Abstract
Endoscopy of the upper aerodigestive tract (UADT) has been influenced by different innovations over the last two decades. The use of chip-on-the-tip rigid or flexible endoscopes was a change in endoscopic function. The next step was the increase of resolution as well as the introduction of high-definition (HD) imaging especially in flexible endoscopes. 4K resolution is almost ready to take the next step. Additionally, endoscopic imaging techniques, e.g., narrowband imaging, autofluorescence, optical coherence tomography, a.o., have been developed to get more details about the pathological changes besides normal white light endoscopy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Endoscopy of the upper aerodigestive tract (UADT ) has been influenced by different innovations over the last two decades. The use of chip-on-the-tip rigid or flexible endoscopes was a change in endoscopic function. The next step was the increase of resolution as well as the introduction of high-definition (HD) imaging especially in flexible endoscopes. 4K resolution is almost ready to take the next step. Additionally, endoscopic imaging techniques, e.g., Narrow Band Imaging (NBI), autofluorescence, optical coherence tomography, a.o., have been developed to get more details about the pathological changes besides normal white light endoscopy.
These imaging techniques are divided in horizontal and vertical imaging techniques. While horizontal imaging techniques look primarily at the surface, vertical images focus on pathological changes underneath the surface. NBI belongs to the horizontal imaging techniques looking at superficial changes of the epithelium and the underlying vessels. In combination with HD, NBI is a powerful endoscopic imaging technique offering a better contrasted picture especially for the assessment of vascular changes. Therefore, it is suitable for the detection of early lesions—either benign or malignant.
Narrow Band Imaging Endoscopy
2004 NBI endoscopy was introduced in the diagnostic workup of the UADT by Muto et al. [1]. At that time it had already proven to be a useful imaging tool in other endoscopic areas, e.g., gastroenterology [2–4] and pulmonology [5].
NBI is a noninvasive imaging technique which can be applied to in- and outpatients during endoscopy by pressing a button, immediately switching from white light to NBI. It enhances the visualization of epithelial and subepithelial microvascular structure and lesions. Especially small lesions can be detected much easier compared to normal white light endoscopy alone.
Compared to white light, superficial epithelial lesions can be detected much better especially due to a more intensive fluorescence of keratin in leukoplakia. Keratotic lesions can be looked at specifically, including their surrounding margins, offering more detailed information about the character of the lesion. Epithelial thickening can be detected much easier. Furthermore, this optical imaging enhancement tool focuses on subepithelial vessels and their different sizes and shapes (Fig. 1).
In oral and oropharyngeal squamous cell carcinoma, Piazza et al. described a sensitivity, specificity, positive, negative predictive values, and accuracy for HDTV white light endoscopy (WLE) of 51 %, 100 %, 100 %, 87 %, and 68 %, respectively, while for HDTV NBI figures were 96 %, 100 %, 100 %, 93 %, and 97 %, respectively [6] (Figs. 2, 3, and 4).
Kraft et al. [7] reported that NBI with WLE showed a significantly higher sensitivity (97 % vs 79 %) and accuracy (97 % vs 90 %) than WLE alone in identifying laryngeal cancer and its precursor lesions. In contrast, the specificity (96 % vs 95 %) was essentially equal for both imaging techniques.
NBI Equipment
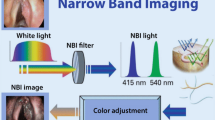
The technical equipment consists of a xenon light source with a special NBI filter narrowing the wavelength of emitted light to 400–430 nm (center wavelength at 415 nm) and 525–555 nm (center wavelength at 540 nm).
Additionally, a high-definition camera in combination with a rigid endoscope (0°–120°) and/or a flexible videoendoscope (30–90 cm length and max. 4 mm in diameter) with chip-on-the-tip technology and a video system unit is needed.
Both wavelengths have special characteristics. 415 nm represents blue light and penetrates about 400 μm into the tissue. In normal mucosa the light beam hits the underlying connective tissue. It leads to better contrasted endoscopic pictures secondary to the corresponding peak absorption spectrum of hemoglobin by enhancement of capillary vessels. The green light centered at the longer wavelength of 540 nm can penetrate deeper into the connective tissue resulting in endoscopic visualization of the submucosal vascular plexus. Additionally, keratin present in leukoplakias is a potential fluorophore emitting bright white light from the epithelial surface, leading to a better depiction of these epithelial changes in contrast to the surrounding green epithelium.
Epithelial Changes in NBI
New endoscopic methods and tools especially NBI have improved the endoscopic diagnosis leading to an early depiction of epithelial but also vascular lesions of the UADT. Therefore, a more differentiated knowledge and assessment of epithelial as well as vascular lesions resulted in a more precise diagnostic workup and therapy [6, 8–18].
Epithelial tumors of the UADT are grouped in papilloma, epithelial dysplasia , and squamous cell carcinoma. These tumors differ in vascular changes, the expression of epithelial changes, as well as the connective tissue architecture being important in the process of preoperative differential diagnosis.
Thin, non-keratinized, stratified squamous epithelium of the larynx and hypopharynx allows good endoscopic visualization of fine vascular lesions during the development of precancerous and cancerous lesions. Especially typical intraepithelial papillary capillary loops (IPCLs) of recurrent respiratory papillomatosis (RRP) (Fig. 5) can be detected by NBI.
In the upper aerodigestive tract, we differentiate between two different epithelial layers—respiratory and squamous cell epithelium. Respiratory epithelium may become metaplastic and change in the lower differentiated squamous cell epithelium. This epithelial change may also result in the development of squamous cell cancer. During NBI endoscopy, an epithelial defect or ulceration can easily be detected by bright red color which is sharply demarcated by the green color of the surrounding tissue covered by epithelium.
In NBI endoscopy, epithelial thickening primarily leads to a darker green appearance of the NBI picture in contrast to the more reddish-green color in normal epithelium. This development is followed by a leukoplakia which contains the fluorophore keratin. The color of the “leukoplakia” becomes more whitish and less transparent leading to the observation of vessel breaks (“umbrella effect”). Leukoplakia is a descriptive term which represents epithelial lesions from hyperkeratosis up to squamous cell cancer. In combination with vascular changes, epithelial lesions can be much better classified by NBI in contrast to white light alone.
In earlier stages leukoplakias are still transparent and have a sharp and regular edge (Fig. 6). Additionally, they present with a more round to oval shape and mostly have a smooth surface. When leukoplakias become dysplastic, they develop an irregular shape and edge. Furthermore, the surface is inhomogeneous and diverse. In carcinoma in situ as well as microinvasive cancer, the epithelial lesion may have a spot-like appearance and goes along with typical vascular changes which will be described in the next section (Figs. 7 and 8). Besides the endoscopic picture of the epithelial lesion during NBI examination, the absence of the mucosal wave during stroboscopy may give additional information about the nature of the lesion. In a progressing lesion, the character of the squamous cell carcinoma is characterized by a recognizable change of the surface and shape secondary to the increase of tumor volume and ulceration (Fig. 9).
Vascular Lesions in NBI
Two groups of vascular lesions can be differentiated-longitudinal and perpendicular. While horizontal vascular lesions are primarily reactive due to ongoing mechanical, chemical, or other external stress factors, vertical lesions develop by a stimulus of the epithelium itself.
Ni et al. [19] have transferred a model coined as “IPCLs” (intraepithelial papillary capillary loops) for the classification of vascular changes of the esophagus seen during NBI examination to precancerous and cancerous lesions of the vocal folds. Kumagai et al. [20] already stated that vessels of the mucosa show characteristic changes according to the invasion depth of cancer. This classification is widely used in gastroenterological endoscopic diagnosis [21, 22] but implies some disadvantages and may not simply be transferred to the description of vascular pattern of the UADT. Additionally the term “papillary loops” is misleading, because regular mucosa of the UADT rarely contains papillary loops (Fig. 1).
Longitudinal Vascular Changes
In the beginning of the pathogenesis of vascular changes, blood vessels often appear enlarged and/or ectatic, especially in inflammation. Ongoing reactive processes and external stimuli lead to angiogenesis and the formation of new vascular structures. Vessels become more visible. The increase of the vessel size is associated with further branching of vessels and anastomoses. These changes may lead to an increased visible vascular density and diameter followed by an increased reddish appearance under white light and a darker green appearance under NBI.
Consideration of such precursor changes of vascular diseases, e.g. vessel ectasia, meander, increasing number and branching of vessels, and change of direction, may lead to more differentiated prognostic statements and adequate therapeutic decisions.
The mentioned vascular changes type I and type II of the IPCL classification [19] correlate to these early vascular changes described. They are primarily induced by mechanical stress on the vessel walls and are typically localized at point of the strongest mechanical forces. The initial changes in the blood vessels are beginning signs of future manifest vascular diseases especially in the vocal folds, like varices, convolutes, polyps (Fig. 10), and hemorrhages.
Perpendicular Vascular Lesions
Perpendicular vascular lesions are characterized by the development of intraepithelial papillary capillary vessel loops (IPCLs) developing secondary to an epithelial stimulus. In HPV-related recurrent respiratory papillomatosis, these loops can endoscopically be recognized as real loops embedded in a three-dimensional warty structure. In contrast precancerous lesions show symmetrical arranged dot-like loops which represent the tip of the loop under the epithelium arising from deeper layers of the mucosa. These typical changes can be detected by NBI.
Perpendicular vessel changes in papillomas, precancerous or cancerous lesions, are induced by an epithelial stimulus. Not only the vessel but also the surrounding tissue is altered by this stimulus. In RRP typical morula-like “warty” changes develop. They may be uni- or bilateral, multicentric, and exophytic with a glassy convex surface, similar to raspberries. There seems to be a local immunological deficit. The epithelial infection stimulus in the underlying connective tissue seems to initiate angiogenesis and a transformation of the connective tissue. Squamous cell carcinoma can also be caused by “high-risk” HPV [23]. Lukes et al. [24] and Tjon Pian Gi et al. [25] highlight that the changes of IPCL and the epithelial surface are essential for differential diagnosis in endoscopic imaging of RRP. Typical features, like central vessel loops in each morula-like bulge usually help to distinguish RRPs from high-grade dysplasia or SCCs.
In the development of precancerous and cancerous lesions, the reddish-appearing effect of an erythroplakia is caused by an increased density of subepithelial vascular structure secondary to neoangiogenesis in combination with a thin and atrophic epithelium. As already described before, leukoplakia is like a chameleon which resembles epithelial changes from benign epithelial thickening to cancerous lesions.
In context of carcinogenesis, perpendicular vascular changes (IPCL) may be recognized during endoscopy at the border of the leukoplakia described above. The combination of a leukoplakia as well as its perilesional vascular changes can give a hint to the character of the disease. Benign hyperkeratotic lesions may go along with vessel ectasia but do not present any kind of vascular loops. These epithelial lesions are reactive and may regress spontaneously. In most cases these lesions are transparent and vessel may be detected underneath the leukoplakia.
As the epithelium becomes dysplastic, the leukoplakia becomes more irregular in shape and size. Longitudinal vascular changes like vessel ectasia transform into perpendicular vascular lesions. Loops become visible in the periphery of the leukoplakia. These loops occur primarily as typical dots representing the top of the loop. In early stages the loops are still visible in a regular symmetrical pattern. When cancer develops, the vascular loops and the epithelial lesion become completely irregular. The irregular tumor surface and shape are caused by vascular malnutrition secondary to chaotic neoangiogenesis (Fig. 11).
NBI, especially in combination with high-definition imaging, is a useful and effective imaging technique in the diagnosis and therapy of pathological changes of the UADT. Especially small lesions characterized by vascular changes can be detected much earlier in contrast to normal white light. It is also suitable for the early diagnosis of papilloma, precancerous and cancerous lesions [6, 17, 19]. NBI can also be used during papilloma or cancer surgery for detection of superficial surgical margins.
NBI in combination with contact endoscopy during microlaryngoscopy, so called “compact endoscopy ” [26], allows us to detect and classify different types of IPCL. Using this technique we are able to distinguish between papilloma, mild or moderate epithelial dysplasia , SIN III, and microinvasive cancer (Figs. 12, 13, 14, and 15).
Therefore, vascular lesions play a significant role in the endoscopic workup of lesions of the UADT. New endoscopes in combination with HD or even 4K imaging as well as NBI permit a detailed observation of the mucosal surface allowing the diagnosis of superficial pathologies with a high probability to anticipate the correct histological examination during preoperative stage. NBI as a new endoscopic tool for rigid as well as flexible endoscopy may be an early step on the way in performing an “optical biopsy.”
References
Muto M, Nakane M, Katada C, et al. Squamous cell carcinoma in situ at oropharyngeal and hypopharyngeal mucosal sites. Cancer. 2004;101:1375–81.
Gono T, Yamazaki K, Doguchi N, et al. Endoscopic observation of tissue by narrowband illumination. Opt Rev. 2003;10:211–5.
Sano Y, Kobayashi M, Hamamoto Y, et al. New diagnostic method based on color imaging using narrow band imaging (NBI) system for gastrointestinal tract. Gastrointest Endosc. 2001;53:AB125.
Yoshida T, Inoue H, Usui S, Satodate H, et al. Narrow-band imaging system with magnifying endoscopy for superficial esophageal lesion. Gastrointest Endosc. 2004;59:288–95.
Shibuya K, Hoshino H, Chiyo M, Iyoda A, et al. High magnification bronchovideoscopy combined with narrow band imaging could detect capillary loops of angiogenic squamous dysplasia in heavy smokers at high risk for lung cancer. Thorax. 2003;58:989–95.
Piazza C, Cocco D, Del Bon F, Mangili S, et al. Narrow band imaging and high definition television in evaluation of oral and oropharyngeal squamous cell cancer: a prospective study. Oral Oncol. 2010;46:307–10.
Kraft M, Fostiropoulos K, Gürtler N, Arnoux A, Davaris N, Arens C. Value of narrow band imaging in the early diagnosis of laryngeal cancer. Head Neck. 2016;38(1):15–20. doi: 10.1002/hed.23838.
Chu PY, Tsai TL, Tai SK, Chang SY. Effectiveness of narrow band imaging in patients with oral squamous cell carcinoma after treatment. Head Neck. 2012;34:155–61.
Ho CY, Lee YL, Chu PY. Use of narrow band imaging in evaluation of possible nasopharyngeal carcinoma. Am J Rhinol Allergy. 2011;25:107–11.
Katada C, Nakayama M, Tanabe S, et al. Narrow band imaging for detecting superficial oral squamous cell carcinoma: a report of two cases. Laryngoscope. 2007;117:1596–9.
Lin YC, Watanabe A, Chen WC, et al. Narrowband imaging for early detection of malignant tumors and radiation effect after treatment of head and neck cancer. Arch Otolaryngol Head Neck Surg. 2010;136:234–9.
Nonaka S, Saito Y. Endoscopic diagnosis of pharyngeal carcinoma by NBI. Endoscopy. 2008;40:347–51.
Takano JH, Yakushiji T, Kamiyama I, et al. Detecting early oral cancer: narrowband imaging system observation of the oral mucosa microvasculature. Int J Oral Maxillofac Surg. 2010;39:208–13.
Ugumori T, Muto M, Hayashi R, Hayashi T, Kishimoto S. Prospective study of early detection of pharyngeal superficial carcinoma with the narrowband imaging laryngoscope. Head Neck. 2009;31:189–94.
Wang WH, Lin YC, Lee KF, Weng H. Nasopharyngeal carcinoma detected by narrow-band imaging endoscopy. Oral Oncol. 2011;47:736–41.
Watanabe A, Tsujie H, Taniguchi M, Hosokawa M, Fujita M, Sasaki S. Laryngoscopic detection of pharyngeal carcinoma in situ with narrowband imaging. Laryngoscope. 2006;116:650–4.
Watanabe A, Taniguchi M, Tsujie H, Hosokawa M, et al. The value of narrow band imaging endoscope for early head and neck cancers. Otolaryngol Head Neck Surg. 2008;138:446–51.
Yang SW, Lee YS, Chang LC, Hwang CC, Chen TA. Diagnostic significance of narrow-band imaging for detecting high-grade dysplasia, carcinoma in situ, and carcinoma in oral leukoplakia. Laryngoscope. 2012;122:2754–61.
Ni XG, He S, Xu ZG, et al. Endoscopic diagnosis of laryngeal cancer and precancerous lesions by narrow band imaging. J Laryngol Otol. 2011;125:288–96.
Kumagai Y, Inoue H, Nagai K, et al. Magnifying endoscopy, stereoscopic microscopy, and the microvascular architecture of superficial esophageal carcinoma. Endoscopy. 2002;34:369–75.
Inoue H, Honda T, Nagai K, et al. Ultra-high magnification endoscopic observation of carcinoma in situ of the esophagus. Dig Endosc. 1997;9:16–8.
Inoue H, Kaga M, Sato Y, Sugaya S, Kudo S. Magnifying endoscopy diagnosis of tissue atypia and cancer invasion depth in the area of pharyngo-esophageal squamous epithelium by NBI enhanced magnification image: IPCL pattern classification. In: Cohen J, editor. Advanced digestive endoscopy: comprehensive atlas of high resolution endoscopy and narrowband imaging. New York, NY: Blackwell Publishing; 2007. p. 49–66.
Zaravinos A. An updated overview of HPV-associated head and neck carcinomas. Oncotarget. 2014;5(12):3956–69.
Lukes P, Zabrodsky M, Lukesova E, et al. The role of NBI HDTV magnifying endoscopy in the prehistologic diagnosis of laryngeal papillomatosis and spinocellular cancer. Biomed. Res. Int. 2014;7 pages. doi: 10.1155/2014/285486.
Tjon Pian Gi REA, Halmos GB, et al. Narrow band imaging is a new technique in visualization of recurrent respiratory papillomatosis. Laryngoscope. 2012;122:1826–30.
Arens C, Voigt-Zimmermann S. Contact endoscopy of the vocal folds in combination with narrow band imaging (compact endoscopy). Laryngorhinootologie. 2015;94(3):150–2.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this chapter
Cite this chapter
Arens, C., Voigt-Zimmermann, S. (2016). Narrow Band Imaging of the Upper Aerodigestive Tract. In: Wong, BF., Ilgner, J. (eds) Biomedical Optics in Otorhinolaryngology. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1758-7_39
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1758-7_39
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1757-0
Online ISBN: 978-1-4939-1758-7
eBook Packages: MedicineMedicine (R0)