Abstract
HIV-positive patients may be effectively treated with highly active antiretroviral therapy and such a strategy is associated with striking immune recovery and viral load reduction to very low levels. Despite undeniable results, the central nervous system (CNS) is commonly affected during the course of HIV infection, with neurocognitive disorders being as prevalent as 20–50 % of treated subjects. This review discusses the pathophysiology of CNS infection by HIV and the barriers to efficacious control of such a mechanism, including the available data on compartmental drug penetration and on pharmacokinetic/pharmacodynamic relationships. In the reviewed articles, a high variability in drug transfer to the CNS is highlighted with several mechanisms as well as methodological issues potentially influencing the observed results. Nevirapine and zidovudine showed the highest cerebrospinal fluid (CSF) to plasma ratios, although target concentrations are currently unknown for the CNS. The use of the composite CSF concentration effectiveness score has been associated with better virological outcomes (lower HIV RNA) but has been inconsistently associated with neurocognitive outcomes. These findings support the CNS effectiveness of commonly used highly antiretroviral therapies. The use of antiretroviral drugs with increased CSF penetration and/or effectiveness in treating or preventing neurocognitive disorders however needs to be assessed in well-designed prospective studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
HIV enters the central nervous system (CNS) early in the natural history of the disease with cerebrospinal fluid (CSF) HIV RNA recovered as early as 8 days after infection [1]. The presence of viral replication (in perivascular macrophages and microglia and, although restricted, in astrocytes) is eventually associated with neuronal damage due to persistent immune activation and cytokines production: the clinical endpoint of untreated CNS HIV infection is the appearance of HIV-associated dementia (HAD) [2, 3]. With the introduction of highly active antiretroviral therapy (HAART), the incidence of dementia significantly declined; nevertheless, cognitive impairment [asymptomatic and moderate according to the impact on everyday life and globally defined as HIV-associated neurocognitive disorders (HAND)] remains highly prevalent [4]. Although several authors highlight the impact of traditional risk factors (age, drug and alcohol abuse, previous head injuries, cardiovascular risk abnormalities, opportunistic infections) [5] on neurocognitive impairment in HIV-positive subjects, the role of neuro-effective HAART is crucial: it is significantly associated with CSF viral control but inconsistently with the prevention and treatment of HAND.
This review analyses the pharmacokinetics and pharmacodynamics of antiretroviral drugs in the CNS, considering the effect on both compartmental viral replication and neurocognitive impairment.
2 Search Strategy
After including studies and reviews on pathogenesis, diagnosis and treatment of neurocognitive disorders in HIV-positive patients, we focused on pharmacokinetic and pharmacodynamic data. The aim was to include all studies containing pharmacokinetic data pertaining to and using the following search terms: [(HIV AND (central nervous system OR cerebrospinal fluid) AND (pharmacokinetics OR pharmacokinetic OR pharmacodynamic OR passage)]. For the pharmacodynamic chapter the following search terms were used: [(HIV AND (CPE OR central nervous system concentration effectiveness score OR HIV RNA)]. Review articles were included for finding references and unpublished conference abstracts. Articles were not restricted based on year of publication or language. Articles identified by the PubMed search were further screened manually by review of the full article text.
3 Pathophysiology of Central Nervous System (CNS) Injury by HIV
The neuropathogenesis of CNS damage is generally considered to be initiated and driven by HIV invasion and replication within the brain parenchyma; productive infection of brain perivascular macrophages and endogenous microglia and restricted infection of astrocytes have been demonstrated [6, 7]. Consequently, neuroinflammation and immune activation of resident glia (macrophages, microglia, astrocytes) have been associated with indirect neuronal injury [2]. With no antiretroviral treatment, activated microglia, infiltrating macrophages, reduced synaptic and dendritic density and neuronal loss are the neuropathological correlates of HAD [8, 9]. With the introduction of HAART, lymphocytes infiltration was markedly reduced (and limited to immune-reconstitution inflammatory syndrome cases) while neuroinflammation was observed in different anatomical sites: while basal ganglia were involved in pre-HAART specimens, in post-HAART samples hippocampus and adjacent parts of entorhinal and temporal cortex were frequently involved [10, 11]. Inflammatory cytokines and chemokines [tumour necrosis factor (TNF)-α, interleukin (IL)-6, IL-10, chemokine C-C motif ligand 2 (CCL-2), C-X-C motif chemokine 10 (CXCL-10)] have been found to be abnormally elevated in HIV-positive patients and have been linked to the alteration of the blood–brain barrier (BBB): viral factors (TAT, gp120) and lipopolysaccharide have been also implicated [3]. The impairment in BBB function has a crucial impact on the pathogenesis of HAD since it facilitates the penetration of HIV-infected monocytes, thus increasing the viral biomass in the CNS [12, 13]. BBB damage may persist despite effective antiretroviral treatment and a low nadir CD4+ T lymphocyte cell count has recently been identified as a predictor of such an event [14–16].
The immune cell trafficking from and toward the CNS has the potential to sustain the persistence of residual viraemia; although the exact origin of the latter is still debated [17], it has been proven that drugs with lower diffusion into tissues [such as protease inhibitors (PIs)] have been associated with either higher residual viraemia or replication in sanctuary sites (such as lymph nodes) [18]. Furthermore, the CNS has been recognised as a site of compartmentalised viral replication, with the possible divergent evolution of HIV quasispecies [19, 20]. Approximately 10 % of patients have detectable HIV RNA in the CSF despite plasma viral control; this ‘CSF escape’ is usually transient and is not associated with neurological sequelae [21]. However, different resistance-associated mutations may be selected in the CSF and cases of symptomatic (and severe) CSF escape have been constantly reported in recent years [22, 23].
The compartmental pharmacokinetic and pharmacodynamic profile of antiretrovirals may be of relevant importance both for HIV control in the CNS and for the reduction of viral biomass in reservoir sites in sight of seeking a functional cure.
4 HIV-Associated Neurocognitive Disorders (HAND)
A consensus research definition of HAND includes the sub-classifications asymptomatic neurocognitive impairment (ANI), mild neurocognitive disorder (MND) and HAD [24]. This categorization relies upon the execution of a full battery of neurocognitive tests (assessing at least five domains, including attention–information processing, language, abstraction–executive, complex perceptual motor skills, memory, simple motor skills or sensory perceptual skills) and upon the determination of functional status (usually self-reported). Patients presenting abnormalities in two cognitive domains [age-adjusted scores 1 standard deviation (SD) lower than the average] are diagnosed with ANI or MND (with no or mild impairment in daily living, respectively); significant deficit in two cognitive domains (with scores lower than 2 SD) and impairment in everyday living are the diagnostic criteria for HAD. Considerable uncertainty is still undeniable in the diagnosis, determinants, prognostic factors and treatment of HAND, although HAART has been associated with significant improvement in symptoms and CSF markers of immune activation and neuronal damage in patients with HAD [4]. One of the key questions is whether the diagnosis of ANI has any relevance in the course of HIV infection: recent data suggest that patients with ANI may progress to MND and that they have a significant impairment in performance-based tests (potentially affecting adherence to medications) [25, 26]. The uncertainty in this area is enhanced by the diagnostic criteria that, to some extent, may overestimate the prevalence of asymptomatic and mild forms of neurocognitive impairment.
Furthermore, several authors highlight the high prevalence of other risk factors for neurocognitive decline such as increasing age, high cardio- and cerebrovascular risk, the often under-diagnosed presence of psychiatric illnesses, the use of psychotropic substances and the prevalence of chronic hepatitis [and specifically hepatitis C virus (HCV)] [5, 27]. The challenge of studying HAND is having an adequate well-matched control group in which all of these confounding factors may be accounted for [28]. Nevertheless, some HIV-associated (CD4 cell count at nadir below 200/mm3, plasmatic or CSF HIV replication, cell-associated HIV DNA) and some other risk factors (age above 50 years, HCV infection, metabolic and glucose abnormalities, cardiovascular risk) have been identified and they may help in selecting patients for accurate neurocognitive screening and follow-up. Finally, a therapeutic approach is not clearly defined since controlling HIV replication may be necessary but not sufficient: neither higher CNS-penetrating combined antiretroviral therapy nor adjuvant treatments have so far proven to be effective in preventing and reversing HAND [29].
5 Mechanisms of Drug Passage to the CNS
To be efficacious, drugs must reach adequate concentrations at the site of action: in the case of CNS infection by HIV, the targets are macrophages, microglia and astrocytes within the brain parenchyma. After intestinal absorption, orally administered antiretrovirals (the vast majority of available drugs, with the exception of intravenous zidovudine and subcutaneous enfuvirtide) are transported by plasma proteins in the bloodstream and distributed to organs and tissues. The CNS is reached by a considerable blood flow (approximately 14 % of cardiac output) but two anatomical barriers can be found that prevent the free passage of drugs into the brain: the BBB and the blood–CSF barrier (BCB). The first one is characterised by endothelial cells connected by tight junctions and by the presence of astrocytic end feet: several substances are restricted from crossing the BBB [30]. Nevertheless, tight junctions are absent in some areas of the brain (hypothalamus, area postrema, subfornical organ) and direct diffusion is possible. Several mechanisms have been identified for crossing the BBB and they affect each compound’s ability to reach the brain tissue: paracellular aqueous pathway, transcellular lipophilic pathway, transport proteins, receptor-mediated transcytosis and adsorptive transcytosis. Therefore, both patients’ and drugs’ characteristics influence antiretrovirals’ passage into the CNS.
The study of the pharmacokinetics of antiretrovirals in the CNS has two key obstacles: the scarce data on tissue concentrations and the intracellular target of action. Obtaining brain tissue concentrations is limited in healthy patients (for obvious ethical reasons) and is associated with potential bias in sick individuals (brain biopsies are usually performed in patients with severe CNS diseases and this may impact the results of measured concentrations): data on autoptic measurements are limited and they may be influenced by the time elapsed from death to the procedure. Furthermore, brain parenchyma concentrations derive from different compartments (averaged as a single measurement per gram of tissue) and they may be influenced by preparation and analysis procedures [31]. Microdyalisis is another option for directly measuring brain extracellular concentrations (through the use of intracranial catheters): however, it is an invasive technique and the results may depend upon the characteristics of the compounds [32, 33]. CSF concentrations are easier to obtain but their reliability as markers of CNS exposure is still debated. CSF is believed to be produced by filtration from blood plasma (two-thirds) and from brain extracellular fluid (one-third), from which it is separated by one layer of ependymal cells; nevertheless, a difference in drug concentrations may be observed if CSF is withdrawn from cisterna magna or from lumbar space [34]. Several animal studies have suggested that CSF is a reliable surrogate marker for most of the studied drugs; although the variability in predicting tissue concentrations was high, it was considerably lower than plasma unbound concentrations and comparable with microdyalisis [33, 35, 36]. As an example, animal data (non-human primates) confirmed the good correlation between zidovudine CSF and brain parenchyma concentrations; [32] data for other antiretrovirals are more variable and have recently been reviewed by Eisfeld et al. [37]. Additionally, drug concentrations in brain tissue are not uniform; they may vary with the distance from the CSF, with the vascularity of brain regions, and between white and grey matter [38]. Since the perivascular areas are probably the main objective of antiretroviral therapy, this may not be relevant in the delivery of drugs to target cells [39].
The second pitfall in the evaluation of CNS exposure is the site of action: with the exception of enfuvirtide and maraviroc, all antiretrovirals have intracellular targets. While non-nucleoside reverse transcriptase inhibitors (NNRTIs), PIs and integrase strand transfer inhibitors (ISTIs) once inside the cells are ready for exerting their activity, nucleoside reverse transcriptase inhibitors (NRTIs) need to be phosphorylated (thrice or twice) to become active and compete with endogenous nucleosides. The direct relationship between plasma and intracellular concentrations support the measurement of the former; however, no data are currently available on the concentrations reached inside CNS macrophages, microglia or astrocytes.
5.1 Patients’ Characteristics and Blood–Brain Barrier Damage
Older age may affect the passage of several drugs into the CNS: reduced blood efflux, permissive BBB and altered CSF flow are some of the potential mechanisms [40]. Being atherosclerosis and cerebrovascular disease common in older HIV-positive patients this may be relevant [41, 42]. Furthermore, as a consequence of declining renal function, plasma concentrations of several antiretrovirals have been shown to increase with increasing age. The only available data suggest that while plasma concentrations of efavirenz and tenofovir are increased in older subjects, efavirenz CSF concentration have a steep increase after 60 years of age [43].
Meningeal inflammation (usually observed in acute infection, rebound encephalitis, CSF escape or with opportunistic infections) has the potential to modulate the penetration of antiretrovirals: this is mediated by blood flow, BBB impairment and pH modifications. The latter mechanism has been identified in bacterial meningitis but may be relevant for drugs very sensitive to pH, such as raltegravir [44].
Finally, BBB impairment has been considered as a key event in the pathogenesis of AIDS dementia complex and other HIV-related neurological complications. BBB alterations were found in 2–22 % of HIV-positive asymptomatic individuals, in about 50 % of patients with AIDS and in 100 % of patients with HAD [45–47]. Furthermore, altered permeability may persist in a subset of patients (mostly those with a low CD4+ T lymphocytes nadir) despite antiretroviral treatment and it has been associated with a higher prevalence of HAND [14–16]. Theoretically, a permissive barrier may allow the passage of both drugs and plasma proteins, thus increasing CSF total concentrations but reducing free drug concentrations: the net effect on antiviral efficacy is currently not known [48]. Tenofovir, emtricitabine and raltegravir CSF concentrations have been shown to be higher in the presence of altered BBB and to be directly proportional to CSF to plasma albumin ratios (CSARs) [49–51].
5.2 Drug Characteristics
Four chemical characteristics that affect drug passage have been identified: molecular weight (the smaller the higher), lipophilicity (the higher the higher, measured as octanol water distribution coefficient, LogP), ionization (the higher the lower) and plasma protein binding (the lower the higher). Table 1 shows the molecular size, LogP and unbound plasma fractions for available antiretrovirals. NRTIs are small, poorly bound molecules with a generally high CSF to plasma ratio; tenofovir is an exception to this example since it is positively charged and thus requires active transport to cross the BBB. Molecular size and lipophilicity can be graphically plotted and an area of optimal characteristics can be drawn, as in a recent paper by Marzolini et al. [52]: drugs with a distribution coefficient (LogD, a measure of pH-dependant lipophilicity) between −1 and +5 and with a cross-sectional area between 20 and 70 (Å2) showed the highest penetration into the CSF.
Protein binding has been classically identified as one of the key characteristics affecting drug distribution into organs and tissues; highly protein bound molecules have less unbound (or free) drug available for exerting the effect or being transported outside the blood stream. The effect of proteins on antiviral effect has been studied in vitro: at higher levels intracellular antiretroviral concentrations are reduced as well as their antiviral effect [53]. This seems to be confirmed in the CSF since a direct relationship between plasma unbound fraction and CSF to plasma ratios has been shown for some antiretrovirals [54–56]. Measuring unbound CSF concentrations has proven to be more challenging due to low drug and protein concentrations: CSF albumin is usually 7.8–40 mg/L in CSF and 35–55 g/L in plasma, with normal CSAR ranging from 6 to 9.5 according to age [57]. Data are available for few compounds: CSF drug concentrations were shown to be very close to plasma unbound ones [56, 58, 59]. Etravirine passage is unexpectedly peculiar: despite very low unbound plasma concentrations (approximately 0.1 %), the total etravirine CSF to plasma ratio was around 4 % [60]. However, etravirine was found to be highly protein bound in the CSF, although the authors were not able to understand the target proteins. This unexpected finding may be explained by specific binding to other plasma/CSF proteins or to the effect of concomitantly administered drugs (since etravirine is often administered with boosted PIs).
5.3 Transporters and Pharmacogenetics
Several transporting proteins have been found to be expressed at the BBB and at the BCB: p-glycoprotein (P-gp), organic anion transporter (OAT) 1, 2 and 3, breast cancer resistance protein (BCRP) and others. P-gp has been extensively studied as it mediates adenosine triphosphate (ATP)-dependant efflux of several drugs towards the bloodstream, thus potentially reducing the amount available for reaching the brain parenchyma; it has also been implicated in refractory epilepsy [61]. Positron emission tomography (PET) techniques were used to quantify (both in animal and in humans) the effect of functionally or pharmacologically inhibited P-gp: several substrates showed a huge increase in brain parenchyma diffusion [62]. Other transporters have been less extensively studied but they are expressed at the brain barriers and, for instance, PIs have been shown to be substrate of OAT1A2 [63, 64].
The importance of understanding drug passage across BBB and BCB lies in the modulatory effects on transporters and on the possible influence of genetic polymorphisms affecting enzyme activity or expression. In non-human primates (using nelfinavir and zosiquidar, a P-gp inhibitor) P-gp blocking was associated with modest increases in CSF concentrations but extensive increments in brain concentrations [65]. Large lipophilic drugs such as PIs have strong binding affinities to drug efflux transporters expressed at the BBB and thus are prevented from entering the brain [52]. When combined, ritonavir (having the highest affinity) will occupy a large proportion of the transporter binding sites and thus slow down the efflux rate of the coadministered PI, thereby facilitating its brain entry. This was confirmed in a study comparing once-daily (800 mg with ritonavir 100 mg) with twice-daily darunavir (600 mg with ritonavir 100 mg twice daily): CSF concentrations (as expected given the lower dose) but also CSF to plasma ratios were lower, possibly because of a reduced ritonavir effect along the dosing interval [66]. Several other drugs are inhibitors or inducers of P-gp and the new pharmacoenhancer cobicistat has the same interacting potential on transporters (P-gp and BCRP) as ritonavir [67, 68].
Several genetic polymorphisms may affect the function or expression of metabolising or transporting enzymes, thus affecting drug exposure. While pharmacogenetic studies have extensively studied plasma pharmacokinetics of antiretrovirals, limited data are available on their effect on CNS exposure. Single nucleotide polymorphisms (SNPs) in cytochrome P450 (CYP) 2B6 have been associated with plasma efavirenz concentrations as well as the occurrence of neuropsychiatric symptoms and withdrawal from treatment [69, 70]. In a limited sample size study, CYP2B6 slow-metabolising children had higher CSF nevirapine concentrations than fast metabolizers [71]. In the aforementioned study on darunavir CSF concentrations, a borderline association was found between polymorphisms in the SLCO1A2 gene (encoding for OAT1A2) and CSF concentrations [66]. Finally, SNPs in the Hepatic Nuclear Factor 4 alpha (HNFalpha4, a nuclear factor implicated in the regulation of OATs) might explain some of the extreme variability observed in raltegravir CSF penetration [51].
5.4 Plasma Concentrations
A direct correlation between plasma and CSF concentrations has been demonstrated for the majority of antiretrovirals (Table 1). Therefore, factors affecting plasma concentrations may potentially influence CNS exposure; for instance, unboosted atazanavir (400 mg without ritonavir) is associated with very low and often undetectable CSF concentrations, as expected from the low plasma exposure observed with such a dosage [72]. Once-daily administered drugs may therefore reach lower concentrations as has been shown for darunavir/ritonavir (800/100 mg): even if no data are available, it may also be relevant for abacavir (for which all data have been derived from the twice-daily dosage) and for maraviroc (studied at 150 mg once daily with boosted PIs) [55, 73–76]. Furthermore, drug-to-drug interactions reducing the plasma exposure of one antiretroviral may significantly affect CNS exposure and efficacy.
6 CNS Penetration of Antiretrovirals
CSF concentrations and pharmacokinetic parameters of antiretrovirals are summarised in Table 1. Here, we briefly describe some of the key pharmacological features of those compounds, according to drug class.
6.1 Nucleoside Reverse Transcriptase Inhibitors
NRTIs are small, hydrophilic molecules, which are poorly bound to plasma proteins and reach very variable CSF exposures. NRTIs are transported by OATs that have been shown to be present at the choroid plexus (OAT1 and OAT3); the modulation of their activity (either by other drugs such as probenecid or by genetic polymorphisms in the encoding genes) may be relevant for zidovudine, stavudine, lamivudine and tenofovir passage [97]. With the exception of didanosine (whose CSF exposure has been found to be undetectable or very low), the other NRTIs have been associated with therapeutic CSF concentrations. Tenofovir is ionised at physiological pH and this limits its uptake by membrane transporters [50, 73, 77–96]. CSF tenofovir concentrations have been described as very low [and with no sample above a concentration of drug producing 50 % inhibition (IC50) of 201 ng/mL]; previous animal data suggested a good CSF passage (through the BCB and OATs independent) but poor penetration into deep brain tissue [98].
6.2 Non-Nucleoside Reverse Transcriptase Inhibitors
NNRTIs show different properties but are small, lipophilic, highly protein bound (with the exception of nevirapine) compounds [51, 58, 60, 71, 87, 99–103]. The neuropsychiatric effects in efavirenz recipients account for its passage into the CNS: nevertheless, as the IC50 is very low (0.5–1.3 ng/mL) and close to the limit of detection of the instruments, a few studies reported poor passage into the CSF. While the data on rilpivirine (one single study) and etravirine (two reports) are still limited, high nevirapine CSF to plasma ratios have been constantly confirmed: the compound properties as well as the in vivo data suggest that nevirapine is one of the antiretrovirals with the highest CSF penetration.
6.3 Protease Inhibitors
PIs are large (with molecular weights above 500 Da), lipophilic, highly protein bound (with the exception of indinavir) compounds with CSF concentrations approximately 1 % of plasma concentrations [54, 56, 59, 72, 104–121]; they have been recognised as substrates of P-gp as well as OAT1A2 and this may limit the drug accumulation into the CNS (as well as into other key tissues such as lymph nodes) [64, 116]. While tipranavir has not been studied, data from first-generation PIs were disappointing, with nelfinavir, saquinavir and amprenavir being undetectable or below the IC50 in most patients. Indinavir CSF exposure was somehow higher, probably due to lower binding to plasma proteins: CSF concentrations were above the concentration of drug producing 95 % inhibition (IC95) and it was mostly unbound (98.6 %). Comparison of the three commonly prescribed PIs (atazanavir, lopinavir and darunavir) favours lopinavir and darunavir since most of atazanavir concentrations were very low or undetectable [117].
6.4 Entry Inhibitors (Fusion Inhibitors and CCR5 Antagonists)
Enfuvirtide is a synthetic 36 amino acid oligopeptide (interacting with viral gp41) with a very large molecular weight: a single study confirmed that CSF concentrations were below the limit of quantification (25 ng/mL), while a case report of emerging enfuvirtide-resistant CSF (and then plasma) viruses reported a CSF concentration of 55 ng/mL [118, 119].
Maraviroc is a small, lipophilic, intermediately protein-bound compound that targets the human co-receptor C-C chemokine receptor type 5 (CCR5) and that is effective in preventing entry of R5-tropic HIV viruses into target cells. It is a substrate of both CYP3A4 and P-gp and drug–drug interactions, potentially affecting CSF penetration, have been reported. The available data have been obtained with twice-daily dosages (150 mg with PIs, 300 mg with NRTIs and nevirapine, and 600 mg with efavirenz or etravirine): CSF concentrations were detectable, 2–3 % of plasma concentrations and in the range of concentrations producing 90 % of maximum effect (EC90) (0.06–10.7 ng/mL) [55, 74–76].
6.5 Integrase Strand Transfer Inhibitors
Integrase inhibitors are the latest antiretroviral drug class and are somehow heterogeneous: while they are small, highly protein-bound molecules, their lipophilicity varies considerably (raltegravir is hydrophilic while elvitegravir is lipophilic). So far no data have been released on elvitegravir CSF exposure, while a single unpublished study reported low CSF to plasma ratios (0.4 %) for dolutegravir but CSF concentrations above IC50 in all samples [122]. Raltegravir pharmacokinetics have peculiar characteristics: very wide inter- and intra-individual variability and an unclear pharmacokinetic/pharmacodynamic relationship [49, 51, 123]. Even if pH-dependant absorption may explain much plasma variability, raltegravir CSF to plasma ratios have been described as varying from 3 to 20 %.
7 Pharmacokinetics/Pharmacodynamics
7.1 Target Concentrations
The study of the pharmacodynamic effect of antiretrovirals in the CNS is complicated by the absence of a clear target. The optimal marker would be the inhibition of HIV tissue replication in the whole brain parenchyma: such a marker is currently not feasible.
The use of CSF HIV RNA as a marker of antiviral activity is the most commonly used one since it decreases with the introduction of HAART and it parallels cognitive improvement in patients with HAD [124–127]. Nevertheless, commercial kits for measuring HIV RNA have not been validated in the CSF and the optimal threshold is currently unknown. Second-generation methods can quantify as low as 20 copies/mL; very sensitive experimental techniques (quantifying 2 copies/mL) have been assessed and residual viraemia (between 2 and 50 copies/mL) was associated with worse cognitive function [128, 129]. The measurement of other CSF markers (such as neopterine or CCL2) may be useful for understanding the pathogenesis of neuronal damage and, potentially, for monitoring changes in immune activation or neuronal function, but it is still not used except for research purposes [3, 130].
The use of magnetic resonance spectroscopy (MRS) and PET has the potential to describe neuronal integrity in different areas of the CNS and have successfully been used to describe antiretroviral effect: nevertheless, these techniques are expensive, time-consuming and not standardised [131]. A recent study using a selective ligand for the translocator protein expressed by activated microglial cells ([11C]-PK11195) showed that HIV-infected patients with long-standing virological suppression on combination antiretroviral therapy and without co-morbidities or drug and alcohol misuse had focal areas of activated microglial cells, indicative of neuroinflammation, in several cortical regions [132].
Finally, one possibility would be to monitor cognitive function after the introduction of antiretrovirals: most studies reported an improvement after antiretroviral treatment initiation or modification [133]. Nevertheless, complete neurocognitive testing is time-consuming and may be influenced by the choice of the control group and by learning effect (patients repeating slightly modified tests may perform better) [28, 134].
Given the inaccessibility of in vivo brain tissue, CSF inhibitory concentrations (IC50, IC90 and IC95) have been used to compare the adequacy of antiretrovirals exposure: these concentrations represents the level at which 50, 90 or 95 % of in vitro viral replication is inhibited (using wild-type viruses). However, these in vitro protein-free concentrations have significantly variable values and the same drug has been judged to reach optimal or insufficient concentrations in different studies when compared with different thresholds [49, 51, 123]. A recent study has quantified both protein-free and protein-corrected inhibitory concentrations of several antiretrovirals using a standardised methodology; [135] our group recently reported better CSF viral control (as CSF HIV RNA below 50 copies/mL and a lower prevalence of CSF escape) when drugs showed higher 95 % inhibitory quotients (as CSF exposure divided by IC95, derived by the aforementioned study) [136].
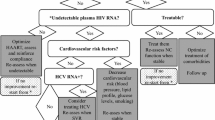
7.2 Cerebrospinal Fluid Escape
In the majority of patients, CSF HIV RNA is lower than plasma HIV RNA (approximately 1 Log10): higher CSF viral loads have been associated with active neurological symptoms and with a shorter time to develop HAND [137]. In some patients, despite plasma viral control CSF HIV RNA may be detectable or 1 Log10 higher: this condition has been defined as ‘CSF escape’. The exact clinical relevance of CSF escape is currently unknown since it may occur in approximately 10 % of patients on HAART and no neurological impairment was observed in a longitudinal study after 5 years of follow-up: this event may therefore be similar to the emergence of plasma ‘blips’ [21, 138]. However, two case series and several case reports have clearly documented the concrete, though uncommon, possibility of symptomatic CSF escape: severe neurological syndromes and neuroradiological findings have been documented [22, 23, 139–142]. In most of the subjects differential viral evolution (with resistance-associated mutation selected in the CSF compartment) was shown and it was explained by asymmetrical penetration of antiretrovirals (with some CSF concentrations below the limit of detection), but this was not confirmed by other reports [143]. In a large longitudinal study factors associated with CSF escape were the presence of CSF pleocytosis, the use of a PI-containing HAART and an ultrasensitive plasma HIV RNA level: [144] the poor CSF to plasma ratios observed with PIs (0–1.4 % with currently used PIs) may possibly explain these results as well as persistent intrathecal immune activation and plasma residual viraemia. In symptomatic patients, switching HAART to more neuro-effective drugs has been shown to improve symptoms and to reduce CSF viral load, and it appears advisable.
7.3 Efficacy of Monotherapy Versus Combination Antiretroviral Treatment
Pharmacodynamic data are available for a few compounds: patients received monotherapy and CSF HIV RNA decay was monitored. While lopinavir/ritonavir and zidovudine had a significant effect on CSF replication, didanosine and saquinavir showed no relevant effect [112, 145]. Abacavir was tested as an adjunctive therapy in patients with HAD: neurocognitive performance and CSF HIV RNA showed no significant change [86]. PI monotherapies have been tested given the need for reducing long-term toxicities and drug expenditure: this strategy is less effective than triple therapy but it is efficacious in the majority of patients. Concerns have been raised on the compartmental activity of low penetrating drugs such as PIs: data on several neurocognitive tests and a review of available data were reassuring on the effect of such strategies [146–149]. Nevertheless, a few patients on darunavir/ritonavir (two from the MONOI study) or lopinavir/ritonavir and several subjects on atazanavir/ritonavir as single agents presented neurological symptoms and elevated CSF HIV RNA despite plasma viral control [three of 20 in the ATARITMO (Atazanavir-Ritonavir Monomaintenance) study with atazanavir] [150, 151]. Furthermore, even in patients with controlled CSF HIV RNA, S100beta (a marker of astrocyte damage) rapidly increased after the interruption of NRTIs [152].
Combination antiretroviral treatment is usually effective in the CNS compartment and a rapid decay in CSF HIV RNA is observed; however, in some cases viral decay in the CSF and blood may differ. Slower decay of CSF HIV RNA has been noted in subjects with HAD and lower CD4 cell counts [125, 126, 153]. Ninety percent of patients with undetectable plasma HIV RNA presented CSF HIV RNA below 50 copies/mL: nevertheless, a compartmental residual viraemia was measurable through sensitive methods. CSF low-level viraemia was associated with neurocognitive impairment and increased immune activation and was unresponsive to intensification strategies (with maraviroc, enfuvirtide or raltegravir) [128, 129, 154, 155].
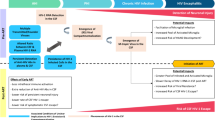
7.4 The CNS Penetration-Effectiveness Score
The CNS Penetration-Effectiveness (CPE) score has been proposed by a large collaborative study group in the USA (the CHARTER group): [156] in the revised 2010 version antiretrovirals were scored 1–4 (where 4 is the most neuro-effective drug) according to drug characteristics, pharmacokinetic and pharmacodynamic properties [157]. The composite CPE (obtained by adding single drug scores to obtain a treatment score) has been used in several studies, leading to conflicting results. Most of the studies found a lower CSF HIV RNA with a higher CPE score, while the effect on immune activation, magnetic resonance imaging cerebral metabolite concentrations and neurocognitive testing were less concordant among studies: the results are summarised in Table 2 [158–170]. Furthermore, while several retrospective studies found an association between higher CPE scores and lower CSF viral loads [125, 152, 171–173], only one study (of three) found a correlation with CSF escape [21, 143, 174]. Some reports tried to define a CPE cut-off: a value of 6 or 7 was found to be associated with heterogeneous CSF outcomes [143, 144, 171, 175].
Some limitations of the CPE score must be highlighted: the limited amount of evidence regarding pharmacodynamic data and standard dosages of drugs, the absence of a clear cut-off, and its validation only in patients receiving triple therapies and with fully sensitive viruses. As an example, a CPE corrected for plasma resistance-associated mutations was a better predictor (compared with standard CPE) of HAND in a cross-sectional study [176]. For these reasons, some authors (and the Italian guidelines) prefer not to use the aggregate CPE, but they suggest that treatment optimisation in patients with CNS diseases may include drugs with an individual elevated neuro-effective score [172, 177].
The CPE score is therefore a valuable and easy to use tool to implement the use of neuro-active drugs, although with some limitations. Nevertheless, a recent review using rigorous methods found that neuroHAART was effective in improving neurocognitive function and decreasing CSF viral load (although only two of those studies were adequately statistically powered): this confirms the possible optimisation of CNS treatment and calls for prospective, randomised, adequately powered studies [178]. A very interesting study (randomised and controlled) was conducted by Ellis et al. [169] but unfortunately it was prematurely interrupted for slow accrual (326 patients screened and 59 enrolled): CNS-targeted HAART was associated with neither virological nor neurocognitive improvements, although in patients with baseline suppressed viral load a trend for improved cognitive performances over time was observed.
7.5 Efficacy in Monocytes, Macrophages and Astrocytes
Given the peculiarity of infected cells in the CNS and several in vitro data, an increasing interest has arisen in antiretroviral activity on monocytes, macrophages and astrocytes. In vitro data suggest that the endogenous nucleoside pool in resting macrophages is smaller than that in activated lymphocytes and therefore that the effective phosphorylated NRTI concentrations required to inhibit HIV replication may be lower [179]. Shikuma et al. [180] used the in vitro effective concentration in acutely infected macrophages (EC50) to calculate a ‘monocyte efficacy score’ (1/EC50 × 1,000): surprising results were observed, with tenofovir being 17 times more efficacious than abacavir (50 vs. 3). In 139 patients the composite score was nicely associated with neurocognitive performance and with the presence of HAND or a minor motor cognitive disorder.
Recent data challenging infected astrocytes with several NRTIs, NNRTIs and raltegravir reported that some drugs (zidovudine, lamivudine and stavudine) may have inadequate inhibitory activity in astrocytes, with the EC90 values exceeding those achievable in the CSF [181].
These preliminary observations warrant further studies on the differential efficacy of antiretrovirals according to target cells: the repeated association between HIV reservoir size (measured as peripheral blood mononuclear cell- or monocyte-associated quantitative HIV DNA) and HAND support the implementation of specific drug strategies in selected patients (for instance, those with low CD4+ cells nadir, high HIV RNA zenith and high cumulative viraemia) [182, 183].
7.6 Potential Adjunctive Effect of Maraviroc in the CNS
Maraviroc is a CCR5 antagonist that binds to the human co-receptor, thus preventing the stable interaction between R5-tropic HIV and target cells: the mechanism of action is therefore peculiar since it blocks an endogenous receptor and has an extracellular target. The compound has been associated with some immunological benefits such as a higher CD4 increase and, although less than expected, reduced immune activation in patients with poor immunological recovery [184]. The drug, used in combination with other antiretrovirals, has been proven to be effective in blocking HIV entry both in naïve and experienced patients. CNS target cells usually express CCR5 and most of the viruses are R5 tropic in the CSF (even if patients harbour X4-tropic viruses); discordant tropism (X4 in CSF samples and R5 in plasma) has been rarely reported, thus suggesting that maraviroc may be effective in treating CNS HIV infection in most patients [185].
While being CNS protective as monotherapy in a macaques model and suppressing CSF HIV RNA in patients with neurological symptoms, three studies evaluated the effects of maraviroc intensification. In one, it was not associated with the control of CSF residual viraemia despite good compartmental penetration [154]. After 14 days of treatment intensification small increases in cerebral metabolite markers of neuronal integrity (N-acetylaspartate/creatine ratios) were observed and they were associated with maraviroc plasma exposure; concomitantly higher plasma concentrations were associated with lower CSF CXCL10 (IP-10), an inflammatory chemokine, concentrations [186, 187]. Both for its activity in CNS target cells and for its non-antiviral properties, maraviroc treatment (either as switch or as intensification) may be an option in neurologically impaired HIV-positive patients with suppressed plasma viral load.
8 Antiretrovial Toxicity in the CNS
It must be highlighted that most antiretrovirals have a well-described toxicity in the peripheral nervous system while little is known on their toxicity profile in CNS neurons. Some in vitro data (immortalised cell lines and peripheral dorsal root ganglia neurons) showed the potential for antiretrovirals to produce neuronal damage: using primary cultures of rat forebrain, Robertson et al. [188–190] showed that several antiretrovirals achieved toxic concentrations in the CSF without any additive effect. Recent data further explored this hypothesis and the production of reactive oxygen species was confirmed in pigtail macaques and rats in vivo (with the exposure to zidovudine, saquinavir and ritonavir) [191].
PIs and efavirenz have been associated with glucose and metabolic disturbances eventually leading to dyslipidaemia, glucose intolerance and abnormal fat distribution (lipodystrophy); the cumulative exposure to PIs has further being implicated in the increasing cardiovascular events observed in HIV-positive patients [192]. Previous studies suggest that HIV-infected patients are at increased risk of ischaemic cerebrovascular disease, potentially caused by infective vasculitis, brain opportunistic diseases, cardiac embolism, hypercoagulopathy or HIV infection itself [193, 194]. Among a variety of brain vessel diseases, cerebral small vessel disease (CVSD) has been associated with ischaemic stroke during life and cerebral infarction at autopsy. Recently, it was demonstrated that mild and moderate/severe small vessel diseases were associated with PI-based HAART exposure and that HAND was associated with mild CSVD (after adjusting for vessel mineralisation, HIV encephalitis, microglial nodular lesions, white matter lesions or older age) [195]. Further to this potentially relevant effect on cerebrovascular disease, PI-based combination treatment has been associated with reduced amyloid phagocytosis and increased neuronal accumulation, justifying some of the shared clinical features with Alzheimer’s dementia [196, 197].
Efavirenz effects in the CNS are well-characterised (abnormal dreams, dizziness) and associated with higher plasma concentrations and to SNPs in genes encoding for proteins involved in the drug metabolism or transport. Furthermore, being on efavirenz treatment was independently associated with the diagnosis of HAND in a cohort of stable HIV-positive patients [198]. One recent study reported that cognition improved for up to 96 weeks in a group of immunologically and virologically stable patients who elected to come off treatment; the improvement was significant in all participants but greater in efavirenz recipients [133].
These results raise the possibility that antiretroviral concentrations may to some extent have detrimental effects: this may be particularly relevant for individuals with specific genetic profiles but it must be compared with the clear beneficial effect of HAART on compartmentalised viral control.
9 Conclusions
HAART is very effective in controlling HIV replication and in increasing patients’ immune systems, thus preventing opportunistic diseases. In the CNS the same rule applies, although persistent immune activation have been demonstrated despite antiviral efficacy. Antiretroviral penetration into the CNS may depend on several drug and patient characteristics: the use of more neuro-effective drugs (high penetration and compartmental activity) has been associated with better CSF viral control and in some, but not all, studies with better neurocognitive performances. Antiretroviral regimens based on neuro-effective drugs may be suggested in patients with increased pharmacological needs (CSF escape, CNS compartmentalised viruses, high intrathecal immune activation) and neurocognitive disorders. The use of antiretroviral drugs with increased CSF penetration and/or effectiveness in treating or preventing neurocognitive disorders however needs to be assessed in well-designed prospective studies addressing antiretroviral neurotoxicity.
References
Valcour V, Chalermchai T, Sailasuta N, et al. Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis. 2012;206(2):275–82.
Gannon P, Khan MZ, Kolson DL. Current understanding of HIV-associated neurocognitive disorders pathogenesis. Curr Opin Neurol. 2011;24(3):275–83.
Zhou L, Saksena NK. HIV associated neurocognitive disorders. Infect Dis Rep. 2013;5(Suppl 1):e8.
Clifford DB, Ances BM. HIV-associated neurocognitive disorder. Lancet Infect Dis. 2013;13(11):976–86.
Bonnet F, Amieva H, Marquant F, et al. Cognitive disorders in HIV-infected patients: are they HIV-related? AIDS. 2013;27(3):391–400.
Burdo TH, Lackner A, Williams KC. Monocyte/macrophages and their role in HIV neuropathogenesis. Immunol Rev. 2013;254(1):102–13.
Carroll-Anzinger D, Kumar A, Adarichev V, Kashanchi F, Al-Harthi L. Human immunodeficiency virus-restricted replication in astrocytes and the ability of gamma interferon to modulate this restriction are regulated by a downstream effector of the Wnt signaling pathway. J Virol. 2007;81(11):5864–71.
Masliah E, Heaton RK, Marcotte TD, et al. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC group. The HIV Neurobehavioral Research Center. Ann Neurol. 1997;42:963–72.
Glass JD, Fedor H, Wesselingh SL, McArthur JC. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol. 1995;38:755–62.
Anthony IC, Bell JE. The neuropathology of HIV/AIDS. Int Rev Psychiatry. 2008;20:15–24.
Kaul M. HIV-1 associated dementia: update on pathological mechanisms and therapeutic approaches. Curr Opin Neurol. 2009;22(3):315–20.
Williams DW, Eugenin EA, Calderon TM, Berman JW. Monocyte maturation, HIV susceptibility, and transmigration across the blood brain barrier are critical in HIV neuropathogenesis. J Leukoc Biol. 2012;91(3):401–15.
Nakagawa S, Castro V, Toborek M. Infection of human pericytes by HIV-1 disrupts the integrity of the blood–brain barrier. J Cell Mol Med. 2012;16(12):2950–7.
Abdulle S, Hagberg L, Gisslén M. Effects of antiretroviral treatment on blood–brain barrier integrity and intrathecal immunoglobulin production in neuroasymptomatic HIV-1-infected patients. HIV Med. 2005;6(3):164–9.
Abdulle S, Hagberg L, Svennerholm B, Fuchs D, Gisslén M. Continuing intrathecal immunoactivation despite two years of effective antiretroviral therapy against HIV-1 infection. AIDS. 2002;16(16):2145–9.
Calcagno A, Alberione MC, Romito A, Imperiale D, Ghisetti V, Audagnotto S, et al. Prevalence and predictors of blood brain barrier damage in the HAART Era. J Neurovirol. Epub 2014 Jun 28.
Shen L, Siliciano RF. Viral reservoirs, residual viremia, and the potential of highly active antiretroviral therapy to eradicate HIV infection. J Allergy Clin Immunol. 2008;122(1):22–8.
Fletcher CV, Staskus K, Wietgrefe SW, et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc Natl Acad Sci U S A. 2014;111(6):2307–12.
Schnell G, Spudich S, Harrington P, Price RW, Swanstrom R. Compartmentalized human immunodeficiency virus type 1 originates from long-lived cells in some subjects with HIV-1-associated dementia. PLoS Pathog. 2009;5(4):e1000395.
Zhang Y, Wei F, Liang Q, et al. High levels of divergent HIV-1 quasispecies in patients with neurological opportunistic infections in China. J Neurovirol. 2013;19(4):359–66.
Edén A, Fuchs D, Hagberg L, et al. HIV-1 viral escape in cerebrospinal fluid of subjects on suppressive antiretroviral treatment. J Infect Dis. 2010;202(12):1819–25.
Canestri A, Lescure FX, Jaureguiberry S, et al. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis. 2010;50(5):773–8.
Peluso MJ, Ferretti F, Peterson J, et al. Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well controlled plasma viral load. AIDS. 2012;26(14):1765–74.
Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–99.
Heaton R, Franlin D, Woods S, et al. Asymptomatic mild HIV-associated neurocognitive disorder increases risk for future symptomatic decline: a CHARTER longitudinal study [abstract]. 19th Conference on Retroviruses and Opportunistic Infections (CROI); 5–8 Mar 2012; Seattle.
Blackstone K, Moore DJ, Heaton RK, et al. Diagnosing symptomatic HIV-associated neurocognitive disorders: self-report versus performance-based assessment of everyday functioning. J Int Neuropsychol Soc. 2012;18(1):79–88.
Cysique LA, Brew BJ. Prevalence of non-confounded HIV-associated neurocognitive impairment in the context of plasma HIV RNA suppression. J Neurovirol. 2011;17(2):176–83.
Winston A, Arenas-Pinto A, Stöhr W, et al. Neurocognitive function in HIV infected patients on antiretroviral therapy. PLoS One. 2013;8(4):e61949.
Spudich S. HIV and neurocognitive dysfunction. Curr HIV/AIDS Rep. 2013;10(3):235–43. doi:10.1007/s11904-013-0171-y.
Varatharajan L, Thomas SA. The transport of anti-HIV drugs across blood–CNS interfaces: summary of current knowledge and recommendations for further research. Antiviral Res. 2009;82(2):A99–109.
Kumar AM, Borodowsky I, Fernandez B, Gonzalez L, Kumar M. Human immunodeficiency virus type 1 RNA Levels in different regions of human brain: quantification using real-time reverse transcriptase-polymerase chain reaction. J Neurovirol. 2007;13(3):210–24.
Fox E, Bungay PM, Bacher J, et al. Zidovudine concentration in brain extracellular fluid measured by microdialysis: steady-state and transient results in rhesus monkey. J Pharmacol Exp Ther. 2002;301(3):1003–11.
Liu X, Van Natta K, Yeo H, et al. Unbound drug concentration in brain homogenate and cerebral spinal fluid at steady state as a surrogate for unbound concentration in brain interstitial fluid. Drug Metab Dispos. 2009;37(4):787–93.
Blaney SM, Daniel MJ, Harker AJ, Godwin K, Balis FM. Pharmacokinetics of lamivudine and BCH-189 in plasma and cerebrospinal fluid of nonhuman primates. Antimicrob Agents Chemother. 1995;39(12):2779–82.
Caruso A, Alvarez-Sánchez R, Hillebrecht A, et al. PK/PD assessment in CNS drug discovery: prediction of CSF concentration in rodents for P-glycoprotein substrates and application to in vivo potency estimation. Biochem Pharmacol. 2013;85(11):1684–99.
de Lange EC. Utility of CSF in translational neuroscience. J Pharmacokinet Pharmacodyn. 2013;40(3):315–26.
Eisfeld C, Reichelt D, Evers S, Husstedt I. CSF penetration by antiretroviral drugs. CNS Drugs. 2013;27(1):31–55.
Enting RH, Hoetelmans RM, Lange JM, et al. Antiretroviral drugs and the central nervous system. AIDS. 1998;12:1941–55.
Soulas C, Conerly C, Kim WK, et al. Recently infiltrating MAC387(+) monocytes/macrophages a third macrophage population involved in SIV and HIV encephalitic lesion formation. Am J Pathol. 2011;178:2121–35.
Minogue AM, Jones RS, Kelly RJ, McDonald CL, Connor TJ, Lynch MA. Age-associated dysregulation of microglial activation is coupled with enhanced blood-brain barrier permeability and pathology in APP/PS1 mice. Neurobiol Aging. 2014;35(6):1442–52.
Vinikoor MJ, Napravnik S, Floris-Moore M, Wilson S, Huang DY, Eron JJ. Incidence and clinical features of cerebrovascular disease among HIV-infected adults in the Southeastern United States. AIDS Res Hum Retroviruses. 2013;29(7):1068–74.
Singer EJ, Valdes-Sueiras M, Commins DL, Yong W, Carlson M. HIV stroke risk: evidence and implications. Ther Adv Chronic Dis. 2013;4(2):61–70.
Croteau D, Best B, Clifford D, et al. Older age is associated with higher ARV concentrations in CSF in HIV+ individuals [abstract]. 19th Conference on Retroviruses and Opportunistic Infections (CROI); 5–8 Mar 2012; Seattle.
Moss DM, Siccardi M, Back DJ, Owen A. Predicting intestinal absorption of raltegravir using a population-based ADME simulation. J Antimicrob Chemother. 2013;68(7):1627–34.
Marshall DW, Brey RL, Butzin CA, et al. Spectrum of cerebrospinal fluid findings in various stages of human immunodeficiency virus infection. Arch Neurol. 1988;45:954–8.
Petito CK, Cash KS. Blood–brain barrier abnormalities in the acquired immunodeficiency syndrome: immunohistochemical localization of serum proteins in postmortem brain. Ann Neurol. 1992;32:658–66.
Andersson LM, Hagbwerg L, Fuchs D, Svennerholm B, Gisslen M. Increased blood brain-barrier permeability in neuroasymptomatic HIV-1-infected individuals-correlation with cerebrospinal fluid HIV-1 RNA and neopterin levels. J Neurovirol. 2001;7:542–7.
Marchi N, Betto G, Fazio V, et al. Blood–brain barrier damage and brain penetration of antiepileptic drugs: role of serum proteins and brain edema. Epilepsia. 2009;50(4):664–77.
Yilmaz A, Gisslén M, Spudich S, et al. Raltegravir cerebrospinal fluid concentrations in HIV-1 infection. PLoS One. 2009;4(9):e6877.
Calcagno A, Bonora S, Simiele M, et al. Tenofovir and emtricitabine cerebrospinal fluid-to-plasma ratios correlate to the extent of blood–brain barrier damage. AIDS. 2011;25(11):1437–9.
Calcagno A, Cusato J, Simiele M, et al. High interpatient variability of raltegravir cerebrospinal fluid concentrations in HIV-positive patients: a pharmacogenetic analysis. J Antimicrob Chemother. 2014;69(1):241–5.
Marzolini C, Mueller R, Li-Blatter X, Battegay M, Seelig A. The brain entry of HIV-1 protease inhibitors is facilitated when used in combination. Mol Pharm. 2013;10(6):2340–9.
Avery LB, Zarr MA, Bakshi RP, Siliciano RF, Hendrix CW. Increasing extracellular protein concentration reduces intracellular antiretroviral drug concentration and antiviral effect. AIDS Res Hum Retroviruses. 2013;29(11):1434–42.
Yilmaz A, Ståhle L, Hagberg L, et al. Cerebrospinal fluid and plasma HIV-1 RNA levels and lopinavir concentrations following lopinavir/ritonavir regimen. Scand J Infect Dis. 2004;36(11–12):823–8.
Croteau D, Best BM, Letendre S, et al. Lower than expected maraviroc concentrations in cerebrospinal fluid exceed the wild-type CC chemokine receptor 5-tropic HIV-1 50 % inhibitory concentration. AIDS. 2012;26(7):890–3.
Croteau D, Rossi SS, Best BM, et al. Darunavir is predominantly unbound to protein in cerebrospinal fluid and concentrations exceed the wild-type HIV-1 median 90 % inhibitory concentration. J Antimicrob Chemother. 2013;68(3):684–9.
Reiber H. Proteins in cerebrospinal fluid and blood: barriers, CSF flow rate and source-related dynamics. Restor Neurol Neurosci. 2003;21(3–4):79–96.
Avery LB, Sacktor N, McArthur JC, Hendrix CW. Protein-free efavirenz concentrations in cerebrospinal fluid and blood plasma are equivalent: applying the law of mass action to predict protein-free drug concentration. Antimicrob Agents Chemother. 2013;57(3):1409–14.
Delille CA, Pruett ST, Marconi VC, et al. Effect of protein binding on unbound atazanavir and darunavir cerebrospinal fluid concentrations. J Clin Pharmacol. 2014;54(9):1063–71. doi:10.1002/jcph.298.
Nguyen A, Rossi S, Croteau D, et al. Etravirine in CSF is highly protein bound. J Antimicrob Chemother. 2013;68(5):1161–8.
Stępień KM, Tomaszewski M, Tomaszewska J, Czuczwar SJ. The multidrug transporter P-glycoprotein in pharmacoresistance to antiepileptic drugs. Pharmacol Rep. 2012;64(5):1011–9.
Kannan P, John C, Zoghbi SS, et al. Imaging the function of P-glycoprotein with radiotracers: pharmacokinetics and in vivo applications. Clin Pharmacol Ther. 2009;86(4):368–77.
Bleasby K, Castle JC, Roberts CJ, et al. Expression profiles of 50 xenobiotic transporter genes in humans and pre-clinical species: a resource for investigations into drug disposition. Xenobiotica. 2006;36(10–11):963–88.
Hartkoorn RC, Kwan WS, Shallcross V, et al. HIV protease inhibitors are substrates for OATP1A2, OATP1B1 and OATP1B3 and lopinavir plasma concentrations are influenced by SLCO1B1 polymorphisms. Pharmacogenet Genomics. 2010;20(2):112–20.
Kaddoumi A, Choi SU, Kinman L, et al. Inhibition of P-glycoprotein activity at the primate blood–brain barrier increases the distribution of nelfinavir into the brain but not into the cerebrospinal fluid. Drug Metab Dispos. 2007;35(9):1459–62.
Calcagno A, Yilmaz A, Cusato J, et al. Determinants of darunavir cerebrospinal fluid concentrations: impact of once-daily dosing and pharmacogenetics. AIDS. 2012;26(12):1529–33.
Zakeri-Milani P, Valizadeh H. Intestinal transporters: enhanced absorption through P-glycoprotein-related drug interactions. Expert Opin Drug Metab Toxicol. 2014;10(6):859-71.
Lepist EI, Phan TK, Roy A, et al. Cobicistat boosts the intestinal absorption of transport substrates, including HIV protease inhibitors and GS-7340, in vitro. Antimicrob Agents Chemother. 2012;56(10):5409–13.
Sánchez Martín A, Cabrera Figueroa S, Cruz Guerrero R, et al. Impact of pharmacogenetics on CNS side effects related to efavirenz. Pharmacogenomics. 2013;14(10):1167–78.
Wyen C, Hendra H, Siccardi M, et al. Cytochrome P450 2B6 (CYP2B6) and constitutive androstane receptor (CAR) polymorphisms are associated with early discontinuation of efavirenz-containing regimens. J Antimicrob Chemother. 2011;66(9):2092–8.
Saitoh A, Sarles E, Capparelli E, et al. CYP2B6 genetic variants are associated with nevirapine pharmacokinetics and clinical response in HIV-1-infected children. AIDS. 2007;21(16):2191–9.
Best BM, Letendre SL, Brigid E, et al. Low atazanavir concentrations in cerebrospinal fluid. AIDS. 2009;23(1):83–7.
Capparelli EV, Letendre SL, Ellis RJ, et al. Population pharmacokinetics of abacavir in plasma and cerebrospinal fluid. Antimicrob Agents Chemother. 2005;49(6):2504–6.
Yilmaz A, Watson V, Else L, Gisslèn M. Cerebrospinal fluid maraviroc concentrations in HIV-1 infected patients. AIDS. 2009;23(18):2537–40.
Melica G, Canestri A, Peytavin G, et al. Maraviroc-containing regimen suppresses HIV replication in the cerebrospinal fluid of patients with neurological symptoms. AIDS. 2010;24(13):2130–3.
Tiraboschi JM, Niubo J, Curto J, Podzamczer D. Maraviroc concentrations in cerebrospinal fluid in HIV-infected patients. J Acquir Immune Defic Syndr. 2010;55(5):606–9.
Burger DM, Kraaijeveld CL, Meenhorst PL, et al. Penetration of zidovudine into the cerebrospinal fluid of patients infected with HIV. AIDS. 1993;7:1581–7.
Lane HC, Falloon J, Walker RE, et al. Zidovudine in patients with human immunodeficiency virus (HIV) infection and Kaposi sarcoma. A phase II randomized, placebo-controlled trial. Ann Intern Med. 1989;111:41–50.
Elovaara I, Poutiainen E, Lahdevirta J, et al. Zidovudine reduces intrathecal immunoactivation in patients with early human immunodeficiency virus type 1 infection. Arch Neurol. 1994;51:943–50.
Balis FM, Pizzo PA, Eddy J, et al. Pharmacokinetics of zidovudine administered intravenously and orally in children with human immunodeficiency virus infection. J Pediatr. 1989;114:880–4.
Hagberg L, Andersson M, Chiodi F, et al. Effect of zidovudine on cerebrospinal fluid in patients with HIV infection and acute neurological disease. Scand J Infect Dis. 1991;23:681–5.
Tozzi V, Narciso P, Galgani S, et al. Effects of zidovudine in 30 patients with mild to end-stage AIDS dementia complex. AIDS. 1993;7:683–92.
Sidtis JJ, Gatsonis C, Price RW, et al. Zidovudine treatment of the AIDS dementia complex: results of a placebo-controlled trial. AIDS Clinical Trials Group. Ann Neurol. 1993;33:343–9.
McDowell JA, Chittick GE, Ravitch JR, et al. Pharmacokinetics of [(14)C]abacavir, a human immunodeficiency virus type 1 (HIV-1) reverse transcriptase inhibitor, administered in a single oral dose to HIV-1-infected adults: a mass balance study. Antimicrob Agents Chemother. 1999;43:2855–61.
McDowell JA, Lou Y, Symonds WS, et al. Multiple-dose pharmacokinetics and pharmacodynamics of abacavir alone and in combination with zidovudine in human immunodeficiency virus-infected adults. Antimicrob Agents Chemother. 2000;44:2061–7.
Brew BJ, Halman M, Catalan J, et al. Factors in AIDS dementia complex trial design: results and lessons from the abacavir trial. PLoS Clin Trials. 2007;2:e13.
Antinori A, Perno CF, Giancola ML, et al. Efficacy of cerebrospinal fluid (CSF)-penetrating antiretroviral drugs against HIV in the neurological compartment: different patterns of phenotypic resistance in CSF and plasma. Clin Infect Dis. 2005;41:1787–93.
Burger DM, Kraayeveld CL, Meenhorst PL, et al. Study on didanosine concentrations in cerebrospinal fluid. Implications for the treatment and prevention of AIDS dementia complex. Pharm World Sci. 1995;17:218–21.
Gisslén M, Norkrans G, Svennerholm B, et al. The effect on human immunodeficiency virus type 1 RNA levels in cerebrospinal fluid after initiation of zidovudine or didanosine. J Infect Dis. 1997;175:434–7.
Best B, Letendre S, Capparelli E, et al. Efavirenz and emtricitabine concentrations consistently exceed wild-type IC50 in cerebrospinal fluid: CHARTER findings [abstract]. 16th Conference on Retroviruses and Opportunistic Infections (CROI); 8–11 Feb 2009; Montreal.
Foudraine NA, Hoetelmans RM, Lange JM, et al. Cerebrospinal-fluid HIV-1 RNA and drug concentrations after treatment with lamivudine plus zidovudine or stavudine. Lancet. 1998;351:1547–51.
Blaschke A, Capparelli E, Ellis R, et al. A population model-based approach for determining lamivudine (3TC) cerebrospinal fluid (CSF) penetration in HIV-infected adults [abstract]. 7th Conference on Retroviruses and Opportunistic Infections (CROI); 30 Jan–2 Feb 2000; San Francisco.
Haworth SJ, Christofalo B, Anderson RD, et al. A single-dose study to assess the penetration of stavudine into human cerebrospinal fluid in adults. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17:235–8.
Brady KA, Boston RC, Aldrich JL, et al. Stavudine entry into cerebrospinal fluid after single and multiple doses in patients infected with human immunodeficiency virus. Pharmacotherapy. 2005;25:10–7.
Zhang L, Price R, Aweeka F, et al. Making the most of sparse clinical data by using a predictive-model-based analysis, illustrated with a stavudine pharmacokinetic study. Eur J Pharm Sci. 2001;12:377–85.
Best BM, Letendre SL, Koopmans P, et al. Low cerebrospinal fluid concentrations of the nucleotide HIV reverse transcriptase inhibitor, tenofovir. J Acquir Immune Defic Syndr. 2012;59(4):376–81.
Takasawa K, Terasaki T, Suzuki H, et al. Distributed model analysis of 3′-azido-3′-deoxythymidine and 2′,3′-dideoxyinosine distribution in brain tissue and cerebrospinal fluid. J Pharmacol Exp Ther. 1997;282:1509–17.
Anthonypillai C, Gibbs JE, Thomas SA. The distribution of the anti-HIV drug, tenofovir (PMPA), into the brain, CSF and choroid plexuses. Cerebrospinal Fluid Res. 2006;3:1.
Tashima KT, Caliendo AM, Ahmad M, et al. Cerebrospinal fluid human immunodeficiency virus type 1 (HIV-1) suppression and efavirenz drug concentrations in HIV-1-infected patients receiving combination therapy. J Infect Dis. 1999;180:862–4.
Best BM, Koopmans PP, Letendre SL, et al. Efavirenz concentrations in CSF exceed IC50 for wild-type HIV. J Antimicrob Chemother. 2010;66:354–7.
van Praag RM, van Weert EC, van Heeswijk RP, et al. Stable concentrations of zidovudine, stavudine, lamivudine, abacavir, and nevirapine in serum and cerebrospinal fluid during 2 years of therapy. Antimicrob Agents Chemother. 2002;46:896–9.
Veldkamp AI, Weverling GJ, Lange JM, et al. High exposure to nevirapine in plasma is associated with an improved virological response in HIV-1-infected individuals. AIDS. 2001;15:1089–95.
Mora-Peris B, Watson V, Vera JH, et al. Rilpivirine exposure in plasma and sanctuary site compartments after switching from nevirapine-containing combined antiretroviral therapy. J Antimicrob Chemother. 2014;69(6):1642-7.
Kravcik S, Gallicano K, Roth V, et al. Cerebrospinal fluid HIV RNA and drug levels with combination ritonavir and saquinavir. J Acquir Immune Defic Syndr. 1999;21:371–5.
Sadler BM, Chittick GE, Polk RE, et al. Metabolic disposition and pharmacokinetics of [14C]-amprenavir, a human immunodeficiency virus type 1 (HIV-1) protease inhibitor, administered as a single oral dose to healthy male subjects. J Clin Pharmacol. 2001;41:386–96.
Murphy R, Currier J, Gerber J, et al. Antiviral activity and pharmacokinetics of amprenavir with or without zidovudine/3TC in the cerebrospinal fluid of HIV-infected adults [abstract]. 7th Conference on Retroviruses and Opportunistic Infections (CROI); 30 Jan–2 Feb 2000; San Francisco.
Saumoy M, Tiraboschi J, Gutierrez M, et al. Viral response in stable patients switching to fosamprenavir/ritonavir monotherapy (the FONT study). HIV Med. 2011;12:438–41.
Yilmaz A, Izadkhashti A, Price RW, et al. Darunavir concentrations in cerebrospinal fluid and blood in HIV-1-infected individuals. AIDS Res Hum Retroviruses. 2009;25:457–61.
Capparelli EV, Holland D, Okamoto C, et al. Lopinavir concentrations in cerebrospinal fluid exceed the 50 % inhibitory concentration for HIV. AIDS. 2005;19:949–52.
DiCenzo R, DiFrancesco R, Cruttenden K, et al. Lopinavir cerebrospinal fluid steady-state trough concentrations in HIV-infected adults. Ann Pharmacother. 2009;43:1972–7.
Lafeuillade A, Solas C, Halfon P, et al. Differences in the detection of three HIV-1 protease inhibitors in non-blood compartments: clinical correlations. HIV Clin Trials. 2002;3:27–35.
Letendre SL, van den Brande G, Hermes A, et al. Lopinavir with ritonavir reduces the HIV RNA level in cerebrospinal fluid. Clin Infect Dis. 2007;45:1511–7.
Yilmaz A, Fuchs D, Hagberg L, et al. Cerebrospinal fluid HIV-1 RNA, intrathecal immunoactivation, and drug concentrations after treatment with a combination of saquinavir, nelfinavir, and two nucleoside analogues: the M61022 study. BMC Infect Dis. 2006;6:63.
Moyle GJ, Sadler M, Buss N. Plasma and cerebrospinal fluid saquinavir concentrations in patients receiving combination antiretroviral therapy. Clin Infect Dis. 1999;28:403–4.
Gisolf EH, Enting RH, Jurriaans S, et al. Cerebrospinal fluid HIV-1 RNA during treatment with ritonavir/saquinavir or ritonavir/saquinavir/stavudine. AIDS. 2000;14:1583–9.
Kim RB, Fromm MF, Wandel C, et al. The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. J Clin Invest. 1998;101:289–94.
Yilmaz A, Price RW, Gisslén M. Antiretroviral drug treatment of CNS HIV-1 infection. J Antimicrob Chemother. 2012;67(2):299–311.
Price RW, Parham R, Kroll JL, et al. Enfuvirtide cerebrospinal fluid (CSF) pharmacokinetics and potential use in defining CSF HIV-1 origin. Antivir Ther. 2008;13:369–74.
van Lelyveld SF, Nijhuis M, Baatz F, et al. Therapy failure following selection of enfuvirtide-resistant HIV-1 in cerebrospinal fluid. Clin Infect Dis. 2010;50:387–90.
Zhou XJ, Havlir DV, Richman DD, et al. Plasma population pharmacokinetics and penetration into cerebrospinal fluid of indinavir in combination with zidovudine and lamivudine in HIV-1-infected patients. AIDS. 2000;14(18):2869–76.
Letendre SL, Capparelli EV, Ellis RJ, McCutchan JA. Indinavir population pharmacokinetics in plasma and cerebrospinal fluid. Antimicrob Agents Chemother. 2000;44(8):2173–5.
Letendre S, Mills A, Tashima K, et al. Distribution and antiviral activity in cerebrospinal fluid (CSF) of the integrase inhibitor, dolutegravir (DTG): ING116070 week 16 results [abstract]. 20th Conference on Retroviruses and Opportunistic Infections (CROI); 3–6 Mar 2013; Atlanta.
Croteau D, Letendre S, Best BM, et al. Total raltegravir concentrations in cerebrospinal fluid exceed the 50-percent inhibitory concentration for wild-type HIV-1. Antimicrob Agents Chemother. 2010;54(12):5156–60.
Staprans S, Marlowe N, Glidden D, et al. Time course of cerebrospinal fluid responses to antiretroviral therapy: evidence for variable compartmentalization of infection. AIDS. 1999;13:1051–61.
Ellis RJ, Gamst AC, Capparelli E, et al. Cerebrospinal fluid HIV RNA originates from both local CNS and systemic sources. Neurology. 2000;54:927–36.
Eggers C, Hertogs K, Sturenburg HJ, et al. Delayed central nervous system virus suppression during highly active antiretroviral therapy is associated with HIV encephalopathy, but not with viral drug resistance or poor central nervous system drug penetration. AIDS. 2003;17:1897–906.
Mellgren A, Antinori A, Cinque P, et al. Cerebrospinal fluid HIV-1 infection usually responds well to antiretroviral treatment. Antivir Ther. 2005;10:701–7.
Gisslen M, Norkrans G, Svennerholm B, et al. HIV-1 RNA detectable with ultrasensitive quantitative polymerase chain reaction in plasma but not in cerebrospinal fluid during combination treatment with zidovudine, lamivudine and indinavir. AIDS. 1998;12:114–6.
Letendre S, McClernon D, Ellis R, et al. Persistent HIV in the central nervous system during treatment is associated with worse ART penetration and cognitive impairment [abstract]. 16th Conference on Retroviruses and Opportunistic Infections (CROI); 8–11 Feb 2009; Montreal.
Yilmaz A, Yiannoutsos CT, Fuchs D, et al. Cerebrospinal fluid neopterin decay characteristics after initiation of antiretroviral therapy. J Neuroinflammation. 2013;10:62.
Masters MC, Ances BM. Role of neuroimaging in HIV-associated neurocognitive disorders. Semin Neurol. 2014;34(1):89–102.
Garvey LJ, Pavese N, Politis M, et al. Increased microglia activation in neurologically asymptomatic HIV-infected patients receiving effective ART. AIDS. 2014;28(1):67–72.
Robertson KR, Su Z, Margolis DM, et al. Neurocognitive effects of treatment interruption in stable HIV-positive patients in an observational cohort. Neurology. 2010;74(16):1260–6.
Grund B, Wright EJ, Brew BJ, et al. Improved neurocognitive test performance in both arms of the SMART study: impact of practice effect. J Neurovirol. 2013;19(4):383–92.
Acosta EP, Limoli KL, Trinh L, et al. Novel method to assess antiretroviral target trough concentrations using in vitro susceptibility data. Antimicrob Agents Chemother. 2012;56(11):5938–45.
Calcagno A, Simiele M, Alberione MC, et al. Cerebrospinal fluid inhibitory quotients of antiretroviral drugs in HIV-positive patients. Clin Infect Dis. 2014. In Press.
Ellis RJ, Moore DJ, Childers ME, et al. Progression to neuropsychological impairment in human immunodeficiency virus infection predicted by elevated cerebrospinal fluid levels of human immunodeficiency virus RNA. Arch Neurol. 2002;59(6):923–8.
Edén A, Hagberg L, Svennerholm B, et al. Longitudinal follow up of Detectable HIV 1 RNA in cerebrospinal fluid in subjects on suppressive antiretroviral therapy [abstract]. 19th Conference on Retroviruses and Opportunistc Infections (CROI); 5–8 Mar 2012; Seattle.
Wendel KA, McArthur JC. Acute meningoencephalitis in chronic human immunodeficiency virus (HIV) infection: putative central nervous system escape of HIV replication. Clin Infect Dis. 2003;37(8):1107–11.
Bogoch II, Davis BT, Venna N. Reversible dementia in a patient with central nervous system escape of human immunodeficiency virus. J Infect. 2011;63(3):236–9.
Bingham R, Ahmed N, Rangi P, et al. HIV encephalitis despite suppressed viraemia: a case of compartmentalized viral escape. Int J STD AIDS. 2011;22(10):608–9.
Khoury MN, Tan CS, Peaslee M, Koralnik IJ. CSF viral escape in a patient with HIV-associated neurocognitive disorder. J Neurovirol. 2013;19(4):402–5.
Cusini A, Vernazza PL, Yerly S, et al. Higher CNS penetration-effectiveness of long-term combination antiretroviral therapy is associated with better HIV-1 viral suppression in cerebrospinal fluid. J Acquir Immune Defic Syndr. 2013;62(1):28–35.
Perez Valero I, Letendre S, Ellis R, et al. Prevalence and risk factors for HIV CSF viral escape: results from the CHARTER and HNRP cohorts. J Int AIDS Soc. 2012;15(Suppl 4):18189.
Gisslén M, Norkrans G, Svennerholm B, Hagberg L. The effect on human immunodeficiency virus type 1 RNA levels in cerebrospinal fluid after initiation of zidovudine or didanosine. J Infect Dis. 1997;175(2):434–7.
Bunupuradah T, Chetchotisakd P, Jirajariyavej S, et al. Neurocognitive impairment in patients randomized to second-line lopinavir/ritonavir-based antiretroviral therapy vs. lopinavir/ritonavir monotherapy. J Neurovirol. 2012;18(6):479–87.
Santos JR, Muñoz-Moreno JA, Moltó J, et al. Virological efficacy in cerebrospinal fluid and neurocognitive status in patients with long-term monotherapy based on lopinavir/ritonavir: an exploratory study. PLoS One. 2013;8(7):e70201.
Pérez-Valero I, González-Baeza A, Estébanez M, et al. Neurocognitive impairment in patients treated with protease inhibitor monotherapy or triple drug antiretroviral therapy. PLoS One. 2013;8(7):e69493.
Perez-Valero I, Bayon C, Cambron I, Gonzalez A, Arribas JR. Protease inhibitor monotherapy and the CNS: peace of mind? J Antimicrob Chemother. 2011;66(9):1954–62.
Katlama C, Valantin MA, Algarte-Genin M, et al. Efficacy of darunavir/ritonavir maintenance monotherapy in patients with HIV-1 viral suppression: a randomized open-label, noninferiority trial, MONOI-ANRS 136. AIDS. 2010;24:2365–74.
Vernazza P, Daneel S, Schiffer V, et al. The role of compartment penetration in PI-monotherapy: the Atazanavir-Ritonavir Monomaintenance (ATARITMO) Trial. AIDS. 2007;21(10):1309–15.
Du Pasquier RA, Jilek S, Kalubi M, et al. Marked increase of the astrocytic marker S100B in the cerebrospinal fluid of HIV-infected patients on LPV/r-monotherapy. AIDS. 2013;27(2):203–10.
Spudich SS, Nilsson AC, Lollo ND, et al. Cerebrospinal fluid HIV infection and pleocytosis: relation to systemic infection and antiretroviral treatment. BMC Infect Dis. 2005;5:98.
Yilmaz A, Verhofstede C, D’Avolio A, et al. Treatment intensification has no effect on the HIV-1 central nervous system infection in patients on suppressive antiretroviral therapy. J Acquir Immune Defic Syndr. 2010;55(5):590–6.
Dahl V, Lee E, Peterson J, et al. Raltegravir treatment intensification does not alter cerebrospinal fluid HIV-1 infection or immunoactivation in subjects on suppressive therapy. J Infect Dis. 2011;204(12):1936–45.
Letendre S, Marquie-Beck J, Capparelli E, et al. Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65:65–70.
Hammond ER, Crum RM, Treisman GJ, et al. The cerebrospinal fluid HIV risk score for assessing central nervous system activity in persons with HIV. Am J Epidemiol. 2014;180(3):297–307.
Cysique LA, Vaida F, Letendre S, et al. Dynamics of cognitive change in impaired HIV-positive patients initiating antiretroviral therapy. Neurology. 2009;73(5):342–8.
Tozzi V, Balestra P, Salvatori MF, et al. Changes in cognition during antiretroviral therapy: comparison of 2 different ranking systems to measure antiretroviral drug efficacy on HIV-associated neurocognitive disorders. J Acquir Immune Defic Syndr. 2009;52(1):56–63.
Marra CM, Zhao Y, Clifford DB, et al. Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS. 2009;23(11):1359–66.
Winston A, Duncombe C, Li PC, et al. Does choice of combination antiretroviral therapy (cART) alter changes in cerebral function testing after 48 weeks in treatment-naive, HIV-1-infected individuals commencing cART? A randomized, controlled study. Clin Infect Dis. 2010;50(6):920–9.
Smurzynski M, Wu K, Letendre S, et al. Effects of central nervous system antiretroviral penetration on cognitive functioning in the ALLRT cohort. AIDS. 2011;25(3):357–65.
Arendt G, Orhan E, Nolting T. Retrospective analysis of the HAART CNS penetration effectiveness (CPE) index on neuropsychological performance of a big neuro-AIDS cohort [abstract]. 18th Conference on Retroviruses and Opportunistic Infections (CROI); 27 Feb–2 Mar 2011; Boston.
Garvey L, Surendrakumar V, Winston A. Low rates of neurocognitive impairment are observed in neuro-asymptomatic HIV-infected subjects on effective antiretroviral therapy. HIV Clin Trials. 2011;12(6):333–8.
Rourke SB, Carvalhal A, Zypurski A, et al. CNS penetration effectiveness of cART and neuropsychological outcomes: cross-sectional results from the OHTN Cohort Study [abstract]. 19th Conference on Retroviruses and Opportunistic Infections; 5–8 Mar 2012; Seattle.
Robertson K, Jiang H, Kumwenda J, et al. Improved neuropsychological and neurological functioning across three antiretroviral regimens in diverse resource-limited settings: AIDS Clinical Trials Group study a5199, the International Neurological Study. Clin Infect Dis. 2012;55(6):868–76.
Ciccarelli N, Fabbiani M, Colafigli M, et al. Revised central nervous system neuropenetration-effectiveness score is associated with cognitive disorders in HIV-infected patients with controlled plasma viraemia. Antivir Ther. 2013;18(2):153–60.
Kahouadji Y, Dumurgier J, Sellier P, et al. Cognitive function after several years of antiretroviral therapy with stable central nervous system penetration score. HIV Med. 2013;14(5):311–5.
Ellis RJ, Letendre S, Vaida F, et al. Randomized trial of central nervous system-targeted antiretrovirals for HIV-associated neurocognitive disorder. Clin Infect Dis. 2014;58(7):1015–22.
Vassallo M, Durant J, Biscay V, et al. Can high central nervous system penetrating antiretroviral regimens protect against the onset of HIV-associated neurocognitive disorders? AIDS. 2014;28(4):493–501.
Antinori A, Lorenzini P, Giancola ML, et al. Antiretroviral CNS penetration-effectiveness (CPE) 2010 ranking predicts CSF viral suppression only in patients with undetectable HIV-1 RNA in plasma [abstract]. 18th Conference on Retroviruses and Opportunistic Infections (CROI); 27 Feb–2 Mar 2011; Boston.
Giancola ML, Lorenzini P, Cingolani A, et al. virological response in cerebrospinal fluid to antiretroviral therapy in a large Italian cohort of HIV-infected patients with neurological disorders. AIDS Res Treat. 2012;2012:708456.
Rawson T, Muir D, Mackie NE, et al. Factors associated with cerebrospinal fluid HIV RNA in HIV infected subjects undergoing lumbar puncture examination in a clinical setting. J Infect. 2012;65(3):239–45.
Pinnetti C, Lorenzini P, Forbici F, et al. CSF viral escape in patients without neurological disorders: prevalence and associated factors [abstract]. 20th Conference on Retroviruses and Opportunistic Infections (CROI); 3–6 Mar 2014; Boston.
Casado JL, Marín A, Moreno A, Iglesias V, Perez-Elías MJ, Moreno S, Corral I. Central nervous system antiretroviral penetration and cognitive functioning in largely pretreated HIV-infected patients. J Neurovirol. 2014;20(1):54–61.
Fabbiani M, Grima P, Milanini B, et al. Central nervous system penetration effectiveness score better correlates with cognitive performance of HIV+ patients after accounting for drug susceptibility of plasma virus [abstract]. 19th Conference on Retroviruses and Opportunistic Infections (CROI); 5–8 Mar 2012; Seattle.
Antinori A, Marcotullio S, Ammassari A, et al. Italian guidelines for the use of antiretroviral agents and the diagnostic-clinical management of HIV-1 infected persons. Update 2011. New Microbiol. 2012;35(2):113–59.
Cysique LA, Waters EK, Brew BJ. Central nervous system antiretroviral efficacy in HIV infection: a qualitative and quantitative review and implications for future research. BMC Neurol. 2011;11:148.
Aquaro S, Svicher V, Schols D, et al. Mechanisms underlying activity of antiretroviral drugs in HIV-1-infected macrophages: new therapeutic strategies. J Leukoc Biol. 2006;80(5):1103–10.
Shikuma CM, Nakamoto B, Shiramizu B, et al. Antiretroviral monocyte efficacy score linked to cognitive impairment in HIV. Antivir Ther. 2012;17(7):1233–42.
Gray LR, Tachedjian G, Ellett AM, et al. The NRTIs lamivudine, stavudine and zidovudine have reduced HIV-1 inhibitory activity in astrocytes. PLoS One. 2013;8(4):e62196.
Kallianpur KJ, Shikuma C, Kirk GR, et al. Peripheral blood HIV DNA is associated with atrophy of cerebellar and subcortical gray matter. Neurology. 2013;80(19):1792–9.
Valcour VG, Ananworanich J, Agsalda M, et al. HIV DNA reservoir increases risk for cognitive disorders in cART-naïve patients. PLoS One. 2013;8(7):e70164.
Rusconi S, Vitiello P, Adorni F, et al. Maraviroc as intensification strategy in HIV-1 positive patients with deficient immunological response: an Italian randomized clinical trial. PLoS One. 2013;8(11):e80157.
Soulié C, Tubiana R, Simon A, et al. Presence of HIV-1 R5 viruses in cerebrospinal fluid even in patients harboring R5X4/X4 viruses in plasma. J Acquir Immune Defic Syndr. 2009;51(1):60–4.
Garvey L, Nelson M, Latch N, et al. CNS effects of a CCR5 inhibitor in HIV-infected subjects: a pharmacokinetic and cerebral metabolite study. J Antimicrob Chemother. 2012;67(1):206–12.
Vera JH, Garvey LJ, Allsop JM, et al. Alterations in cerebrospinal fluid chemokines are associated with maraviroc exposure and in vivo metabolites measurable by magnetic resonance spectroscopy. HIV Clin Trials. 2012;13(4):222–7.
Cui L, Locatelli L, Xie MY, Sommadossi JP. Effect of nucleoside analogs on neurite regeneration and mitochondrial DNA synthesis in PC-12 cells. J Pharmacol Exp Ther. 1997;280:1228–34.
Werth JL, Zhou B, Nutter LM, Thayer SA. 2′,3′-Dideoxycytidine alters calcium buffering in cultured dorsal root ganglion neurons. Mol Pharmacol. 1994;45:1119–24.
Robertson K, Liner J, Meeker RB. Antiretroviral neurotoxicity. J Neurovirol. 2012;18(5):388–99.
Akay C, Cooper M, Odeleye A, et al. Antiretroviral drugs induce oxidative stress and neuronal damage in the central nervous system. J Neurovirol. 2014;20(1):39–53.
Friis-Møller N, Thiébaut R, Reiss P, et al. Predicting the risk of cardiovascular disease in HIV-infected patients: the data collection on adverse effects of anti-HIV drugs study. Eur J Cardiovasc Prev Rehabil. 2010;17(5):491–501.
Cruse B, Cysique LA, Markus R, Brew BJ. Cerebrovascular disease in HIV-infected individuals in the era of highly active antiretroviral therapy. J Neurovirol. 2012;18:264–76.
Ortiz G, Koch S, Romano JG, Forteza AM, Rabinstein AA. Mechanisms of ischemic stroke in HIV-infected patients. Neurology. 2007;68:1257–61.
Soontornniyomkij V, Umlauf A, Chung SA, et al.HIV protease inhibitor exposure predicts cerebral small vessel disease. AIDS. 2014;28(9):1297-306.
Green DA, Masliah E, Vinters HV, et al. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS. 2005;19(4):407–11.
Giunta B, Ehrhart J, Obregon DF, et al. Antiretroviral medications disrupt microglial phagocytosis of β-amyloid and increase its production by neurons: implications for HIV-associated neurocognitive disorders. Mol Brain. 2011;4(1):23.
Ciccarelli N, Fabbiani M, Di Giambenedetto S, et al. Efavirenz associated with cognitive disorders in otherwise asymptomatic HIV-infected patients. Neurology. 2011;76(16):1403–9.
Conflict of interest
No funding has been received for the preparation of this review.
A. Calcagno has received travel grants or speaker’s honoraria from Abbott, Bristol-Myers Squibb (BMS), Merck Sharp & Dohme (MSD) and Janssen-Cilag. S. Bonora has received grants, travel grants and consultancy fees from Abbott, Boehringer-Inghelheim, BMS, Gilead-Sciences, GlaxoSmithKline (GSK), MSD, Pfizer and Janssen-Cilag. G. Di Perri has received grants, travel frants and consultancy fees from Abbott, Boehringer-Inghelheim, BMS, Gilead-Sciences, GSK, MSD, Pfizer, Roche and Tibotec (Johnson & Johnson).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Calcagno, A., Di Perri, G. & Bonora, S. Pharmacokinetics and Pharmacodynamics of Antiretrovirals in the Central Nervous System. Clin Pharmacokinet 53, 891–906 (2014). https://doi.org/10.1007/s40262-014-0171-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-014-0171-0