Abstract
The central nervous system (CNS) can act as a compartment in which HIV can replicate independently from plasma. HIV infection of the brain and the ongoing immune response leads to neurocognitive impairment. Therefore, besides optimally suppressing HIV viral load in the plasma, it is essential to reach adequate concentrations of antiretrovirals (ARVs) in the CNS to prevent and improve neurocognitive impairments. ARVs have limitations in their ability to enter the brain due the blood-brain barrier (BBB) and different efficient efflux mechanisms. Moreover, ARVs exposed to the CNS show variability and distinctiveness of their characteristics including molecular weight, lipophilicity, protein binding, and on their capacity as substrates for efflux transporters.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara Core MessageIn the era of combination antiretroviral therapy (cART), advanced neurologic complications of HIV infection are less common, than pre-cART. There is evidence that antiretroviral therapy prevents, delays, and may reverse neurocognitive complications of HIV infection. However, clinical trials leading to antiretroviral drug approval primarily measure HIV in the plasma as an indicator of therapy efficacy. In this chapter we assemble and present data on CNS exposure and penetration of antiretroviral drugs.
17.1 Introduction
Prior to the introduction of combination antiretroviral therapy (cART), neurologic complications of HIV infection were common, termed overall HIV-associated neurocognitive disorders (HAND) . HAND ranges from asymptomatic neurocognitive impairment (ANI) , to a mild neurocognitive disorder (MND) , to full-blown HIV-associated dementia (HAD) and often exhibited as HIV encephalitis (HIVE) [1, 2]. The incidence of HAND decreased over time due to the use of cART. Especially, HAD is now less common, and it is rare in patients who are clinically stable on cART. The incidence of HAD per 1000 person-years reduced from 6.49 in 1997 to 0.66 during the years 2003–2006; this was associated with the introduction of highly active antiretroviral therapy (HAART) or cART, as mentioned [3]. HIV-1 invades the CNS early and can cause persistent infection and inflammation [4]. HAND shows decreased association with immune activation and has a more diffuse range of neuropsychological deficits that may overlap other brain diseases and, at times, with continued association with suppressed virus loads [4, 5]. Moreover, despite the advent of cART, an overall neurologic impairment is still prevalent, in some studies; for those patients on cART, it occurs greater than 80%, especially in its milder form. Reduction of HIV viral load in the CSF alone perhaps is not the only clinical indicator of treatment efficacy for HAND [4, 6,7,8,9]. For example, there was a lack of association between neurocognitive impairment with virologic and immunological factors that indicates ongoing neural injury regardless of the success of antiretroviral therapy based on these laboratory measures [8]. In addition to HIV load levels, several studies suggest other factors including immunological, aging, persistent HIV replication in the CNS including macrophages, evolution of highly neurovirulent HIV strains, and the long-term neurotoxicity of cART [9, 10].
In this chapter, we will discuss further the effects of antiretroviral therapy on HAND and the interaction between ARVs and the brain. Most data, when indicated, were studied in HIV-1 infection.
17.2 The CNS
17.2.1 The CNS Barriers
The CNS is surrounded by the BBB and the blood-cerebrospinal fluid barrier (BCB) . These barriers prevent most molecules from entering the CNS and maintain a stable environment. The BBB inhibits the free diffusion of water-soluble molecules by complex tight junctions that interconnect endothelial cells within CNS microvessels. They lack intercellular pores and have low pinocytosis activity. The endothelial cells and pericytes are enclosed by basement membranes and are almost completely surrounded by astrocyte foot processes [11]. A functional BBB has numerous active transport systems , specifically expressed by brain capillary endothelial cells to ensure the transport of nutrients into the CNS and block potentially offending molecules from CNS entry. Moreover, BBB is the passage for HIV, viral products, infected cells, and activated immune cells to penetrate to the CNS [12]. On the other hand, the BCB is formed by choroid plexus epithelial cells and found at the apical tight junctions between the choroid plexus epithelial cells. The BCB inhibits paracellular diffusion of water-soluble molecules across this barrier. In addition, it has a secretory function and produces CSF. With CNS pathology, these barrier characteristics are disrupted, leading to edema and inflammation entry into the CNS [11]. Molecular diffusion and exchange can occur in both directions and additionally via the perivascular space as well as the CSF [13].
HIV is neurotropic, as indicated by neurocognitive impairment with HIV infection of the brain, on the one hand, and by decreasing neurocognitive impairment in patients with viral suppressions from cART, on the other hand. Moreover, HIV brain invasion results in neuronal loss, synaptic and dendritic damage, astrogliosis, microgliosis, and multinucleated giant cell formation [14, 15]. HIV infection of the CNS occurs by cell-free and HIV-infected cells that migrate from peripheral blood into the CNS. Cell-free HIV particles pass through the BBB using mannose-5-phosphate receptor and tight junction dissolution [16]. Moreover, HIV proteins and HIV infection activate T cells and monocytes resulting in immune cell trafficking across the BBB. Gp120 protein is a potent neurotoxin. Circulating gp120 increases BBB permeability by downregulating tight junction proteins [17]. Tat protein itself causes oxidative stress leading to compromised BBB integrity. Nef protein not only facilitates downregulation of CD4 and increases HIV replication; it also stimulates apoptosis and induces disruption of the BBB [18]. Lastly, viral protein R (Vpr) increases permeability of the BBB and recruits monocytes and macrophages into the CNS by dysregulating the astrocyte compartment [19].
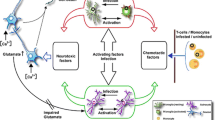
Pericytes of the BBB endothelium secrete cytokines and increase HIV penetration of the BBB [16]. LPS stimulation , as a result of the increased gut microbial translocation [20], may facilitate HIV entry into the brain by inducing production and release of inflammatory mediators by brain endothelial cells. Additionally, cell-to-cell interaction facilitates HIV transmission from T lymphocytes to astrocytes. It was observed that virologic synapses formed by filopodial extensions binding of either cell type could be inhibited by anti-C-X-C chemokine receptor type 4 (CXCR4) antibody [18]. The interaction between HIV with the brain microvascular endothelial cells increases BBB leakiness, as a result, it increases brain-to-blood efflux of antiretroviral drugs [12, 18] (Fig. 17.1).
Illustrates the mechanism of viral and cellular migration from peripheral blood into the brain. HIV, HIV proteins (gp120, tat), and HIV-infected cells can cross the BBB via transcytosis and infected microglia and astrocytes which then will be activated and release inflammatory cytokines, further activating microglia and astrocytes (Adapted from Hong and Banks [12])
Penetrability of the ARVs across the BBB is facilitated by low molecular weight, high lipophilicity, degree of ionization, active transport pump, cerebral blood flow, and the degree of local inflammation [21, 22]. Many antiretroviral drugs, including protease inhibitors such as ritonavir and indinavir , are substrates for brain-to-blood transporters like P-glycoprotein (P-gp) [23]. Such transporters can pump the substrates out of the brain and prevent drugs from reaching therapeutic concentrations within the CNS [24].
17.2.2 The CNS as a Compartment
As mentioned above, the CNS is one of the compartments that HIV infects, and it provides sanctuary and allows independent replications. Occasionally, HIV escapes the CSF despite suppression in the plasma. It suggests that low-grade central nervous system infection may continue in treated patients and can cause further neurocognitive impairment [25, 26]. Different and inadequate penetration of antiretroviral agents can cause resistant mutations and distinct genetic profiles compared to HIV in plasma [27, 28].
17.3 cART
There seems to be an inverse correlation between concentration of antiretroviral agents in the CSF and the HIV CSF viral loads [22, 29]. Generally, the levels of ARVs in the CSF are low compared to plasma. There are questions if those ARVs in CSF are actually adequate to inhibit HIV replication. ARV concentration in the brain parenchyma is not uniform [30]. The ideal way to measure the ARV concentration in the CNS is the actual measurement of tissue concentration or the fluids (e.g., sinuses) from different parts of the brain. Such measurements are impractical in clinical settings. Most studies, however, use the ARV concentration in CSF as an indirect measurement of drug exposure, although it is unclear whether CSF concentrations accurately reflect parenchymal ARV concentrations [13].
Suppression of HIV replication requires a minimum drug concentration above the inhibitory concentrations (ICs) . ICs are concentrations based on in vitro findings using HIV strains susceptible to the drug. For example, the concentration of the drug necessary to inhibit 50% (IC50), 90% (IC90), or 95% (IC95) of viral replication is reported. For improved in vivo estimates, the corrected IC is derived by taking into account drug binding to plasma proteins, and the effective concentration (EC) is then calculated [31].
Frequently, IC50 of ARVs for wild-type HIV is often used as a reference and compared with half-maximal effective concentrations (EC50) to represent the plasma concentration [32]. However concentration of the drug necessary to inhibit 90% or 95% (IC90, IC95) offers greater accuracy. For example, for protease inhibitors , which are typically highly protein bound, the IC50 in CSF can be overestimated. It needs caution to compare IC50 values in plasma and in CSF [31, 33]. The measurement may vary depending on the methods to determine it, cell types, chronicity of infection, and HIV strains [34, 35]. Clinically, it also depends on the overall cART regimen, which may have additive or synergistic effects due to the use of multiple drugs. This rationale may be useful to treat CNS infection and to ensure that the cART regimen has adequate CNS drug levels. CNS escape, however, may occur as a result of inadequate treatment (suboptimal drug concentrations) of the CNS-compartmentalized HIV. Later, it may lead to the development of resistance mutations and additional neurologic complications [36,37,38]. Unfortunately, it is very difficult to compare drug levels and their IC values, because the results can vary with the protein concentrations as well as drug protein-binding ability [31]. Letendre et al. developed a quantification rank system of ARVs in CSF, which can be useful in selecting ARVs for patients with neurocognitive impairment [29, 39].
17.4 CNS Penetration and Effects of Current ARVs
17.4.1 Nucleoside/Nucleotide Reverse Transcriptase Inhibitors(NRTIs)
NRTIs are effective CSF concentrations because of their small molecular weights and low plasma protein-binding capacities; however, they have elevated hydrophilicity, which does not favor crossing the BBB. The optimum way to analyze the CNS concentration of NRTIs is to measure their intracellular triphosphate metabolites, which is not practical in the clinical setting [31]. Moreover, NRTIs are transported by organic anion transporter (OAT) at the choroid plexus [40]. Strazielle et al. studied delivery of ARVs and found that zidovudine (AZT) is the best among the NRTIs followed by stavudine, didanosine, and lamivudine [41]. Following is summary of the available data on CNS penetration of each NRTI.
17.4.1.1 Zidovudine (AZT)
AZT has the highest partition coefficient, which determines the ability of AZT to distribute, in the brain and CNS tissue [41]. It is the substrate for P-glycoprotein (P-gp) , multidrug resistance-associated protein (MRP)-4 and MRP-5 [42]. Since it was approved, pharmacokinetics of AZT was studied extensively [43,44,45]. In the largest study, Burger et al. had 39 patient participants and studied CSF concentrations of AZT. The CSF/plasma ratio increased linearly over time without significant relationship between AZT dose and CSF levels of AZT. This suggests that CSF penetration of AZT is dose independent. This finding may be an explanation for the efficacy of AZT in the prevention and treatment of HIV-related neurological diseases despite in low doses. Moreover, Burger et al. demonstrated that AZT reached therapeutic levels in CSF and was able to decrease CSF HIV viral load. Thus, it improved neurocognitive dysfunction as a single agent [43].
It is also associated with improved neuropsychological functioning in children with progressive encephalopathy by reduction of HIV viral load in CSF and improves neurocognitive performance in children with HIV encephalopathy as found early in the HIV epidemic to the present [46,47,48,49]. In addition, ART improves neurocognitive outcomes in HIV-infected children when applied early in their disease process [47, 49].
17.4.1.2 Stavudine (d4T)
The main side effect of d4T is mitochondrial toxicity; this led to a drastic reduction of d4T use in current clinical practice. However, in the early HIV epidemic, the CSF concentration of d4T was studied extensively [50, 51]. In patients with long-term use of d4T, its concentration in CSF ranged from 0 to 109.9 ng/ml with the mean d4T concentration of 51.6 ng/ml that exceeds the EC50 of clinical isolates of HIV (230 nM, 52 ng/ml) [50]. Stavudine uses organic anion-transporting polypeptide (OATP)-like transporter for its uptake [52].
17.4.1.3 Didanosine (ddI)
Didanosine chemical structure contains hypoxanthine. It has less than 5% protein binding [53]. In HIV-infected patients, ddI reaches a negligible level in the CSF. In patients who had been chronically taking ddI, average CSF concentration at 4 h after administration was 0.16 mumol/l in the CSF and 0.70 mumol/l in plasma. CSF concentration primarily uses the OATP-2-like transporter [53]. In one study, it had median CSF/plasma ratios of 0 in five patients [54]. However, unlike AZT, the use of ddI monotherapy did not reduce HIV viral load in CSF [55].
17.4.1.4 Lamivudine (3TC)
3TC has optimum uptake from blood to choroid plexus using the dioxin-sensitive transporters and organic cation transporters [56]. The concentrations of 3TC were studied in combination with either AZT or d4T. All subjects had detectable HIV viral load in CSF. However, there was no correlation between plasma and cerebrospinal fluid HIV-1 RNA concentrations; after 12 weeks of treatment, none of the subject had detectable CSF HIV viral load even when the subjects did not achieve complete plasma HIV viral load suppression. In this study, 3TC had the highest CSF concentration followed by d4T and AZT. Drug concentrations in plasma declined rapidly, while drug concentrations in CSF reduced more slowly. CSF to plasma concentration for d4T, AZT, and 3TC increased over time, and the time-dependent CSF to plasma drug/penetration ratios was highest for AZT followed by d4T and 3TC. And the 3TC level was well above the IC50 (range 66–80 ng/ml) [57].
17.4.1.5 Abacavir (ABC)
ABC has optimum CSF penetration with moderate plasma protein binding and lipophilicity. In animal model, it crosses BBB without influence of other drugs [58]. A human study of 54 subjects demonstrated its CSF/plasma ratio that is enhanced by dose escalation, and the CSF concentration is adequate to inhibit HIV replication. Subjects received ABC 300 mg twice daily as part of cART. The median CSF ABC concentration was 128 ng/mL (range 37–384 ng/ml). Predicted CSF trough concentrations exceeded the IC50 (70 ng/mL) for 85% of the dose interval. The CSF/plasma ABC ratio is approximately 31–44% [59]. However, its lacking in the active efflux mechanism and having P-gp as its major transporter limit CNS penetration [60].
There are no studies to demonstrate a virological or clinical effect in the CNS. Adding high-dose ABC for HAD patients on stable cART did not improve performance scores or reduce CSF HIV RNA levels more than placebo [61].
17.4.1.6 Tenofovir
According to the DHHS guideline, tenofovir has been the preferred agent in cART component (www.aidsinfo.nih.gov). The initial FDA-approved formulation, tenofovir disoproxil fumarate (TDF) , demonstrated efficacy and persistency, with concerns of nephrotoxicity, osteopenia, and osteoporosis [62, 63]. To minimize such concerns, tenofovir alafenamide (TAF) was developed. Both TDF and TAF are prodrugs of tenofovir diphosphate. And clinically, TAF has been replacing TDF in the existing combination formulas [63].
CSF tenofovir concentration has been described to be very low due to its limited uptake by membrane transporters [64]. A study to determine tenofovir CSF penetration using random plasma and CSF samples from 183 subjects who were on tenofovir found median plasma, and CSF tenofovir concentrations were 96 ng/mL and 5.5 ng/mL, respectively. Thirty-four of 231 plasma (14.7%) and 9 of 77 CSF samples (11.7%) were below detection. CSF/plasma concentration ratio from paired samples was 0.057. Median CSF to wild-type 50% inhibitory concentration ratio was 0.48 (IQR 0.24–0.98). Moreover, 77% of CSF concentrations were below the tenofovir wild-type IC50. The tenofovir concentrations in the CSF are only 5% of plasma concentrations , which suggest limited transfer into the CSF and possibly active transport out of the CSF. Therefore, tenofovir may not effectively inhibit viral replication in the CSF [65].
17.4.2 Non-nucleoside Reverse Transcriptase Inhibitors (NNRTIs)
17.4.2.1 Efavirenz (EFV)
Despite its common CNS side effects, it has limited CNS penetration. One of the reasons is that EFV bounded extensively by plasma albumin left a small fraction of unbound EFV passively penetrated into CNS. The CNS EFV is not bound well by CSF protein; both unbounded EFV concentrations are similar resulting in distortion of CSF/plasma EFV ratio [66]. The first published study of the CNS penetration of EFV of ten patients showed a mean CSF concentration of 11.1 ng/mL (range 2.1–18.6 ng/mL) and a CSF/plasma ratio of 0.61% [67]. A pharmacokinetic study of EFV showed its CSF penetration of 0.44% of plasma concentration [31]. A recent larger study of 80 CSF samples reported a median CSF EFV concentration of 13.9 ng/mL with all except two samples that were above the IC50 (0.51 ng/mL). Its CSF concentration is only about 0.5–1% of plasma concentration. However, the CSF concentration offers the CSF concentration above IC95 of HIV wild type. This suggests the potent ability of EFV in inhibiting HIV in the CSF in such a low CSF concentration [68]. Its efflux mechanism uses P-gp expression and function. It also concentration-dependently inhibits MRP-1, MRP-2, and MRP-3 [69]. Moreover, EFV major haptic metabolite, 8-hydroxyefavirenz, neither has significant association with EFV plasma concentration nor association with CYP2B6 genotype; therefore, it reaches the 0.01 μM toxicity threshold [54, 70]. There are increasing evidences of EFV-related CNS toxicities. We describe cART especially EFV toxicities in Sect. 17.6 of this chapter.
17.4.2.2 Nevirapine (NVP)
NVP crosses BBB well, has stable CSF concentration, has highest CSF/plasma penetrability rate, and offers the highest penetrability rank in Latendre classification [39, 54, 71, 72]. It has median CSF/plasma ratio of 0.63 [54]. In a study of nine HIV-infected patients, the median CSF NVP concentration was 932 ng/mL (range 219–1837 ng/mL), which exceed the CSF IC50 by tenfolds [72, 73]. Therefore, it is suitable to use in patients with neurocognitive impairment.
17.4.2.3 Etravirine (ETV)
ETV has a CSF/plasma ratio of 4%. In one study, all 17 CSF concentrations exceeded the wild-type IC50 by a median of 13.6-fold [31]. In another study of 12 patients, the median ETV concentration in plasma was 611.5 ng/mL with the median CSF concentration of 7.24 ng/mL, which was above the IC(50) range (0.39–2.4 ng/mL). The median ETV CSF/plasma ratio was 0.01. All but one patient had undetectable CSF viral load. This study suggested that ETV use was associated with virus suppression in CSF and plasma and may help control HIV in the CNS [31, 72, 74].
17.4.2.4 Rilpivirine (RPV)
RPV CSF drug penetration has been limitedly studied. In a NVP to RPV switch study, CSF drug concentration was measured. The mean plasma RVP trough concentration was 29.7 ng/mL with the mean CSF RVP concentration of 0.8 ng/mL (95% CI: 0.7–1.0), resulting in a CSF/plasma ratio of 1.4 the protein. It was shown that switching NVP to RVP was safe with reassuring drug levels both in plasma and in CSF [75].
17.4.3 Protease Inhibitors (PIs)
Due to their lipophilicity , PIs are expected to have good CNS concentrations but have low CSF concentrations due to the efflux mechanism (all PIs are P-gp substrates) and high protein-binding capacity (except indinavir) [76]. Using ritonavir to boost PIs also increases CSF penetrability [29].
17.4.3.1 Ritonavir (RTV)
Currently, RTV is used exclusively to pharmacokinetic enhance (aka boost) other PIs rather than as a primary antiviral drug. It is a cytochrome P450 3A isoenzyme inhibitor and increases plasma areas under the curve (AUC), drug half-lives, and trough concentrations (lowest drug concentration at steady state) of others. It has large molecular weight, is highly protein bound, and is also a P-gp inhibitor. Therefore RTV can enhance CSF levels of other drugs both by increasing plasma concentrations and by inhibiting efflux [23].
A cross-sectional study of 28 subjects on saquinavir/RTV therapy was evaluated and resulted in a strong correlation between plasma and CSF HIV viral loads. Low CSF drug levels of both saquinavir (<2 ng/ml) and ritonavir (<25 ng/ml) with low CSF/plasma concentration ratio of <0.005 suggested that CSF ritonavir and saquinavir levels are consistent with the estimated known fraction of unbound drug in plasma (<2%), and suppression of plasma viremia can indicate low CSF HIV RNA levels. Likewise, CSF virologic breakthrough was the result of plasma virology failure [77].
17.4.3.2 Indinavir (IDV)
Currently, IDV is only rarely used because of its dosing frequency and renal toxicity; however, it offers the best CNS penetrations among the PIs because of its low protein-binding capacity [78, 79]. It is the only PI that has a CSF concentration that attains its IC95 (18–71 ng/mL) [78, 80, 81]. It is also the only PI that achieves therapeutic concentrations in CSF without RTV boosting (dosing 800 mg three times daily). Using it in a boosted fashion with RTV, there are even higher CSF concentrations with mean CSF IDV concentrations of 203 ng/mL. This is well above the IC95 (18–71 ng/mL) [79]. Using IDV showed clinically improved neurocognitive dysfunction [81].
17.4.3.3 Lopinavir (LPV)
The median CSF concentrations of LPV were 11.2–17.0 ng/mL based on three studies with the CSF/plasma LPV ratio that was approximately 0.2% in all of them [82,83,84]. All the CSF samples were above the median IC50 (1.9 ng/mL) for wild-type virus. RTV-boosted LPV (LPV/r) has CSF levels that exceed the IC50 [82]. It has been shown that LPV/r both monotherapy and as a component of cART reduces CSF HIV replication and immune activation [84]. However, a recent study with patients on effective cART randomized to LPV/r monotherapy (n1/429) or continued treatment (n1/431) had to be terminated prematurely because of the high rate of failures in the monotherapy arm. A total of four out of six patients with plasma virologic failure developed neurological symptoms, and all of them were on monotherapy. In five of the failing patients, all had elevated CSF HIV RNA levels (3.1–5.1 log10 copies/mL). In addition, 8 of 25 patients who consented to a lumbar puncture at study termination had detectable HIV RNA in the CSF. All these patients were on monotherapy at the time of study termination, whereas none of 15 patients in the continued treatment arm had detectable HIV RNA in their CSF. The use of LPV/r may benefit patients with neurocognitive disorders as part of cART, not as monotherapy [38].
17.4.3.4 Amprenavir (APV) and Fosamprenavir (FPV)
Boosted APV and, its prodrug, FPV (both in unboosted and boosted forms) reaches their IC50 rapidly after oral administration. FPV is almost completely hydrolyzed to APV prior to reaching systemic circulation. A study of 119 matched CSF-plasma pairs from 75 subjects found that APV concentrations were 5.6 ng/ml compared to the IC50 for wild-type HIV. The APV concentrations in CSF exceeded the median IC50 for wild-type HIV in more than 97% of CSF specimens with detectable APV by a median of 4.4-fold (IQR, 2.9–7.9). This showed that FPV may control HIV replication in the CNS as a cART component [85]. With a single dose of 630 mg of APV, only one of five CSF samples collected from healthy males had detectable CSF APV levels [86].
In a study of boosted FPV monotherapy (FPV 700/RTV 100 mg twice daily), 20 patients entered the study with 9 patients (45%) had therapeutic failure. Hence, this study ended prematurely and the use of boosted FPV monotherapy was discouraged. The CSF APV concentration was well above the IC50. Despite virologic failure, APV levels and undetectable HIV RNA levels in CSF were documented in all samples evaluated [87].
17.4.3.5 Nelfinavir (NFV)
NFV did not reach therapeutic CSF concentration and was below the detection limit [88]. However, in two studies, NFV was quantifiable in 9 of 15 samples and 8 of 18 samples, respectively. Some of the concentrations were in the range of the IC50, but most of them were below it. When used as a single agent for 17 days (in three patients), NFV failed to suppress the CSF viral load [89, 90]. Another study measuring NFV concentration in both plasma and CSF in 6 study subjects, even though NFV was not detected in any of the CSF specimen, there was a significant reduction of HIV RNA PCR in CSF of patients who were treated with NFV-containing regimen. This finding demonstrated that reduction of CSF HIV RNA correlated to the reduction in plasma HV RNA [91].
17.4.3.6 Saquinavir (SQV)
SQV did not reach therapeutic CSF concentration and was below the detection limit even with ritonavir boosting [39, 77].
17.4.3.7 Atazanavir (ATV)
CSF RTV concentrations increase when used with ATV boosting. In a study of 68 patients on a treatment regimen with boosted ATV (ATV 300–400 mg/RTV 100 mg once daily), the median CSF ATV concentration was 10.3 ng/mL (range, 5–38 ng/mL) and the CSF/plasma ratio was 0.9% [92]. Fifty-four percent of the samples were below the IC50 with plasma (11 ng/mL) and 24% were close to the IC50 determined without human proteins (1.0 ng/mL). The authors concluded that ATV did not reach therapeutic CSF concentration and that it might not protect against HIV replication in the CSF. Moreover, it has highly variable CSF concentrations and more than 100-fold lower than the plasma concentration even with RTV boosting. Its concentration did not consistently exceed the IC50 for the wild-type virus [39, 92]. To evaluate the effect of monotherapy with boosted ATV on the CSF viral load, lumbar punctures were performed on 20 patients who had received this regimen as maintenance therapy for 24 weeks. Two patients (7%) failed this regimen. Excluding failing patients, individual measurements of HIV RNA in patients showed occasional viral “blips” in five patients. Samples with elevated HIV RNA greater than 500 copies/ml in CSF were all wild type. The mean ATV drug concentration ratio was 0.9%. This finding supports potential use of PI-based mono-maintenance therapies. However, their results in CSF caution against the uncontrolled use of PI-based monotherapies that can lead to CSF escape [36].
17.4.3.8 Darunavir (DRV)
DRV has detectable and stable levels in the CSF that exceed levels needed to inhibit HIV replication. In a study of 14 samples from eight treatment-experienced HIV-infected patients receiving 600 mg/100 mg of DRV/RTV twice daily plus optimized background therapy, the median CSF DRV concentration was 34.2 ng/mL (range 15.9–212 ng/mL); all of them had CSF DRV levels well above the IC50. The finding suggests DRV contributes to viral suppression in the CNS [93].
DRV/RTV monotherapy was investigated in 225 patients, and three patients developed virologic failure on DRV/RTV monotherapy, and none failed on DTV/RTV triple-drug therapy. No resistance to protease inhibitor emerged in patients with plasma viral load above 50 copies/ml. The patients failing on DRV/RTV monotherapy had no emergence of new DRV resistance mutations preserving future treatment options [37]. Another study of DRV/RTV monotherapy at 48 weeks HIV RNA was less than 50 copies/ml – 86.2 versus 87.8% in the monotherapy and triple therapy arms, respectively. One patient per arm showed at least one protease inhibitor mutation, and one patient in the triple therapy arm showed an NRTI mutation. In both studies, switching to DRV/r monotherapy showed noninferior efficacy versus triple antiretroviral therapy [94]. The addition of two NRTIs led to improvements of symptoms and reductions of CSF viral load.
17.4.3.9 Tipranavir (TPV)
There was no published data on the CSF concentration of TPV.
17.4.4 Integrase Inhibitors (Integrase Strand Transfer Inhibitors, INSTIs)
Although raltegravir, elvitegravir, and dolutegravir are the three agents in this class that are currently approved by the FDA, there are limited published data available for CSF penetration.
17.4.4.1 Raltegravir (RAL)
RAL is a substrate for P-gp in vitro [95]. A study of 18 subjects who took RAL-containing regimens demonstrated a median concentration in plasma that was 260.9 ng/ml, with a median CSF/plasma ratio of 0.058. RAL concentrations in CSF exceeded IC50 for wild-type HIV-1 (3.2 ng/ml) by a median of 4.5-fold. Its presence in CSF was sufficiently high concentrations to inhibit wild-type HIV in all subjects [96]. Another study showed that in patients who were on RAL-based cART regimen, the median CSF/plasma ratio was 0.20 and correlated with plasma and CSF trough concentration. Despite variability of RAL penetration into CSF, the concentrations were well above wild-type HIV IC50s in all patients and above IC95 in 28.6% of the patients [97]. Moreover, in another study, 50% of the CSF specimens, concentrations exceeded the IC95 levels reported to inhibit HIV-1 strains without resistance to INSTIs [93]. Based on its CNS penetrability, as a component of cART , RAL likely contributes to the control of HIV replication in the nervous system as well as being neuroprotective by suppression of HIV-infected inflammatory cytokine, IL-8, production [98].
17.4.4.2 Elvitegravir (EGV)
An ongoing CSF pharmacokinetic study to determine the CSF concentrations of EGV along with tenofovir and TAF (NCT 02251236) has completed subject recruitment; data are not yet available (https://clinicaltrials.gov/ct2/show/record/NCT02251236).
17.4.4.3 Dolutegravir (DGV)
In 12 treatment-naive subjects, using 50 mg doses of DGV in combination of 3TC and ABC, the median DGV concentration in CSF was 13.2 ng/ml, 2–6 h post dose after 16 weeks of treatment. However, clinical relevance of this information is not established (https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Tivicay/pdf/TIVICAY-PI-PIL.PDF).
17.4.5 Entry Inhibitors
17.4.5.1 Enfuvirtide
Enfuvirtide is entry inhibitor acting on the process of fusion. Its chemical structure suggests that it would not reach effective drug concentrations in the brain due to high molecular weight (4492 Dalton), high protein binding (92%), and lack of lipid solubility [31, 99]. Its CSF concentrations were below the lower limit of quantification (25 ng/mL) in 18 out of 18 CSF samples [100]. A report of a patient who developed virologic failure while on enfuvirtide-containing regimen, using genotypic analysis of CSF-derived HIV RNA, V38A mutation was detected in the CSF but not in plasma. This finding illustrated the selection of enfuvirtide-resistant virus in CSF, causing subsequent loss of viral suppression in plasma [101].
17.4.5.2 Maraviroc
To date, maraviroc is the only approved CCR5 coreceptor antagonist for the treatment of HIV-1 infection. It is also a substrate for P-gp [102]. In one study, maraviroc was detectable in all seven CSF samples with a median concentration of 3.6 ng/mL (range 1.8–12.2 ng/mL) [93] and 2.6 ng/mL (range, 0.5–7.2 ng/mL) in another study [103] with median CSF/plasma ratios of 3% and 2.2%, respectively. All CSF samples contained ≥3-fold maraviroc concentration above the median EC90 (0.57 ng/mL) [103] (Table 17.1).
17.5 The CNS Penetration Effectiveness (CPE) Score
The CNS penetration effectiveness (CPE) score has been proposed as a method for estimating the combined CNS effectiveness of cART regimens. The study was done as part of the CHARTER (CNS HIV Antiretroviral Therapy Effects Research) study. Eight hundred and thirty-three HIV-positive individuals had enrolled, and 467 (71%) met eligibility criteria for CPE analysis by ARV drug use report and HIV viral load in both plasma and CSF measured. ARVs were classified into three categories based on chemical properties (molecular weight, protein binding, lipophilicity, charge at physiological pH), pharmacokinetic data (mainly CSF concentrations compared with inhibitory concentrations for wild-type HIV-1), and pharmacodynamic data (effectiveness in CNS in clinical studies). For this initial version of the CPE score, individual ARV drugs were assigned a penetration rank of 0 (low), 0.5 (intermediate), or 1 (high) based on their chemical properties, concentrations in CSF, and/or effectiveness in the CNS in clinical studies. The CPE rank was calculated by summing the individual penetration ranks for each ARV in the regimen. The findings noted that the median CPE rank was 1.5. Lower CPE ranks correlated with higher CSF viral loads even after adjusting for the total number of ARV drugs, ARV drug adherence, plasma viral load, duration and type of the current regimen, and CD4 count [29]. In the revised 2010 version of this ranking system, individual antiretroviral drugs are assigned a penetration score of 1 (none), 2 (low), 3 (intermediate), or 4 (high) [39]. CPE rank has been shown to correlate with improvements in cognitive performance and with CSF viral loads in some studies [29, 108], while other studies have found no correlation with neurocognitive improvement, detectable CSF viral loads, or level of intrathecal immune activation [25, 81, 109]. This suggests that using a simple categorical scale still has limitations that might not be sufficient in judging CNS efficacy. Moreover, data for some drugs is very limited and do not take into account possible genotypic resistance. A study of 64 subjects focusing on the effects of CPE score on neuropsychological performance showed that CPE score is not related to cognitive outcomes [110]. In recent studies, there was evidence of worsening neurocognitive function in patients who were on a high CPE regimen. For example, in the HIV-CAUSAL collaborative, 51,938 patients were followed, and they were compared based on high (>9) vs. low (<8) CPE regimens (with regimen CPE scores range 4–16). The patients who were on high CPE score regimens had increased risk of HAD (hazard ratio 1.74), while there was a difference in the risk of developing cryptococcal meningitis, CNS toxoplasmosis, and progressive multifocal leukoencephalopathy [111]. Other studies suggested that high CPE regimens may be neurotoxic. Using NRTIs can cause mitochondrial toxicities by inhibiting mitochondrial DNA polymerase gamma. N-acetyl-aspartate (NAA) was used as a surrogate marker for neuronal integrity and mitochondrial function and can be measured by magnetic resonance spectroscopy (MRS) . Robertson et al. used this technique in 18 patients and found that patients who were on d4T and ddI had depleted NAA levels [112]. Efavirenz is also found to be neurotoxic and damaged dendritic cells [113]. The antiretroviral drug score (ΣCPE) was investigated in a small study for its potential correlation with brain atrophy. The investigators used ventricular/brain ratio, calculated by lateral ventricular area divided by the brain area at the same level in T2 transversal MRI slices, as an index of overall brain atrophy. The ΣCPE scores were done in 2010 and 2008 version. ΣCPE 2010 version was found to be correlated with atrophy than the 2008 version [114]. Future revision as more information available perhaps helps to better correlate with clinical findings (Table 17.2).
This table shows the CNS penetration effectiveness (CPE) ranking of the currently available antiretroviral. The initial CPE rank proposed by Latendre et al. was based on physiochemical characteristics, CSF concentrations, and efficacy data [29]. However, the recent cross-sectional data that included CSF vs. plasma viral load studies from the CHARTER cohort led to a revised CPE ranking system. The new system reflects stronger associations with CSF viral load analysis by incorporating recent pharmacokinetic and pharmacodynamic data. A higher number estimates better penetration in the CNS [39].
Drug transporters involved in each antiretroviral distribution at the BBB are mentioned. These transporters are ATP-binding cassette (ABC) superfamily including P-glycoprotein (P-gp) , multidrug resistance-associated protein (MRP) , and breast cancer resistance protein (BCRP) . Solute-carrier superfamily (SLC) includes organic anion-transporting polypeptide (OATP) , organic anion transporter (OAT) , and organic cation transporter (OCT) . Nucleoside transporters include equilibrative nucleoside transporter (ENT) and concentrative nucleoside transporter (CNT) (adapted from Shapshak et al. [3]).
Gao et al. proposed a method to predict drug BBB permeability that can apply to both small compounds and macromolecules by various mechanisms besides passive diffusion. The curated drugs with known BBB permeability dataset were extracted from SIDER database. They built classification models with support vector machine (SVM) algorithms using data mining software, evaluated the performance of the model independently from the dataset, and conducted de novo prediction for each drug in the SIDER database. This method uses clinical phenotypes including drug side effects and indications, using dataset of 213 drugs, which has three antiretrovirals including EFV, AZT, and RTV. In this model it was found that it was predicted of BBB permeability in AZT but not EFV and RTV. This dataset is found to have an accuracy of 85.5% and can potentially serve as a point to commence further CNS drug repositioning and combinatorial research [132].
17.6 Nanobiology and CNS ARV Drug Delivery
To date, available antiretroviral drugs are effective primarily in decreasing the viral load in the peripheral system but do not as yet eradicate virus in the CNS reservoir. The primary impediment is the BBB; drug delivery is still a challenge. HIV neurotherapeutics through nanocarrier-based delivery of the antiretroviral drugs through the BBB is a promising methodology against HIV cure by possibly eradicating persistent and latent HIV infection in the CNS. Delivery systems experimented include liposomes and magnetic liposomes, nanoART, cationic trans-activating transcriptor (TAT) nanoparticles, and polymer-based nanoparticles. All of these approaches created an improved ARV delivery to the CNS and are potentially applied to all ARV classes [133]. The global call of HIV eradication promotes research in nanocarriers and noninvasive strategies to deliver drugs through BBB such as site-specific release of ARVs, nanoformulations to eradicate HIV reservoirs, and diagnostic tools to detect and monitor HIV infection. Such developments will help to develop personalized nanomedicines toward HIV cure [134].
17.6.1 Liposomes
Liposome-based nanoformulation was benchmarked by nanoformulation of foscarnet, which was also employed with other ARVs [135, 136]. However, this delivery method seems to have shorter half-life by reticuloendothelial cell sequestration [137].
17.6.2 NanoART
NanoART was explored by Kuo and Chen by incorporating AZT and 3TC into polybutylcyanoacrylate and methylmethacrylate-sulfopropylmethacrylate nanoparticles. This formulation increases BBB permeability [138]. Additionally, they investigated the electromagnetic field-regulated transport of cationic solid lipid nanoparticles across human brain endothelial cells. Using this technique, the permeability of SQV across the brain endothelial cell monolayer was increased about 17- to 22-folds [139]. Later, this strategy was exploited further to deliver IDV nanoparticle loaded into bone marrow macrophages in mice with HIV-1 encephalitis. IDV was detected in the brain suggesting nanoparticles deliver IDV across BBB [140]. ATV and RTV crystalline nanoART and nanoART using monocyte-derived macrophages as Trojan horses (ATV, RTV, IDV, and EFV) are proven to increase ARV penetration through BBB [141, 142]. Additionally, magnetic nanoART was developed using a magnetically guided layer-by-layer technique coloads TDF and vorinostat. This formulation provides sustained drug release with acceptable BBB penetrations [143].
17.6.3 TAT-Nanoparticles
TAT-nanopeptides are the most commonly used cell-penetrating peptides. This demonstrated delivery of RTV to the brain by escaping the P-glycoprotein efflux without disruption of BBB integrity [133].
17.6.4 Actively Targeting Nanoparticles
Actively targeting nanoparticles have been studied in ARV drug deliveries. These include (1) PEGylated albumin nanoparticles encapsulating AZT [144]; (2) methylmethacrylate-sulfopropylmethacrylate nanoparticle functionalized with the bradykinin analogue, RMP-7, to increase permeation of D4T, SQV, and delavirdine (DLV) across the BBB [145]; and (3) brain-specific nano-NRTIs decorated with the peptide apolipoprotein E receptor, which provided low neurotoxicity and enhanced anti-HIV activities [146].
17.6.5 Polymer-Based Nanoparticles
Poly(dl-lactide-co-glycolide) (PLGA) nanoparticles were demonstrated to increase the peak concentrations of RTV, LPV, and EFV in mice brain [147]. Enfuvirtide conjugated with iron oxide nanoparticles coated with an amphiphilic polymer increases enfuvirtide penetrations across the BBB and increases its efflux into brain parenchyma [148].
17.7 cART and CNS Toxicities
Among antiretroviral drugs, EFVs have been well known to cause neurological and neuropsychiatric adverse reactions . Patients treated with EFVs present a wide range of symptoms including nightmares, dizziness, insomnia, nervousness, lack of concentration, as well as more severe symptoms including depression, suicidal ideation, or even psychosis. Moreover, EFVs have recently been shown to associate with mild/moderate neurocognitive impairment.
In fact, neuropsychiatric side effects are the most common cause of EFV discontinuation [149,150,151]. These side effects can occur as early as the first dose of therapy and likely to subside within the first month. However, some patients might experience them several months or years post-therapy, requiring switching to alternative agents [151,152,153,154,155,156]. Although the mechanisms of these adverse reactions were unclear, there has been increased evidence of mitochondrial function disturbances in the brain and the bioenergetic systems [70].
In addition, potential EFV neurotoxicity mechanisms include the following:
-
1.
Upregulation of pro-inflammatory cytokines (IL-1β and TNF-α) in blood cells exposed to pro-inflammatory stimuli. In animal models, this is associated with anxious behaviors and impaired cognitive performance, causing spatial memory deficits and increased stress susceptibilities. Moreover, EFVs upregulate these pro-inflammatory cytokines [157, 158].
-
2.
Increase in 5-HT levels occurring in parallel to a reduced activity of tryptophan 2,3-dioxygenase. Apostolova et al. found that EFV-exposed rats showed downregulation of serotonin via tryptophan 2,3-dioxygenase resulting in increased serotonin activities [158].
-
3.
EFV acting as a partial agonist of the serotonin receptors 5-HT2C and, particularly, 5-HT2A [159].
-
4.
EFVs significantly inhibiting creatinine kinase activities in the cerebellum, hippocampus, striatum, and cortex in a mouse model. EFVs affect mitochondrial function of the brain by depleting ADP that leads to cognitive impairment as well as increased seizure susceptibilities in EFV-treated mice [160, 161];
-
5.
It was also shown that EFV reduced creatinine kinase activity in the mouse brain by a specific inhibition of complex IV (cytochrome c oxidase) of the electron transport chain in the cerebral cortex, striatum, and hippocampus, but not in the cerebellum [162].
-
6.
EFV, not other NNRTIs, increased endothelial permeability by inducing reduction in, and disrupting localization of, a tight junction protein, claudin-5 [163].
-
7.
EFV impacts mitochondrial function and neural bioenergetics . EFV was found to increase soluble amyloid beta, promote increased β-secretase-1 expression, and decrease clearance of the amyloid beta peptides, resulting in mitochondrial stress [164].
In addition, the impact of EFV on biogenetics especially in the neurons and glial cells has been shown in in vitro studies. It disrupts mitochondrial function by direct inhibition of complex I activity of the mitochondrial electron transport chain , leading to a decrease in total oxygen consumption, an increase in the production of reactive oxidase species, and a decrease in mitochondrial membrane potential [165, 166]. The action of EFV on the mitochondria of glial cells and neurons is similar. EFVs directly inhibit complex I resulting in reduction of mitochondrial respiration both in neurons and glial cells. However, the bioenergetic responses of reduction of mitochondrial respiration in glial and neurons are different. In glial cells, the increase in the AMP/ATP ratio induced by mitochondrial dysfunction causes the activation of AMPK (by phosphorylation forming P-AMPK), leading to upregulation of glycolysis (increased extracellular lactate) and consequently to increased intracellular levels of ATP. Glycolysis activation is not observed in neurons causing reduction in intracellular ATP [166,167,168].
Bioenergetic interference also plays a role in nitric oxide synthesis in glial cells. The decrease in respiration and the increase in glycolysis and mitochondrial reactive oxygen species generation were partially reversed when inducible nitric oxide synthase activity was inhibited in glial cells. Additionally, while EFV produced a decrease in complex I activity in both neurons and glial cells, a slight inhibition of complex IV activity was detected only in glial cells, which is consistent with an inhibitory action of nitric oxide on this mitochondrial complex. EFV-treated mice display inhibition of complex IV activity in different areas of the brain in EFV-treated mice, and no alteration of complex I activity was observed [158, 162].
Direct toxicities from EFV primary metabolite, 8-hydroxyefavirenz, have been observed by stimulation of glycolytic flux and decreased mitochondrial oxygen consumption. Moreover, increase in calcium into cells mediated by L-type voltage-operated calcium channels damages dendritic spines and induces apoptosis [113].
17.8 Conclusions
Using cART helps to alleviate and prevent HAND. However, the efficacy of each ARV compound in the CNS should be considered specifically as well as in concert with others in choosing cART regimens. Early diagnosis of neurocognitive impairment is needed to appropriately select cART regimen with good CSF penetrability rank that proffers neuroprotection.
Another consideration of adequate suppression of HIV replication in the brain is to prevent CNS escape. Compartmentalization of HIV infection is associated with genetic differences between plasma and CSF strains in terms of resistance. Efficacy of cART in the CNS sanctuary requires awareness on ARV penetrability, resistance mutations in CSF, factors as modification of BBB, drug interactions, additive or synergistic effects of cART components, and comorbidities. Unfortunately, technology and data are helpful when assessing individual agents, but more difficult evaluating each regimen. Nanotechnologies are the promising areas to develop efficient drug delivery to eradicate HIV CNS reservoirs. Further studies are needed to discover improved assays to measure cART regimen efficacy in viral compartments and to find drugs that can assure a good balance between therapeutic effect and neurotoxicity, leading to HIV eradication.
References
Portegies P, de Gans J, Lange JM, Derix MM, Speelman H, Bakker M et al (1989) Declining incidence of AIDS dementia complex after introduction of zidovudine treatment. BMJ 299:819–821
Brew BJ, Sidtis JJ, Rosenblum M, Price RW (1988) AIDS dementia complex. J R Coll Physicians Lond 22:140–144
Shapshak P, Kangueane P, Fujimura RK, Commins D, Chiappelli F, Singer E et al (2011) Editorial neuroAIDS review. AIDS 25:123–141
Cinque P, Brew BJ, Gisslen M, Hagberg L, Price RW (2007) Cerebrospinal fluid markers in central nervous system HIV infection and AIDS dementia complex. Handb Clin Neurol 85:261–300
Simioni S, Cavassini M, Annoni JM, Rimbault Abraham A, Bourquin I, Schiffer V et al (2010) Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS 24:1243–1250
Giancola ML, Lorenzini P, Balestra P, Larussa D, Baldini F, Corpolongo A et al (2006) Neuroactive antiretroviral drugs do not influence neurocognitive performance in less advanced HIV-infected patients responding to highly active antiretroviral therapy. J Acquir Immune Defic Syndr 41:332–337
Tozzi V, Balestra P, Bellagamba R, Corpolongo A, Salvatori MF, Visco-Comandini U et al (2007) Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr 45:174–182
Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J et al (2007) The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS 21:1915–1921
Heaton RK, Clifford DB, Franklin DR Jr, Woods SP, Ake C, Vaida F et al (2010) HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 75:2087–2096
Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S et al (2011) HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 17:3–16
Engelhardt B, Sorokin L (2009) The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Semin Immunopathol 31:497–511
Hong S, Banks WA (2014) Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav Immun 45:1–2
Groothuis DR, Levy RM (1997) The entry of antiviral and antiretroviral drugs into the central nervous system. J Neurovirol 3:387–400
Budka H, Wiley CA, Kleihues P, Artigas J, Asbury AK, Cho ES et al (1991) HIV-associated disease of the nervous system: review of nomenclature and proposal for neuropathology-based terminology. Brain Pathol 1:143–152
Everall I, Luthert P, Lantos P (1993) A review of neuronal damage in human immunodeficiency virus infection: its assessment, possible mechanism and relationship to dementia. J Neuropathol Exp Neurol 52:561–566
Dohgu S, Banks WA (2013) Brain pericytes increase the lipopolysaccharide-enhanced transcytosis of HIV-1 free virus across the in vitro blood-brain barrier: evidence for cytokine-mediated pericyte-endothelial cell crosstalk. Fluids Barriers CNS 10:23
Kanmogne GD, Primeaux C, Grammas P (2005) HIV-1 gp120 proteins alter tight junction protein expression and brain endothelial cell permeability: implications for the pathogenesis of HIV-associated dementia. J Neuropathol Exp Neurol 64:498–505
Atluri VS, Hidalgo M, Samikkannu T, Kurapati KR, Jayant RD, Sagar V et al (2015) Effect of human immunodeficiency virus on blood-brain barrier integrity and function: an update. Front Cell Neurosci 9:212
Ferrucci A, Nonnemacher MR, Wigdahl B (2013) Extracellular HIV-1 viral protein R affects astrocytic glyceraldehyde 3-phosphate dehydrogenase activity and neuronal survival. J Neurovirol 19:239–253
Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S et al (2006) Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 12:1365–1371
Oldendorf WH (1974) Lipid solubility and drug penetration of the blood brain barrier. Proc Soc Exp Biol Med 147:813–815
Letendre SL, McCutchan JA, Childers ME, Woods SP, Lazzaretto D, Heaton RK et al (2004) Enhancing antiretroviral therapy for human immunodeficiency virus cognitive disorders. Ann Neurol 56:416–423
Kim RB, Fromm MF, Wandel C, Leake B, Wood AJ, Roden DM et al (1998) The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. J Clin Invest 101:289–294
Hayashi K, Pu H, Andras IE, Eum SY, Yamauchi A, Hennig B et al (2006) HIV-TAT protein upregulates expression of multidrug resistance protein 1 in the blood-brain barrier. J Cereb Blood Flow Metab 26:1052–1065
Eden A, Fuchs D, Hagberg L, Nilsson S, Spudich S, Svennerholm B et al (2010) HIV-1 viral escape in cerebrospinal fluid of subjects on suppressive antiretroviral treatment. J Infect Dis 202:1819–1825
Canestri A, Lescure FX, Jaureguiberry S, Moulignier A, Amiel C, Marcelin AG et al (2010) Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis 50:773–778
Cunningham PH, Smith DG, Satchell C, Cooper DA, Brew B (2000) Evidence for independent development of resistance to HIV-1 reverse transcriptase inhibitors in the cerebrospinal fluid. AIDS 14:1949–1954
Smit TK, Brew BJ, Tourtellotte W, Morgello S, Gelman BB, Saksena NK (2004) Independent evolution of human immunodeficiency virus (HIV) drug resistance mutations in diverse areas of the brain in HIV-infected patients, with and without dementia, on antiretroviral treatment. J Virol 78:10133–10148
Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC et al (2008) Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol 65:65–70
Thomas SA, Segal MB (1998) The transport of the anti-HIV drug, 2′,3′-didehydro-3′-deoxythymidine (D4T), across the blood-brain and blood-cerebrospinal fluid barriers. Br J Pharmacol 125:49–54
Yilmaz A, Price RW, Gisslen M (2012) Antiretroviral drug treatment of CNS HIV-1 infection. J Antimicrob Chemother 67:299–311
Ene L, Duiculescu D, Ruta S (2011) How much do antiretroviral drugs penetrate into the central nervous system? J Med Life 4:432–439
Condra JH, Petropoulos CJ, Ziermann R, Schleif WA, Shivaprakash M, Emini EA (2000) Drug resistance and predicted virologic responses to human immunodeficiency virus type 1 protease inhibitor therapy. J Infect Dis 182:758–765
Parkin NT, Hellmann NS, Whitcomb JM, Kiss L, Chappey C, Petropoulos CJ (2004) Natural variation of drug susceptibility in wild-type human immunodeficiency virus type 1. Antimicrob Agents Chemother 48:437–443
Acosta EP, Limoli KL, Trinh L, Parkin NT, King JR, Weidler JM et al (2012) Novel method to assess antiretroviral target trough concentrations using in vitro susceptibility data. Antimicrob Agents Chemother 56:5938–5945
Vernazza P, Daneel S, Schiffer V, Decosterd L, Fierz W, Klimkait T et al (2007) The role of compartment penetration in PI-monotherapy: the Atazanavir-Ritonavir Monomaintenance (ATARITMO) Trial. AIDS 21:1309–1315
Katlama C, Valantin MA, Algarte-Genin M, Duvivier C, Lambert-Niclot S, Girard PM et al (2010) Efficacy of darunavir/ritonavir maintenance monotherapy in patients with HIV-1 viral suppression: a randomized open-label, noninferiority trial, MONOI-ANRS 136. AIDS 24:2365–2374
Gutmann C, Cusini A, Gunthard HF, Fux C, Hirschel B, Decosterd LA et al (2010) Randomized controlled study demonstrating failure of LPV/r monotherapy in HIV: the role of compartment and CD4-nadir. AIDS 24:2347–2354
Letendre SL, Ellis RJ, Ances BM, McCutchan JA (2010) Neurologic complications of HIV disease and their treatment. Top HIV Med 18:45–55
Calcagno A, Di Perri G, Bonora S (2014) Pharmacokinetics and pharmacodynamics of antiretrovirals in the central nervous system. Clin Pharmacokinet 53:891–906
Strazielle N, Ghersi-Egea JF (2005) Factors affecting delivery of antiviral drugs to the brain. Rev Med Virol 15:105–133
Varatharajan L, Thomas SA (2009) The transport of anti-HIV drugs across blood-CNS interfaces: summary of current knowledge and recommendations for further research. Antivir Res 82:A99–109
Burger DM, Kraaijeveld CL, Meenhorst PL, Mulder JW, Koks CH, Bult A et al (1993) Penetration of zidovudine into the cerebrospinal fluid of patients infected with HIV. AIDS 7:1581–1587
Elovaara I, Poutiainen E, Lahdevirta J, Hokkanen L, Raininko R, Mattinen S et al (1994) Zidovudine reduces intrathecal immunoactivation in patients with early human immunodeficiency virus type 1 infection. Arch Neurol 51:943–950
Balis FM, Pizzo PA, Eddy J, Wilfert C, McKinney R, Scott G et al (1989) Pharmacokinetics of zidovudine administered intravenously and orally in children with human immunodeficiency virus infection. J Pediatr 114:880–884
Simpson DM (1999) Human immunodeficiency virus-associated dementia: review of pathogenesis, prophylaxis, and treatment studies of zidovudine therapy. Clin Infect Dis 29:19–34
Crowell CS, Huo Y, Tassiopoulos K, Malee KM, Yogev R, Hazra R et al (2015) Early viral suppression improves neurocognitive outcomes in HIV-infected children. AIDS 29:295–304
Kinai E, Kato S, Hosokawa S, Sadatsuki M, Gatanaga H, Kikuchi Y et al (2016) High plasma concentrations of zidovudine (AZT) do not parallel intracellular concentrations of AZT-triphosphates in infants during prevention of mother-to-child HIV-1 transmission. J Acquir Immune Defic Syndr 72:246–253
Crowell C (2016) Viral suppression and neurocognition in children. In: Global virology II, HIV and neuroAIDS. Edited by P S: Springer Publ. New York
Haworth SJ, Christofalo B, Anderson RD, Dunkle LM (1998) A single-dose study to assess the penetration of stavudine into human cerebrospinal fluid in adults. J Acquir Immune Defic Syndr Hum Retrovirol 17:235–238
Brady KA, Boston RC, Aldrich JL, MacGregor RR (2005) Stavudine entry into cerebrospinal fluid after single and multiple doses in patients infected with human immunodeficiency virus. Pharmacotherapy 25:10–17
Gibbs JE, Thomas SA (2002) The distribution of the anti-HIV drug, 2′3′-dideoxycytidine (ddC), across the blood-brain and blood-cerebrospinal fluid barriers and the influence of organic anion transport inhibitors. J Neurochem 80:392–404
Burger DM, Kraayeveld CL, Meenhorst PL, Mulder JW, Hoetelmans RM, Koks CH et al (1995) Study on didanosine concentrations in cerebrospinal fluid. Implications for the treatment and prevention of AIDS dementia complex. Pharm World Sci 17:218–221
Antinori A, Perno CF, Giancola ML, Forbici F, Ippolito G, Hoetelmans RM et al (2005) Efficacy of cerebrospinal fluid (CSF)-penetrating antiretroviral drugs against HIV in the neurological compartment: different patterns of phenotypic resistance in CSF and plasma. Clin Infect Dis 41:1787–1793
Gisslen M, Norkrans G, Svennerholm B, Hagberg L (1997) The effect on human immunodeficiency virus type 1 RNA levels in cerebrospinal fluid after initiation of zidovudine or didanosine. J Infect Dis 175:434–437
Minuesa G, Purcet S, Erkizia I, Molina-Arcas M, Bofill M, Izquierdo-Useros N et al (2008) Expression and functionality of anti-human immunodeficiency virus and anticancer drug uptake transporters in immune cells. J Pharmacol Exp Ther 324:558–567
Foudraine NA, Hoetelmans RM, Lange JM, de Wolf F, van Benthem BH, Maas JJ et al (1998) Cerebrospinal-fluid HIV-1 RNA and drug concentrations after treatment with lamivudine plus zidovudine or stavudine. Lancet 351:1547–1551
Thomas SA, Bye A, Segal MB (2001) Transport characteristics of the anti-human immunodeficiency virus nucleoside analog, abacavir, into brain and cerebrospinal fluid. J Pharmacol Exp Ther 298:947–953
Capparelli EV, Letendre SL, Ellis RJ, Patel P, Holland D, McCutchan JA (2005) Population pharmacokinetics of abacavir in plasma and cerebrospinal fluid. Antimicrob Agents Chemother 49:2504–2506
Giri N, Shaik N, Pan G, Terasaki T, Mukai C, Kitagaki S et al (2008) Investigation of the role of breast cancer resistance protein (Bcrp/Abcg2) on pharmacokinetics and central nervous system penetration of abacavir and zidovudine in the mouse. Drug Metab Dispos 36:1476–1484
Brew BJ, Halman M, Catalan J, Sacktor N, Price RW, Brown S et al (2007) Factors in AIDS dementia complex trial design: results and lessons from the abacavir trial. PLoS Clin Trials 2:e13
Kearney BP, Flaherty JF, Shah J (2004) Tenofovir disoproxil fumarate: clinical pharmacology and pharmacokinetics. Clin Pharmacokinet 43:595–612
Gibson AK, Shah BM, Nambiar PH, Schafer JJ (2016) Tenofovir alafenamide: a review of its use in the treatment of HIV-1 infection. Ann Pharmacother 50:942–952
Anthonypillai C, Gibbs JE, Thomas SA (2006) The distribution of the anti-HIV drug, tenofovir (PMPA), into the brain, CSF and choroid plexuses. Cerebrospinal Fluid Res 3:1
Best BM, Letendre SL, Koopmans P, Rossi SS, Clifford DB, Collier AC et al (2012) Low cerebrospinal fluid concentrations of the nucleotide HIV reverse transcriptase inhibitor, tenofovir. J Acquir Immune Defic Syndr 59:376–381
Eisfeld C, Reichelt D, Evers S, Husstedt I (2013) CSF penetration by antiretroviral drugs. CNS Drugs 27:31–55
Tashima KT, Caliendo AM, Ahmad M, Gormley JM, Fiske WD, Brennan JM et al (1999) Cerebrospinal fluid human immunodeficiency virus type 1 (HIV-1) suppression and efavirenz drug concentrations in HIV-1-infected patients receiving combination therapy. J Infect Dis 180:862–864
Best BM, Koopmans PP, Letendre SL, Capparelli EV, Rossi SS, Clifford DB et al (2010) Efavirenz concentrations in CSF exceed IC50 for wild-type HIV. J Antimicrob Chemother 66:354–357
Weiss J, Theile D, Ketabi-Kiyanvash N, Lindenmaier H, Haefeli WE (2007) Inhibition of MRP1/ABCC1, MRP2/ABCC2, and MRP3/ABCC3 by nucleoside, nucleotide, and non-nucleoside reverse transcriptase inhibitors. Drug Metab Dispos 35:340–344
Apostolova N, Funes HA, Blas-Garcia A, Galindo MJ, Alvarez A, Esplugues JV (2015) Efavirenz and the CNS: what we already know and questions that need to be answered. J Antimicrob Chemother 70:2693–2708
Glynn SL, Yazdanian M (1998) In vitro blood-brain barrier permeability of nevirapine compared to other HIV antiretroviral agents. J Pharm Sci 87:306–310
van Praag RM, van Weert EC, van Heeswijk RP, Zhou XJ, Sommadossi JP, Jurriaans S et al (2002) Stable concentrations of zidovudine, stavudine, lamivudine, abacavir, and nevirapine in serum and cerebrospinal fluid during 2 years of therapy. Antimicrob Agents Chemother 46:896–899
Veldkamp AI, Weverling GJ, Lange JM, Montaner JS, Reiss P, Cooper DA et al (2001) High exposure to nevirapine in plasma is associated with an improved virological response in HIV-1-infected individuals. AIDS 15:1089–1095
Tiraboschi JM, Niubo J, Vila A, Perez-Pujol S, Podzamczer D (2012) Etravirine concentrations in CSF in HIV-infected patients. J Antimicrob Chemother 67:1446–1448
Mora-Peris B, Watson V, Vera JH, Weston R, Waldman AD, Kaye S et al (2014) Rilpivirine exposure in plasma and sanctuary site compartments after switching from nevirapine-containing combined antiretroviral therapy. J Antimicrob Chemother 69:1642–1647
Polli JW, Jarrett JL, Studenberg SD, Humphreys JE, Dennis SW, Brouwer KR et al (1999) Role of P-glycoprotein on the CNS disposition of amprenavir (141W94), an HIV protease inhibitor. Pharm Res 16:1206–1212
Kravcik S, Gallicano K, Roth V, Cassol S, Hawley-Foss N, Badley A et al (1999) Cerebrospinal fluid HIV RNA and drug levels with combination ritonavir and saquinavir. J Acquir Immune Defic Syndr 21:371–375
Polis MA, Suzman DL, Yoder CP, Shen JM, Mican JM, Dewar RL et al (2003) Suppression of cerebrospinal fluid HIV burden in antiretroviral naive patients on a potent four-drug antiretroviral regimen. AIDS 17:1167–1172
Haas DW, Johnson B, Nicotera J, Bailey VL, Harris VL, Bowles FB et al (2003) Effects of ritonavir on indinavir pharmacokinetics in cerebrospinal fluid and plasma. Antimicrob Agents Chemother 47:2131–2137
Zhou XJ, Havlir DV, Richman DD, Acosta EP, Hirsch M, Collier AC et al (2000) Plasma population pharmacokinetics and penetration into cerebrospinal fluid of indinavir in combination with zidovudine and lamivudine in HIV-1-infected patients. AIDS 14:2869–2876
Marra CM, Zhao Y, Clifford DB, Letendre S, Evans S, Henry K et al (2009) Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS 23:1359–1366
Capparelli EV, Holland D, Okamoto C, Gragg B, Durelle J, Marquie-Beck J et al (2005) Lopinavir concentrations in cerebrospinal fluid exceed the 50% inhibitory concentration for HIV. AIDS 19:949–952
DiCenzo R, DiFrancesco R, Cruttenden K, Donnelly J, Schifitto G (2009) Lopinavir cerebrospinal fluid steady-state trough concentrations in HIV-infected adults. Ann Pharmacother 43:1972–1977
Yilmaz A, Stahle L, Hagberg L, Svennerholm B, Fuchs D, Gisslen M (2004) Cerebrospinal fluid and plasma HIV-1 RNA levels and lopinavir concentrations following lopinavir/ritonavir regimen. Scand J Infect Dis 36:823–828
Croteau D, Letendre S, Best BM, Rossi SS, Ellis RJ, Clifford DB et al (2012) Therapeutic amprenavir concentrations in cerebrospinal fluid. Antimicrob Agents Chemother 56:1985–1989
Sadler BM, Chittick GE, Polk RE, Slain D, Kerkering TM, Studenberg SD et al (2001) Metabolic disposition and pharmacokinetics of [14C]-amprenavir, a human immunodeficiency virus type 1 (HIV-1) protease inhibitor, administered as a single oral dose to healthy male subjects. J Clin Pharmacol 41:386–396
Saumoy M, Tiraboschi J, Gutierrez M, Niubo J, Domingo P, Vila A et al (2011) Viral response in stable patients switching to fosamprenavir/ritonavir monotherapy (the FONT Study). HIV Med 12:438–441
Yilmaz A, Verhofstede C, D’Avolio A, Watson V, Hagberg L, Fuchs D et al (2010) Treatment intensification has no effect on the HIV-1 central nervous system infection in patients on suppressive antiretroviral therapy. J Acquir Immune Defic Syndr 55:590–596
Yilmaz A, Fuchs D, Hagberg L, Nillroth U, Stahle L, Svensson JO et al (2006) Cerebrospinal fluid HIV-1 RNA, intrathecal immunoactivation, and drug concentrations after treatment with a combination of saquinavir, nelfinavir, and two nucleoside analogues: the M61022 study. BMC Infect Dis 6:63
Karlstrom O, Stahle L, Perrin L, Tegude H, Sonnerborg A (2006) Efficacy of nelfinavir-based treatment in the central nervous system of HIV-1 infected patients. Scand J Infect Dis 38:371–374
Aweeka F, Jayewardene A, Staprans S, Bellibas SE, Kearney B, Lizak P et al (1999) Failure to detect nelfinavir in the cerebrospinal fluid of HIV-1--infected patients with and without AIDS dementia complex. J Acquir Immune Defic Syndr Hum Retrovirol 20:39–43
Best BM, Letendre SL, Brigid E, Clifford DB, Collier AC, Gelman BB et al (2009) Low atazanavir concentrations in cerebrospinal fluid. AIDS 23:83–87
Yilmaz A, Gisslen M, Spudich S, Lee E, Jayewardene A, Aweeka F et al (2009) Raltegravir cerebrospinal fluid concentrations in HIV-1 infection. PLoS One 4:e6877
Arribas JR, Horban A, Gerstoft J, Fatkenheuer G, Nelson M, Clumeck N et al (2009) The MONET trial: darunavir/ritonavir with or without nucleoside analogues, for patients with HIV RNA below 50 copies/ml. AIDS 24:223–230
Kassahun K, McIntosh I, Cui D, Hreniuk D, Merschman S, Lasseter K et al (2007) Metabolism and disposition in humans of raltegravir (MK-0518), an anti-AIDS drug targeting the human immunodeficiency virus 1 integrase enzyme. Drug Metab Dispos 35:1657–1663
Croteau D, Letendre S, Best BM, Ellis RJ, Breidinger S, Clifford D et al (2010) Total raltegravir concentrations in cerebrospinal fluid exceed the 50-percent inhibitory concentration for wild-type HIV-1. Antimicrob Agents Chemother 54:5156–5160
Calcagno A, Cusato J, Simiele M, Motta I, Audagnotto S, Bracchi M et al (2014) High interpatient variability of raltegravir CSF concentrations in HIV-positive patients: a pharmacogenetic analysis. J Antimicrob Chemother 69:241–245
Tatro ET, Soontornniyomkij B, Letendre SL, Achim CL (2014) Cytokine secretion from brain macrophages infected with human immunodeficiency virus in vitro and treated with raltegravir. BMC Infect Dis 14:386
Moore JP, Doms RW (2003) The entry of entry inhibitors: a fusion of science and medicine. Proc Natl Acad Sci U S A 100:10598–10602
Price RW, Parham R, Kroll JL, Wring SA, Baker B, Sailstad J et al (2008) Enfuvirtide cerebrospinal fluid (CSF) pharmacokinetics and potential use in defining CSF HIV-1 origin. Antivir Ther 13:369–374
van Lelyveld SF, Nijhuis M, Baatz F, Wilting I, van den Bergh WM, Kurowski M et al (2010) Therapy failure following selection of enfuvirtide-resistant HIV-1 in cerebrospinal fluid. Clin Infect Dis 50:387–390
Thomas SA (2004) Drug transporters relevant to HIV therapy. J HIV Ther 9:92–96
Tiraboschi JM, Niubo J, Curto J, Podzamczer D (2010) Maraviroc concentrations in cerebrospinal fluid in HIV-infected patients. J Acquir Immune Defic Syndr 55:606–609
Cottrell ML, Hadzic T, Kashuba AD (2013) Clinical pharmacokinetic, pharmacodynamic and drug-interaction profile of the integrase inhibitor dolutegravir. Clin Pharmacokinet 52:981–994
Lee JSF, Calmy A, Andrieux-Meyer I, Ford N (2012) Review of the safety, efficacy, and pharmacokinetics of elvitegravir with an emphasis on resource-limited settings. HIV AIDS (Auckl) 4:5–15
Nwogu JN, Ma Q, Babalola CP, Adedeji WA, Morse GD, Taiwo B (2016) Pharmacokinetic, pharmacogenetic, and other factors influencing CNS penetration of antiretrovirals. AIDS Res Treat 2016:1–13
Shimura K, Kodama E, Sakagami Y, Matsuzaki Y, Watanabe W, Yamataka K et al (2008) Broad antiretroviral activity and resistance profile of the novel human immunodeficiency virus integrase inhibitor elvitegravir (JTK-303/GS-9137)▿†. J Virol 82:764–774
Tozzi V, Balestra P, Salvatori MF, Vlassi C, Liuzzi G, Giancola ML et al (2009) Changes in cognition during antiretroviral therapy: comparison of 2 different ranking systems to measure antiretroviral drug efficacy on HIV-associated neurocognitive disorders. J Acquir Immune Defic Syndr 52:56–63
Hagberg L, Cinque P, Gisslen M, Brew BJ, Spudich S, Bestetti A et al (2010) Cerebrospinal fluid neopterin: an informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res Ther 7:15
Baker LM, Paul RH, Heaps-Woodruff JM, Chang JY, Ortega M, Margolin Z et al (2015) The effect of central nervous system penetration effectiveness of highly active antiretroviral therapy on neuropsychological performance and neuroimaging in HIV infected individuals. J Neuroimmune Pharmacol 10:487–492
Caniglia EC, Cain LE, Justice A, Tate J, Logan R, Sabin C et al (2014) Antiretroviral penetration into the CNS and incidence of AIDS-defining neurologic conditions. Neurology 83:134–141
Robertson KR, Su Z, Margolis DM, Krambrink A, Havlir DV, Evans S et al (2010) Neurocognitive effects of treatment interruption in stable HIV-positive patients in an observational cohort. Neurology 74:1260–1266
Tovar-y-Romo LB, Bumpus NN, Pomerantz D, Avery LB, Sacktor N, McArthur JC et al (2012) Dendritic spine injury induced by the 8-hydroxy metabolite of efavirenz. J Pharmacol Exp Ther 343:696–703
Novakovic M, Turkulov V, Maric D, Kozic D, Rajkovic U, Bjelan M et al (2015) Prediction of brain atrophy using three drug scores in neuroasymptomatic HIV-infected patients with controlled viremia. Braz J Infect Dis 19:503–509
Gollapudi S, Gupta S (1990) Human immunodeficiency virus I-induced expression of P-glycoprotein. Biochem Biophys Res Commun 171:1002–1007
Yao SY, Ng AM, Sundaram M, Cass CE, Baldwin SA, Young JD (2001) Transport of antiviral 3′-deoxy-nucleoside drugs by recombinant human and rat equilibrative, nitrobenzylthioinosine (NBMPR)-insensitive (ENT2) nucleoside transporter proteins produced in Xenopus oocytes. Mol Membr Biol 18:161–167
Pan G, Giri N, Elmquist WF (2007) Abcg2/Bcrp1 mediates the polarized transport of antiretroviral nucleosides abacavir and zidovudine. Drug Metab Dispos 35:1165–1173
Gibbs JE, Jayabalan P, Thomas SA (2003) Mechanisms by which 2′,3′-dideoxyinosine (ddI) crosses the guinea-pig CNS barriers; relevance to HIV therapy. J Neurochem 84:725–734
Baldwin SA, Beal PR, Yao SY, King AE, Cass CE, Young JD (2004) The equilibrative nucleoside transporter family, SLC29. Pflugers Arch 447:735–743
Jung N, Lehmann C, Rubbert A, Knispel M, Hartmann P, van Lunzen J et al (2008) Relevance of the organic cation transporters 1 and 2 for antiretroviral drug therapy in human immunodeficiency virus infection. Drug Metab Dispos 36:1616–1623
Stormer E, von Moltke LL, Perloff MD, Greenblatt DJ (2002) Differential modulation of P-glycoprotein expression and activity by non-nucleoside HIV-1 reverse transcriptase inhibitors in cell culture. Pharm Res 19:1038–1045
Kim AE, Dintaman JM, Waddell DS, Silverman JA (1998) Saquinavir, an HIV protease inhibitor, is transported by P-glycoprotein. J Pharmacol Exp Ther 286:1439–1445
Lee LS, Soon GH, Shen P, Yong EL, Flexner C, Pham P (2010) Darunavir/ritonavir and efavirenz exert differential effects on MRP1 transporter expression and function in healthy volunteers. Antivir Ther 15:275–279
Fujimoto H, Higuchi M, Watanabe H, Koh Y, Ghosh AK, Mitsuya H et al (2009) P-glycoprotein mediates efflux transport of darunavir in human intestinal Caco-2 and ABCB1 gene-transfected renal LLC-PK1 cell lines. Biol Pharm Bull 32:1588–1593
Vierling P, Greiner J (2003) Prodrugs of HIV protease inhibitors. Curr Pharm Des 9:1755–1770
Eilers M, Roy U, Mondal D (2008) MRP (ABCC) transporters-mediated efflux of anti-HIV drugs, saquinavir and zidovudine, from human endothelial cells. Exp Biol Med (Maywood) 233:1149–1160
Zastre JA, Chan GN, Ronaldson PT, Ramaswamy M, Couraud PO, Romero IA et al (2009) Up-regulation of P-glycoprotein by HIV protease inhibitors in a human brain microvessel endothelial cell line. J Neurosci Res 87:1023–1036
Gupta A, Zhang Y, Unadkat JD, Mao Q (2004) HIV protease inhibitors are inhibitors but not substrates of the human breast cancer resistance protein (BCRP/ABCG2). J Pharmacol Exp Ther 310:334–341
Perloff MD, von Moltke LL, Fahey JM, Greenblatt DJ (2007) Induction of P-glycoprotein expression and activity by ritonavir in bovine brain microvessel endothelial cells. J Pharm Pharmacol 59:947–953
Dumond JB, Vourvahis M, Rezk NL, Patterson KB, Tien HC, White N et al (2010) A phenotype-genotype approach to predicting CYP450 and P-glycoprotein drug interactions with the mixed inhibitor/inducer tipranavir/ritonavir. Clin Pharmacol Ther 87:735–742
Walker DK, Bowers SJ, Mitchell RJ, Potchoiba MJ, Schroeder CM, Small HF (2008) Preclinical assessment of the distribution of maraviroc to potential human immunodeficiency virus (HIV) sanctuary sites in the central nervous system (CNS) and gut-associated lymphoid tissue (GALT). Xenobiotica 38:1330–1339
Gao Z, Chen Y, Cai X, Xu R (2016) Predict drug permeability to blood-brain-barrier from clinical phenotypes: drug side effects and drug indications. Bioinformatics 33:901–908
Fiandra L, Capetti A, Sorrentino L, Corsi F (2017) Nanoformulated antiretrovirals for penetration of the central nervous system: state of the art. J Neuroimmune Pharmacol 12:17–30
Kaushik A, Jayant RD, Nair M (2016) Advancements in nano-enabled therapeutics for neuroHIV management. Int J Nanomedicine 11:4317–4325
Canestri A, Ghosn J, Wirden M, Marguet F, Ktorza N, Boubezari I et al (2006) Foscarnet salvage therapy for patients with late-stage HIV disease and multiple drug resistance. Antivir Ther 11:561–566
Delory T, Papot E, Rioux C, Charpentier C, Auge-Courtoi C, Michard F et al (2016) Foscarnet, zidovudine and dolutegravir combination efficacy and tolerability for late stage HIV salvage therapy: a case-series experience. J Med Virol 88:1204–1210
Torchilin VP (2005) Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov 4:145–160
Kuo YC, Chen HH (2006) Effect of nanoparticulate polybutylcyanoacrylate and methylmethacrylate-sulfopropylmethacrylate on the permeability of zidovudine and lamivudine across the in vitro blood-brain barrier. Int J Pharm 327:160–169
Kuo YC, Chen HH (2010) Effect of electromagnetic field on endocytosis of cationic solid lipid nanoparticles by human brain-microvascular endothelial cells. J Drug Target 18:447–456
Dou H, Grotepas CB, McMillan JM, Destache CJ, Chaubal M, Werling J et al (2009) Macrophage delivery of nanoformulated antiretroviral drug to the brain in a murine model of neuroAIDS. J Immunol 183:661–669
Dash PK, Gendelman HE, Roy U, Balkundi S, Alnouti Y, Mosley RL et al (2012) Long-acting nanoformulated antiretroviral therapy elicits potent antiretroviral and neuroprotective responses in HIV-1-infected humanized mice. AIDS 26:2135–2144
Kanmogne GD, Singh S, Roy U, Liu X, McMillan J, Gorantla S et al (2012) Mononuclear phagocyte intercellular crosstalk facilitates transmission of cell-targeted nanoformulated antiretroviral drugs to human brain endothelial cells. Int J Nanomedicine 7:2373–2388
Jayant RD, Atluri VS, Agudelo M, Sagar V, Kaushik A, Nair M (2015) Sustained-release nanoART formulation for the treatment of neuroAIDS. Int J Nanomedicine 10:1077–1093
Mishra V, Mahor S, Rawat A, Gupta PN, Dubey P, Khatri K et al (2006) Targeted brain delivery of AZT via transferrin anchored pegylated albumin nanoparticles. J Drug Target 14:45–53
Kuo YC, Lee CL (2012) Methylmethacrylate-sulfopropylmethacrylate nanoparticles with surface RMP-7 for targeting delivery of antiretroviral drugs across the blood-brain barrier. Colloids Surf B Biointerfaces 90:75–82
Gerson T, Makarov E, Senanayake TH, Gorantla S, Poluektova LY, Vinogradov SV (2014) Nano-NRTIs demonstrate low neurotoxicity and high antiviral activity against HIV infection in the brain. Nanomedicine 10:177–185
Destache CJ, Belgum T, Goede M, Shibata A, Belshan MA (2010) Antiretroviral release from poly(DL-lactide-co-glycolide) nanoparticles in mice. J Antimicrob Chemother 65:2183–2187
Fiandra L, Colombo M, Mazzucchelli S, Truffi M, Santini B, Allevi R et al (2015) Nanoformulation of antiretroviral drugs enhances their penetration across the blood brain barrier in mice. Nanomedicine 11:1387–1397
Carr A (2004) Antiretroviral therapy for previously untreated HIV-1-infected adults: 2NN, or just one? Lancet 363:1248–1250
Gutierrez F, Navarro A, Padilla S, Anton R, Masia M, Borras J et al (2005) Prediction of neuropsychiatric adverse events associated with long-term efavirenz therapy, using plasma drug level monitoring. Clin Infect Dis 41:1648–1653
Clifford DB, Evans S, Yang Y, Acosta EP, Goodkin K, Tashima K et al (2005) Impact of efavirenz on neuropsychological performance and symptoms in HIV-infected individuals. Ann Intern Med 143:714–721
Blanch J, Martinez E, Rousaud A, Blanco JL, Garcia-Viejo MA, Peri JM et al (2001) Preliminary data of a prospective study on neuropsychiatric side effects after initiation of efavirenz. J Acquir Immune Defic Syndr 27:336–343
Munoz-Moreno JA, Fumaz CR, Ferrer MJ, Gonzalez-Garcia M, Molto J, Negredo E et al (2009) Neuropsychiatric symptoms associated with efavirenz: prevalence, correlates, and management. A neurobehavioral review. AIDS Rev 11:103–109
Treisman GJ, Kaplin AI (2002) Neurologic and psychiatric complications of antiretroviral agents. AIDS 16:1201–1215
Gazzard B, Duvivier C, Zagler C, Castagna A, Hill A, van Delft Y et al (2011) Phase 2 double-blind, randomized trial of etravirine versus efavirenz in treatment-naive patients: 48-week results. AIDS 25:2249–2258
Nguyen A, Calmy A, Delhumeau C, Mercier I, Cavassini M, Mello AF et al (2011) A randomized cross-over study to compare raltegravir and efavirenz (SWITCH-ER study). AIDS 25:1481–1487
O’Mahony SM, Myint AM, Steinbusch H, Leonard BE (2005) Efavirenz induces depressive-like behaviour, increased stress response and changes in the immune response in rats. Neuroimmunomodulation 12:293–298
Apostolova N, Funes HA, Blas-Garcia A, Alegre F, Polo M, Esplugues JV (2015) Involvement of nitric oxide in the mitochondrial action of efavirenz: a differential effect on neurons and glial cells. J Infect Dis 211:1953–1958
Gatch MB, Kozlenkov A, Huang RQ, Yang W, Nguyen JD, Gonzalez-Maeso J et al (2013) The HIV antiretroviral drug efavirenz has LSD-like properties. Neuropsychopharmacology 38:2373–2384
Jost CR, Van Der Zee CE, In ’t Zandt HJ, Oerlemans F, Verheij M, Streijger F et al (2002) Creatine kinase B-driven energy transfer in the brain is important for habituation and spatial learning behaviour, mossy fibre field size and determination of seizure susceptibility. Eur J Neurosci 15:1692–1706
Streck EL, Scaini G, Rezin GT, Moreira J, Fochesato CM, Romao PR (2008) Effects of the HIV treatment drugs nevirapine and efavirenz on brain creatine kinase activity. Metab Brain Dis 23:485–492
Streck EL, Ferreira GK, Scaini G, Rezin GT, Goncalves CL, Jeremias IC et al (2011) Non-nucleoside reverse transcriptase inhibitors efavirenz and nevirapine inhibit cytochrome C oxidase in mouse brain regions. Neurochem Res 36:962–966
Bertrand L, Dygert L, Toborek M (2016) Antiretroviral treatment with efavirenz disrupts the blood-brain barrier integrity and increases stroke severity. Sci Rep 6:39738
Brown LA, Jin J, Ferrell D, Sadic E, Obregon D, Smith AJ et al (2014) Efavirenz promotes beta-secretase expression and increased Abeta1-40,42 via oxidative stress and reduced microglial phagocytosis: implications for HIV associated neurocognitive disorders (HAND). PLoS One 9:e95500
Brown LA, Scarola J, Smith AJ, Sanberg PR, Tan J, Giunta B (2014) The role of tau protein in HIV-associated neurocognitive disorders. Mol Neurodegener 9:40
Funes HA, Apostolova N, Alegre F, Blas-Garcia A, Alvarez A, Marti-Cabrera M et al (2014) Neuronal bioenergetics and acute mitochondrial dysfunction: a clue to understanding the central nervous system side effects of efavirenz. J Infect Dis 210:1385–1395
Almeida A, Moncada S, Bolanos JP (2004) Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat Cell Biol 6:45–51
Bolanos JP, Almeida A, Moncada S (2010) Glycolysis: a bioenergetic or a survival pathway? Trends Biochem Sci 35:145–149
Acknowledgment
We would like to thank Suzi Hong, PhD (University of California, San Diego, CA), for her assistance in the reproduction of graphics in Fig. 17.1.
Conflict of interest The authors report no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Science+Business Media LLC
About this chapter
Cite this chapter
Somboonwit, C., Montero, J.A., Sinnott, J.T., Shapshak, P. (2017). Antiretroviral Therapy: Brain Penetration. In: Shapshak, P., et al. Global Virology II - HIV and NeuroAIDS. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-7290-6_17
Download citation
DOI: https://doi.org/10.1007/978-1-4939-7290-6_17
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-7288-3
Online ISBN: 978-1-4939-7290-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)