Keypoints
-
1.
Different functional imaging methods, such as SPECT, PET, and fMRI, have been used for investigating tinnitus.
-
2.
Neuroimaging methods have provided windows to the brain that allow detection of the localization of tinnitus-related changes in the brain.
-
3.
Such studies have shown signs of abnormalities in many parts of the brain, including auditory brain regions but also nonauditory brain areas involved in sensory integration, in attention, or in emotional evaluation.
-
4.
New treatment strategies have evolved from fMRI and PET findings of abnormal neuronal activity in the auditory cortex.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Tinnitus
- Neuroimaging
- Electroencephalography
- Magnetoencephalography
- Functional magnetic resonance tomography
- Positron emission tomography
- Diagnosis
- Pathophysiology
Introduction
Tinnitus-related functional changes of neural activity have been investigated with functional imaging techniques such as single photon emission computed tomography (SPECT), positron emission tomography (PET), and functional magnetic resonance imaging (fMRI). The different methods and the results of their use in the investigation of subjects with tinnitus will be presented in detail in the following sections. Finally, we will discuss how the different techniques have contributed to identify the anatomical location of the functional abnormalities that cause some forms of tinnitus.
Single Photon Emission Computed Tomography (SPECT)
SPECT (single photon emission computed tomography) scanning makes use of a radioactive tracer emitting gamma rays to measure blood flow in regions of the brain (regional cerebral blood flow, rCBF). The emission of photons is recorded by a camera that provides a 3D image of the anatomical location of indicators of neural activity. To obtain a SPECT scan, the individual person receives an injection of a small amount of a radio-labeled compound, e.g., Technetium-HMPAO. The distribution of this compound is related to blood flow and is used as a measure for local neural activity.
A study of rCBF using SPECT [1] in 45 depressed individuals, of whom 27 had severe tinnitus, found decreased CBF in the right frontal lobe Brodmann area 45 (Broca, pars triangularis), the left parietal lobe area 39 (angular gyrus, part of Wernicka’s area), and the left visual association cortex area 18 (secondary visual cortex, V2) in tinnitus patients compared with nontinnitus patients. In patients with tinnitus, the CBF was increased in the primary, secondary, and auditory association areas of the temporal lobe (Brodmann’s area 41, primary auditory cortex; area 21, middle temporal gyrus; area 22, superior temporal gyrus) as compared to gender-matched controls and depressed patients who did not have tinnitus. The study also showed signs that the superior temporal gyrus bilaterally (primary and secondary auditory cortex) and three further brain areas were more active in depressed patients who had tinnitus than in depressed patients without tinnitus: Brodmann areas 18 (V2), 39 (inferior parietal, angular gyrus), and 45 (Broca’s homologue, VLPFC) [1]. Another study in two individuals who had tinnitus found differences in the temporal, frontal, parietal, hippocampal, and amygdala regions when compared with normative Tc-HMPAO SPECT data [2].
Positron Emission Tomography
PET has a similarity to SPECT and makes use of a radioactive tracer (a short-lived radioactive isotope) to identify the anatomical location of indicators of neural activity such as blood flow or glucose metabolism.
As the radioactive atoms in the compound decay, they release positively charged positrons. When a positron collides with a negatively charged electron, they are both annihilated and two photons are emitted. The photons move in opposite directions and are detected by the sensor ring of the PET scanner. Reconstruction of the three-dimensional paths of the articles provides information about the maximum accumulation or metabolism of the short-lasting radio-labeled isotope at a higher resolution than obtained with a SPECT scan (1 cm for SPECT).
PET retains unique advantages in studies of auditory processing over fMRI because it is not associated with any noticeable noise, as are fMRI machines, which produce up to 130 dB noise at the location where the person who is scanned is placed. Unlike fMRI, PET can be used to study individuals with cochlear implants or other kinds of implanted electrodes, which do not allow use of fMRI [3]. PET is also much less sensitive to body movements, such as those from arterial pulsations in the brainstem. On the other hand, PET is not widely available, is relatively expensive, and is always associated with exposure to ionized radiation, which precludes repetitive imaging sessions.
The two PET methods have been used in the investigation of tinnitus: one uses a radioactively labeled glucose (FDG PET), which reflects metabolic activity, and the other type uses radioactively labeled water ([15O]-H2O PET), which provides a measure for cerebral blood flow.
Studies Using [15O]-H2O PET
Estimates of changes in rCBF using PET with radioactively labeled water ([15O]-H2O PET) have been used as an indicator of changes in neural activity during transient reduction of tinnitus loudness, e.g., by the administration of lidocaine [4, 5].
Several PET studies of rCRB took advantage of the fact that few individuals can modulate their tinnitus by orofacial movements [6, 7] or eye movements, a condition which may occur after surgical operations in the cerebellopontine angle [8]. Thus, Giraud et al. [9] found that such forms of tinnitus are associated with an increase in CBF bilaterally, especially in auditory temporoparietal association areas.
Individuals with unilateral tinnitus who could alter the loudness of their tinnitus by orofacial movements showed indications that neural activity in areas adjacent to the contralateral auditory cortex increased and decreased in parallel to the reported change in loudness of tinnitus [10]. In contrast, auditory stimulation in the same individuals resulted in bilateral activation of the auditory cortex, suggesting that the abnormal neural activity that caused the sensation of tinnitus originated in the central auditory system rather than the cochlea [10]. When investigating subjects with gaze-evoked tinnitus, Lockwood et al. [11] found signs of CBF alterations in a large part of the frontal, parietal, and temporal cortex, as well as the lateral pontine tegmentum and the primary auditory cortex. Whereas lateral gaze reduced rCBF in the temporal lobe in control subjects, this was not the case in individuals with tinnitus whose condition worsened during lateral gaze. This finding suggests that gaze-evoked tinnitus may be caused by reduced gaze-evoked inhibition of the auditory cortex [11].
Tinnitus was also associated with more widespread activation of neural structures in the brain not activated by sound stimulation, including activation of limbic structures, which indicated that plastic changes of the auditory nervous system had occurred (see Chap. 12).
Several investigators using PET scans have shown indications that intravenous administration of lidocaine can modulate tinnitus [4, 5, 12, 13]. Most studies of the effect of lidocaine on tinnitus have been done in individuals where lidocaine decreased the loudness of the tinnitus. In such a study, Reyes et al. [5] found that the decrease in the loudness of the tinnitus was associated with changes in the neural activity in the right auditory association cortex. These findings were confirmed and extended by Plewnia et al. [4], who found changes in CBF in a broad region of the auditory cortex (middle temporal gyrus), including areas involved in the integration of sensory stimuli (gyrus angularis) and cognitive processing (posterior cingulated cortex) of sensory stimuli.
In a recent study, Andersson et al. [14] showed evidence that reduction of tinnitus loudness during a cognitive task (silent backward counting) is accompanied by reduced CBF in auditory cortex.
Taken together, measurements of rCBF with [15O]-H2O PET have consistently provided evidence for tinnitus-related increases of neural activity in auditory pathways as well as in some nonauditory neural systems. However, the use of this technique depends on the ability to influence the loudness of the tinnitus by specific interventions, which means that it can only be used in individuals who can modulate their tinnitus.
Studies Using FDG-PET
Another, and perhaps more direct method for getting estimates of neural activity uses measurements of regional glucose uptake (FDG-PET) that is related to metabolic activity and, in turn, is a marker for steady-state neuronal activity. This technique has been applied to measure steady-state brain activity in individuals with tinnitus [15–17].
This method does not depend on the ability to change the tinnitus as does the [15O]-H2O PET and can therefore be used for diagnostic purposes in almost every tinnitus patient. The results of using this form of imaging in individuals with tinnitus was first described by Arnold et al. in 1996 [15]. These investigators found asymmetric activation of the auditory cortex, predominantly on the left side and independent of tinnitus perceived laterality in tinnitus patients as compared to controls. Nine out of ten patients with tinnitus (two right sided, six left sided, and two with tinnitus centered in the head) had signs of significantly increased metabolic activity in the left primary auditory cortex (Brodmann area 41, primary auditory cortex), and one had increased activity in the right cortex. In one patient, in whom the severity of the tinnitus fluctuated up and down, repeated PET scans showed that the metabolic activity of the left primary auditory cortex changed in a similar way as the loudness of the tinnitus changed. These results were confirmed by a case series [18] and two studies involving larger sample sizes [16, 17] all of which demonstrated asymmetry in the auditory cortices of tinnitus patients with higher levels of spontaneous neuronal activity predominantly on the left side, irrespective of tinnitus laterality. An example for a FDG PET scan of a tinnitus patient is given in Fig. 18.1
The unilateral activation pattern resembles findings from Lookwood and colleagues, who observed unilateral auditory cortex activation in individuals with tinnitus using a different method [10]. A major limitation of all published FDG PET studies of tinnitus patients is that data analysis has been restricted to the auditory cortex by using a region of interest approach [19].
Functional Magnetic Resonance Imaging (fMRI)
fMRI is a specialized form of MRI that is used for identifying regions of the brain, where neural activity increases in response to neural stimulation, such as sensory stimulation. The use of fMRI is based on the finding that magnetic properties of hemoglobin depend on its oxygenation level and the observation that blood flow and blood oxygenation is closely related to neural activity. Regional changes in hemoglobin oxygenation occur because of local neuronal activation e.g., in response to a stimulus or during a specific task. Blood oxygenation level-dependent (BOLD) contrast is the basis of brain mapping using fMRI. BOLD contrast provides in vivo real-time maps of blood deoxygenation in the brain under normal physiological conditions. Brain regions of increased oxygen consumption are depicted by comparison of two MRI images: one at rest and one with increased oxygen consumption due to a specific task.
The use of fMRI offers several advantages over PET. First, participants are not exposed to ionized radiation; second fMRI is easier to perform, less expensive, and more widely available. Furthermore, fMRI provides high spatial resolution (1 × 1 × 1 mm) [20].
The fMRI obtained in individuals with bilateral tinnitus show symmetrical activation in all investigated areas of the auditory pathways (auditory cortex, thalamus, and inferior colliculus) while all published studies show that in individuals with unilateral tinnitus altered activation patterns only of structures contralateral to where the tinnitus is perceived were observed [21–24]. Lanting et al. found an increased sensitivity of the contralateral inferior colliculus to the loudness of the presented acoustic stimuli, whereas Melcher and Smits observed reduced neuronal activation in the contralateral inferior colliculus in individuals with tinnitus.
For the correct interpretation of these data, it is important to remember that functional MRI activation always represents a comparison between two activation states. Energy usage by the brain depends largely on firing rate [25], and it has been shown that high-frequency activity in the gamma range correlates with the BOLD signal in the auditory cortex [26, 27]. Thus, increased spontaneous neural activity, such as postulated in tinnitus patients, may imply only a limited increase in activity during stimulation with sound due to a ceiling effect. According to this saturation model, hypoactivation in fMRI has been interpreted as a possible indicator of pathologically increased neuronal spontaneous activity.
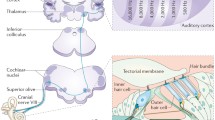
Functional MRI can also provide information about the tonotopic organization of the auditory cortex (Fig. 18.2)
. In individuals with pure-tone tinnitus, fMRI during presentation of a tone with the frequency of the tinnitus has made it possible to determine the anatomical localization of the representation of the tinnitus frequency in the primary and secondary auditory cortices. The fMRI technique has been used for finding the best placement of the stimulating electrode for epidural cortical stimulation [28]. However, there are no fMRI studies available that investigated systematically potential alterations of the tonotopic map in tinnitus patients.
In a recent study, fMRI studies were combined with an emotional paradigm in order to identify tinnitus-related changes in emotional processing (Rosengarth et al., personal communication). The study showed signs of abnormal neural activity in the hippocampus, the parahippocampal gyrus, and the amygdala that were independent from depressive comorbidity. These results provide further experimental evidence for the involvement of limbic brain structures in tinnitus [29, 30].
Summary of the Use of Imaging Methods for Studies of Tinnitus
A summary of the findings of functional imaging studies in tinnitus is given in table 1 and in Fig. 18.3
PET studies have shown which areas of the brain are involved in the tinnitus network: primary [10, 15, 17, 31] and secondary auditory cortex, extending into the temporoparietal junction (=auditory association area) [1, 2, 9, 31]; (para)hippocampus [10]; medial geniculate body [10]; anterior [32] and posterior cingulate cortex [4, 33]; and precuneus and inferior lateral parietal cortex [12]. Voxel-based morphometry adds the subgenual anterior cingulate cortex extending into the nucleus accumbens area [34], and both VBM and fMRI add inferior colliculus [21, 24, 35]. Most of the neural networks activated by tinnitus overlap with brain regions that are involved in attention to and processing of normal sounds include the primary and secondary auditory cortex, parahippocampus, amygdala, as well as the right superior, middle, and inferior dorsolateral prefrontal cortex [33, 36] have the important difference that tinnitus-related activity seems to be predominantly unilateral. More recent studies have shown that other brain areas are activated by aversive sound stimulation and are related to the reward and emotional system, such as nucleus accumbens and insula [37].
New treatment strategies have evolved from fMRI and PET findings of abnormal neuronal activity in the auditory cortex [38, 39]. The results of several pilot studies using low-frequency transcranial magnetic stimulation (see Chap. 88) and direct electrical stimulation of the auditory cortex (see Chap. 90), which have been based on findings in imaging studies, have shown promising results for treatment of some forms of tinnitus. The application of brain stimulation for treatment of tinnitus patients most likely requires guidance by neuroimaging studies. Areas of the auditory cortex that have shown signs of increased metabolic activity have been identified as targets for stimulation. PET scan is now used for guidance of treatment. Preliminary findings suggest that the results of PET scanning may also serve as a predictor of the outcome of treatment [17, 32, 40].
Even data that come from relatively small sample sizes emphasize the value of fMRI, SPECT, and PET for developing new therapeutic strategies, but also show the potential of these procedures to become clinical tools for the diagnostic differentiation between different forms of tinnitus and for the assessment of treatment outcome.
Abbreviations
- FDG-PET:
-
Fluor-deoxy-glucose PET
- fMRI:
-
Functional Magnetic Resonance Imaging
- PET:
-
Positron emission tomography
- rCBF:
-
Regional cerebral blood flow
- SPECT:
-
Single positron emission computed tomography
- [15O]-H2O PET:
-
Positron emission tomography with radioactively labeled water
References
Gardner, A, Pagani, M, Jacobsson, H, Lindberg, G, Larsson, SA, Wagner, A, et al. Differences in resting state regional cerebral blood flow assessed with 99mTc-HMPAO SPECT and brain atlas matching between depressed patients with and without tinnitus. Nucl Med Commun. 2002;23(5):429–39
Shulman, A. A final common pathway for tinnitus – the medial temporal lobe system. Int Tinnitus J. 1995;1(2):115–26
Johnsrude, IS, Giraud, AL, Frackowiak, RS. Functional imaging of the auditory system: the use of positron emission tomography. Audiol Neurootol. 2002;7(5):251–76
Plewnia, C, Reimold, M, Najib, A, Brehm, B, Reischl, G, Plontke, SK, et al. Dose-dependent attenuation of auditory phantom perception (tinnitus) by PET-guided repetitive transcranial magnetic stimulation. Hum Brain Mapp. 2007;28(3):238–46
Reyes, SA, Salvi, RJ, Burkard, RF, Coad, ML, Wack, DS, Galantowicz, PJ, et al. Brain imaging of the effects of lidocaine on tinnitus. Hear Res. 2002;171:43–50
Levine, RA, Abel, M, Cheng, H. CNS somatosensory-auditory interactions elicit or modulate tinnitus. Exp Brain Res. 2003;153(4):643–8
Sanchez, TG, Guerra, GC, Lorenzi, MC, Brandao, AL, Bento, RF. The influence of voluntary muscle contractions upon the onset and modulation of tinnitus. Audiol Neurootol. 2002;7(6):370–5
Cacace, AT, Lovely, TJ, McFarland, DJ, Parnes, SM, Winter, DF. Anomalous cross-modal plasticity following posterior fossa surgery: some speculations on gaze-evoked tinnitus. Hear Res. 1994;81:22–32
Giraud, AL, Chery-Croze, S, Fischer, G, Fischer, C, Vighetto, A, Gregoire, MC, et al. A selective imaging of tinnitus. Neuroreport. 1999;10:1–5
Lockwood, AH, Salvi, RJ, Coad, ML, Towsley, ML, Wack, DS, Murphy, BW. The functional neuroanatomy of tinnitus: evidence for limbic system links and neural plasticity. Neurology. 1998;50:114–20
Lockwood, AH, Wack, DS, Burkard, RF, Coad, ML, Reyes, SA, Arnold, SA, et al. The functional anatomy of gaze-evoked tinnitus and sustained lateral gaze. Neurology. 2001;56:472–80
Mirz, F, Pedersen, B, Ishizu, K, Johannsen, P, Ovesen, T, Stodkilde-Jorgensen, H, et al. Positron emission tomography of cortical centers of tinnitus. Hear Res. 1999;134:133–44
Andersson, G, Lyttkens, L, Hirvela, C, Furmark, T, Tillfors, M, Fredrikson, M. Regional cerebral blood flow during tinnitus: a PET case study with lidocaine and auditory stimulation. Acta Otolaryngol. 2000;120:967–72
Andersson, G, Juris, L, Classon, E, Fredrikson, M, Furmark, T. Consequences of suppressing thoughts about tinnitus and the effects of cognitive distraction on brain activity in tinnitus patients. Audiol Neurootol. 2006;11(5):301–9
Arnold, W, Bartenstein, P, Oestreicher, E, Romer, W, Schwaiger, M. Focal metabolic activation in the predominant left auditory cortex in patients suffering from tinnitus: a PET study with [18F]deoxyglucose. ORL J Otorhinolaryngol Relat Spec. 1996;58:195–9
Wang, H, Tian, J, Yin, D. Positron emission tomography of tinnitus-related brain areas. Zhonghua Er Bi Yan Hou Ke Za Zhi. 2000;35(6):420–4
Langguth, B, Eichhammer, P, Kreutzer, A, Maenner, P, Marienhagen, J, Kleinjung, T, et al. The impact of auditory cortex activity on characterizing and treating patients with chronic tinnitus–first results from a PET study. Acta Otolaryngol Suppl. 2006;(556):84–8
Smith, JA, Mennemeier, M, Bartel, T, Chelette, KC, Kimbrell, T, Triggs, W, et al. Repetitive transcranial magnetic stimulation for tinnitus: a pilot study. Laryngoscope. 2007;117(3):529–34
Eichhammer, P, Hajak, G, Kleinjung, T, Landgrebe, M, Langguth, B. Functional imaging of chronic tinnitus: the use of positron emission tomography. Prog Brain Res. 2007;166:83–8
Yacoub, E, Duong, TQ, Van De Moortele, PF, Lindquist, M, Adriany, G, Kim, SG, et al. Spin-echo fMRI in humans using high spatial resolutions and high magnetic fields. Magn Reson Med. 2003;49(4):655–64
Melcher, JR, Sigalovsky, IS, Guinan, JJ, Jr, Levine, RA. Lateralized tinnitus studied with functional magnetic resonance imaging: abnormal inferior colliculus activation. J Neurophysiol. 2000;83:1058–72
Lanting, CP, De, KE, Bartels, H, Van, DP. Functional imaging of unilateral tinnitus using fMRI. Acta Otolaryngol. 2008;128(4):415–21
Londero, A, Lefaucheur, JP, Malinvaud, D, Brugieres, P, Peignard, P, Nguyen, JP, et al. Magnetic stimulation of the auditory cortex for disabling tinnitus: preliminary results. Presse Med. 2006;35(2 Pt 1):200–6
Smits, M, Kovacs, S, De, RD, Peeters, RR, Van, HP, Sunaert, S. Lateralization of functional magnetic resonance imaging (fMRI) activation in the auditory pathway of patients with lateralized tinnitus. Neuroradiology. 2007;49(8):669–79
Attwell, D, Laughlin, SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21(10):1133–45
Mukamel, R, Gelbard, H, Arieli, A, Hasson, U, Fried, I, Malach, R. Coupling between neuronal firing, field potentials, and FMRI in human auditory cortex. Science. 2005;309(5736):951–4
Nir, Y, Fisch, L, Mukamel, R, Gelbard-Sagiv, H, Arieli, A, Fried, I, et al. Coupling between neuronal firing rate, gamma LFP, and BOLD fMRI is related to interneuronal correlations. Curr Biol. 2007;17(15):1275–85
De Ridder, D, De Mulder, G, Menovsky, T, Sunaert, S, Kovacs, S. Electrical stimulation of auditory and somatosensory cortices for treatment of tinnitus and pain. Prog Brain Res. 2007;166:377–88
Jastreboff, PJ. Phantom auditory perception (tinnitus): mechanisms of generation and perception. Neurosci Res. 1990;8:221–54
Møller, AR. Pathophysiology of tinnitus. Otolaryngol Clin North Am. 2003;36:249–66
Lockwood, AH, Salvi, RJ, Burkard, RF, Galantowicz, PJ, Coad, ML, Wack, DS. Neuroanatomy of tinnitus. Scand Audiol Suppl. 1999;51:47–52
Plewnia, C, Reimold, M, Najib, A, Reischl, G, Plontke, SK, Gerloff, C. Moderate therapeutic efficacy of positron emission tomography-navigated repetitive transcranial magnetic stimulation for chronic tinnitus: a randomised, controlled pilot study. J Neurol Neurosurg Psychiatry. 2007;78(2):152–6
Mirz, F, Gjedde, A, Ishizu, K, Pedersen, CB. Cortical networks subserving the perception of tinnitus–a PET study. Acta Otolaryngol Suppl. 2000;543:241–3
Muhlau, M, Rauschecker, JP, Oestreicher, E, Gaser, C, Rottinger, M, Wohlschlager, AM, et al. Structural brain changes in tinnitus. Cereb Cortex. 2006;16(9):1283–8
Landgrebe M, Langguth B, Rosengarth K, Braun S, Koch A, Kleinjung T, et al. Structural brain changes in tinnitus: Grey matter decrease in auditory and nonauditory brain areas. Neuroimage. 2009;46(1):213–8
Voisin, J, Bidet-Caulet, A, Bertrand, O, Fonlupt, P. Listening in silence activates auditory areas: a functional magnetic resonance imaging study. J Neurosci. 2006;26(1):273–8
Zald, DH, Pardo, JV. The neural correlates of aversive auditory stimulation. Neuroimage. 2002;16:746–53
De Ridder, D, De Mulder, G, Walsh, V, Muggleton, N, Sunaert, S, Møller, A. Magnetic and electrical stimulation of the auditory cortex for intractable tinnitus Case report. J Neurosurg. 2004;100(3):560–4
Langguth, B, Eichhammer, P, Wiegand, R, Marienhegen, J, Maenner, P, Jacob, P, et al. Neuronavigated rTMS in a patient with chronic tinnitus effects of 4 weeks treatment. Neuroreport. 2003;14:977–80
Richter, GT, Mennemeier, M, Bartel, T, Chelette, KC, Kimbrell, T, Triggs, W, et al. Repetitive transcranial magnetic stimulation for tinnitus: a case study. Laryngoscope. 2006;116(10):1867–72
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Langguth, B., De Ridder, D. (2011). Functional Neuroimaging. In: Møller, A.R., Langguth, B., De Ridder, D., Kleinjung, T. (eds) Textbook of Tinnitus. Springer, New York, NY. https://doi.org/10.1007/978-1-60761-145-5_18
Download citation
DOI: https://doi.org/10.1007/978-1-60761-145-5_18
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-60761-144-8
Online ISBN: 978-1-60761-145-5
eBook Packages: MedicineMedicine (R0)