Abstract
Among geographical factors causing variations in animal population abundance, elevation gradients have received little attention. For nonhuman primates in particular, very scattered information is available. We have investigated changes in population abundance across an elevational gradient in four nonhuman primate species (Udzungwa red colobus, Angolan colobus, Sykes’ monkeyand Sanje mangabey) in the Udzungwa Mountains of Tanzania, an area of global importance for biodiversity conservation and biological endemisms. The study focuses on Mwanihana forest, one of the largest forest blocks in the area, with unique, continuous forest cover from 300 to 2,300 m a.s.l. Relative abundance data were collected from a grid of 43 transects that were 2 km in length. About 80 km of transects were walked and 117 sightings of primate groups were recorded. Encounter rates (groups seen per km walked) were highest for Udzungwa red colobus and smallest for Sanje mangabey. For all species, encounter rates decreased markedly with increasing elevation. Using multivariate analysis, we assessed the influence of elevation together with a set of vegetation parameters (i.e., total basal area (TBA), mean basal area (MBA), and species richness). We found that elevation has a negative significant effect on the species tested; TBA and MBA have, respectively, a negative and positive effect on abundance; TBA increases linearly with elevation, while MBA is greater in lowland and submontane forest. Conversely, tree species’ richness does not have any meaningful effect. We discuss the implications as especially linked to variation in forest habitat suitability to different species.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Since Darwin (1839) and von Humboldt (1849) identified elevation gradients as geographical factors causing ecological variations, this topic has still received little attention (MacArthur 1972), especially when compared to the conspicuous literature associated with latitudinal gradients (Stevens 1989). In 2001, however, a volume of Global Ecology and Biogeography (Rahbek 1995) was dedicated to elevation gradients of species richness in mammals. Rahbek (1995) showed that most studies detected the highest species richness at lower and some at mid elevations. Several hypotheses on the cause of species richness and gradient relationship have been proposed since, but none of these has yet received clear empirical support (Brown 2001; Heaney 2001; Lomolino 2001). Species diversity may peak at intermediate elevation gradients because of overlapping ranges of species distributions that are bounded by the ends of the gradient (Colwell and Hurtt 1994; Rahbek 1995, 1997; Kessler 2001; Lomolino 2001; Rahbek 2005). This hypothesis fits with standard ecological theory on diversity and geographic ranges, in that temperature declines with elevation, leading to low productivity and consequent changes in habitat diversity and biotic interactions (Givnish 1999; Heaney 2001; Lomolino 2001). A second geographical component which is more poorly understood is the relationship between population density and elevation gradient. At present, it is not clear whether a density–elevation gradient relationship exists within a single species, since only one study is known to have investigated such an effect (Hanya et al. 2004).
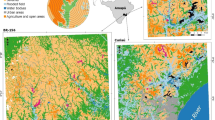
The present study aims to assess the changes of population abundance with elevational gradient in four nonhuman primate species from the Udzungwa Mountains of Tanzania. The Udzungwa Mountains are part of the Eastern Arc Mountains chain, an area of global importance for biodiversity and endemism (Burgess et al. 2007). In particular, the Udzungwa represents one of the most important areas in Africa for primate diversity and endemisms, with two strictly endemic monkeys, the Udzungwa red colobus (Procolobus gordonorum) and the Sanje mangabey [Cercocebus (galeritus) sanjei], and the near endemic kipunji (Rungwecebus kipunji; Rovero et al. 2009). Of all the Eastern Arc Mountains blocks, the Udzungwa have the largest forested area, widest elevation gradient, and greatest habitat diversity, making the area an excellent model for studying changes of primate abundance with elevation. The study focuses on Mwanihana forest, one of the largest forest blocks and one of the only two forests (the other being the Udzungwa Scarp Forest Reserve) that have continuous forest cover from 300 to 2,300 m a.s.l.
A detailed study in woody vegetation along elevation gradients of the Mwanihana forest found that basal area, species’ richness, and diversity increase with elevation (Lovett et al. 2006). In contrast, previous work on elevational variation in primate abundance is very limited. It either relates to studies conducted on a limited range of the overall forest elevational gradient, such as the long-term monitoring conducted in Mwanihana and Udzungwa Scarp Forest Reserve from lowland (300 m a.s.l.) to mid elevation (1,000 m a.s.l.; Rovero et al. 2006, 2012); or it relates to comparisons of abundance among forests with different elevation gradients (Marshall et al. 2005; Rovero and Marshall 2005). This latter approach would likely introduce biases from comparing forests which differ in overall area, floristic and/or structural tree community features, as well as for differences related to anthropological effects (e.g., hunting and habitat fragmentation). Nevertheless, these comparisons show that both diversity and relative abundance of primate populations were higher at lower elevation (e.g., Mwanihana Forest: 300–800 m) than at mid to high elevation (e.g., Ndundulu Forest: 1,400–2,100 m; Struhsaker et al. 2004; Rovero and Marshall 2005). The present study aims to (a) assess variation in primate abundance along the entire elevational gradient (300–2,300 m a.s.l.) within the same forest; and (b) correlate variations in primate abundance to elevation and vegetation parameters such as mean and total basal area, tree species’ richness, and canopy cover.
Methods
Study Site
The Udzungwa Mountains are in south–central Tanzania and extends over 10,000 km2. Overall elevations span from 290 (Kilombero valley on the eastern side) to 2,600 m a.s.l. (Mount Luhomero). The focal area for this study, Mwanihana forest (centered on 7°46’S, 36°43’E; 177 km2), covers a steep and east facing escarpment slope within the eastern side of the Udzungwa Mountains National Park (Fig. 1). This forest is a key site for conservation of the two endemic monkeys, the Udzungwa red colobus and Sanje mangabey (Dinesen et al. 2001; Rovero et al. 2009). For the latter, Mwanihana holds one of the two existing populations; the other one is in the southern Uzungwa Scarp Forest Reserve. Mwanihana forest is important for a range of plant and fauna species, including gray-faced sengi, or elephant-shrew (Rhynchocyon udzungwensis), Abbott’s duiker (Cephalophus spadix), and rufous-wing sunbird (Nectarinia rufipennis).
Forest cover at Mwanihana is continuous from 300 to almost 2,300 m a.s.l. and consists of different habitat types roughly corresponding to elevation zones (Lovett 1993; Lovett et al. 2006), from lowland deciduous ‘miombo’ forest, and semideciduous forest to submontane and montane evergreen forest, the latter including upper montane, bamboo-dominated forest (Lovett 1993; Lovett et al. 2006).
The forest is under the direct climatic influence of the east blowing Indian Ocean winds, and rainfall is therefore higher than in the surroundings, varying from 2,000 to 2,500 mm per year, usually distributed in two periods: November–December and March–May.
Primate Species
Four primate species out of the 13 primates (of which seven diurnal) found within the Udzungwa Mountains occur in Mwanihana forest and are considered in the current analysis (Fig. 2).
Subfamily Colobinae:
-
(1)
Udzungwa red colobus (P. gordonorum)
This IUCN-Vulnerable monkey is one of the Udzungwa endemic primates. It occurs throughout the Udzungwa Mountains range but because of habitat destruction for farming and settlements, its presence in small forest fragments has decreased drastically in recent years. They live in multi-male/multi-female groups and range in size from 3 to 85 individuals (Struhsaker et al. 2004; Struhsaker 2010). Extensive work by Struhsaker and colleagues has shown that group size is affected by several habitat parameters, including tree density, degree of deciduousness, and forest size (Struhsaker et al. 2004). Thus, groups are bigger in size in the large blocks of mature, moist, mixed evergreen, and semideciduous forest (such as Mwanihana) but size does not appear correlated with elevation (Struhsaker et al. 2004; Marshall et al. 2005, 2010).
Like other colobine monkeys, the species is arboreal and is rarely seen on the ground. Colobus feed on leaves, especially young leaves (Rovero 2003; Pucci and Rovero 2004). Thus, the richness of food plant species in the forest is an important variable correlating with the abundance of red colobus (Rovero and Struhsaker 2007). Red colobus in the Udzungwas associate with all sympatric diurnal monkeys with the exception of yellow baboons, and are most frequently observed in association with Angolan colobus (Struhsaker 2010).
-
(2)
Angolan colobus (Colobus angolensis palliatus)
This species occurs throughout the Udzungwa Mountains (Marshall et al. 2010) and, like red colobus monkeys, has decreased in recent years at heavily disturbed sites (Rovero et al. 2012). The distribution of Angolan colobus monkeys is very similar to that of red colobus monkeys, and it appears to be relatively more common at higher elevations, in the upper montane forest zone (Marshall et al. 2005). Like the red colobus, it is negatively affected by forest degradation, but to a lesser degree (Marshall et al. 2005; Rovero and Struhsaker 2007; Rovero et al. 2012).
This colobus species lives in one-male/multi-female groups (occasionally with 2 or 3 males) ranging in size from 2 to at least 14 individuals. Similarly to the pattern observed for the red colobus, group size decreases in degraded forests (Marshall et al. 2005). They are typically arboreal and spend large amounts of time resting, and feed predominantly on mature leaves.
-
Subfamily Cercopithecinae:
-
(3)
Sanje mangabey [Cercocebus (galeritus) sanjei]
This endemic and IUCN-endangered monkey was discovered in 1979 (Homewood and Rodgers 1981) and it is present only in two forests, Mwanihana and Udzungwa Scarp Forest Reserve (Fig. 1). Habituation of two groups in the Mwanihana forest suggests that Sanje mangabeys live in multi-male/multi-female groups of approximately 40–60 individuals, with up to five adult males. Group home range size is approximately 4–6 km2 and can encompass habitats including primary and secondary forest, which are all exploited over the year. Fruits form the greatest component of their diet, which also includes flowers, seeds, fungi, invertebrates, and small vertebrates. All strata of the forest are exploited; however, up to 70 % of time is spent for aging and traveling on the forest floor. Sanje mangabeys form polyspecific associations in Mwanihana with Sykes’ monkeys and the two colobines (Jones and Laizzer unpublished data).
-
(4)
Sykes’ monkey (Cercopithecus mitis spp. moloneyi/monoides)
This species occurs throughout the Udzungwa Mountains range. In Mwanihana forest, it prefers secondary, regenerating, and semideciduous forest zones (Rovero et al. 2006); however, it is also common in higher elevation forests (e.g., Marshall et al. 2005) and it does not seem to be clearly negatively influenced by habitat degradation as the two colobines (Rovero et al. 2012).
This monkey lives in uni-male/multi-female groups ranging in size from 2 to at least 22 individuals (Rovero et al. 2006). It is an opportunistic species that uses all vertical forest strata and feeds predominantly on fruits. Sykes’ monkeys are found in polyspecific association with Sanje mangabeys, Angolan colobus and Udzungwa red colobus.
Yellow baboons (Papio cynocephalus) also occur in Mwanihana but confined along the lower, miombo woodland, and thus not relevant for the study purpose. Vervet monkeys (Chlorocebus pygerythrus) are also sporadically present along forest edges.
Primate Census
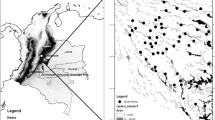
Primate abundance data were collected in Mwanihana forest between July and November 2011 through line transects. A grid of 2 km-long transects, spaced 1 km apart (along both longitude and latitude) and oriented North–South was randomly overlapped with a satellite forest cover map (Fig. 1a). A set of 43 transects deemed feasible was chosen based on accessibility and actual forest cover (i.e., excluding areas with rock cliffs or large forest gaps). The transect areas were presurveyed to assess feasibility of conducting censuses, and the majority of transects did not involve any vegetation cutting, with exception of lower elevation transects that crossed secondary, regenerating vegetation that could not be properly walked without minimum precutting.
The line transect method is very well established and routinely deployed for forest monkeys (review in Marshall et al. 2008; see also Buckland et al. 2010). Transects were walked by a team of one observer and two assistants at an average speed of about 1 km per h, starting in the early morning (between 07:00 and 07:30) and completed in 2–3 h, depending on vegetation density (average: 2 h:53 m; range: 1 h:02 m–4 h:33 m). For each primate sighting, the following data were noted: Observer’s position from the beginning of the transect (measured through a hip chain), species observed, position, and elevation were recorded by using a hand-held GPS unit (Garmin Map60 CSx). Solitary monkeys were also scored, but we included only social groups in the analysis. We did not analyze groups that were heard but not seen. An additional set of information was taken for other purposes than the present study, such as the number of individuals, if countable, the perpendicular distance from transect to the first animal seen, and the animal observer distance.
Vegetation Sampling Along Transects
To assess the relationship between primate abundance and arboreal vegetation structure and composition, after each census walk the field team conducted tree sampling on the way back to the starting point. Four, 25 by 25 m square plots centered on the line transect were established every 500 m along transects (at 250, 750, 1,250, and 1,750 m). All tree and woody climber species above 10 cm of diameter at breast height (DBH) within the plot were identified and measured by using a DBH measuring tape. In addition, we visually estimated the extent of canopy cover above each plot by using 5 classes: open canopy (0 %), semi-open (25 %), half-closed (50 %), semi-closed (75 %), and closed (100 %).
Data Analysis
We computed a primates’ encounter rate (ER) as the number of groups seen per km of transect walked, and considered it an index of relative abundance, in accordance with several other primate studies (e.g., Chapman et al. 2000; Mitani et al. 2000; Rovero et al. 2006; Linder and Oates 2011; Lwanga et al. 2011). To assess variation of ER with elevation, we used the elevation of each sighting (or mean elevation when multiple sightings were scored per transect) to attribute the ER from each transect to three elevation classes. By dividing in approximately equal portions the overall elevation range (433–1,850 m a.s.l.) of primate sightings, we identified three classes of elevation: lowland (400–700 m a.s.l), submontane (700–1,200), and montane (1,200–1,800). Due to the steep terrain of the forest, transects inevitably crossed a range of elevation (minimum and max elevation gradient within each transect: 15–545 m). Thus, we considered it more accurate to classify the ER from each transect by the elevation of sightings rather than by the average transect elevation. However, when no sightings were scored per transect, we used the average transect elevation as the mean elevation of the four vegetation plots. By using the Kruskal–Wallis test, we then tested for differences in ER across elevation zones. We also used Pearson’s correlation test to further assess the relationship between primates’ ERs and elevation on a continuous scale. Correlation tests were also used to assess relationship between vegetation variables and elevation. Data from vegetation plots were used to compute the following variables: DBH values from each tree were converted into basal area, and used to compute both mean basal area (MBA) and total basal area (TBA); species’ richness was computed as the total number of species per plot; and canopy cover was the index of canopy closure above the plot.
The influence of both elevation and vegetation variables on primates’ relative abundance was assessed using generalized linear modeling (GLM), with Poisson error distribution that is appropriate when the response variables are counts (Maindonald and Braun 2003). Because of data overdispersion, we used a quasi-Poisson error distribution (Zuur et al. 2009). We implemented GLMs in R software version 2.10 (http://www.r-project.org). Vegetation variables (MBA, TBA, canopy cover, and species’ richness) were used as the mean values per transect from the four vegetation plots. Variables were first checked for collinearity through a correlation matrix and using a threshold of Pearson’s r > 0.7. As a result, canopy cover was not presented to GLMs because of positive, significant correlation with TBA (r = 0.71, p < 0.001). We preferred TBA instead of canopy cover because the latter is an estimate and, as such, it is subject to observer bias. TBA and MBA were also significantly auto-correlated (r = 0.75, p < 0.001). However, we retained both variables because they provide complementary information; TBA reflects the overall amount of tree cover, while MBA reflects the average size of trees. Species’ richness and TBA were also auto-correlated (r = 0.59, p < 0.001) and both retained for regression analysis.
Results
Primate Density and Elevation Gradients
We walked a total of 80.05 km of transects from the 43 transects. Some transects could not be completed due to harsh terrain (range of transect length: 859–2,000 m). This sample size yielded 117 sightings of monkeys, which consisted of 43 Udzungwa red colobus monkeys, 38 Angolan colobus monkeys, 31 Sykes’ monkeys, and 5 Sanje mangabeys. Accordingly, mean ER from all transects were highest for Udzungwa red colobus (0.54 groups per km walked), followed by Angolan colobus (0.49), Sykes’ monkey (0.38), and Sanje mangabey (0.06; Fig. 3).
For all species, ER varied markedly across elevation zones (Fig. 4). Udzungwa red colobus’ ER decreased steadily and significantly from lowland to montane (Kruskal–Wallis test: χ2 = 12.67, p < 0.005), while Angolan colobus’ ER was similar in lowland and submontane zones but decreased in the montane zone, and the overall difference was significant (χ2 = 6.61, p < 0.05). Sykes’ monkey’s ER also decreased steadily and significantly (χ2 = 19.47, p < 0.001). Sanje mangabeys were not sighted in the lowland zone, and sightings were almost equally divided between the submontane and montane zones. However, sample size was too small for statistical analyses. As noted in other studies (e.g., Rovero et al. 2012), line transects are not an efficient method to census Sanje mangabeys, as they move predominantly on the ground and are very elusive.
Correlative analysis of relative abundance values from each transect against elevation of sightings confirms the trends presented above: the correlation was negative and significant for the three species, with highest coefficient for the Sykes’ monkey (r = −0.63, p < 0.001), followed by the Udzungwa red colobus, (r = –0.50 p < 0.001) and Angolan colobus (r = –0.35, p < 0.05).
Effect of vegetation parameters and elevation on primate abundance
Correlation tests between vegetation parameters (using mean values from the four plots per transect) and mean transect elevation show that positive and significant correlations emerged between: (1) elevation and TBA (r = 0.43, p < 0.005; Fig. 5a), (2) elevation and canopy cover (r = 0.46, p < 0.005), while the correlation was low and not significant between, (3) elevation and MBA (r = 0.25, p = 0.10; Fig. 5b), and (4) elevation and species richness (r = 0.21, p = 0.17). In particular, while TBA increases throughout the elevation gradient, MBA increases less steeply than TBA and appears to peak at elevation gradients of 1,000–1,500 m (Fig. 5).
Multivariate regression analysis on the effect of vegetation parameters and elevation on primate relative abundance resulted in elevation, TBA, and MBA being retained by the models for all three species tested (deviance explained by the model: Udzungwa red colobus 31.5 %, Angolan colobus 32.6 %, Sykes’ monkey 36.9 %; Table 1). Canopy cover was not used a priori (see methods) and species’ richness was manually excluded during model selection as it did not improve the fit in any model. As expected, elevation had a significant, negative effect on all three species. MBA had a positive effect on all species, which was relatively more marked for the colobines than the Sykes’ monkey. TBA, on the contrary, had a negative effect on all species, but it was significant only for the colobines and it appeared of less importance than the effect of MBA.
Discussion
Results of the current study clearly show that relative abundance of diurnal primates in Mwanihana forest varies sharply with elevation. ER decreases with increasing elevation, according to different patterns in the four investigated species. These results confirm the trend inferred in previous studies (Marshall et al. 2005; Rovero et al. 2006) by addressing, for the first time, an elevation gradient spanning about 1,500 m (433–1,850 m a.s.l.) and occurring within the same forest. Even though all target species in Udzungwa Mountains range well up to above 2,000 m a.s.l. in the western, Ndundulu-Luhomero forest (review in Rovero et al. 2009), this study indicates that highest abundances are recorded within the lowland, or lowland to submontane forest, for the two colobus monkey species and Syke’s monkey. The Sanje mangabey, in contrast, appears to prefer submontane and montane altitudinal zones, even though counts were too few to draw definitive conclusions.
In accordance to another study on density–elevation gradient relationship (Hanya et al. 2004), also Udzungwa primates show a preference for lowland altitude. Dietary preference of red colobus for young leaves (Waterman and Kool 1994; Usongo and Amubode 2001; Rovero 2003; Chapman et al. 2004; Struhsaker 2010) may explain its preference in lowland forest where trees are deciduous and semideciduous, and therefore availability of young leaves is greater (Chapman et al. 2002). Angolan colobus, on the contrary, is known to be able to better digest mature leaves (Bocian and Anderson 2013), which may explain their sustained abundance in the evergreen, submontane forest (e.g., Fimbel et al. 2001). Sykes’ monkey, instead, is an omnivore and opportunistic species, not influenced by habitat degradation, thus capable of using all vertical forest strata (Butysnki 1990). As already suggested for primates residing in the Udzungwas (Marshall et al. 2005), as well as from other areas (Durham 1975; Caldecott 1980; Fimbel et al. 2001; Chapman et al. 2002), knowledge on species’ diets, and information on food quality and quantity available at different elevations, are required to test potential hypotheses. Physiological effects of altitude may also directly impact species’ abundance and/or group sizes, such as through the increased nutritional demands of thermoregulation and locomotion in colder habitat (Caldecott 1980; Bryant et al. 1983; Dudt and Shure 1994).
Red colobus are dependent upon the richness of food plant species (Rovero and Struhsaker 2007) which may be related to overall tree species’ richness. However, species richness as such does not seem to be of importance, as richness slightly increases over the altitude range investigated, an opposite effect from the increasing red colobus’ abundance trend. Tree species diversity is likely to be more relevant but further studies are required. An approximation of forest structure (i.e., defined by the community of trees having DBH above 10 cm) appears to have a clear influence on abundance changes with elevation. In agreement with findings from focal vegetation studies (Lovett et al. 2006), the negative effect of TBA on primate abundance is consistent with the almost linear increase of TBA with elevation (the relation has however a weak explanatory power: R 2 = 0.15). Interestingly, this is slightly in contrast with previous studies that found positive influence of TBA on both Angolan colobus (Rovero and Struhsaker 2007; Rovero et al. 2012) and red colobus abundance (Rovero et al. 2012). However, previous studies focused only on low to mid elevation (300–1,000 m a.s.l.) and concluded that within that range, which includes optimal habitat, TBA is a proxy for closed-canopy cover, selected by typical arboreal primates as the colobines. The positive and significant influence of MBA on the abundance of colobines also fits well with the relationship between MBA and altitude; the relationship is not significant but shows a slight increase of MBA up to submontane forest, which is the higher end of the preferred altitudinal range for both species. A positive influence of MBA on red colobus was found at lower to mid elevation in Rovero and Struhsaker (2007), and can be explained by the species’ preference for old-growth, mature forest habitat. The different pattern found for the Sykes’ monkey is not surprising, given this opportunistic frugivorous-omnivore species’ preference for secondary, regenerating vegetation (Butynski 1990) that is characterized by a high presence of woody climbers (Lawes et al. 2013). This degraded type of habitat is almost exclusively present along the lower forest belt (Rovero et al. 2006, 2012). Therefore, the non-significant effect of TBA and MBA is easily explained.
Red colobus monkeys appear to prefer lowland forests, with species that range nearly exclusively at low elevation, including the western red colobus (Procolobus badius) (0–900 m a.s.l.; Butysnki et al. 2013a), Preuss’s red colobus (P. preussi) (50–1,079 m; Butynski and Kingdon 2013), and Pennant’s red colobus (P. pennantii) (0–800 m; Butynski et al. 2013b). The higher altitudinal range of the Udzungwa red colobus resembles that of Central African red colobus (P. rufomitratus tephrosceles) that ranges up to 2,420 m in SW Tanzania (Struhsaker and Grubb 2013). For the Angolan colobus, the highest altitudinal range of 2,200 m in Udzungwa (Rovero et al. 2009) is only slightly smaller than the species’ upper limit of 2,415 reported from Nyungwe, Rwanda (Bocian and Anderson 2013). On the contrary, the range reported for the C. mitis group of monkeys is much wider than in the Udzungwa Mountains, extending up to 3,800 m on Rwenzori Mountains, Uganda (Lawes et al. 2013).
Conclusions and Conservation Implications
This study is a first assessment of primate groups’ relative abundance across an altitudinal gradient throughout Mwanihana forest. The pattern observed is unambiguous. From an intensive demographic study previously conducted, no differences in group size, among the Udzungwa red colobus, was found with elevation in mixed semideciduous and evergreen forests (Struhsaker et al. 2004). Thus, the pattern for the Udzungwa red colobus should remain unchanged when group size (number of individuals per group) is included into the analysis. At high elevation, Marshall and colleagues (2005) found smaller group size in the western Ndundulu forest, but they interpreted the results as driven by human disturbance and vegetation degradation effects rather than elevation (Struhsaker et al. 2004). Detailed dietary and habitat quality assessments, in parallel with an assessment of energy and climate gradients, will be necessary to understand causation in the relationships found.
The findings have relevant implications in conservation management, especially given the threatened status of both endemic monkeys and the documented decline of primates reported for the least protected forests in the Udzungwas, including the southern Udzungwa Scarp Forest Reserve (Rovero et al. 2012). The importance of large forest blocks, with large portions of old-growth, mixed evergreen, and semideciduous forest has been highlighted before (Struhsaker et al. 2004). This study stresses that the presence of original forest cover from the lowland belt of the mountains is especially critical, particularly for the Udzungwa red colobus. The greatest forest loss in recent decades (1955–2000) in the Eastern Arc Mountains has occurred in the lowland zone (200–800 m, 55 %) and in the submontane zone (800–1,200 m, 41 %), relative to montane zone (1,200–1,800 m, 20 %: Hall et al. 2009). The Udzungwa Mountains hold some of the few forests (Mwanihana and Udzungwa Scarp Forest Reserve) in the Eastern Arc retaining forest cover from lowland to montane. Besides the greater size of these forests relative to most of the Eastern Arc forests, such persistence of lowland forest may be the single, most important reason for the exceptional importance of Udzungwa for primate diversity and conservation. The near absence of Sanje mangabeys at lower elevation may well be an effect of disturbance caused by human activities. The lowland forest has faced historically, and may currently be subject to the greatest human pressure through timber and pole cutting, as especially documented in the southern, less protected Udzungwa Scarp Forest Reserve (Rovero et al. 2012). Increased protection of these forests is of great importance.
References
Bocian, C. M., & Anderson, J. (2013). Colobus angolensis (Angolan colobus). In: T. M. Butynski, J. Kingdon & J. Kalina (Eds.), The mammals of Africa (Vol. 2). Primates. London: Bloomsbury Publishing.
Brown, J. H. (2001). Mammals on mountainsides: elevational patterns of diversity. Global Ecology and Biogeography, 10, 101–109.
Bryant, J. P., Chapin, F. S, I. I. I., & Klein, D. P. (1983). Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos, 40, 357–368.
Buckland, S. T., Plumptre, A. J., Thomas, L., & Rexstad, E. A. (2010). Design and analysis of line transect surveys for primates. International Journal of Primatology, 31, 833–847.
Burgess, N. D., Butynski, T. M., Cordeiro, N. J., Doggart, N., Fjeldsa, J., Howell, K. M., et al. (2007). The biological importance of the Eastern Arc mountains of Tanzania and Kenya. Biological Conservation, 134, 209–231.
Butynski, T. M. (1990). Comparative ecology of blue monkeys (Cercopithecus mitis) in highand low-density subpopulations. Ecological Monographs, 60, 1–26.
Butynski, T. M., & Kingdon, J. (2013). Procolobus preussi (Preuss’s red colobus). In: T. M. Butynski, J. Kingdon & J. Kalina (Eds.), The mammals of Africa (Vol. 2). Primates. London: Bloomsbury Publishing.
Butynski, T. M., Grubb, P., & Kingdon, J. (2013a). Procolobus badius (Western red colobus). In: T. M. Butynski, J. Kingdon & J. Kalina (Eds.), The Mammals of Africa, (Vol. 2). Primates. London: Bloomsbury Publishing.
Butynski, T. M., Grubb, P., & Kinhdon, J. (2013b). Procolobus pennantii (Pennant’s red colobus). In: T. M. Butynski, J. Kingdon & J. Kalina (Eds.), The mammals of Africa (Vol. 2). Primates. London: Bloomsbury Publishing.
Caldecott, J. O. (1980). Habitat quality and populations of two sympatric gibbons (Hylobatidae) on a mountain in Malaya. Folia Primatologica, 33, 291–309.
Chapman, C. A., Balcomb, S. R., Gillespie, T. R., Skorupa, J. P., & Struhsaker, T. T. (2000). Long-term effects of logging on African primate communities: a 28 year comparison from Kibale National Park, Uganda. Conservation Biology, 14, 207–217.
Chapman, C. A., Chapman, L. J., Bjorndal, K. A., & Onderdonk, D. A. (2002). Application of protein-to-fiber ratios to predict colobine abundance on different spatial scales. International Journal of Primatology, 23, 283–310.
Chapman, C. A., Chapman, L. J., Naughton-Trevers, L., Lawes, M. J., & Mcdowell, L. R. (2004). Predicting folivorous primate abundance: validation of a nutritional model. American Journal of Primatology, 62, 55–69.
Colwell, R. K., & Hurtt, G. C. (1994). Non-biological gradients in species richness and a spurious rapoport effect. The American Naturalist, 144, 570–595.
Darwin, C. (1839). Journal of the researches into the geology and natural history of various countries visited by the H.M.S. Beagle, under the command of captain fitzroy, R.N. from 1832 to 1836. London: Henry Colburn.
Dinesen, L., Lehmberg, T., Rahner, M. C., & Fjeldsa, J. (2001). Conservation priorities for the forests of the Udzungwa Mountains, Tanzania, based on primates, duikers and birds. Biological Conservation, 99, 223–236.
Dudt, J. F., & Shure, D. J. (1994). The influence of light and nutrients on foliar phenolics and insect herbivory. Ecology, 75, 86–98.
Durham, N. M. (1975). Some ecological, distributional, and group behavioral features of Atelinae in southern Peru: with commetns on interspecific relations. In R. H. Tuttle (Ed.), Socioecology and psychology of primates (pp. 87–101). The Hague: Mouton Publishers.
Fimbel, C., Vedder, A., Dierenfeld, E., & Mulindahabi, F. (2001). An ecological basis for large group size in Colobus angolensis in the Nyungwe Forest, Rwanda. African Journal of Ecology, 39, 83–92.
Givnish, T. J. (1999). On the causes of gradients in tropical tree diversity. Journal of Ecology, 87, 193–210.
Hall, J., Burgess, N. D., Lovett, J., Mbilinyi, B., & Gereau, R. E. (2009). Conservation implications of deforestation across an elevational gradient in the Eastern Arc Mountains, Tanzania. Biological Conservation, 142, 2510–2521.
Hanya, G., Yoshihiro, S., Zamma, K., Matsubara, H., Ohtake, M., Kubo, R., et al. (2004). Environmental determinants of the altitudinal variations in relative group densities of Japanese macaques on Yakushima. Ecological Research, 19, 485–493.
Heaney, L. R. (2001). Small mammal diversity along elevational gradients in the Philippines: an assessment of patterns and hypotheses. Global Ecology and Biogeography, 10, 15–39.
Homewood, K. M., & Rodgers, W. A. (1981). A previously undescribed mangabey from southern Tanzania. International Journal of Primatology, 2, 47–55.
von Humboldt, A. (1849). Aspects of nature in different lands and different climates, with scientific elucidations. Translated by M. Sabine. London: Longman, Brown, Green, and Longman.
Kessler, M. (2001). Patterns of diversity and range size of selected plant groups along an elevational transect in the Bolivian Andes. Biodiversity and Conservation, 10, 1897–1921.
Lawes, M. J., Cords, M., & Lehn, C. (2013). Cercopithecus mitis (Blue Monkey, Sykes’ Monkey). In: T. M. Butynski, J. Kingdon & J. Kalina (Eds.), The mammals of Africa (Vol. 2). Primates. London: Bloomsbury Publishing.
Linder, J. M., & Oates, J. F. (2011). Differential impact of bushmeat hunting on monkey species and implications for primate conservation in Korup National Park, Cameroon. Biological Conservation, 144, 738–745.
Lomolino, M. V. (2001). Elevation gradients of species-density: historical and prospective views. Global Ecology and Biogeography, 10, 3–13.
Lovett, J. C. (1993). Eastern Arc moist forest flora. In J. C. Lovett & S. K. Wasser (Eds.), Biogeography and ecology of the rain forests of eastern Africa (pp. 33–55). New York: Cambridge University Press.
Lovett, J. C., Marshall, A. R., & Carr, J. (2006). Changes in tropical forest vegetation along an altitudinal gradient in the Udzungwa Mountains National Park, Tanzania. African Journal of Ecology, 44, 478–490.
Lwanga, J. S., Struhsaker, T. T., Struhsaker, P. J., Butynski, T. M., & Mitani, J. C. (2011). Primate population dynamics over 32.9 years at Ngogo, Kibale National Park. Uganda. American Journal of Primatology, 73, 997–1011.
MacArthur, R. M. (1972). Geographical ecology. New York: Harper and Row.
Maindonald, J., & Braun, J. (2003). Data analysis and graphics using R: an example-based approach. New York: Cambridge University Press.
Marshall, A. R., Topp-Jørgensen, J. E., Brink, H., & Fanning, E. (2005). Monkey abundance and social structure in two high elevation forests in the Udzungwa Mountains of Tanzania. International Journal of Primatology, 26, 127–145.
Marshall, A. R., Lovett, J. C., & White, P. C. L. (2008). Selection of line-transect methods for estimating the density of group-living animals: lessons from the primates. American Journal of Primatology, 70, 452–462.
Marshall, A. R., Jørgensbye, H. I. O., Rovero, F., Platts, P. J., White, P. C. L., & Lovett, J. C. (2010). The species–area relationship and confounding variables in a threatened monkey community. American Journal of Primatology, 72, 325–336.
Mitani, J. C., Struhsaker, T. T., & Lwanga, J. S. (2000). Primate community dynamics in old growth forest over 23.5 years at Ngogo, Kibale National Park, Uganda: implications for conservation and census methods. International Journal of Primatology, 21, 269–286.
Pucci, A., & Rovero, F. (2004). Observations on the food habits of the endemic Udzungwa red colobus (Piliocolobus gordonorum) from different forest sites in the Udzungwa Mountains, Tanzania. Folia Primatologica, 75, 406 (abstract).
Rahbek, C. (1995). The elevational gradient of species richness: a uniform pattern? Ecography, 18, 200–205.
Rahbek, C. (1997). The relationship among area, elevation, and regional species richness in Neotropical birds. The American Naturalist, 149, 875–902.
Rahbek, C. (2005). The role of spatial scale and the perception of large-scale species-richness patterns. Ecology Letters, 8, 224–239.
Rovero, F. (2003). Conservation Biology of the Udzungwa Red Colobus and the Sanje Mangabey in the Udzungwa Mountains, Tanzania: the Effect of Habitat Quality and Human Activities on Population Density and emography. Unpublished report to The Whitley Laing Foundation for International Nature Conservation/Rufford Small Grants.
Rovero, F., & Marshall, A. R. (2005). Diversity and abundance of diurnal primates and forest antelopes in relation to habitat quality: a case study from the Udzungwa Mountains of Tanzania. In B.A. Huber, B.J. Sinclair & K-H. Lampe (Eds.), African biodiversity: molecules, organisms, ecosystems. Springer, Berlin.
Rovero, F., & Struhsaker, T. T. (2007). Vegetative predictors of primate abundance: utility and limitations of a fine-scale analysis. American Journal of Primatology, 69, 1242–1257.
Rovero, F., Struhsaker, T. T., Marshall, A. R., Rynne, T. A., Pedersen, U. B., Ehardt, C. L., et al. (2006). Abundance of diurnal primates in Mwanihana Forest, Udzungwa mountains, Tanzania: a multi-observer comparison of line-transect data. International Journal of Primatology, 27, 675–697.
Rovero, F., Marshall, A. R., Jones, T., & Perkin, A. (2009). The primates of the Udzungwa Mountains: diversity, ecology and conservation. Journal of Anthropological Sciences, 87, 93–126.
Rovero, F., Mtui, A. S., Kitegile, A. S., & Nielsen, M. R. (2012). Hunting or habitat degradation? Decline of primate populations in Udzungwa mountains, Tanzania: an analysis of threats. Biological Conservation, 146, 89–96.
Stevens, G. C. (1989). The latitudinal gradient in geographical range: how so many species coexist in the tropics. The American Naturalist, 133, 240–256.
Struhsaker, T. T. (2010). The red colobus monkeys: variation in demography, behavior, and ecology of endangered species. Oxford: Oxford University Press.
Struhsaker T. T., & Grubb P. (2013). Procolobus rufomitratus (Eastern red colobus). In: T. M. Butynski, J. Kingdon & J. Kalina (Eds.), The mammals of Africa (Vol. 2). Primates. London: Bloomsbury Publishing.
Struhsaker, T. T., Marshall, A. R., Detwiler, K. M., Siex, K., Ehardt, C. L., Lisbjerg, D. D., et al. (2004). Demographic variation among the Udzungwa Red colobus (Procolobus gordonorum) in relation to gross ecological and sociological Parameters. International Journal of Primatology, 25, 615–658.
Usongo, L., & Amubode, F. O. (2001). Nutritional ecology of Preuss’s red colobus monkey (Colobus badius preussi Rahm 1970) in Korup National Park, Cameroon. African Journal of Ecology, 39, 121–125.
Waterman, P. G., & Kool, K. M. (1994). Colobine food selection and plant chemistry. In A. G. Davies & J. F. Oates (Eds.), Colobine monkeys, their ecology, behaviour and evolution. Cambridge: Cambridge University Press.
Zuur, A. F., Ieno, E. N., Walker, N., Saveliev, A. A., & Smith, G. M. (2009). Mixed effects models and extensions in ecology with R (First edition ed.). Berlin: Springer.
Acknowledgments
We are very grateful to the Tanzania Commission for Science and Technology (COSTECH), Tanzania Wildlife Research Institute (TAWIRI), and Tanzania National Parks (TANAPA) for granting us permissions to conduct the study (Costech Permit No. 2011-85-NA-2011-33 and 2011-84-NA-2011-33). We thank the Tanzanian field assistants, the warden, and staff of Udzungwa Mountains National Park for their valuable assistance throughout the study. We would also like to thank K. Hodges, T. Struhsaker, and two anonymous reviewers for comments on the manuscript. Financial support was from the Provincia Autonoma di Trento and the EU (Marie Curie Actions COFUND) post-doctoral grant to C. Barelli, MUSE—Science Museum, and the German Primate Center (DPZ).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Barelli, C., Gallardo Palacios, J.F., Rovero, F. (2014). Variation in Primate Abundance Along an Elevational Gradient in the Udzungwa Mountains of Tanzania. In: Grow, N., Gursky-Doyen, S., Krzton, A. (eds) High Altitude Primates. Developments in Primatology: Progress and Prospects. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-8175-1_12
Download citation
DOI: https://doi.org/10.1007/978-1-4614-8175-1_12
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-8174-4
Online ISBN: 978-1-4614-8175-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)