Abstract
Rheumatoid arthritis is an autoimmune disease that causes pain, swelling, and deformity of the joints and may result in other systemic effects. In animal models, vitamin D has been shown to suppress autoimmunity; however, in humans the role of vitamin D is less clear as to the effects on rheumatoid arthritis. There do appear to be suppressive effects of specific disease mechanisms; however, epidemiologic results on the effect of vitamin D on the development of the disease are contradictory. A better understanding of the rheumatoid arthritis immune system and the effects of vitamin D is needed before conclusions can be drawn.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Key Words
1 Introduction
Rheumatoid arthritis is an autoimmune disease, a disease in which the body identifies “self” as being foreign and initiates an attack on itself. Rheumatoid arthritis is characterized by chronic, painful symmetrical joint inflammation, joint stiffness, synovial swelling, and deformed joints due to bone and cartilage destruction. The disease may affect other organs and tissues in the body, such as the eyes and lungs. Rheumatoid arthritis is a disease which has existed for thousands of years (1). The prevalence of rheumatoid arthritis among the general population is slightly less than 1% (2). It is a complex disorder in which both genetic and environmental factors contribute. Rheumatoid arthritis is more common in women, most often develops between the ages of 30–50, and increases in prevalence with increasing age in both men and women. Potential factors that have been identified as contributing to rheumatoid arthritis include smoking, hormones (oral contraception use, pregnancy, lactation, hormone replacement therapy), and viruses (3–5). The disease is characterized by periods of remission and flares. Additionally, rheumatoid arthritis has been associated with an increased mortality (6).
2 Rheumatoid Arthritis Disease Mechanism
The normal immune response is regulated so as not to perpetuate a reaction once a foreign pathogen has been contained, as well as to have a “memory” for a pathogen and a recognition of “self.” However, in rheumatoid arthritis, as with other autoimmune diseases, the immune system is dysfunctional and continues the assault on the body’s tissues. In the case of rheumatoid arthritis, the assault is concentrated in the synovium of joints. What initiates and perpetuates the inflammation in rheumatoid arthritis and other autoimmune diseases is very complex and not completely understood. However, studying other autoimmune and inflammatory diseases as well as the constituents of synovial tissue in established disease has provided insight into the disease mechanism of rheumatoid arthritis.
2.1 Genetic Involvement
As a disease that has both genetic and environmental contributions, one genetic area that has been identified as contributing to rheumatoid arthritis is the major histocompatibility complex (MHC) on chromosome 6. The MHC contains the human leukocyte antigen (HLA) genes that have a role in antigen presentation in rheumatoid arthritis. These genes have a function in the immune system for the recognition of “self” (7). One of the antigen-presenting cells (APCs) expressing the MHC molecule is the dendritic cell. Maturation of the dendritic cell has been shown to be inhibited by 1,25-dihydroxyvitamin D3[1,25(OH)2D] (8, 9). The genes for the vitamin D receptor (VDR), which is found in the cells of the immune system, are located on chromosome 12 and have been associated with rheumatoid arthritis and bone loss (10), osteoporosis (11), and disease severity (12). The VDR is activated by 1,25(OH)2D and acts with other factors to alter the rate of transcription in genes (8).
2.2 Rheumatoid Arthritis Immune System Involvement
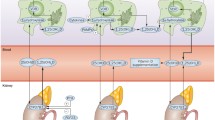
Rheumatoid arthritis involves a very complex mechanism that is not completely understood, although it has been theorized that innate immune mechanisms may initiate the disease which then proceeds to acquire adaptive immunity mechanisms that may differ at each phase of the disease process (7). Promonocytes are cells made in bone marrow, released into the blood as monocytes, and differentiated into macrophages or dendritic cells and are associated with innate immunity. Macrophages can be concentrated in synovial tissue and osteoclasts and can mature into dendritic cells. Lymphocyte (B and T cells) cells are produced in bone marrow. B cells produce antibodies and cells that differentiate along this line are considered a TH2 (T helper type 2) immune response subtype. The T cells, on the other hand, get sensitized in the thymus and upon presentation of antigens differentiate into helper, regulating, or suppressor cells, depending on the antigen. These are non-antibody producing and are associated with acquired immunity. Cells that differentiate along this line are considered a TH1 (T helper type 1) immune response subtype. Rheumatoid arthritis is a cell-mediated disease because of the predominating effects of the TH1 immune response.
Once the disease has been initiated in response to a pathogen, macrophages and lymphocytes infiltrate the synovial tissue and produce pro-inflammatory cytokines and chemokines which regulate the disease process that ultimately results in joint damage (13). There are many regulatory factors and mediators involved in the disease mechanism that may activate or suppress multiple cellular structures (14). The inflammatory response and cytokine milieu has been an area of intense interest that has resulted in treatments for the disease and helped lead to a better understanding of the disease process.
T-cell immune responses have classically been described as differentiated into the TH1 immune response subtype (cell-mediated immunity) or the TH2 subtype (antibody-mediated immunity). Differentiation into the TH1 subtype, generally associated with rheumatoid arthritis, is regulated by the interaction of naïve CD4+ T cells and antigen-presenting cells (dendritic or macrophage) that express class II MHC molecules in the presence of co-stimulatory molecules that stimulate macrophages, synovial fibroblasts, and monocytes to produce cytokines (15). The TH1 subtype results in the production of the cytokines, IL-2, tumor necrosis factor α (TNFα), and interferon-γ (INF-γ) (16). IL-12 produced by activated antigen-presenting cells is a necessary cytokine for the differentiation into the TH1 subtype, whereas IL-4 is necessary for differentiation into the TH2 subtype (17). It has been theorized that TH1 cytokines prevent naïve T cells from differentiating into TH2 cells and thus prevent the release of cytokines that suppress the inflammatory response (such as IL-4, IL-6, IL-10) (7).
More recently, another immune response subtype, classified as TH17, has been identified which produces inflammatory responses and cytokines more consistent with that seen in rheumatoid arthritis (18). This subtype also has considerable overlap with the TH1 subtype (19). The cytokines IL-1β, IL-6, and IL-23 promote T-cell differentiation into the TH17 subtype response. This new line of immune subtype response is important because it has been found to be involved in the differentiation of cells and thus cytokines that promote excessive osteoclast activity and helps explain the lack of INF-γ and IL-2 in synovial joints (20). Osteoclast activity is inhibited by INF-γ and enhanced by IL-17, which is dependent on TH17 helper cells producing IL-17. Since INF-γ is produced with the TH1 subtype, the inhibition of osteoclast activity is not consistent with that subtype (21). Osteoclastogenesis is a normal function of bone resorption. However, the excessive osteoclastogenesis associated with rheumatoid arthritis results in the bone destruction, which is a hallmark of the disease. In addition to the above subtypes, naïve T cells can also differentiate into regulatory T cells (Treg) that regulate the helper T cells and can have a role in immune tolerance (16).
3 Vitamin D And Rheumatoid Arthritis
3.1 Background
The effects vitamin D might exert in rheumatoid arthritis disease pathogenesis were recognized in the early 1940s when it was noticed that patients with rheumatoid arthritis were prone to an increased susceptibility to bone fractures (22). Vitamin D was also used as a treatment for rheumatoid arthritis (23). In 1974, a vitamin D deficiency in postmenopausal women with rheumatoid arthritis who had suffered fractures compared with postmenopausal women with rheumatoid arthritis who had not suffered fractures was reported (24). The disparity was attributed to a deficiency in the dietary consumption of vitamin D as well as a lack of exposure to sun since the patients were housebound due to their disease. Other studies showing similar effects were also reported (25). The use of corticosteroids in the treatment of rheumatoid arthritis was also known to contribute to corticosteroid-induced osteoporosis (26), with vitamin D often prescribed to prevent this side effect. The foundation for the relationship of vitamin D with rheumatoid arthritis was the recognition that many people with rheumatoid arthritis had problems with their bone mineralization. In addition to these studies associated with rheumatoid arthritis, studies of the effects of vitamin D on other diseases affected by immune system problems such as tuberculosis, sarcoidosis, multiple sclerosis, psoriasis, and cancer were being reported (27, 28 ).
3.1.1 Immune System Effects of Vitamin D and Rheumatoid Arthritis
Rheumatoid arthritis is a disease in which the immune system is aberrant. The finding that rheumatoid arthritis patients had more receptors for 1,25(OH2)D in circulating lymphocytes compared to controls established a link between the disease and vitamin D (29). The discovery of receptors for 1,25(OH2)D in thymus and lymphocyte tissue (30) and in activated lymphocytes (31) provided further support for the idea that the immune system had a role in the production of 1,25(OH2)D and that vitamin D was not just a regulator of bone mineralization and calcium homeostasis.
Using the knowledge that vitamin D had a function with the immune system and that rheumatoid arthritis resulted in bone and cartilage destruction, researchers looked for vitamin D metabolites in the synovium in order to try and understand the function of vitamin D in the pathogenesis of the disease. The fact that measureable amounts of the vitamin D metabolites 24(OH)D and 24,25-(OH)2D were obtainable from synovial fluid and were also found in serum from patients with rheumatoid arthritis as well as other rheumatic conditions (32) provided a starting point for assessing the vitamin D–synovium relationship.
The synthesis of the active form, 1,25(OH2)D, in the synovial fluid of subjects with inflammatory arthritis compared to those with non-inflammatory arthritis suggested that macrophages appeared to be the source of production and thus played a role in the inflammatory arthritis (33, 34). Since macrophages are found in abundance in synovial tissue, these findings were significant in helping to try and determine the role of vitamin D in the pathogenesis of rheumatoid arthritis. The finding that the enzyme 25-OHD3-lα-hydroxylase was expressed in a diverse distribution of tissue implied a role for peripheral synthesis of vitamin D and likely modulation of the immune system (35).
Sophisticated tools were developed that allowed researchers to advance their understanding of the immune system at the molecular level. Additionally, concurrent research conducted in other specialized fields provided insight into the mechanisms that might be involved in the immune system and the actions of vitamin D. Some of this research showed that an infiltration of T cells and macrophages, commonly found in synovial tissue of rheumatoid arthritis patients, produced cytokines that were involved in the disease process. The association of vitamin D levels in synovial tissue with cytokine production has also been studied with the report of significantly increased levels of vitamin D metabolites in rheumatoid arthritis patients compared to osteoarthritis patients. This finding strongly suggested an extra-renal synthesis of vitamin D in synovial tissues which was stimulated by the pro-inflammatory cytokines IL-1 and/or IL-2 (36). IL-1 is also one of the macrophage-produced cytokines involved in the rheumatoid arthritis disease process.
In the immune system, vitamin D stimulates macrophages and suppresses T lymphocytes (37). Vitamin D also downregulates the expression of MCH class II groups, thus proliferation of T cells is decreased (38–42). In addition to vitamin D effects on several cytokines, one action of T-cell suppression occurs due to the inhibitory effects on IL-12 and IL-2 and INF production which suppress the TH1 subtype (43, 44). Vitamin D, through effects on naïve T cells, also promotes TH2 development (i.e., the subtype that suppresses the rheumatoid arthritis inflammatory process) (45–53).
3.2 Joint Destruction in Rheumatoid Arthritis
With the immune actions of vitamin D in mind, studying the effects of vitamin D, cytokines, and bone metabolism associated with rheumatoid arthritis was undertaken. Postmenopausal women were divided into varying levels of disease severity. The results showed that in women with high disease activity, low serum levels of 1,25(OH2)D were inversely related to markers of T-cell activation, but this same marker had a positive correlation with disease activity. Thus the researchers speculated that the increased binding of 1,25(OH)2D by the vitamin D receptor on activated T cells reduced serum levels of 1,25(OH)2D. This study also found that the pro-inflammatory cytokine IL-6 was the main determinant of increased bone resorption in postmenopausal women, thus contributing to osteoporosis (54). IL-6 was also the cytokine cited as being involved in synovial joint destruction (55).
Bones undergo a remodeling process in which osteoblasts form new bone tissue and osteoclasts resorp old bone tissue. Bone remodeling usually occurs in a balanced manner. The destruction of bone and cartilage in the joints of patients with rheumatoid arthritis has been the focus of much research in trying to understand the disease and its process. Macrophages in the synovial joints may differentiate into dendritic cells or osteoclasts. Excessive osteoclast activity (osteoclastogenesis) results in the bone destruction, which is a hallmark of rheumatoid arthritis. Osteoclast activity is dependent on TH17 helper cells producing IL17 (21). In the rheumatoid synovial joint, in the presence of excessive levels of cytokines, osteoclast precursors differentiate into mature osteoclasts, while they are also activated to produce cytokines that amplify the inflammatory response (56). The cytokine receptor activator necessary for osteoclast differentiation (RANKL) is expressed on activated T cells. The pro-inflammatory cytokines associated with rheumatoid arthritis can induce RANKL, thus initiating osteoclastogenesis (57). 1,25(OH2)D along with the vitamin D receptor, is also a critical component for osteoclastogenesis by stimulating RANKL which promotes osteoblast maturation to osteoclasts and inhibits a decoy receptor for RANKL – osteopoteregin – which suppresses osteoclastogenesis (45). It is not too ill conceived, therefore, that an action of 1,25(OH2)D is a possible modulator of the disease mechanism involved in the TH17 immune response subtype in rheumatoid arthritis. However, future research will have to elucidate that role.
3.3 Clinical, Animal, and Epidemiologic Studies of Vitamin D and Rheumatoid Arthritis
The remissions and flares associated with rheumatoid arthritis have also been studied in relation to further the understanding of the disease process. Women with rheumatoid arthritis have reported remission of their symptoms during pregnancy which often flare postpartum. An analysis of various cytokines and modulators of the immune system was performed and found that cytokines and substances that suppress immune function (IL-12, cortisol and 1,25-dihydroxyvitamin D3) were higher in the third trimester of pregnancy compared to postpartum and TNFα (an immune function stimulator) was lower in the third trimester and rebounded postpartum (58).
Since studies of other autoimmune conditions have shown a latitude effect with prevalence, and the fact that vitamin D levels fluctuate with seasons, researchers looked at these issues as well as the relationship with rheumatoid arthritis severity. Latitude and seasonal effects were observed in both winter and summer with the northern latitude having lower serum levels of 25(OH)D in rheumatoid arthritis patients compared to controls. Additionally, both patients and controls had lower levels in the winter compared to the summer. However, mixed effects with regard to disease activity as measured by a rating instrument were observed in this same study (59). A prospective cohort study that followed over 83,000 nurses for 28 years reported on the geographic variation and migration patterns of subjects with new on-set rheumatoid arthritis. Place of residence at birth, age 15, and age 30 was assessed. Subjects living in the Western United States at all time periods had the lowest risk for rheumatoid arthritis and those in the Midwest and Northeast had the highest. One possible explanation was UV exposure, which stimulates the production of vitamin D (60).
In a study using c-reactive protein levels as the measure of disease severity, the c-reactive protein levels were worse for subjects with low levels of 1,25(OH2)D compared to those with higher levels, whereas no relationship was seen for 25(OH)D. This was true irrespective of whether subjects were taking corticosteroids or were not taking corticosteroids (61). Controlling for disease treatment and duration, similar results were obtained in another study looking at vitamin D metabolism. This same study found a seasonal difference in serum vitamin D levels (62). Patients with recently diagnosed rheumatoid arthritis were also assessed for serum levels of 25(OH)D and 1,25(OH)2D and early disease activity. Mean levels of both vitamin D metabolites were lower for patients satisfying American College of Rheumatology criteria for rheumatoid arthritis. Patients deficient in 25(OH)D at baseline had worse disease activity and severity at baseline and 1 year follow-up. 25(OH)D showed a stronger relationship than did 1,25(OH)2D for disease activity and severity (63).
Rodent models have produced supporting evidence showing the suppressive inflammatory effects of vitamin D in relation to rheumatoid arthritis. Cantorna, who had studied the effects of the administration of vitamin D analogs in mice with experimental autoimmune encephalomyelitis (a multiple sclerosis-like disease), extended her studies to mice with collagen-induced arthritis (an inflammatory disease with similarities to rheumatoid arthritis). The incidence of the arthritis in the mice treated with vitamin D was reduced by 50% and those who did get arthritis had milder symptoms (64). Similarly, in a study by Larsson, Mattsson et al. (65) they administered a vitamin D analog at various time points to rats that were immunized to induce collagen arthritis. Collagen-induced arthritis was inhibited in rats that were injected prior to or around the time of immunization and in those that were administered the vitamin D analog after immunization had a reduction in the severity of their disease. This study suggested that the timing of exposure of vitamin D relative to the pathogen exposure was important on the outcome. The animal studies further confirmed the suppressive effects of vitamin D on the immune system.
Recognizing the benefits of vitamin D not only for bone mineralization but also for suppression of immune activity, vitamin D has also been studied as a treatment for rheumatoid arthritis. Administration of alpha calcidiol for 3 months resulted in an improvement of disease activity in 89% of patients (66). Another assessment of the nutritional status of patients with “general rheumatology” compared to patients with osteoarthritis showed lower serum levels of 25(OH)D, suggesting that new guidelines and greater attention to the assessment of nutritional status of rheumatology patients with regard to vitamin D need to be enacted (67). Based on the lack of rheumatoid arthritis prevalence in sub-Saharan Africans, McCarty advocated a vegan diet rich in omega-3-rich fish, along with supplementation with vitamin D as a prevention strategy for rheumatoid arthritis (68). Similar to the suggestions of the previous findings, a review article on the topic suggested that a more widely advocated assessment of vitamin D status and treatment in the clinical setting was warranted, given the weight of evidence of low vitamin D status in rheumatoid arthritis patients and the potential benefits of suppressing the immune response (69).
There are very few epidemiologic studies that have assessed the association between vitamin D and rheumatoid arthritis. Two prospective cohort studies that have been published have produced contradictory results. Both of these studies were based on self-report of dietary intake of vitamin D. The Iowa Women’s Health Study had 11 years of follow-up of 29, 368 postmenopausal women who were over the age of 55 at baseline and developed documented rheumatoid arthritis after baseline. This study found a protective association with increasing levels of dietary/supplemental vitamin D intake but did not include any measures of sun exposure (70). The Nurses’ Health Study had 22 years of follow-up of 186,389 women who were aged 30–55 at baseline and developed documented rheumatoid arthritis after baseline. This study did not find any effect of dietary/supplemental vitamin D intake and did include a proxy measure for sun exposure early in life (71). A third study from Amsterdam that measured serum levels of 25(OH)D in blood samples of 79 patients and matched controls collected prior to onset of rheumatoid arthritis looked at the association of serum levels of vitamin D and newly diagnosed cases of rheumatoid arthritis. They looked at three timeframes prior to onset and found no association with the development of rheumatoid arthritis when compared to matched controls (72). Studies on the relationship of vitamin D in the etiology of rheumatoid arthritis are contradictory and need further investigation.
Vitamin D has a role in specific mechanisms of the immune system of rheumatoid arthritis patients. However, a comprehensive understanding of the individual mechanisms on the complete activation of the immune system is not clear. There are multiple possible areas of action in which vitamin D may impact on the pathogenesis of rheumatoid arthritis, especially given the extensive effects of this hormone. Vitamin D has been shown to suppress T-cell activity through several actions and to modulate cytokines that can alter the course of the disease. Whether the effects of vitamin D are limited to disease severity/progression or have a role in the etiology is not completely clear. Ongoing research is leading to a better understanding of the immune system with regard to rheumatoid arthritis. The knowledge gained will provide better information, in the future, on the role of vitamin D in the pathogenesis of rheumatoid arthritis.
References
Rothschild B, Woods R, Rothschild C et al (1992) Geographic distribution of rheumatoid arthritis in ancient North America: implications for pathogenesis. Semin Arthritis Rheum 22:181–187
Helmick C, Felson D, Lawrence R et al (2008) Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum 58:15–25
Hernandez-Avila M, Liang M, Willett W et al (1990) Reproductive factors, smoking, and the risk for rheumatoid arthritis. Epidemiol 1:285–291
Silman A, Newman J, MacGregor A (1996) Cigarette smoking increases the risk of rheumatoid arthritis. Results from a nationwide study of disease-discordant twins [see comments]. Arthritis Rheum 39:732–735
Criswell L, Merlino L, Cerhan J et al (2002) Cigarette smoking and the risk of rheumatoid arthritis among postmenopausal women: results from the Iowa Women’s Health Study. Am J Med 112:465–471
Mikuls T, Saag K, Criswell L et al (2002) Mortality risk associated with rheumatoid arthritis in a prospective cohort of older women: results from the Iowa Women’s Health Study. Ann Rheum Dis 61:994–999
Harris E, Budd R, Genovese M, Firestein G, Sargent J, Ruddy S, Sledge C (2005) Kelley’s textbook of rheumatology, 7th edn. Saunders, Philadelphia
Griffin M, Xing N, Kumar R (2003) Vitamin D and its analogs as regulators of immune activation and antigen presentation. Annu Rev Nutr 23:117–145
Piemonti L, Monti P, Sironi M et al (2000) Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol 164:4443–4451
Gough A, Sambrook P, Devlin J et al (1998) Effect of vitamin D receptor gene alleles on bone loss in early rheumatoid arthritis. J Rheumatol 25:864–868
Rass P, Pakozdi A, Lakatos P et al (2006) Vitamin D receptor gene polymorphism in rheumatoid arthritis and associated osteoporosis. Rheumatol Int 26:964–971
Gomez-Vaquero C, Fiter J, Enjuanes A et al (2007) Influence of the BsmI polymorphism of the vitamin D receptor gene on rheumatoid arthritis clinical activity. J Rheumatol 34:1823–1826
Firestein G (2007) Evolving concepts of rheumatoid arthritis. Nature 423:356–361
Choy E, Panayi G (2001) Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med 344:907–916
Feldmann M, Maini S (2008) Role of cytokines in rheumatoid arthritis: an education in pathophysiology and therapeutics. Immunol Rev 223:7–19
Boissier M, Assier E, Falgarone G et al (2008) Shifting the imbalance from Th1/Th2 to Th17/treg: the changing rheumatoid arthritis paradigm. Joint Bone Spine 75:373–375
Park H, Li Z, Yang XO et al (2005) A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 6:1133–1141
McGeachy M, Cua D (2008) Th17 cell differentiation: the long and winding road. Immunity 28:445–453
Harrington L, Hatton R, Mangan P et al (2005) Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 6:1123–1132
Sato K (2008) Th17 cells and rheumatoid arthritis -from the standpoint of osteoclast differentiation. Allergol Int 57:109–114
Nakashima T, Takayanagi H (2008) The dynamic interplay between osteoclasts and the immune system. Arch Biochem Biophys 473:166–171
Baer GJ (1941) Fractures in chronic arthritis. Ann Rheum Dis 2:269–273
Addis H, Currie R (1950) Hypercalcaemia during vitamin D treatment of rheumatoid arthritis. Br Med J 1:877–879
Maddison P, Bacon P (1974) Vitamin D deficiency, spontaneous fractures, and osteopenia in rheumatoid arthritis. Br Med J 4:433–435
O’Driscoll S, O’Driscoll M (1980) Osteomalacia in rheumatoid arthritis. Ann Rheum Dis 39:1–6
Demartini F, Grokoest A, Ragan C (1952) Pathological fractures in patients with rheumatoid arthritis treated with cortisone. J Am Med Assoc 149:750–752
Hayes C, Cantorna M, DeLuca H (1997) Vitamin D and multiple sclerosis. Proc Soc Exp Biol Med 216:21–27
Garland C, Garland F, Gorham E (1999) Calcium and vitamin D. Their potential roles in colon and breast cancer prevention. Ann NY Acad Sci 889:107–119
Manolagas S, Werntz D, Tsoukas C et al (1986) 1,25-Dihydroxyvitamin D3 receptors in lymphocytes from patients with rheumatoid arthritis. J Lab Clin Med 108:596–600
Provvedini D, Rulot C, Sobol R et al (1987) 1 alpha,25-Dihydroxyvitamin D3 receptors in human thymic and tonsillar lymphocytes. J Bone Miner Res 2:239–247
Bhalla A, Amento E, Serog B et al (1984) 1,25-Dihydroxyvitamin D3 inhibits antigen-induced T cell activation. J Immunol 133:1748–1754
Fairney A, Straffen A, May C et al (1987) Vitamin D metabolites in synovial fluid. Ann Rheum Dis 46:370–374
Hayes M, Denton J, Freemont A et al (1989) Synthesis of the active metabolite of vitamin D, 1,25(OH)2D3, by synovial fluid macrophages in arthritic diseases. Ann Rheum Dis 48:723–729
Smith S, Hayes M, Selby P et al (1999) Autocrine control of vitamin D metabolism in synovial cells from arthritic patients. Ann Rheum Dis 58:372–378
Zehnder D, Bland R, Williams M et al (2001) Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab 86:888–894
Inaba M, Yukioka K, Furumitsu Y et al (1997) Positive correlation between levels of IL-1 or IL-2 and 1,25(OH)2D/25-OH-D ratio in synovial fluid of patients with rheumatoid arthritis. Life Sci 61:977–985
Lemire J (1992) Immunomodulatory role of 1,25-dihydroxyvitamin D3. J Cell Biochem 49:26–31
Tsoukas C, Watry D, Escobar S et al (1989) Inhibition of interleukin-1 production by 1,25-dihydroxyvitamin D3. J Clin Endocrinol Metab 69:127–133
Tokuda N, Mizuki N (1992) 1,25 Dihydroxyvitamin D3 down regulation of HLA-DR on human peripheral blood monocytes. Immunology 75:349–354
Rigby W, Waugh M (1992) Decreased accessory cell function and co-stimulatory activity by 1,25 dihydroxyvitamin D3 treated monocytes. Arthritis Rheum 35:110–119
Tokuda N, Kano M, Meiri H et al (2000) Calcitriol therapy modulates the cellular immune responses in hemodialysis patients. Am Nephrol 20:129–137
Clavreul A, D’Hellencourt C, Montero-Menei C et al (1998) Vitamin D differentially regulates B7.1 and B7.2 expression on human peripheral blood monocytes. Immunology 95:272–277
Manetti R, Parronchi P, Guidizi M (1993) Natural killer cell stimulations factor [IL-12] induces T helper type 1 (TH1) specific immune responses and inhibits the development of IL-4 producing Th cells. J Exp Med 177:1199–1204
Mattner F, Smiroldo S, Galbiati F et al (2000) Inhibition of Th1 development and treatment of chronic-relapsing experimental allergic encephalomyelitis by a non-hypercalcemic analogue of 1,25-dihydroxyvitamin D(3). Eur J Immunol 30:498–508
Dusso A, Brown A, Slatopolsky E (2005) Vitamin D. Am J Physiol Renal Physiol 289:F8–F28
Reichel H, Koeffler H, Tobler A (1987) 1a,25 dihydroxyvitamin D3 inhibits gamma interferon synthesis by normal human peripheral blood lymphocytes. Proc Natl Acad Sci USA 84:3385–3389
Gepner P, Amor B, Fournier C (1989) 1,25-dihydroxyvitamin D3 potentiates the in vitro inhibitory effects of cyclosporin A on T cells from rheumatoid arthritis patients. Arthritis Rheum 32:31–36
Rigby W, Hamilton B, Waugh M (1990) 1,25 Dihydroxyvitamin D3 modulates the effects of interleukin 2 independent of IL2 receptor binding. Cell Immunol 125:396–414
al Janaki M, al-Balla S, al-Dalaan A, et al (1993) Cytokine profile in systemic lupus erythematosus, rheumatoid arthritis, and other rheumatic diseases. J Clin Immunol 13:58–66
Muller K, Odun N, Bendtzen K (1993) 1,25 Dihydroxyvitamin D3 selectively reduces interleukin 2 levels and proliferation of human T cell lines in vitro. Immunology 35(Lett.):177–178
Spiegelberg J, Beck L, Stevenson D (1994) Recognition of T cell epitopes and lymphokine secretion by rye grass allergen - specific human T cell clones. J Immunol 152:4706–4711
Jirapongsananuruk O, Melamed I, Leung DY (2000) Additive immunosuppressive effects of 1,25-dihydroxyvitamin D3 and corticosteroids on TH1, but not TH2, responses. J Allergy Clin Immunol 106:981–985
Rausch-Fan X, Leutmezer F, Willheim M et al (2002) Regulation of cytokine production in human peripheral blood mononuclear cells and allergen-specific Th cell clones by 1alpha,25-dihydroxyvitamin D3. Int Arch Allergy Immunol 128:33–41
Oelzner P, Franke S, Muller A et al (1999) Relationship between soluble markers of immune activation and bone turnover in post-menopausal women with rheumatoid arthritis. Rheumatology 38:841–847
van Leeuwen M, Westra J, Limburg P et al (1995) Interleukin-6 in relation to other proinflammatory cytokines, chemotactic activity and neutrophil activation in rheumatoid synovial fluid. Ann Rheum Dis 54:33–38
Boyce B, Schwarz E, Xing L (2006) Osteoclast precursors: cytokine-stimulated immunomodulators of inflammatory bone disease. Curr Opin Rheumatol 18:427–432
Sato K, Takayanagi H (2006) Osteoclasts, rheumatoid arthritis and osteoimmunology. Curr Opin Rheumatol 18:419–426
Elenkov I, Wilder R, Bakalov V et al (2001) IL-12, TNF-alpha, and hormonal changes during late pregnancy and early postpartum: implications for autoimmune disease activity during these times. J Clin Endocrinol Metab 86:4933–4938
Cutolo M, Otsa K, Laas K et al (2006) Circannual vitamin d serum levels and disease activity in rheumatoid arthritis: Northern versus Southern Europe. Clin Exp Rheumatol 24:702–704
Costenbader K, Chang S, Laden F et al (2008) Geographic variation in rheumatoid arthritis incidence among women in the United States. Arch Intern Med 168:1664–1670
Oelzner P, Muller A, Deschner F et al (1998) Relationship between disease activity and serum levels of vitamin D metabolites and PTH in rheumatoid arthritis. Calcif Tissue Int 62:193–198
Kroger H, Penttila I, Alhava E (1993) Low serum vitamin D metabolites in women with rheumatoid arthritis. Scan J Rheumatol 22:172–177
Patel S, Farragher T, Berry J et al (2007) Association between serum vitamin D metabolite levels and disease activity in patients with early inflammatory polyarthritis. Arthritis Rheum 56:2143–2149
Cantorna M, Munsick C, Bermiss C et al (2000) 1,25 Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr 130:2648–2652
Larsson P, Mattsson L, Klareskog L et al (1998) A vitamin D analogue (MC 1288) has immunomodulatory properties and suppresses collagen-induced arthritis (CIA) without causing hypercalcaemia. Clin Exp Immunol 114:277–283
Andjelkovic Z, Vojinovic J, Pejnovic N et al (1999) Disease modifying and immunomodulatory effects of high dose 1 alpha (OH) D3 in rheumatoid arthritis patients. Clin Exp Rheum 17:453–456
Mouyis M, Ostor A, Crisp A et al (2008) Hypovitaminosis D among rheumatology outpatients in clinical practice. Rheumatology 47:1348–1351
McCarty M (2001) Upregulation of lymphocyte apoptosis as a strategy for preventing and treating autoimmune disorders: a role for whole-food vegan diets, fish oil and dopamine agonists. Med Hypotheses 57:258–275
Leventis P, Patel S (2008) Clinical aspects of vitamin D in the management of rheumatoid arthritis. Rheumatology 47:1617–1621
Merlino L, Curtis J, Mikuls T et al (2004) Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women’s Health Study. Arthritis Rheum 50:72–77
Costenbader K, Feskanich D, Holmes M et al (2008) Vitamin D intake and risks of systemic lupus erythematosus and rheumatoid arthritis in women. Ann Rheum Dis 67:530–553
Nielen M, van Schaardenburg D, Lems W et al (2006) Vitamin D deficiency does not increase the risk of rheumatoid arthritis: comment on the article by Merlino et al. Arthritis Rheum 54:3719–3720
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Merlino, L.A. (2010). Role of Vitamin D in Rheumatoid Arthritis. In: Holick, M. (eds) Vitamin D. Nutrition and Health. Humana Press. https://doi.org/10.1007/978-1-60327-303-9_54
Download citation
DOI: https://doi.org/10.1007/978-1-60327-303-9_54
Published:
Publisher Name: Humana Press
Print ISBN: 978-1-60327-300-8
Online ISBN: 978-1-60327-303-9
eBook Packages: MedicineMedicine (R0)