Abstract
Various image-enhanced endoscopic systems are available in clinical practice. For effective detection and histological prediction of lesions, endoscopists should understand the advantages and limitations of each imaging technology. Narrowband imaging (NBI) illuminates the tissue surface by using optical filters that narrow the respective red-green-blue bands. I-scan and flexible spectral imaging color enhancement (FICE) have proprietary image processing algorithm applied on white light images based on spectral emission methods. Herein, this chapter focuses on understanding the detailed principle and clinical applications of advanced imaging technologies such as NBI, i-scan, and FICE. With recent advancement in endoscopic imaging technology, optical biopsy has been enabled as real-time histologic prediction of tumor. The improvement of endoscopic resolution can facilitate the accurate and detailed endoscopic findings. Moreover, new imaging technologies may make the endoscopists walk a big step in the field of diagnostic and therapeutic gastrointestinal endoscopy. In the future, further studies are needed for evaluation of cost-effectiveness and diagnostic accuracy.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Flexible Spectral Imaging Color Enhancement (FICE)
- Narrowband Images
- Histology Prediction
- Therapeutic Gastrointestinal Endoscopy
- Emission Spectral Method
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
31.1 Narrow band Imaging (NBI)
31.1.1 Principles
NBI is an innovative optical technology that modifies the wavelengths and bandwidths of an endoscope’s light into narrow band illumination of 415 ± 30 nm and 540 ± 30 nm [1]. In this endoscopy system that uses the red (R), green (G), and blue (B) sequential imaging system, the gastrointestinal mucosa is illuminated sequentially with R, G, and B lights through a rotating RGB filter wheel [2]. When endoscopist presses the button at the handle part of the scope, a narrow band filter is inserted between the lamp and the RGB filter. Red with a long wavelength diffuses widely and deeply, whereas blue with a short wavelength diffuses within a smaller range and shallowly. Because such light with a short wavelength has strong reflection at the epithelial surface, it is suitable for visualizing the mucosal surface morphology. Accordingly, the application of this system has advantages in producing sharp images of microvascular architecture and microsurface structure.
31.1.2 Clinical Applicability
NBI with magnification has been widely evaluated in the diagnosis of esophageal lesions (Fig. 31.1). In patients with Barrett’s esophagus (BE), NBI with magnification showed the high diagnostic reproducibility and accuracy [3]. When esophageal neoplasia was assessed in a high-risk population, NBI was suitable for esophageal screening [4]. Compared to the Lugol chromoendoscopy, NBI presented the same detection rate of high-grade dysplasia and esophageal squamous cell carcinoma. In the differential diagnosis between esophageal neoplasia and other mucosal lesions, NBI was superior to Lugol chromoendoscopy.
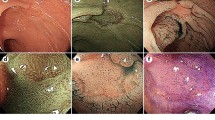
Esophageal lesions observed by NBI system. (a) Ectopic gastric mucosa. An oval, brownish lesion was noted at the upper esophagus. Between the lesion and esophageal squamous mucosa, the border was distinct and smooth. (b) High-grade dysplasia. A small lesion (10 × 5 mm) was detected during endoscopic screening. When esophageal mucosa is examined, the detection rate of early stage neoplasia may increase with NBI endoscopy. (c) Magnifying endoscopic finding. Within the lesion, intra-papillary capillary loop (IPCL) type IV was observed. (d) Squamous cell carcinoma. A large lesion (31 × 25 mm) was located at the middle portion of esophagus. Without Lugol chromoendoscopy, a brownish area with irregular border was seen clearly
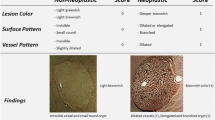
NBI system has been also applied in the further diagnosis of gastric lesions (Figs. 31.2 and 31.3). In the non-inflamed mucosa of gastric body, magnifying NBI endoscopy can observe the regular arrangement of collecting venules and subepithelial capillary network. The presence of these patterns was shown as good indicator of normal stomach without Helicobacter pylori infection. Magnifying NBI (M-NBI) endoscopic findings of early gastric cancer are (1) the presence of demarcation line between a lesion and the background mucosa and (2) the presence of an irregular microvascular pattern within the lesion. In conjunction with white light endoscopy (WLE), M-NBI identified small, depressed gastric mucosal cancers with high diagnostic accuracy [5]. Furthermore, M-NBI allows reliable delineation of the horizontal extent of early gastric cancer (EGC) before endoscopic submucosal dissection [6].
Gastric dysplasia examined by NBI endoscopy. NBI system can allow the dysplastic lesions to be clarified within the background mucosa of intestinal metaplasia. (a) A small tubular adenoma at the gastric angle. (b) A high grade dysplasia (24 x 13 mm) at the gastric angle.(c) A well-differentiated adenocarcinoma (15 x 9 mm) at the posterior side of gastric antrum. (d) A large tubulovillous adenoma (46 x 36 mm) at the lesser curvature side of gastric mid-body
In patients undergoing screening or surveillance colonoscopy, NBI did not increase the yield of colon polyps, adenomas, or flat adenomas [7]. NBI might be better than standard-definition WLC (white light colonoscopy) and equal to high-definition WLC for detection of patients with colorectal polyps or colorectal adenomas [8]. Accurate optical characterization of small colorectal polyps (<10 mm) at colonoscopy would allow only adenomas to be removed [9]. However, NBI optical diagnosis cannot currently be recommended for application in routine clinical practice.
31.2 I-Scan
31.2.1 Principles
I-scan technology is the newly developed image-enhanced endoscopy technology from PENTAX (Tokyo, Japan) [10]. This consists of three types of algorithms: surface enhancement (SE), contrast enhancement (CE), and tone enhancement (TE). SE allows a detailed inspection of a mucosal surface structure. CE allows close observation of subtle irregularities and mucosal vascular pattern around the surface. SE and CE modes are suitable for screening endoscopy to detect an early stage gastrointestinal tumor. TE consists of three modes such as TE-e for the esophagus, TE-g for the stomach, and TE-c for the colon. TE is mainly proper for additional characterization of the detected lesions during a screening endoscopy. Three modes (SE, CE, and TE) are serially converted by pressing a button, and it enables to apply two or more modes simultaneously.
31.2.2 Clinical Applicability
In comparison with chromoendoscopy with Lugol’s solution, i-scan is simple and useful for evaluating the reflux esophagitis-associated lesions. Compared to WLE, i-scan endoscopy increased the diagnostic yield of reflux esophagitis by detecting more minimal changes in the squamocolumnar junction of the esophagus [11]. For colon polyps, diagnostic accuracy of i-scan was similar to chromoendoscopy using methylene blue [12]. Compared to standard colonoscopy, i-scan colonoscopy detected significantly more patients with colorectal neoplasia [13]. Also, i-scan was effective for histologic prediction of colorectal polyps [14]. After a short training session and a review of standardized images, a learning curve may be obtained rapidly [15]. In patients with inflammatory bowel diseases (IBD), i-scan has the potential to increase assessment of disease severity and extent, leading to new implications for decision making in management of IBD [16]. When superficial gastric lesion of less than 1 cm was detected during WLE, i-scan endoscopy with magnification was performed for diagnosis of gastric neoplasia (Fig. 31.4) [17]. However, only limited data using i-scan for gastric lesions is available. Further studies will be required to confirm its value.
31.3 Flexible Spectral Imaging Color Enhancement (FICE)
31.3.1 Principles
FICE, also known as multiband imaging, is based on a spectral image processing technology. NBI uses physical filters, whereas FICE system takes an ordinary endoscopic image from the video processor and arithmetically processes, estimates, and produces an image of a given, dedicated wavelength of light. By switching a button on the endoscope, FICE system can select an optimal wavelength between 400 and 700 nm for various mucosa of gastrointestinal tract. Single-wavelength images are randomly selected and assigned to red, green, and blue to build and display a virtually enhanced color images.
31.3.2 Clinical Applicability
When BE is diagnosed by endoscopy, palisade vessels is more clearly observed in BE mucosa with FICE than WLE [18]. Demarcation line between whitish BE mucosa and brownish gastric mucosa was more distinct by FICE images, thereby leading to accurate diagnosis of BE. When the extent of EGC is diagnosed, FICE system can be used to evaluate the demarcation lines between a depressed-type and an elevated-type EGC and surrounding tissue (Fig. 31.5). Both expert and non-expert endoscopist determined the tumor margins with higher accuracy, compared to WLE. Also, FICE may be useful for the diagnosis of gastric lesions such as nonneoplastic lesion, adenoma, and cancer [19]. For the diagnosis of colon adenomas of less than 10 mm, the overall accuracy achieved by FICE with/without magnification was 87.0% and 80.4%, respectively [20]. For identifying adenomas, sensitivity and diagnostic accuracy by FICE were significantly higher than those of WLE and comparable to those of conventional chromoendoscopy [21].
FICE endoscopic images of early gastric cancer. (a) White-light endoscopy. (b) FICE endoscopy [22]
References
Gono K, Obi T, Yamaguchi M, et al. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J Biomed Opt. 2004;9:568–77.
Yao K, Takaki Y, Matsui T, et al. Clinical application of magnification endoscopy and narrow-band imaging in the upper gastrointestinal tract: new imaging techniques for detecting and characterizing gastrointestinal neoplasia. Gastrointest Endosc Clin N Am. 2008;18:415–33.
Kato M, Goda K, Shimizu Y, et al. Image assessment of Barrett’s esophagus using the simplified narrow band imaging classification. J Gastroenterol. 2017;52:466–75.
Morita FH, Bernardo WM, Ide E, et al. Narrow band imaging versus lugol chromoendoscopy to diagnose squamous cell carcinoma of the esophagus: a systematic review and meta-analysis. BMC Cancer. 2017;17:54.
Ezoe Y, Muto M, Uedo N, et al. Magnifying narrow-band imaging is more accurate than conventional white-light imaging in diagnosis of gastric mucosal cancer. Gastroenterology. 2011;141:2017–25.
Nagahama T, Yao K, Maki S, et al. Usefulness of magnifying endoscopy with narrow-band imaging for determining the horizontal extent of early gastric cancer when there is an unclear margin by chromoendoscopy (with video). Gastrointest Endosc. 2011;74:1259–67.
Pasha SF, Leighton JA, Das A, et al. Comparison of the yield and miss rate of narrow band imaging and white light endoscopy in patients undergoing screening or surveillance colonoscopy: a meta-analysis. Am J Gastroenterol. 2012;107:363–70.
Nagorni A, Bjelakovic G, Petrovic B. Narrow band imaging versus conventional white light colonoscopy for the detection of colorectal polyps. Cochrane Database Syst Rev. 2012;(1):CD008361.
Rees CJ, Rajasekhar PT, Wilson A, et al. Narrow band imaging optical diagnosis of small colorectal polyps in routine clinical practice: the Detect Inspect Characterise Resect and Discard 2 (DISCARD 2) study. Gut. 2017;66:887–95.
Kodashima S, Fujishiro M. Novel image-enhanced endoscopy with i-scan technology. World J Gastroenterol. 2010;16:1043–9.
Kang HS, Hong SN, Kim YS, et al. The efficacy of i-SCAN for detecting reflux esophagitis: a prospective randomized controlled trial. Dis Esophagus. 2013;26:204–11.
Hoffman A, Kagel C, Goetz M, et al. Recognition and characterization of small colonic neoplasia with high-definition colonoscopy using i-Scan is as precise as chromoendoscopy. Dig Liver Dis. 2010;42:45–50.
Hoffman A, Sar F, Goetz M, et al. High definition colonoscopy combined with i-Scan is superior in the detection of colorectal neoplasias compared with standard video colonoscopy: a prospective randomized controlled trial. Endoscopy. 2010;42:827–33.
Hong SN, Choe WH, Lee JH, et al. Prospective, randomized, back-to-back trial evaluating the usefulness of i-SCAN in screening colonoscopy. Gastrointest Endosc. 2012;75:1011–21.
Neumann HN, Vieth M, Fry LC, et al. Learning curve of virtual chromoendoscopy for the prediction of hyperplastic and adenomatous colorectal lesions: a prospective 2-center study. Gastrointest Endosc. 2013;78:115–20.
Neumann H, Vieth M, Günther C, et al. Virtual chromoendoscopy for prediction of severity and disease extent in patients with inflammatory bowel disease: a randomized controlled study. Inflamm Bowel Dis. 2013;19:1935–42.
Li CQ, Li Y, Zuo XL, et al. Magnified and enhanced computed virtual chromoendoscopy in gastric neoplasia: a feasibility study. World J Gastroenterol. 2013;19:4221–7.
Osawa H, Yamamoto H, Yamada N, et al. Diagnosis of endoscopic Barrett’s esophagus by transnasal flexible spectral imaging color enhancement. J Gastroenterol. 2009;44:1125–32.
Jung SW, Lim KS, Lim JU, et al. Flexible spectral imaging color enhancement (FICE) is useful to discriminate among non-neoplastic lesion. Dig Dis Sci. 2011;56:2879–86.
Kim YS, Kim D, Chung SJ, et al. Differentiating small polyp histologies using real-time screening colonoscopy with Fuji Intelligent Color. Clin Gastroenterol Hepatol. 2011;9:744–9.
Pohl J, Nguyen-Tat M, Pech O, May A, Rabenstein T, Ell C. Computed virtual chromoendoscopy for classification of small colorectal lesions: a prospective comparative study. Am J Gastroenterol. 2008;103:562–9.
Mouri R, et al. Evaluation and validation of computed virtual chromoendoscopy in early gastric cancer. Gastrointest Endosc. 2009;69(6):1057.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Cho, JH. (2018). Image-Enhanced Endoscopy. In: Chun, H., Yang, SK., Choi, MG. (eds) Clinical Gastrointestinal Endoscopy. Springer, Singapore. https://doi.org/10.1007/978-981-10-4995-8_31
Download citation
DOI: https://doi.org/10.1007/978-981-10-4995-8_31
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-4994-1
Online ISBN: 978-981-10-4995-8
eBook Packages: MedicineMedicine (R0)