auditory information to the medial temporal lobe memory system, where salient information is encoded into long-term memory [184]. The left parahippocampal gyrus along with left inferior frontal and left superior temporal regions are specifically associated with listening to meaningful sounds [186]. The parahippocampal area has also been linked to the unpleasantness of the auditory information [302], in contrast to the left amygdale, which is related to the salience of the aversive auditory (verbal) information [190].
Based on visual system data, it has been suggested that the parahippocampal cortex may play a broad role in contextual association [303, 304]. If complex auditory phantom phenomena (such as auditory hallucinations) and simple auditory phantom phenomena (such as tinnitus) share common pathophysiological mechanisms, it is of interest to note that at onset of auditory hallucinations, the parahippocampus becomes deactivated as well as the anterior cingulate [188]. Furthermore, when analyzing the difference between responders and non-responders to auditory cortex stimulation by means of LORETA EEG, non-responders demonstrate increased theta activity in the left parahippocampus, whereas responders have increased gamma band (30–40 Hz) activity in the (left) parahippocampal area t(9) = 1.98; p < 0.05 (van der Loo, unpublished data). Perception involves the processing of sensory stimuli and their translation into conscious experience. A novel percept can, once synthesized, be maintained or discarded from awareness. Visual perception is associated with distributed bilateral activation in the posterior thalamus and regions in the occipito-temporal, parietal, and frontal cortices. In contrast, sustained perception is associated with activation of the left prefrontal cortex and left (para)hippocampus [305]. Thus, if tinnitus is considered a sustained auditory perception, it could explain why amytal tests of the amygdalohippocampal area are capable of suppressing tinnitus in chronic unilateral tinnitus [306].
The Tinnitus Network: A Summary
A stimulus only makes sense if it is related to and incorporated the person’s self-percept. Therefore, the self-perception network, consisting of the ACC-vmPFC, PCC–precuneus, superior frontal-parietal, and STS, has to be activated for the tinnitus to be consciously perceived (Fig. 21.6). This is supported by the data from PVS patients.
The tinnitus intensity is related to auditory cortex activity, which might be controlled by dACC–insula baseline activity, expressing that the tinnitus is salient.
The tinnitus percept, per se, might not be encoded in the auditory cortex but be represented by DLPFC–premotor activity, connected to the self-perception network via the PCC–precuneus activity. This could be analogous to the somatosensory processing, where stimulus intensity is encoded by somatosensory cortex activity and the conscious percept in the frontal cortex. The parahippocampus might serve as an entry to auditory memory, pulling the missing information due to deafferentation from memory (Fig. 21.6).
The Tinnitus Network: Future Perspectives
Since the recent development of network science [307–311] to study complex adaptive systems (CAS), these analyses have been introduced in brain science [312–318] as well. The underlying idea is that CAS, whether it is the internet, ant societies, social interactions, the weather, or economy, are structured by similar universal rules [319].
Network topology describes how different nodes in a network are connected or linked. It was initially assumed that networks predominantly form randomly, in which each node is connected to another node randomly, characterized by a Poisson distribution of its connectivity [307]. All nodes are equal in this network. More recently, scale-free networks have been described [311], in which some nodes are more connected and more clustered (i.e., have a shorter path length, turning them into hubs). This suggests that some nodes are clearly more critical with regard to the robustness of the network. Both random and scale-free networks are very robust to random errors, but scale-free networks are more sensitive to attacks on hubs. Eighty percent of nodes can be removed in scale-free networks without failure, but if some critically important hubs are removed, the network system fails. Most likely, these scale-free networks become incorporated into hierarchical networks [320], permitting incorporation of modularity and scale-free behavior of the network.
The approach to studying complex adaptive systems has recently been extended to the human brain, as the brain clearly fulfils the criteria of a complex adaptive system [312, 313, 315]. The topological network approach can be applied to brain anatomy [318, 321], electrical [317, 322] and magnetic brain activity [314], and blood oxygenation changes (fMRI) [312, 313].
The entire brain is not ruled by one network, but most likely different topologies exist depending on the brain area and functional state of the brain. The brainstem is organized like a small world, but is not scale free [323]. The cerebellum seems to be structured like a regular or strictly local network [324], the hippocampus more like a random [324] and small world network, and the cortex has both small world [312, 325] and scale-free [326, 327] properties. These different network systems might be integrated in a hierarchical system of functional modules [320].
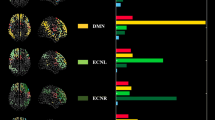
This network approach to studying the brain of patients with tinnitus could benefit the future neuromodulation management for individuals with this condition. Based on this short introduction to network analysis, it becomes clear that if tinnitus is related to scale-free hub disability, neuromodulation makes sense, as with limited targeting the persistent tinnitus network might be normalized again. This will, however, be impossible in random networks and will not be useful in regular networks. A recent study demonstrates that the hubs in tinnitus might consist of the PCC, dACC, and sgACC, extending into the OFC and parahippocampal area [328]. More similar studies with higher resolution will permit future pathophysiologically-based hub targeting in tinnitus.
Conclusion
There is insufficient literature to develop an evidence-based neuropathophysiological model of tinnitus, but a heuristic model can be conceived when available tinnitus data are supplemented by knowledge from other sensory systems, as well as the limbic, autonomic, and motor systems. Since it has been suggested that plasticity uses similar mechanisms in all sensory areas, extrapolating information from other sensory systems seems acceptable.
Tinnitus intensity is correlated with increased gamma activity in the contralateral auditory cortex, possibly as a reaction on reduced auditory input via thalamocortical or reverse thalamocortical dysrhythmia, resulting in lack of inhibition and increased synchrony, which in turn may lead to topographic map reorganization in the auditory cortex.
The tinnitus percept, per se, is almost certainly not related to isolated synchronous gamma band activity in the auditory cortex, but requires co-activation of the ill-defined global workspace or a self-perception network.
The distress some tinnitus patients perceive seems to be correlated to increased activity in the amygdala, anterior cingulate, and right anterior insula. Tinnitus distress might also be the result of synchronization of auditory thalamocortical dysrhythmia and distress network activation.
In time, the neural generators of tinnitus might change, possibly only by spectral modifications within the tinnitus global space network, hypothetically based on an allostatic mechanism.
Future studies, applying techniques from network science might demonstrate which hubs are critical for maintaining the tinnitus percept and therefore could be good targets for tinnitus neuromodulation treatments.
Abbreviations
- AC:
-
Auditory cortex
- ACC:
-
Anterior cingulate cortex
- BA:
-
Brodman area
- BOLD:
-
Blood oxygen level dependent
- BPS:
-
Band pass small
- BPW:
-
Band pass wide
- BRAI²N:
-
Brain research center antwerp for innovative & interdisciplinary neuromodulation
- CAS:
-
Complex adaptive systems
- DACC:
-
Dorsal part of ACC
- DLPFC:
-
Dorsolateral prefrontal cortex
- EEG:
-
Electroencephalography
- ERP:
-
Event related potential
- FMRI:
-
Functional magnetic resonance imaging
- Hz:
-
Hertz
- IC:
-
Inferior colliculus
- ICA:
-
Independent component analysis
- IPS:
-
Intraparietal sulcus
- IEEG:
-
Intracranial EEG
- LORETA:
-
Low resolution electro tomography
- LTP:
-
Long term potentiation
- MCS:
-
Minimally conscious state
- MD:
-
Mediodorsal
- MEG:
-
Magnetoencephalography
- MGB:
-
Medial geniculate body
- NB:
-
Nucleus basalis
- OF:
-
Other frequency
- PET:
-
Positron emission tomography
- PCC:
-
Posterior cingulate cortex
- PVS:
-
Persistent vegetative state
- RTMS:
-
Repetitive transcranial magnetic stimulation
- SMA:
-
Supplementary motor area
- SPL:
-
Superior parietal lobule
- STG:
-
Superior temporal gyrus
- STS:
-
Superior temporal sulcus
- TF:
-
Tinnitus frequency
- TQ:
-
Tinnitus questionnaire
- TPJ:
-
Temporoparietal junction
- TRI:
-
Tinnitus research initiative
- VMPFC:
-
Ventromedial prefrontal cortex
- VTA:
-
Ventral tegmental area
References
Jones EG (2001) The thalamic matrix and thalamocortical synchrony. Trends Neurosci 24:595–601.
Jones EG (1998) Viewpoint: the core and matrix of thalamic organization. Neuroscience 85:331–45.
Molinari M, ME Dell’Anna, E Rausell et al (1995) Auditory thalamocortical pathways defined in monkeys by calcium-binding protein immunoreactivity. J Comp Neurol 362:171–94.
Munkle MC, HJ Waldvogel and RL Faull (2000) The distribution of calbindin, calretinin and parvalbumin immunoreactivity in the human thalamus. J Chem Neuroanat 19:155–73.
Møller AR (2003) Sensory systems: anatomy and physiology. Amsterdam: Academic Press.
He J and B Hu (2002) Differential distribution of burst and single-spike responses in auditory thalamus. J Neurophysiol 88:2152–6.
Hu B, V Senatorov and D Mooney (1994) Lemniscal and non-lemniscal synaptic transmission in rat auditory thalamus. J Physiol 479 (Pt 2):217–31.
Chacron MJ, A Longtin and L Maler (2004) To burst or not to burst? J Comput Neurosci 17:127–36.
Oswald AM, MJ Chacron, B Doiron et al (2004) Parallel processing of sensory input by bursts and isolated spikes. J Neurosci 24:4351–62.
De Ridder D, T Menovsky and P Van de Heyning, eds. Tinnitus as a central auditory processing disorder. Current controversies in central auditory processing disorder, ed. AT Cacace and DJ McFarland. 2008, Plural Publishing: San Diego. 291–305.
Strominger NL, LR Nelson and WJ Dougherty (1977) Second order auditory pathways in the chimpanzee. J Comp Neurol 172:349–65.
Parvizi J and AR Damasio (2003) Differential distribution of calbindin D28k and parvalbumin among functionally distinctive sets of structures in the macaque brainstem. J Comp Neurol 462:153–67.
Tennigkeit F, DW Schwarz and E Puil (1996) Mechanisms for signal transformation in lemniscal auditory thalamus. J Neurophysiol 76:3597–608.
McCormick DA and HR Feeser (1990) Functional implications of burst firing and single spike activity in lateral geniculate relay neurons. Neuroscience 39:103–13.
Jones EG (2003) Chemically defined parallel pathways in the monkey auditory system. Ann N Y Acad Sci 999:218–33.
Chiry O, E Tardif, PJ Magistretti et al (2003) Patterns of calcium-binding proteins support parallel and hierarchical organization of human auditory areas. Eur J Neurosci 17:397–410.
Bordi F and JE LeDoux (1994) Response properties of single units in areas of rat auditory thalamus that project to the amygdala. I. Acoustic discharge patterns and frequency receptive fields. Exp Brain Res 98:261–74.
Calford MB (1983) The parcellation of the medial geniculate body of the cat defined by the auditory response properties of single units. J Neurosci 3:2350–64.
Sherman SM and C Koch (1998) The synaptic organization of the brain. ed. G Shepherd. Oxford: Oxford University Press.
Disterhoft JF and J Olds (1972) Differential development of conditioned unit changes in thalamus and cortex of rat. J Neurophysiol 35:665–79.
Bordi F, J LeDoux, MC Clugnet et al (1993) Single-unit activity in the lateral nucleus of the amygdala and overlying areas of the striatum in freely behaving rats: rates, discharge patterns, and responses to acoustic stimuli. Behav Neurosci 107:757–69.
Bartlett EL and PH Smith (1999) Anatomic, intrinsic, and synaptic properties of dorsal and ventral division neurons in rat medial geniculate body. J Neurophysiol 81:1999–2016.
Mooney DM, L Zhang, C Basile et al (2004) Distinct forms of cholinergic modulation in parallel thalamic sensory pathways. Proc Natl Acad Sci USA 101:320–4.
Sherman SM (2001) A wake-up call from the thalamus. Nat Neurosci 4:344–6.
Sherman SM (2001) Tonic and burst firing: dual modes of thalamocortical relay. Trends Neurosci 24:122–6.
Swadlow HA and AG Gusev (2001) The impact of ‘bursting’ thalamic impulses at a neocortical synapse. Nat Neurosci 4:402–8.
Ramcharan EJ, CL Cox, XJ Zhan et al (2000) Cellular mechanisms underlying activity patterns in the monkey thalamus during visual behavior. J Neurophysiol 84:1982–7.
Tardif E, O Chiry, A Probst et al (2003) Patterns of calcium-binding proteins in human inferior colliculus: identification of subdivisions and evidence for putative parallel systems. Neuroscience 116:1111–21.
Syka J (2002) Plastic changes in the central auditory system after hearing loss, restoration of function, and during learning. Physiol Rev 82:601–36.
Forster CR and RB Illing (2000) Plasticity of the auditory brainstem: cochleotomy-induced changes of calbindin-D28k expression in the rat. J Comp Neurol 416:173–87.
Caicedo A, C d’Aldin, M Eybalin et al (1997) Temporary sensory deprivation changes calcium-binding proteins levels in the auditory brainstem. J Comp Neurol 378:1–15.
Garcia MM, R Edward, GB Brennan et al (2000) Deafferentation-induced changes in protein kinase C expression in the rat cochlear nucleus. Hear Res 147:113–24.
Itoh K, H Kamiya, A Mitani et al (1987) Direct projections from the dorsal column nuclei and the spinal trigeminal nuclei to the cochlear nuclei in the cat. Brain Res 400:145–50.
Møller AR (2000) Hearing: its physiology and pathophysiology. San Diego: Academic Press.
Szczepaniak WS and AR Møller (1993) Interaction between auditory and somatosensory systems: a study of evoked potentials in the inferior colliculus. Electroencephalogr Clin Neurophysiol 88:508–15.
Leinonen L, J Hyvarinen and AR Sovijarvi (1980) Functional properties of neurons in the temporo-parietal association cortex of awake monkey. Exp Brain Res 39:203–15.
Kawaguchi Y and Y Kubota (1993) Correlation of physiological subgroupings of nonpyramidal cells with parvalbumin- and calbindinD28k-immunoreactive neurons in layer V of rat frontal cortex. J Neurophysiol 70:387–96.
Kawaguchi Y (2001) Distinct firing patterns of neuronal subtypes in cortical synchronized activities. J Neurosci 21:7261–72.
Solbach S and MR Celio (1991) Ontogeny of the calcium binding protein parvalbumin in the rat nervous system. Anat Embryol 184:103–24.
Baimbridge KG, MR Celio and JH Rogers (1992) Calcium-binding proteins in the nervous system. Trends Neurosci 15:303–8.
Caillard O, H Moreno, B Schwaller et al (2000) Role of the calcium-binding protein parvalbumin in short-term synaptic plasticity. Proc Natl Acad Sci USA 97:13372–7.
Rausell E, CG Cusick, E Taub et al (1992) Chronic deafferentation in monkeys differentially affects nociceptive and nonnociceptive pathways distinguished by specific calcium-binding proteins and down-regulates gamma-aminobutyric acid type A receptors at thalamic levels. Proc Natl Acad Sci USA 89:2571–5.
De Ridder D, E van der Loo, K Van der Kelen et al (2007) Do tonic and burst TMS modulate the lemniscal and extralemniscal system differentially? Int J Med Sci 4:242–6.
Phillips ML, WC Drevets, SL Rauch et al (2003) Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry 54:504–14.
Phan KL, T Wager, SF Taylor et al (2002) Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage 16:331–48.
Dalgleish T (2004) The emotional brain. Nat Rev Neurosci 5:583–9.
Ghashghaei HT, CC Hilgetag and H Barbas (2007) Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage 34:905–23.
Baxter MG and EA Murray (2002) The amygdala and reward. Nat Rev Neurosci 3:563–73.
Craig AD (2002) How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 3:655–66.
Kringelbach ML (2005) The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci 6:691–702.
Ikemoto S (2007) Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev 56:27–78.
Summerfield C, T Egner, M Greene et al (2006) Predictive codes for forthcoming perception in the frontal cortex. Science 314:1311–4.
Ullsperger M and DY von Cramon (2003) Error monitoring using external feedback: specific roles of the habenular complex, the reward system, and the cingulate motor area revealed by functional magnetic resonance imaging. J Neurosci 23:4308–14.
Behrens TE, MW Woolrich, ME Walton et al (2007) Learning the value of information in an uncertain world. Nat Neurosci 10:1214–21.
Critchley HD (2005) Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol 493:154–66.
Kennerley SW, ME Walton, TE Behrens et al (2006) Optimal decision making and the anterior cingulate cortex. Nat Neurosci 9:940–7.
Margulies DS, AM Kelly, LQ Uddin et al (2007) Mapping the functional connectivity of anterior cingulate cortex. Neuroimage 37:579–88.
Mottaghy FM, K Willmes, B Horwitz et al (2006) Systems level modeling of a neuronal network subserving intrinsic alertness. Neuroimage 29:225–33.
Critchley HD, DR Corfield, MP Chandler et al (2000) Cerebral correlates of autonomic cardiovascular arousal: a functional neuroimaging investigation in humans. J Physiol 523 (Pt 1):259–70.
Critchley HD, RN Melmed, E Featherstone et al (2002) Volitional control of autonomic arousal: a functional magnetic resonance study. Neuroimage 16:909–19.
Critchley HD, CJ Mathias and RJ Dolan (2001) Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron 29:537–45.
Boly M, E Balteau, C Schnakers et al (2007) Baseline brain activity fluctuations predict somatosensory perception in humans. Proc Natl Acad Sci USA 104:12187–92.
Sridharan D, DJ Levitin and V Menon (2008) A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA 105:12569–74.
Craig AD (2003) Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol 13:500–5.
Schultz W (1998) Predictive reward signal of dopamine neurons. J Neurophysiol 80:1–27.
Schultz W, P Dayan and PR Montague (1997) A neural substrate of prediction and reward. Science 275:1593–9.
Critchley HD, S Wiens, P Rotshtein et al (2004) Neural systems supporting interoceptive awareness. Nat Neurosci 7:189–95.
Sander D, J Grafman and T Zalla (2003) The human amygdala: an evolved system for relevance detection. Rev Neurosci 14:303–16.
Hansel A and R von Kanel (2008) The ventro-medial prefrontal cortex: a major link between the autonomic nervous system, regulation of emotion, and stress reactivity? Biopsychosoc Med 2:21.
Vermetten E, C Schmahl, SM Southwick et al (2007) Positron tomographic emission study of olfactory induced emotional recall in veterans with and without combat-related posttraumatic stress disorder. Psychopharmacol Bull 40:8–30.
Etkin A and TD Wager (2007) Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 164:1476–88.
Schlee W, N Weisz, O Bertrand et al (2008) Using auditory steady state responses to outline the functional connectivity in the tinnitus brain. PLoS One 3:e3720.
Goebel G and W Hiller (1994) [The tinnitus questionnaire. A standard instrument for grading the degree of tinnitus. Results of a multicenter study with the tinnitus questionnaire]. HNO 42:166–72.
Plewnia C, M Reimold, A Najib et al (2007) Moderate therapeutic efficacy of positron emission tomography-navigated repetitive transcranial magnetic stimulation for chronic tinnitus: a randomised, controlled pilot study. J Neurol Neurosurg Psychiatr 78:152–6.
Lockwood AH, RJ Salvi, ML Coad et al (1998) The functional neuroanatomy of tinnitus: evidence for limbic system links and neural plasticity. Neurology 50:114–20.
Oppenheimer S (1993) The anatomy and physiology of cortical mechanisms of cardiac control. Stroke 24:I3–5.
Critchley HD, R Elliott, CJ Mathias et al (2000) Neural activity relating to generation and representation of galvanic skin conductance responses: a functional magnetic resonance imaging study. J Neurosci 20:3033–40.
Cerqueira JJ, OF Almeida and N Sousa (2008) The stressed prefrontal cortex. Left? Right! Brain Behav Immun 22:630–8.
Hilz MJ, O Devinsky, H Szczepanska et al (2006) Right ventromedial prefrontal lesions result in paradoxical cardiovascular activation with emotional stimuli. Brain 129:3343–55.
Hilz MJ, M Dutsch, K Perrine et al (2001) Hemispheric influence on autonomic modulation and baroreflex sensitivity. Ann Neurol 49:575–84.
Pascual-Marqui RD, CM Michel and D Lehmann (1994) Low resolution electromagnetic tomography: a new method for localizing electrical activity in the brain. Int J Psychophysiol 18:49–65.
Moisset X and D Bouhassira (2007) Brain imaging of neuropathic pain. Neuroimage 37 Suppl 1:S80–8.
Price DD (2000) Psychological and neural mechanisms of the affective dimension of pain. Science 288:1769–72.
Kulkarni B, DE Bentley, R Elliott et al (2005) Attention to pain localization and unpleasantness discriminates the functions of the medial and lateral pain systems. Eur J Neurosci 21:3133–42.
Landgrebe M, W Barta, K Rosengarth et al (2008) Neuronal correlates of symptom formation in functional somatic syndromes: a fMRI study. Neuroimage 41:1336–44.
von Leupoldt A, T Sommer, S Kegat et al (2009) Dyspnea and pain share emotion-related brain network. Neuroimage 48:200–6.
Bavelier D and H Neville (2002) Developmental neuroplasticity. in Encyclopedia of the human brain, V Ramachandran, Editor. Academic Press: Amsterdam. 561–78.
Whitehead MC and DK Morest (1985) The development of innervation patterns in the avian cochlea. Neuroscience 14:255–76.
Kandel ER (1991) Cellular mechanisms of hearing and the biological basis of individiuality. in Principles of neural science, E Kandel, J Schwartz and T Jessell, Editors. Appleton & Lange: Norwalk, CT. 1009–31.
Snyder RL and PA Leake (1997) Topography of spiral ganglion projections to cochlear nucleus during postnatal development in cats. J Comp Neurol 384:293–311.
Staecker H, V Galinovic-Schwartz, W Liu et al (1996) The role of the neurotrophins in maturation and maintenance of postnatal auditory innervation. Am J Otol 17:486–92.
Rubsamen R (1992) Postnatal development of central auditory frequency maps. J Comp Physiol A 170:129–43.
Harrison RV, D Ibrahim and RJ Mount (1998) Plasticity of tonotopic maps in auditory midbrain following partial cochlear damage in the developing chinchilla. Exp Brain Res 123:449–60.
Sininger YS, KJ Doyle and JK Moore (1999) The case for early identification of hearing loss in children. Auditory system development, experimental auditory deprivation, and development of speech perception and hearing. Pediatr Clin North Am 46:1–14.
Deacon T, (1997) Evolution and intelligence: beyond the argument from design. in The origin and evolution of intelligence, A Scheibel and J Schopf, Editors. 1997, Jones and Bartlett: Boston. 103–36.
Sanes DH, J Song and J Tyson (1992) Refinement of dendritic arbors along the tonotopic axis of the gerbil lateral superior olive. Brain Res Dev Brain Res 67:47–55.
Gao E and N Suga (1998) Experience-dependent corticofugal adjustment of midbrain frequency map in bat auditory system. Proc Natl Acad Sci USA 95:12663–70.
Suga N, E Gao, Y Zhang et al (2000) The corticofugal system for hearing: recent progress. Proc Natl Acad Sci USA 97:11807–14.
Weinberger NM and JS Bakin (1998) Learning-induced physiological memory in adult primary auditory cortex: receptive fields plasticity, model, and mechanisms. Audiol Neurootol 3:145–67.
Recanzone GH, CE Schreiner and MM Merzenich (1993) Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci 13:87–103.
Cohen YE and JC Saunders (1994) The effect of acoustic overexposure on the tonotopic organization of the nucleus magnocellularis. Hear Res 81:11–21.
Dietrich V, M Nieschalk, W Stoll et al (2001) Cortical reorganization in patients with high frequency cochlear hearing loss. Hear Res 158:95–101.
Muhlnickel W, T Elbert, E Taub et al (1998) Reorganization of auditory cortex in tinnitus. Proc Natl Acad Sci USA 95:10340–3.
Chowdhury SA and N Suga (2000) Reorganization of the frequency map of the auditory cortex evoked by cortical electrical stimulation in the big brown bat. J Neurophysiol 83:1856–63.
Zhang Y and N Suga (2000) Modulation of responses and frequency tuning of thalamic and collicular neurons by cortical activation in mustached bats. J Neurophysiol 84:325–33.
Zhang Y, N Suga and J Yan (1997) Corticofugal modulation of frequency processing in bat auditory system. Nature 387:900–3.
Ma CL, JB Kelly and SH Wu (2002) AMPA and NMDA receptors mediate synaptic excitation in the rat’s inferior colliculus. Hear Res 168:25–34.
Xiao Z and N Suga (2002) Modulation of cochlear hair cells by the auditory cortex in the mustached bat. Nat Neurosci 5:57–63.
Kilgard MP and MM Merzenich (1998) Cortical map reorganization enabled by nucleus basalis activity. Science 279:1714–8.
Bao S, VT Chan and MM Merzenich (2001) Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature 412:79–83.
Ji W and N Suga (2007) Serotonergic modulation of plasticity of the auditory cortex elicited by fear conditioning. J Neurosci 27:4910–8.
Eggermont JJ and H Komiya (2000) Moderate noise trauma in juvenile cats results in profound cortical topographic map changes in adulthood. Hear Res 142:89–101.
Norena AJ, M Tomita and JJ Eggermont (2003) Neural changes in cat auditory cortex after a transient pure-tone trauma. J Neurophysiol 90:2387–401.
Norena AJ and JJ Eggermont (2005) Enriched acoustic environment after noise trauma reduces hearing loss and prevents cortical map reorganization. J Neurosci 25:699–705.
Seki S and JJ Eggermont (2003) Changes in spontaneous firing rate and neural synchrony in cat primary auditory cortex after localized tone-induced hearing loss. Hear Res 180:28–38.
Norena AJ and JJ Eggermont (2003) Changes in spontaneous neural activity immediately after an acoustic trauma: implications for neural correlates of tinnitus. Hear Res 183:137–53.
Norena AJ and JJ Eggermont (2006) Enriched acoustic environment after noise trauma abolishes neural signs of tinnitus. Neuroreport 17:559–63.
Flor H, T Elbert, S Knecht et al (1995) Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature 375:482–4.
Rajan R (1998) Receptor organ damage causes loss of cortical surround inhibition without topographic map plasticity. Nat Neurosci 1:138–43.
Chen GD and PJ Jastreboff (1995) Salicylate-induced abnormal activity in the inferior colliculus of rats. Hear Res 82:158–78.
Eggermont JJ and M Kenmochi (1998) Salicylate and quinine selectively increase spontaneous firing rates in secondary auditory cortex. Hear Res 117:149–60.
Eggermont JJ (2003) Central tinnitus. Auris Nasus Larynx 30 Suppl:S7–12.
Jeanmonod D, M Magnin and A Morel (1996) Low-threshold calcium spike bursts in the human thalamus. Common physiopathology for sensory, motor and limbic positive symptoms. Brain 119 (Pt 2):363–75.
Gopal KV and GW Gross (2004) Unique responses of auditory cortex networks in vitro to low concentrations of quinine. Hear Res 192:10–22.
Møller AR (1984) Pathophysiology of tinnitus. Ann Otol Rhinol Laryngol 93:39–44.
Cazals Y, KC Horner and ZW Huang (1998) Alterations in average spectrum of cochleoneural activity by long-term salicylate treatment in the guinea pig: a plausible index of tinnitus. J Neurophysiol 80:2113–20.
Martin WH, JW Schwegler, J Scheibelhoffer et al (1993) Salicylate-induced changes in cat auditory nerve activity. Laryngoscope 103:600–4.
Ochi K and JJ Eggermont (1996) Effects of salicylate on neural activity in cat primary auditory cortex. Hear Res 95:63–76.
Ochi K and JJ Eggermont (1997) Effects of quinine on neural activity in cat primary auditory cortex. Hear Res 105:105–18.
Brozoski TJ, CA Bauer and DM Caspary (2002) Elevated fusiform cell activity in the dorsal cochlear nucleus of chinchillas with psychophysical evidence of tinnitus. J Neurosci 22:2383–90.
Zhang JS and JA Kaltenbach (1998) Increases in spontaneous activity in the dorsal cochlear nucleus of the rat following exposure to high-intensity sound. Neurosci Lett 250:197–200.
Zacharek MA, JA Kaltenbach, TA Mathog et al (2002) Effects of cochlear ablation on noise induced hyperactivity in the hamster dorsal cochlear nucleus: implications for the origin of noise induced tinnitus. Hear Res 172:137–43.
Kaltenbach JA and CE Afman (2000) Hyperactivity in the dorsal cochlear nucleus after intense sound exposure and its resemblance to tone-evoked activity: a physiological model for tinnitus. Hear Res 140:165–72.
Kaltenbach JA, DA Godfrey, JB Neumann et al (1998) Changes in spontaneous neural activity in the dorsal cochlear nucleus following exposure to intense sound: relation to threshold shift. Hear Res 124:78–84.
Kaltenbach JA, MA Zacharek, J Zhang et al (2004) Activity in the dorsal cochlear nucleus of hamsters previously tested for tinnitus following intense tone exposure. Neurosci Lett 355:121–5.
Jastreboff PJ (1990) Phantom auditory perception (tinnitus): mechanisms of generation and perception. Neurosci Res 8:221–54.
Jastreboff PJ, JF Brennan and CT Sasaki (1988) An animal model for tinnitus. Laryngoscope 98:280–6.
Jastreboff PJ and CT Sasaki (1986) Salicylate-induced changes in spontaneous activity of single units in the inferior colliculus of the guinea pig. J Acoust Soc Am 80:1384–91.
Gerken GM (1996) Central tinnitus and lateral inhibition: an auditory brainstem model. Hear Res 97:75–83.
Komiya H and JJ Eggermont (2000) Spontaneous firing activity of cortical neurons in adult cats with reorganized tonotopic map following pure-tone trauma. Acta Otolaryngol 120:750–6.
Ulanovsky N, L Las and I Nelken (2003) Processing of low-probability sounds by cortical neurons. Nat Neurosci 6:391–8.
Naatanen R, P Paavilainen, H Tiitinen et al (1993) Attention and mismatch negativity. Psychophysiology 30:436–50.
Zeman A (2002) Consciousness, a user’s guide. 2002, New Haven: Yale University Press. 77–110.
Steriade M (2000) Corticothalamic resonance, states of vigilance and mentation. Neuroscience 101:243–76.
Gray CM, P Konig, AK Engel et al (1989) Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature 338:334–7.
Gray CM and W Singer (1989) Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Natl Acad Sci USA 86:1698–702.
Tiitinen H, J Sinkkonen, K Reinikainen et al (1993) Selective attention enhances the auditory 40-Hz transient response in humans. Nature 364:59–60.
Joliot M, U Ribary and R Llinas (1994) Human oscillatory brain activity near 40 Hz coexists with cognitive temporal binding. Proc Natl Acad Sci USA 91:11748–51.
Llinas R, U Ribary, D Contreras et al (1998) The neuronal basis for consciousness. Philos Trans R Soc Lond B Biol Sci 353:1841–9.
Llinas R, U Ribary, M Joliot et al (1994) Content and context in temporal thalamocortical binding. in Temporal coding in the brain, G Buzsaki, R Llinas and W singer, Editors. Springer-Verlag: Berlin. 251–72.
Ribary U, AA Ioannides, KD Singh et al (1991) Magnetic field tomography of coherent thalamocortical 40-Hz oscillations in humans. Proc Natl Acad Sci USA 88:11037–41.
Crone NE, D Boatman, B Gordon et al (2001) Induced electrocorticographic gamma activity during auditory perception. Brazier Award-winning article, 2001. Clin Neurophysiol 112:565–82.
Steriade M, F Amzica and D Contreras (1996) Synchronization of fast (30–40 Hz) spontaneous cortical rhythms during brain activation. J Neurosci 16:392–417.
MacDonald KD and DS Barth (1995) High frequency (gamma-band) oscillating potentials in rat somatosensory and auditory cortex. Brain Res 694:1–12.
Menon V, WJ Freeman, BA Cutillo et al (1996) Spatio-temporal correlations in human gamma band electrocorticograms. Electroencephalogr Clin Neurophysiol 98:89–102.
Llinas R, FJ Urbano, E Leznik et al (2005) Rhythmic and dysrhythmic thalamocortical dynamics: GABA systems and the edge effect. Trends Neurosci 28:325–33.
Gaillard R, S Dehaene, C Adam et al (2009) Converging intracranial markers of conscious access. PLoS Biol 7:e61.
Melloni L, C Molina, M Pena et al (2007) Synchronization of neural activity across cortical areas correlates with conscious perception. J Neurosci 27:2858–65.
Freeman WJ (2003) The wave packet: an action potential for the 21st century. J Integr Neurosci 2:3–30.
Freeman WJ and LJ Rogers (2002) Fine temporal resolution of analytic phase reveals episodic synchronization by state transitions in gamma EEGs. J Neurophysiol 87:937–45.
Van der Loo E, M Congedo, M Plazier et al (2007) Correlation between independent components of scalp EEG and intra-cranial EEG (iEEG) time series. International Journal of Bioelectromagnetism 9:270–5.
Weisz N, S Muller, W Schlee et al (2007) The neural code of auditory phantom perception. J Neurosci 27:1479–84.
Llinas RR, U Ribary, D Jeanmonod et al (1999) Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci USA 96:15222–7.
Hughes SW and V Crunelli (2005) Thalamic mechanisms of EEG alpha rhythms and their pathological implications. Neuroscientist 11:357–72.
van der Loo E, S Gais, M Congedo et al (2009) Tinnitus intensity dependent gamma oscillations of the contralateral auditory cortex. PLoS One 4:e7396.
Lisman J and G Buzsaki (2008) A neural coding scheme formed by the combined function of gamma and theta oscillations. Schizophr Bull 34:974–80.
Weisz N, S Voss, P Berg et al (2004) Abnormal auditory mismatch response in tinnitus sufferers with high-frequency hearing loss is associated with subjective distress level. BMC Neurosci 5:8.
Eggermont JJ and LE Roberts (2004) The neuroscience of tinnitus. Trends Neurosci 27:676–82.
El-Amamy H and PC Holland (2007) Dissociable effects of disconnecting amygdala central nucleus from the ventral tegmental area or substantia nigra on learned orienting and incentive motivation. Eur J Neurosci 25:1557–67.
Holland PC (2007) Disconnection of the amygdala central nucleus and the substantia innominata/nucleus basalis magnocellularis disrupts performance in a sustained attention task. Behav Neurosci 121:80–9.
Maddux JM, EC Kerfoot, S Chatterjee et al (2007) Dissociation of attention in learning and action: effects of lesions of the amygdala central nucleus, medial prefrontal cortex, and posterior parietal cortex. Behav Neurosci 121:63–79.
Oades RD and GM Halliday (1987) Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res 434:117–65.
Onn SP and XB Wang (2005) Differential modulation of anterior cingulate cortical activity by afferents from ventral tegmental area and mediodorsal thalamus. Eur J Neurosci 21:2975–92.
Seeley WW, V Menon, AF Schatzberg et al (2007) Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–56.
Taylor KS, DA Seminowicz and KD Davis (2009) Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp. 30:2731–45.
Mulert C, G Leicht, O Pogarell et al (2007) Auditory cortex and anterior cingulate cortex sources of the early evoked gamma-band response: relationship to task difficulty and mental effort. Neuropsychologia 45:2294–306.
Montaron MF, JJ Bouyer, A Rougeul et al (1982) Ventral mesencephalic tegmentum (VMT) controls electrocortical beta rhythms and associated attentive behaviour in the cat. Behav Brain Res 6:129–45.
Bamiou DE, FE Musiek and LM Luxon (2003) The insula (Island of Reil) and its role in auditory processing. Literature review. Brain Res Brain Res Rev 42:143–54.
Engelien A, D Silbersweig, E Stern et al (1995) The functional anatomy of recovery from auditory agnosia. A PET study of sound categorization in a neurological patient and normal controls. Brain 118 ( Pt 6):1395–409.
Habib M, G Daquin, L Milandre et al (1995) Mutism and auditory agnosia due to bilateral insular damage – role of the insula in human communication. Neuropsychologia 33:327–39.
Fifer RC (1993) Insular stroke causing unilateral auditory processing disorder: case report. J Am Acad Audiol 4:364–9.
Lisman JE and AA Grace (2005) The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron 46:703–13.
Boutros NN, R Mears, ME Pflieger et al (2008) Sensory gating in the human hippocampal and rhinal regions: regional differences. Hippocampus 18:310–6.
Engelien A, E Stern, N Isenberg et al (2000) The parahippocampal region and auditory-mnemonic processing. Ann N Y Acad Sci 911:477–85.
Muller BW, M Juptner, W Jentzen et al (2002) Cortical activation to auditory mismatch elicited by frequency deviant and complex novel sounds: a PET study. Neuroimage 17:231–9.
Engelien A, O Tuscher, W Hermans et al (2006) Functional neuroanatomy of non-verbal semantic sound processing in humans. J Neural Transm 113:599–608.
Tanaka E, K Inui, T Kida et al (2008) A transition from unimodal to multimodal activations in four sensory modalities in humans: an electrophysiological study. BMC Neurosci 9:116.
Hoffman RE, AW Anderson, M Varanko et al (2008) Time course of regional brain activation associated with onset of auditory/verbal hallucinations. Br J Psychiatry 193:424–5.
Shergill SS, MJ Brammer, E Amaro et al (2004) Temporal course of auditory hallucinations. Br J Psychiatry 185:516–7.
Anderson AK and EA Phelps (2001) Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature 411:305–9.
Mulert C, G Juckel, M Brunnmeier et al (2007) Rostral anterior cingulate cortex activity in the theta band predicts response to antidepressive medication. Clin EEG Neurosci 38:78–81.
Kurthen M, P Trautner, T Rosburg et al (2007) Towards a functional topography of sensory gating areas: invasive P50 recording and electrical stimulation mapping in epilepsy surgery candidates. Psychiatry Res 155:121–33.
Norena A, C Micheyl, S Chery-Croze et al (2002) Psychoacoustic characterization of the tinnitus spectrum: implications for the underlying mechanisms of tinnitus. Audiol Neurootol 7:358–69.
Ramachandran VS and W Hirstein (1998) The perception of phantom limbs. The D. O. Hebb lecture. Brain 121 (Pt 9):1603–30.
De Ridder D and P Van de Heyning (2007) The Darwinian plasticity hypothesis for tinnitus and pain. Prog Brain Res 166:55–60.
Ogawa S, TM Lee, AR Kay et al (1990) Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA 87:9868–72.
Brookes MJ, AM Gibson, SD Hall et al (2005) GLM-beamformer method demonstrates stationary field, alpha ERD and gamma ERS co-localisation with fMRI BOLD response in visual cortex. Neuroimage 26:302–8.
Nir Y, L Fisch, R Mukamel et al (2007) Coupling between neuronal firing rate, gamma LFP, and BOLD fMRI is related to interneuronal correlations. Curr Biol 17:1275–85.
Mukamel R, H Gelbard, A Arieli et al (2005) Coupling between neuronal firing, field potentials, and FMRI in human auditory cortex. Science 309:951–4.
Smits M, S Kovacs, D De Ridder et al (2004) Lateralization of signal change in the auditory pathway in patients with lateralized tinnitus studied with functional Magnetic Resonance Imaging (fMRI). Radiology 233, supplement:abstract 12-06.
Crick F and C Koch (2003) A framework for consciousness. Nat Neurosci 6:119–26.
Crick F and C Koch (1990) Toward a neurobiological theory of consciousness. Semin Neurosci 2:263–75.
Crick F and C Koch (1995) Are we aware of neural activity in primary visual cortex? Nature 375:121–3.
Laureys S (2007) Eyes open, brain shut. Sci Am 296:84–9.
Laureys S, S Goldman, C Phillips et al (1999) Impaired effective cortical connectivity in vegetative state: preliminary investigation using PET. Neuroimage 9:377–82.
Laureys S, ME Faymonville, A Luxen et al (2000) Restoration of thalamocortical connectivity after recovery from persistent vegetative state. Lancet 355:1790–1.
Laureys S, M Boly and P Maquet (2006) Tracking the recovery of consciousness from coma. J Clin Invest 116:1823–5.
Boly M, ME Faymonville, P Peigneux et al (2004) Auditory processing in severely brain injured patients: differences between the minimally conscious state and the persistent vegetative state. Arch Neurol 61:233–8.
Boly M, ME Faymonville, P Peigneux et al (2005) Cerebral processing of auditory and noxious stimuli in severely brain injured patients: differences between VS and MCS. Neuropsychol Rehabil 15:283–9.
Baars BJ (1993) How does a serial, integrated and very limited stream of consciousness emerge from a nervous system that is mostly unconscious, distributed, parallel and of enormous capacity? Ciba Found Symp 174:282–90; discussion 91–303.
Dehaene S, M Kerszberg and JP Changeux (1998) A neuronal model of a global workspace in effortful cognitive tasks. Proc Natl Acad Sci USA 95:14529–34.
Dehaene S and L Naccache (2001) Towards a cognitive neuroscience of consciousness: basic evidence and a workspace framework. Cognition 79:1–37.
Dehaene S, JP Changeux, L Naccache et al (2006) Conscious, preconscious, and subliminal processing: a testable taxonomy. Trends Cogn Sci 10:204–11.
Edelman GM (1993) Neural Darwinism: selection and reentrant signaling in higher brain function. Neuron 10:115–25.
Liegeois-Chauvel C, A Musolino, JM Badier et al (1994) Evoked potentials recorded from the auditory cortex in man: evaluation and topography of the middle latency components. Electroencephalogr Clin Neurophysiol 92:204–14.
Yvert B, A Crouzeix, O Bertrand et al (2001) Multiple supratemporal sources of magnetic and electric auditory evoked middle latency components in humans. Cereb Cortex 11:411–23.
Zouridakis G, PG Simos and AC Papanicolaou (1998) Multiple bilaterally asymmetric cortical sources account for the auditory N1m component. Brain Topogr 10:183–9.
Loveless N, S Levanen, V Jousmaki et al (1996) Temporal integration in auditory sensory memory: neuromagnetic evidence. Electroencephalogr Clin Neurophysiol 100:220–8.
Jaaskelainen IP, J Ahveninen, G Bonmassar et al (2004) Human posterior auditory cortex gates novel sounds to consciousness. Proc Natl Acad Sci USA 101:6809–14.
Saletu M, P Anderer, GM Saletu-Zyhlarz et al (2008) Event-related-potential low-resolution brain electromagnetic tomography (ERP-LORETA) suggests decreased energetic resources for cognitive processing in narcolepsy. Clin Neurophysiol 119:1782–94.
Korzyukov O, ME Pflieger, M Wagner et al (2007) Generators of the intracranial P50 response in auditory sensory gating. Neuroimage 35:814–26.
Grunwald T, NN Boutros, N Pezer et al (2003) Neuronal substrates of sensory gating within the human brain. Biol Psychiatry 53:511–9.
Frot M, F Mauguiere, M Magnin et al (2008) Parallel processing of nociceptive A-delta inputs in SII and midcingulate cortex in humans. J Neurosci 28:944–52.
Rosburg T, P Trautner, OA Korzyukov et al (2004) Short-term habituation of the intracranially recorded auditory evoked potentials P50 and N100. Neurosci Lett 372:245–9.
Clementz BA, LD Blumenfeld and S Cobb (1997) The gamma band response may account for poor P50 suppression in schizophrenia. Neuroreport 8:3889–93.
Grau C, L Fuentemilla and J Marco-Pallares (2007) Functional neural dynamics underlying auditory event-related N1 and N1 suppression response. Neuroimage 36:522–31.
Meyer M, S Baumann and L Jancke (2006) Electrical brain imaging reveals spatio-temporal dynamics of timbre perception in humans. Neuroimage 32:1510–23.
Atcherson SR, HJ Gould, MA Pousson et al (2006) Long-term stability of N1 sources using low-resolution electromagnetic tomography. Brain Topogr 19:11–20.
Potts GF, J Dien, AL Hartry-Speiser et al (1998) Dense sensor array topography of the event-related potential to task-relevant auditory stimuli. Electroencephalogr Clin Neurophysiol 106:444–56.
Volpe U, A Mucci, P Bucci et al (2007) The cortical generators of P3a and P3b: a LORETA study. Brain Res Bull 73:220–30.
Mulert C, O Pogarell, G Juckel et al (2004) The neural basis of the P300 potential. Focus on the time-course of the underlying cortical generators. Eur Arch Psychiatry Clin Neurosci 254:190–8.
Frisch S, SA Kotz, DY von Cramon et al (2003) Why the P600 is not just a P300: the role of the basal ganglia. Clin Neurophysiol 114:336–40.
Andreassi J (2000) Psychophysiology, human behavior and physiological response. Mahwah, NJ: Lawrence Erlbaum Associates.
Cromwell HC, RP Mears, L Wan et al (2008) Sensory gating: a translational effort from basic to clinical science. Clin EEG Neurosci 39:69–72.
Vincent JL, I Kahn, AZ Snyder et al (2008) Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol 100:3328–42.
Vincent JL, AZ Snyder, MD Fox et al (2006) Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol 96:3517–31.
Fox MD, M Corbetta, AZ Snyder et al (2006) Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci USA 103:10046–51.
Fox MD, AZ Snyder, JL Vincent et al (2005) The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102:9673–8.
Buckner RL, JR Andrews-Hanna and DL Schacter (2008) The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38.
Raichle ME, AM MacLeod, AZ Snyder et al (2001) A default mode of brain function. Proc Natl Acad Sci USA 98:676–82.
Kahn I, JR Andrews-Hanna, JL Vincent et al (2008) Distinct cortical anatomy linked to subregions of the medial temporal lobe revealed by intrinsic functional connectivity. J Neurophysiol 100:129–39.
Greicius MD and V Menon (2004) Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. J Cogn Neurosci 16:1484–92.
Miller EK (2000) The prefrontal cortex and cognitive control. Nat Rev Neurosci 1:59–65.
Stein JL, LM Wiedholz, DS Bassett et al (2007) A validated network of effective amygdala connectivity. Neuroimage 36:736–45.
Maquet P, P Ruby, A Maudoux et al (2005) Human cognition during REM sleep and the activity profile within frontal and parietal cortices: a reappraisal of functional neuroimaging data. Prog Brain Res 150:219–27.
Corbetta M, G Patel and GL Shulman (2008) The reorienting system of the human brain: from environment to theory of mind. Neuron 58:306–24.
Manes F, B Sahakian, L Clark et al (2002) Decision-making processes following damage to the prefrontal cortex. Brain 125:624–39.
Dehaene S, C Sergent and JP Changeux (2003) A neuronal network model linking subjective reports and objective physiological data during conscious perception. Proc Natl Acad Sci USA 100:8520–5.
de Lafuente V and R Romo (2005) Neuronal correlates of subjective sensory experience. Nat Neurosci 8:1698–703.
de Lafuente V and R Romo (2006) Neural correlate of subjective sensory experience gradually builds up across cortical areas. Proc Natl Acad Sci USA 103:14266–71.
de Lafuente V and R Romo (2002) A hidden sensory function for motor cortex. Neuron 36:785–6.
O’Regan K and A Noë (2001) A sensorimotor account of vision and visual consciousness. Behav Brain Sci 24:883–917.
Noë A (2004) Action in Perception. Cambridge, MA: MIT Press.
Aquinas ST (1268) Commentaries on Aristotle’s on sense and what is sensed and on memory and recollection. Washington, DC: The Catholic University of America Press.
Freeman WJ (1999) How brains make up their minds. London: Phoenix.
Attias J, D Urbach, S Gold et al (1993) Auditory event related potentials in chronic tinnitus patients with noise induced hearing loss. Hear Res 71:106–13.
Attias J, V Furman, Z Shemesh et al (1996) Impaired brain processing in noise-induced tinnitus patients as measured by auditory and visual event-related potentials. Ear Hear 17:327–33.
Shiraishi T, K Sugimoto, T Kubo et al (1991) Contingent negative variation enhancement in tinnitus patients. Am J Otolaryngol 12:267–71.
Delb W, DJ Strauss, YF Low et al (2008) Alterations in Event Related Potentials (ERP) associated with tinnitus distress and attention. Appl Psychophysiol Biofeedback 33:211–21.
Norena A, H Cransac and S Chery-Croze (1999) Towards an objectification by classification of tinnitus. Clin Neurophysiol 110:666–75.
Hoke M, H Feldmann, C Pantev et al (1989) Objective evidence of tinnitus in auditory evoked magnetic fields. Hear Res 37:281–6.
Pantev C, M Hoke, B Lutkenhoner et al (1989) Tinnitus remission objectified by neuromagnetic measurements. Hear Res 40:261–4.
Jacobson GP, BK Ahmad, J Moran et al (1991) Auditory evoked cortical magnetic field (M100–M200) measurements in tinnitus and normal groups. Hear Res 56:44–52.
Arnold W, P Bartenstein, E Oestreicher et al (1996) Focal metabolic activation in the predominant left auditory cortex in patients suffering from tinnitus: a PET study with [18F]deoxyglucose. ORL J Otorhinolaryngol Relat Spec 58:195–9.
Lockwood AH, RJ Salvi, RF Burkard et al (1999) Neuroanatomy of tinnitus. Scand Audiol Suppl 51:47–52.
Eichhammer P, G Hajak, T Kleinjung et al (2007) Functional imaging of chronic tinnitus: the use of positron emission tomography. Prog Brain Res 166:83–8.
Langguth B, P Eichhammer, A Kreutzer et al (2006) The impact of auditory cortex activity on characterizing and treating patients with chronic tinnitus – first results from a PET study. Acta Otolaryngol Suppl 84–8.
Giraud AL, S Chery-Croze, G Fischer et al (1999) A selective imaging of tinnitus. Neuroreport 10:1–5.
Plewnia C, M Reimold, A Najib et al (2007) Dose-dependent attenuation of auditory phantom perception (tinnitus) by PET-guided repetitive transcranial magnetic stimulation. Hum Brain Mapp 28:238–46.
Mirz F, A Gjedde, K Ishizu et al (2000) Cortical networks subserving the perception of tinnitus – a PET study. Acta Otolaryngol Suppl 543:241–3.
Mirz F, T Ovesen, K Ishizu et al (1999) Stimulus-dependent central processing of auditory stimuli: a PET study. Scand Audiol 28:161–9.
Muhlau M, JP Rauschecker, E Oestreicher et al (2006) Structural brain changes in tinnitus. Cereb Cortex 16:1283–8.
Landgrebe M, B Langguth, K Rosengarth et al (2009) Structural brain changes in tinnitus: grey matter decrease in auditory and non-auditory brain areas. Neuroimage 46:213–8.
Melcher JR, IS Sigalovsky, JJ Guinan, Jr. et al (2000) Lateralized tinnitus studied with functional magnetic resonance imaging: abnormal inferior colliculus activation. J Neurophysiol 83:1058–72.
Smits M, S Kovacs, D De Ridder et al (2007) Lateralization of functional magnetic resonance imaging (fMRI) activation in the auditory pathway of patients with lateralized tinnitus. Neuroradiology 49:669–79.
Weisz N, S Moratti, M Meinzer et al (2005) Tinnitus perception and distress is related to abnormal spontaneous brain activity as measured by magnetoencephalography. PLoS Med 2:e153.
Mirz F, A Gjedde, H Sodkilde-Jrgensen et al (2000) Functional brain imaging of tinnitus-like perception induced by aversive auditory stimuli. Neuroreport 11:633–7.
Zald DH and JV Pardo (2002) The neural correlates of aversive auditory stimulation. Neuroimage 16:746–53.
De Ridder D, H Ryu, G De Mulder et al (2005) Frequency specific hearing improvement in microvascular decompression of the cochlear nerve. Acta Neurochir (Wien) 147:495–501; discussion 501.
De Ridder D, K Heijneman, B Haarman et al (2007) Tinnitus in vascular conflict of the eighth cranial nerve: a surgical pathophysiological approach to ABR changes. Prog Brain Res 166:401–11.
Møller MB, AR Møller, PJ Jannetta et al (1993) Vascular decompression surgery for severe tinnitus: selection criteria and results. Laryngoscope 103:421–7.
De Ridder D, H Ryu, AR Møller et al (2004) Functional anatomy of the human cochlear nerve and its role in microvascular decompressions for tinnitus. Neurosurgery 54:381–8; discussion 8–90.
Jannetta P, (1997) Outcome after microvascular decompression for typical trigeminal neuralgia, hemifacial spasm, tinnitus, disabling positional vertigo, and glossopharyngeal neuralgia. in Clinical neurosurgery, S Grady, Editor. 1997, Williams and Wilkins: Baltimore. 331–84.
Ryu H, S Yamamoto, K Sugiyama et al (1998) Neurovascular compression syndrome of the eighth cranial nerve. What are the most reliable diagnostic signs? Acta Neurochir (Wien) 140:1279–86.
Brookes GB (1996) Vascular-decompression surgery for severe tinnitus. Am J Otol 17:569–76.
De Ridder D, E Verstraeten, K Van der Kelen et al (2005) Transcranial magnetic stimulation for tinnitus: influence of tinnitus duration on stimulation parameter choice and maximal tinnitus suppression. Otol Neurotol 26:616–9.
Kleinjung T, T Steffens, P Sand et al (2007) Which tinnitus patients benefit from transcranial magnetic stimulation? Otolaryngol Head Neck Surg 137:589–95.
Khedr EM, JC Rothwell, MA Ahmed et al (2008) Effect of daily repetitive transcranial magnetic stimulation for treatment of tinnitus: comparison of different stimulus frequencies. J Neurol Neurosurg Psychiatr 79:212–5.
Schlee W, T Hartmann, B Langguth et al (2009) Abnormal resting-state cortical coupling in chronic tinnitus. BMC Neurosci 10:11.
Sterling P and J Eyer (1988) Allostasis: a new paradigm to explain arousal pathology. in Handbook of life stress, cognition and health, S Fisher and J Reason, Editors. 1988, Wiley: New York. 629–49
McEwen BS (2007) Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 87:873–904.
McEwen BS (2008) Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol 583:174–85.
Cannon W (1929) Organization for physiological homeostasis. Physiol Rev 9:399–431.
Bernard C (1865) Introduction a l’Etude de la Médicine Expérimentale. Paris: JB Baillière.
Koob GF and M Le Moal (2008) Addiction and the brain antireward system. Annu Rev Psychol 59:29–53.
Koob GF and M Le Moal (2001) Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 24:97–129.
Korte SM, JM Koolhaas, JC Wingfield et al (2005) The Darwinian concept of stress: benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci Biobehav Rev 29:3–38.
McEwen BS (2002) Protective and damaging effects of stress mediators: the good and bad sides of the response to stress. Metabolism 51:2–4.
Celerier E, JP Laulin, JB Corcuff et al (2001) Progressive enhancement of delayed hyperalgesia induced by repeated heroin administration: a sensitization process. J Neurosci 21:4074–80.
Walton ME, PL Croxson, TE Behrens et al (2007) Adaptive decision making and value in the anterior cingulate cortex. Neuroimage 36 Suppl 2:T142–54.
Holroyd CB, OE Krigolson, R Baker et al (2009) When is an error not a prediction error? An electrophysiological investigation. Cogn Affect Behav Neurosci 9:59–70.
Gosselin N, S Samson, R Adolphs et al (2006) Emotional responses to unpleasant music correlates with damage to the parahippocampal cortex. Brain 129:2585–92.
Eichenbaum H and PA Lipton (2008) Towards a functional organization of the medial temporal lobe memory system: role of the parahippocampal and medial entorhinal cortical areas. Hippocampus 18:1314–24.
Aminoff E, N Gronau and M Bar (2007) The parahippocampal cortex mediates spatial and nonspatial associations. Cereb Cortex 17:1493–503.
Portas CM, BA Strange, KJ Friston et al (2000) How does the brain sustain a visual percept? Proc Biol Sci 267:845–50.
De Ridder D, H Fransen, O Francois et al (2006) Amygdalohippocampal involvement in tinnitus and auditory memory.Acta Otolaryngol Suppl 50–3.
Strogatz SH (2001) Exploring complex networks. Nature 410:268–76.
Watts DJ and SH Strogatz (1998) Collective dynamics of ‘small-world’ networks. Nature 393:440–2.
Albert R and AL Barabasi (2000) Topology of evolving networks: local events and universality. Phys Rev Lett 85:5234–7.
Albert R and AL Barabasi (2000) Dynamics of complex systems: scaling laws for the period of boolean networks. Phys Rev Lett 84:5660–3.
Barabasi AL and R Albert (1999) Emergence of scaling in random networks. Science 286:509–12.
Achard S, R Salvador, B Whitcher et al (2006) A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J Neurosci 26:63–72.
Bassett DS, E Bullmore, BA Verchinski et al (2008) Hierarchical organization of human cortical networks in health and schizophrenia. J Neurosci 28:9239–48.
Bassett DS, A Meyer-Lindenberg, S Achard et al (2006) Adaptive reconfiguration of fractal small-world human brain functional networks. Proc Natl Acad Sci USA 103:19518–23.
Bullmore E and O Sporns (2009) Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10:186–98.
Salvador R, J Suckling, C Schwarzbauer et al (2005) Undirected graphs of frequency-dependent functional connectivity in whole brain networks. Philos Trans R Soc Lond B Biol Sci 360:937–46.
Stam CJ and JC Reijneveld (2007) Graph theoretical analysis of complex networks in the brain. Nonlinear Biomed Phys 1:3.
van den Heuvel MP, CJ Stam, M Boersma et al (2008) Small-world and scale-free organization of voxel-based resting-state functional connectivity in the human brain. Neuroimage 43:528–39.
Barabasi AL (2009) Scale-free networks: a decade and beyond. Science 325:412–3.
Oltvai ZN and AL Barabasi (2002) Systems biology. Life’s complexity pyramid. Science 298:763–4.
Hagmann P, L Cammoun, X Gigandet et al (2008) Mapping the structural core of human cerebral cortex. PLoS Biol 6:e159.
Ponten SC, L Douw, F Bartolomei et al (2009) Indications for network regularization during absence seizures: weighted and unweighted graph theoretical analyses. Exp Neurol 217:197–204.
Humphries MD, K Gurney and TJ Prescott (2006) The brainstem reticular formation is a small-world, not scale-free, network. Proc Biol Sci 273:503–11.
Buzsaki G (2006) Rhythms of the brain. Oxford: Oxford University Press.
Yu S, D Huang, W Singer et al (2008) A small world of neuronal synchrony. Cereb Cortex 18:2891–901.
Eguiluz VM, DR Chialvo, GA Cecchi et al (2005) Scale-free brain functional networks. Phys Rev Lett 94:018102.
Miller KJ, LB Sorensen, JG Ojemann et al (2009) Power-law scaling in the brain surface electric potential. PLoS Comput Biol 5:e1000609.
Schlee W, N Mueller, T Hartmann et al (2009) Mapping cortical hubs in tinnitus. BMC Biol 7:80.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
De Ridder, D. (2011). A Heuristic Pathophysiological Model of Tinnitus. In: Møller, A.R., Langguth, B., De Ridder, D., Kleinjung, T. (eds) Textbook of Tinnitus. Springer, New York, NY. https://doi.org/10.1007/978-1-60761-145-5_21
Download citation
DOI: https://doi.org/10.1007/978-1-60761-145-5_21
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-60761-144-8
Online ISBN: 978-1-60761-145-5
eBook Packages: MedicineMedicine (R0)