Abstract
The purpose of this chapter is to provide a basic introduction to biofeedback and to discuss how it relates to the treatment of excessive stress. Although being applied for more than 40 years, biofeedback may still be considered by some to be “high-technology” therapy that may be used to (1) engender a relaxation response, thus treating the stress response itself, or (2) alter target-organ activity, thus treating the symptoms of excessive stress arousal. Indeed, it can help to do this and possibly more by restoring and even enhancing balance and control to the systems involved.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Attention Deficit Hyperactivity Disorder
- Heart Rate Variability
- Chronic Fatigue Syndrome
- Galvanic Skin Response
- Pelvic Floor Muscle Training
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
The purpose of this chapter is to provide a basic introduction to biofeedback and to discuss how it relates to the treatment of excessive stress. Although being applied for more than 40 years, biofeedback may still be considered by some to be “high-technology” therapy that may be used to (1) engender a relaxation response, thus treating the stress response itself, or (2) alter target-organ activity, thus treating the symptoms of excessive stress arousal. Indeed, it can help to do this and possibly more by restoring and even enhancing balance and control to the systems involved.
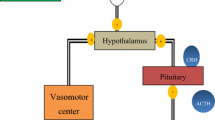
Bios from the Greek for “life” and “feedback” defines the concept of providing focused information that enables performance enhancement. Biofeedback may, therefore, be conceptualized as a procedure in which data regarding an individual’s biological activity are collected, processed, and conveyed back to the person so that he or she can modify that activity. In essence, biofeedback allows for the construction of a “feedback loop,” which is illustrated in Fig. 14.1. Such feedback loops exist in almost all functions of the human body, from the rate-modifying feedback loops concerned with the most elementary biochemical reactions to the most complex human endeavors. Information regarding the result of any event is necessary at some level if it is to be modified.
Thus, the concept underlying biofeedback is fundamental in biology, and is widely employed in the therapeutic sciences. In the traditional medical model, the patient presents a physiological problem, data regarding his or her physiological functioning are collected by the clinician, then the clinician diagnoses the condition, and institutes appropriate interventions. The patient in this model has a basically passive role. This interaction, as shown in Fig. 14.2, represents an indirect closed loop of information, starting and ending with the patient and including information-gathering devices, the clinician, and therapeutic devices.
As can be seen in a comparison of Figs. 14.1 and 14.2, the principle on which biofeedback is based involves the active participation of the individual in the modification of his or her condition. Consider the case of a function such as breathing, which is unique in the sense that we can control it voluntarily, but fortunately, occurs without conscious awareness. It is as if there are priorities for the human brain, with many functions occurring at subcortical levels—especially those that must be maintained in an ongoing fashion, such as heartbeat and biochemical reactions. Although this may be the most efficient way to function, it keeps the organism from being able to monitor many of its autonomic functions consciously and thus actively change them. This is what biofeedback provides for the individual—the potential to exert additional control over autonomic biological activity.
Given the information provided with biofeedback, we have repeatedly found that we can learn to alter bodily functions that were once thought to be inaccessible, including greater finite control over the activities of both the voluntary and the autonomic nervous systems. As will be discussed later in this chapter, the possible alterations that can be controlled range from voluntary muscle tension to more autonomic functions, such as blood flow and brain waves.
The purpose of this chapter is to expand the principles on which biofeedback is based and to describe how it may be beneficial in the treatment of the stress response. We also review some of the historical trends that have led to the present state of the art and then discuss some of the more traditional biofeedback modalities, as well as the more current trends in the field. Finally, we examine the role of the therapist in the biofeedback paradigm.
History
The term biofeedback was reportedly coined at the first annual meeting of the Biofeedback Research Society in 1969, as a shortened version of “biological feedback.” Although the term itself may have been new, its foundations are not.
The historical development of biofeedback can be traced back to the early 1900s, and the work of Pavlov and Watson on the one hand and Thorndike on the other. Pavlov and Watson’s research on classical conditioning of the autonomic nervous system (ANS) was thought to be discretely separate from the work of Thorndike on operant conditioning of the musculoskeletal system. Early researchers were convinced that conditioning that affected the ANS had to be accomplished through a classical conditioning paradigm (an S(stimulus)R(response) model involving conditioning on the basis of association rather than as a function of behavioral consequence as in an operant model). In fact, Kimble (1961), in his edited textbook Conditioning and Learning, stated unequivocally that autonomically mediated behavior could only be modified by classical, not operant conditioning.
However, in a discipline like psychology, assertions such as Kimble’s often serve as challenges for others. For example, according to a review by Gatchel and Price (1979), case reports existed of individuals who reportedly could voluntarily alter autonomic functioning (see Lindsley & Sassaman, 1938; Luria, 1958; McClure, 1959; Ogden & Shock, 1939). Interestingly, Edmund Jacobson, the originator of progressive relaxation training (see Chap. 12), performed some of the earliest clinical biofeedback work in the 1930s using an oscilloscope to measure forearm tension in progressive relaxation trainees. However, since he used a raw electromyograph (EMG) signal, most people had difficulty understanding how to interpret the information, and Jacobson apparently discarded the method (Schneider, 1989). Following Kimble’s published work, other researchers reported the use of operant conditioning of heart rate to avoid mild electrical shocks (Frazier, 1966; Shearn, 1962). Supporters of Kimble’s may have argued, however, that these types of studies, in which changes in autonomically mediated responses (such as heart rate) were modified by responses under voluntary control (such as altered breathing), were actually consistent with a classical conditioning paradigm (Blanchard & Epstein, 1978).
Therefore, the animal studies of Miller (1969) and DiCara and Miller (1968a, 1968b, 1968c) in which laboratory rats were given injections of curare, a drug that produces complete muscle paralysis, provided additional and clearer support for the effect of operant conditioning of autonomic responses. These rats were kept alive via artificial respiration, and stimulation of an electrode implanted in the pleasure center of the hypothalamus served as a reinforcer. Using this research design, DiCara and Miller (1968a, 1968b, 1968c) successfully demonstrated operant conditioning of heart rate, blood pressure, and urine formation. Although later attempts to replicate these findings were not supported (Miller & Dworkin, 1974), this type of basic research, along with the pioneering work of other researchers such as Green, who built EMG, temperature, GSR, and EEG biofeedback equipment to assess self-control of the ANS (see Green & Green, 1977), helped to define and legitimize the field of biofeedback. In fact, in a review, Schwartz (1995) noted that “some professionals view biofeedback as essentially instrumental [italics added] conditioning of visceral responses” (p. 5).
Basmajian (1963), another originator in the field, reported on the ability of patients to control single motor-unit activity. In the late 1960s, Budzynski and Stoyva began utilizing behavioral therapy techniques to enhance efficacy of biofeedback protocols for general relaxation and for the treatment of tension headaches [see Stoyva and Budzynski (1974), for an early review]. The early work of Kamiya (1969) and Brown (1977) in EEG biofeedback gained widespread attention for applications in relaxation and alteration of consciousness, although it was (Sterman, 1973; Sterman & Friar, 1972) work in clinical applications in the treatment of epilepsy that appeared to have the most clinical utility.
In recent years, biofeedback has shown potential applicability to a variety of clinical problems, including decubitus ulcers (pressure sores) (Verbunt & Bartneck, 2010), PTSD (Staples, Abdel Atti, & Gordon, 2011; Tan, Dao, Farmer, Sutherland, & Gevirtz, 2011; Wood, Wiederhold, & Spira, 2010), chronic pain disorders (Angoules et al., 2008; Caro & Winter, 2011; Hallman, Olsson, von Schéele, Melin, & Lyskov, 2011; Palermo, Eccleston, Lewandowski, Williams, & Morley; 2010), GI disorders (Chiarioni & Whitehead, 2008), epilepsy (Nagai, Goldstein, Fenwick, & Trimble, 2004; Sterman & Egner, 2006), stroke (Doğan-Aslan, Nakipoğlu-Yüzer, Doğan, Karabay, & Özgirgin, 2010; Drużbicki, Kwolek, Depa, & Przysada, 2010;Varoqui, Froger, Pélissier, &Bardy, 2011), attention-deficit disorders (Arns, de Ridder, Strehl, Breteler, &Coenen, 2009; Gevensleben, Moll, & Heinrich, 2010; Monastra, 2005), urinary incontinence (Imamura et al., 2010; Palmer, 2010), migraine headaches (McGrath, 1999; Reid & McGrath, 1996), and rehabilitation (Miller & Chang, 1999; Richards & Pohl, 1999).
As noted, biofeedback is not a new endeavor; the technology for its use has been available for decades. What is reasonably new, however, is the speed, precision, and range of applications available from today’s computers in acquiring, storing, analyzing, and displaying data in virtual environments (Wong, 2008). The use of virtual reality, including the advances in dynamic three-dimensional (3D) technology, has been implemented with moderate success across a range of clinical applications, including generalize anxiety, balance in elderly patients who experienced traumatic brain injury (TBI), and PTSD (Bisson, Contant, Sveistrup, Lajoie, 2007; Gorini et al., 2010; Pallavicini, Algeri, Repetto, Gorini, & Riva, 2009; Repetto et al., 2009; Wiederhold & Rizzo, 2005; Wood et al., 2010). In addition to the potential uses of virtual reality environments to enhance therapeutic effects, collaborative and integrative approaches with primary care physicians may be a potentially burgeoning area where the implementation of biofeedback may address some of the aforementioned disorders and positively impact patient care (Gevirtz, 2006;Glick & Greco, 2010;Isler, 2006; Lynch, McGrady, Nagel, & Wahl, 2007; Ryan &Gevirtz, 2004).
Biofeedback Modalities
In this section, we briefly review several types of biofeedback, focusing on their nature and potential utility.
Electromyographic (EMG) Biofeedback
Description
The EMG instrument used in biofeedback detects minute electrical impulses through special sensors (electrodes), which are applied to the skin with electrode jelly used as a conducting medium. The strength of the electrical impulse is amplified, processed by the instrument and then fed back in ways that allow the data to be easily interpreted. This feedback incorporates a potential myriad of possible creative signals; numerical data, displays of lights, deflections of a meter, sounds, etc. that correlate with the magnitude of the signal, or any combination of these. Since measurement is of electrical correlates of muscle contraction, the numerical display of data in EMG biofeedback is expressed in volts, or more specifically, in microvolts, one-millionth of a volt. The displayed data serve as information to be processed by the client in order to modify function—in this case, muscle tension. The key here is that the data displayed are many times more sensitive than what a person can feel so that the instrument actually extends the person’s awareness of minute changes that would otherwise be imperceptible.
The words stress and tension are often used interchangeably, and muscle tension itself is an obvious component of the fight-or-flight response. When a threat is perceived, any muscle throughout the body may tense; however, some do so in a characteristic way. For example, the muscles in the back of the neck characteristically become tense, as if in an effort to keep the head erect to aid in vigilance. Back, shoulder, and jaw muscles tense when the individual perceives him- or herself as being threatened, or when he or she is under stress. Other muscle tension is less obvious such as changes in intestinal motility or alterations in blood vessel diameter.
Because we are describing striated muscle, it would seem that control would be voluntary, and therefore easily responsive to learning. The difficulty arises when the contraction increases so slowly and imperceptibly that the individual is not aware of increased muscle tension until the muscles are already in spasm. The EMG apparatus allows the individual to become aware of small increments of change in muscle tension, thus allowing him or her to learn to relax the muscles involved. As noted by Basmajian (1967), the EMG signal indicates the status of the muscle as well as the status of the nervous system serving the muscle.
One can place the EMG sensors over virtually any striated muscle available to either skin or needle electrodes. Frontalis muscle biofeedback has traditionally been used for low-arousal training (Field, 2009; Stoyva & Budzynski, 1993). However, the effectiveness of frontalis placement for generalized relaxation has been questioned (Graham et al., 1986; Jones & Evans, 1981), and other evidence suggests that multiple- and reactive-site EMG biofeedback may be as effective as frontalis biofeedback in reducing sympathetic arousal (Mariela, Matt, &Burish, 1992). One of the other common EMG placements is the cervical paraspinal muscles (Donaldson, Donaldson, & Snelling, 2003).
Indications
For the purposes of this chapter, EMG biofeedback is used to treat the stress response in primarily two ways: first, it allows the patient to learn to relax a particular set of muscles (e.g., the masseter muscles in bruxism—teeth grinding), and second, it may be used to produce a more generalized state of relaxation and decreased arousal (e.g., frontalis or paraspinal muscle EMG biofeedback), thus affecting the stress response more centrally (see Donaldson et al., 2003; Mariela et al., 1992; Stoyva & Budzynski, 1993; see also Chap. 9).
Historically, two of the most commonly encountered, specific muscle contraction problems have been muscle tension-type headaches and bruxism. In a meta-analysis of 53 studies, results revealed that EMG biofeedback in combination with relaxation training yielded a large mean effect size (0.32) and was the most effective biofeedback modality in treating tension-type headaches (Nestoriuc, Rief, & Martin, 2008). In a subsequent comprehensive efficacy review, which incorporated the above meta-analysis along with a 2007 meta-analysis (Nestoriuc & Martin, 2007), Nestoriuc, Martin, Rief, and Andrasik (2008) reported a large effect size for EMG biofeedback that corresponded with a 69 % success rate for biofeedback compared with a 31 % improved rate in an untreated control group. In one of the studies used in these analyses, Rokicki and colleagues (1997) suggested that increase in self-efficacy as a result of EMG biofeedback training might be what accounts for the treatment’s effectiveness.
Biondi and Picardi (1998) and Foster (2004) have reported on the benefits of biofeedback in the treatment of bruxism. In a meta-analysis, Crider and Glaros (1999) reported on the practical application of biofeedback to tempromandibular disorders (TMDs), disorders of the jaw muscles often related to bruxism. Jadidi, Castrillon, and Svensson (2008) have reported that “biofeedback with electrical pulses does not cause major disruption in sleep and is associated with pronounced reduction in temporalis EMG activity during sleep” (p. 181).
Sometimes the problem is loss of control or weakening of muscle function and EMG feedback can be used to strengthen muscle function. For instance, within the past two decades, the use of EMG biofeedback for the treatment of urinary incontinence has expanded. Using surface abdominal EMG, pelvic floor EMG, and rectal pressure, patients with urinary incontinence have been shown to learn successfully how to strengthen pelvic floor muscles and inhibit abdominal muscle contractions (Butler et al., 1999; McDowell et al., 1999; Weatherall, 1999). In a sample of 390 patients with stress or mixed urinary incontinence that was treated with EMG-biofeedback assisted pelvic floor muscle training (PFMT), Dannacker, Wolf, Raab, Hepp, and Anthuber (2005) reported an improvement in EMG potentials and self-report of incontinence symptoms, both short- and long term (avg. 2.8 years). In 2011, Huebner and colleagues reported on the benefits of PFMT using three different strategies of EMG biofeedback (PFMT with conventional and dynamic electrical stimulation, and no electrical stimulation). In a systematic review of the clinical effectiveness of non-surgical treatments for women with stress urinary incontinence, including survey, meta-analysis, and economic modeling (used to discern which combinations of treatments were most cost-effective) Imamura and colleagues (2010) reported that delivering PFMT more intensely, either through more sessions or augmented with biofeedback, appeared to be the most effective treatment. EMG biofeedback has also been shown to effectively treat urinary incontinence (e.g., secondary to dysfunctional voiding or giggling) in children (Palmer, 2010; Richardson & Palmer, 2009).
As noted in Chap. 5, and alluded to in this chapter, the frontalis muscles appear to have value in the treatment of the human stress response because of their potential ability to serve as an indicator of generalized arousal, for example, SNS arousal (Rubin, 1977), by virtue of what appears to be their dual neurological constituency, that is, alpha motor neuron innervation and sympathetic neural innervation (see Everly & Sobelman, 1987). When using the frontalis muscles as a means of engendering the relaxation response, it is important to keep in mind that it may first be necessary to have the patient learn to relax the frontalis specifically before expecting any generalizability to the ANS. That is to say, the frontalis muscles may serve as indicators of sympathetic activity only when they are in a relaxed state. Donaldson, Donaldson & Snelling (2003) and Hermens, Freriks, Disselhorst-Klug, & Rau (2000) note other issues related to skin preparation and instrument specifications in the use of EMG biofeedback. The EMG biofeedback paradigm has demonstrated its scientific integrity and clinical utility in the hands of competent, well-trained professionals (Schwartz & Montgomery, 2003) and should still be considered in the treatment of the human stress response (McKee, 2008; Sharpley& Rogers, 1984).
Temperature Biofeedback
Description
The use of temperature biofeedback is based on the fact that peripheral skin temperature is a function of vasodilatation and constriction. Thus, when the peripheral blood vessels are dilated and more blood flows through them, the skin is warmer. By measuring the temperature in the extremities, it is possible to get an indication of the amount of blood vessel constriction. Also, since constriction and dilation are controlled by the sympathetic portion of the ANS, one can get an indirect measurement of the amount of sympathetic activity (Peek, 2003).
The equipment used in thermal biofeedback has the same basic function as the EMG biofeedback equipment described earlier—that is, a sensor, a processor, and a display. The sensor is a thermistor, a small thermal sensor, or thermometer sensing device that is usually attached to the dorsal side of the finger. The thermistor and associated transducer transform the electrical signal, and amplify and processes it so that it can be presented as feedback though lights, sounds, or a change in meter reading show small increments of rising or lowering temperature in varying time intervals. Because skin temperature can be raised only to the theoretical high of core body temperature, 98.6 °F, there are practical limits to how much change can occur. Therefore, rather than set a specific temperature goal, more often the change in temperature is measured from the original or baseline reading and is used as a “change score from baseline” to gauge success of thermal biofeedback. For example, a patient with a baseline skin temperature of 75 °F will have a greater possible warming change than one with a baseline temperature of 94 °F. Response time, which indicates how rapidly a change in skin temperature occurs, absolute accuracy, or how closely the displayed temperature corresponds to the actual or “true” temperature, and resolution, which refers to the smallest temperature change that the thermistor may pick up and display, are other parameters of temperature biofeedback devices (Peek, 2003).
Indications
Temperature feedback has been useful for treating individuals with functional vascular disease or circulatory problems, such as Raynaud’s disease (see Karavidas, Tsai, Yucha, McGrady, & Lehrer, 2006 for a comprehensive review), or advanced heart failure (Moser, Dracup, Woo, & Stevenson, 1997). It has also been used for patients with endocrine diseases, particularly diabetes (McGinnis, McGrady, Cox, & Grower-Dowling, 2005; McGrady, Graham, & Bailey, 1996; Rice, 2007), and in the treatment of migraine headaches in adults and children (Allen & Shriver, 1997; Biondi, 2005; Herman, Blanchard, & Flor, 1997; Holroyd & Penzien, 1994; Landy, 2004; Scharff et al. 2002), hypertension (Blanchard et al., 1996; Linden & Moseley, 2006), and in those instances when control over sympathetic activity is sought (e.g., asthma; Meany et al., 1988). Temperature biofeedback was also used as part of a comprehensive group treatment program for PTSD in 129 children in Gaza (Staples et al., 2011). Thermal biofeedback has also been used in psychotherapy to help determine areas of prominent sympathetic arousal and to address the issue of treatment resistance. As noted above, one minor difficulty involved in the interface between physiology and technology is the short but relevant delay of several seconds between the time of sympathetic discharge, vasoconstriction, and lowering of temperature in the extremity. This measurable reduction in temperature may be displayed several seconds after the event that caused the sympathetic discharge has passed. As a result, the measurements are often tracked and graphed over time to better display the type and rate of temperature change.
Temperature biofeedback has a clear role in the treatment of the stress response in that it is a good indicator of general SNS arousal. Therefore, it is a useful teaching tool for general relaxation, because individuals are instructed to try to raise their skin temperature. This mode of therapy may be used alone, alternatively with EMG, or in combination with it. See Lehrer and colleagues (1994) and Peek (2003) for a practical review of thermal biofeedback.
Electroencephalographic (EEG) Biofeedback
Description
The brain’s electrical activity is continuous, most likely the result of discharges at synapses. In 1924, Hans Berger developed a graphic method for recording that electrical brain-wave activity. What appears to be recorded by the EEG are those synapses closest to the surface of the brain. There are many ascending pathways to the cortex; however, it is believed that the most highly represented area on the outermost surface of the cortex is the reticular activating system (Hall, 2011). These data are, however, difficult to analyze, because a single neuron may have as many as a thousand branchings in the cortex. Therefore, although data attained on the EEG are fairly nonspecific, it is generally agreed that various wave patterns do correlate with various states of consciousness and reflect activity, particularly in the reticular activating system.
Brain waves have been divided into four categories, depending on their predominant frequency and amplitude. The term frequency refers to the cycles/s, or per minute, and reflects the number of firings of neurons per unit of time. Brain-wave frequency on the surface of the scalp ranges from 1 every few seconds to 50 or more per second (Hall, 2011). The amplitude refers to the amount of electricity generated and reflects the number of neurons firing synchronously.
Brain waves are classified as alpha, beta, theta, and delta waves. Alpha waves are characterized by a frequency of 8–13 cycles/s and an amplitude of 20–100 + μv. These rhythmic waves are related to an awake, relaxed state characterized by calmness and passive attention. Alpha waves do not occur when participants are asleep, or when they have their attention focused (Hall, 2011). Beta waves occur at a frequency of 14 or more cycles per second to as high as 80 cycles/s and have low amplitude. They are characteristic of an awake, attentive state when the subject is focusing his or her thoughts, or is aroused or have tense. Theta waves occur at a frequency of 4–7 cycles/s, with a usual amplitude of 20 μv or less. They are often considered part of the daydreaming state. Last, delta wave frequencies are from 0.5 to less than 4 cycles/s and are associated with deep sleep. Thus, when one is resting, dominant EEG activity is in the alpha and theta ranges; however, excitement shifts brain-wave activity toward the beta range. It is also of note that as we grow older, the relative proportion of beta-wave activity increases, whereas theta-wave activity decreases (Lubar, 1991).
Indications
Predicated on the work of Sterman and colleagues (Sterman, 1973; Sterman & Friar, 1972) involving sensorimotor rhythm (SMR) training, which is thought to capture activity of the sensorimotor cortex, one of the first areas investigated for use of EEG biofeedback training occurred in an attempt to manage epileptic seizures (Seifert & Lubar, 1975). This research expanded to include treatment of hyperkinetic children, in which EEG biofeedback was used to increase SMR production and inhibit theta-wave production (Lubar & Shouse, 1976; Shouse & Lubar, 1978). Lubar and colleagues (see Lubar, 1991; Lubar & Deering, 1981) later added enhanced beta-wave production via EEG biofeedback in the treatment of Attention Deficit Hyperactivity Disorder (ADHD). The premise behind the use of EEG biofeedback, which is also referred to as neurofeedback, neurotherapy, and alpha-theta feedback, is that ADHD is thought to be associated with neurological dysfunction at the cortical level, involving primarily the prefrontal lobes and central theta activity, which is marked by underarousal and decreased cortical activity (Chabot & Serfontein, 1996; El-Sayed, Larsson, Persson, & Rydelius, 2002; Lubar, 1995). The use of EEG neurofeedback is thought to normalize cortical function, which leads to normalization of behavior and overall academic and social adjustment (Lubar, 1995). More recently, the development of Quantitative EEG (QEEG) techniques has helped to more accurately assess EEG activity. QEEG is a computerized statistical procedure in which electrophysiological activity is rapidly and precisely measured and then converted using digital technology into forms that allow for more exact pattern recognition of amplitude, frequency, spectral plots, topographic maps, or functional connectivity maps (Kaiser, 2006). The QEEG information is in essence a helpful diagnostic adjunct that helps to facilitate more targeted sensor placement when using neurofeedback treatment techniques.
The clinical application of neurofeedback for the treatment of ADHD has flourished in the past decade (Holtmann & Stadler, 2006; Loo & Barkley, 2005); however, research support remains somewhat equivocal, due in part to the “scientific provincialism that is evident when new treatment paradigms are introduced” (Monastra, 2003, p. 438). To help address this issue, Arns and colleagues (2009) conducted a meta-analysis, which included randomized controlled trials, and concluded that neurofeedback for the treatment of ADHD is efficacious, and noted a high effect size for inattention and impulsivity and a medium effect size for hyperactivity.
QEEG-guided neurofeedback has been reported to be useful in the treatment of recurrent migraine headaches (Walker, 2011), children with histories of abuse and neglect (Huang-Storms, Bodenhamer-Davis, Davis, & Dunn, 2007), and in psychotherapy to treat patients diagnosed with antisocial personality disorder (Surmeli & Ertem, 2009) and schizophrenia (Monastra, 2003). Neurofeedback has also been used in the treatment of fibromyalgia (Kayiran, Dursun, Dursun, Ermutlu & Karamürsel, 2010), and in developing peak alpha frequency (PAF) (discrete frequency with the highest magnitude in the alpha range) in the elderly (Angelakis et al., 2007). Case studies also have shown EEG biofeedback to be effective in the treatment of Lyme disease (Packard & Ham, 1996), chronic fatigue syndrome (James & Folen, 1996), and depression (Baehr, Rosenfeld, & Baehr, 1997). Moreover, there has been use of alpha–theta EEG neurofeedback therapy and QEEG in the treatment of alcoholism and other addictive disorders [see Peniston and Kulkosky, (1999) and Sokhadze, Cannon, & Trudeau (2008) for reviews].
Particularly relevant to this text has been the use of topographic EEG mapping of Benson’s relaxation response on 20 novice participants (Jacobs, Benson, & Friedman, 1996). Using a controlled, within-subjects design, the data revealed that elicitation of the relaxation response resulted in statistically significant reductions in frontal EEG beta activity, which reflects reduced cortical activation in anterior brain regions. EEG biofeedback has been shown to augment stress management training in helping to ameliorate aversive responses to infant crying (Tyson, 1996). EEG changes, including an increase in delta waves and a decrease in alpha and beta activity, as well as a shift toward left frontal activation (all are indicative of a relaxation response) have been observed in participants receiving moderate massage therapy (Diego, Field, Sanders, & Hernandez-Reif, 2004). There has also been a case study purporting the advantages of the innovative use of integrating imagery/video/EEG biofeedback (i.e., diaphragmatic breathing, coordination exercises, visual exercises to improve tracking, internal visual imagery, and video imagery) in mitigating anxiety and improving performance of a 21-year-old collegiate baseball player who suffered a fractured cheekbone and eye socket after being hit by a pitch (Davis & Sime, 2005).
Sensor placement in neurofeedback is usually standardized based on the International 10–20 System of electrode placement. For the neurotherapy systems typically in use, an experienced therapist requires only about 2 min to connect the sensors to the scalp. Training sessions include a baseline assessment to determine the average microvolt level of the brain waves being investigated. Reward criteria are then established by an amplitude “window,” which sets high and low microvolt levels that are reinforced or inhibited. For example, in the beta–theta training used in treating ADHD, beta thresholds may be raised 1 μv higher, or theta levels may be set 1–2 μv lower (Lubar 1995; see Neumann Strehl & Birbaumer (2003) for a primer on EEG instrumentation). Rewards are typically auditory and visual, and given today’s advancements in technology, the effects can be quite elaborate.
Electrodermal (EDR) Biofeedback
Description
Electrodermal is a generic term that refers to the electrical characteristics of the skin. There are numerous measurement options available when considering this type of biofeedback. The oldest and most commonly used is the galvanic skin response (GSR); the name attributed to Galvani’s discovery of electrical activity in nerves and muscles. Generally, variation of the skin’s electrical characteristics appears to be a function of sympathetic neural activity; therefore, when using EDR biofeedback, the patient appears to be training to affect sympathetic neural arousal. More specifically, what is being measured is the conductance and resistance of sweat gland activity and the units measured are micromhos or the newer term micro-siemens (Peek, 2003).
Indications
The major use for EDR is to reduce levels of sympathetic tone and reactivity. It has been used in conjunction with other biofeedback modalities in the treatment of hypertension (Khumar, Kaur, &Kaur, 1992; Patel & Marmot, 1988), asthma (Meany et al., 1988), epilepsy (Nagai, Critchley, Rothwell, Duncan, & Trimble, 2009), and Tourette syndrome (Nagai, Cavanna, & Critchley, 2009, and has also been used as an adjunct in psychotherapy. For example, EDR has been used for systematic desensitization, the theory being that relaxation and arousal cannot happen concurrently, and that phobias and anxiety would respond to this type of treatment. EDR has also been used as a tool for exploration in psychotherapy, and in “lie detector” equipment (Peek, 2003). The appeal of this modality is that the changes in response can be extremely rapid and relatively easy to measure.
Heart Rate Variability (HRV) Biofeedback
Within the past decade there has been enhanced development of clinical applications of heart rate variability (HRV) biofeedback, a technique credited to Russian physiologist Evgney Vaschillo in the 1970s (Moss, 2008). Heart rate variability in essence means changes in the interval or distance between one beat of the heart and the next (i.e., interbeat interval or IBI) (Moss & Shaffer, 2009). In his work Vaschillo found that while participants could not consistently raise or lower heart rate using biofeedback, they could produce high-amplitude oscillations or variability. This variability, which was typically brought about by breathing controlled and slowly (i.e., diaphragmatically, see Chap. 11) around five to seven times per minute, is associated with improvement in a wide range of health and cognitive functioning. These improvements included athletic performance (Iellamo et al., 2002; Manzi et al., 2009), stress-related chronic pain (Hallman et al., 2011), ulcerative colitis (Maunder et al., 2012), asthma (Lehrer et al., 2006; Tsai, Lai, Chen, Jeng, 2011), trauma (Gevirtz & Dalenberg, 2008; Whitehouse & Heller, 2008), and depression (Karavidas et al., 2007). A more recent exploratory study did not find quantitative support for HRV biofeedback in the treatment of PTSD or depression in a sample of 49 active-duty military participants, even though many of them commented favorably on the biofeedback experience (Lande, Williams, Francis, Gragnani, & Morin, 2010). However, a recent pilot study suggests the benefit of incorporating HRV biofeedback in Acceptance and Commitment therapy (Kleen & Reitsma, 2011). Conversely, decreased heart rate variability has been associated with poor health outcomes (Gevirtz & Lehrer, 2003) including behavior problems in children (Calkins, Graziano, & Keane, 2007), depression in adults (Rechlin, Weis, Spitzer, & Kaschka, 1994), generalized anxiety in adults (Friedman, 2007), diabetes (Lindmark, Burén, & Eriksson, 2006), and alcoholism (Ingjaldsson, Laberg, & Thayer, 2003). Overall, and relative to one’s age, abnormally low HRV is associated with all causes of mortality (Levy, Slade, Kunkel, & Kasl, 2002).
According to Lehrer and Vaschillo (2008), the mechanism surrounding HRV remains uncertain, due mostly to the complex nature of the autonomic nervous system, but theoretical and empirical support suggest that it is mediated more parasympathetically. What seems more important to these researchers regarding putative mechanisms associated with HRV is the resonance characteristics of the cardiovascular system, or the frequency at which heart rate variability is at its greatest. More specifically, the homeostatic balance of the sympathetic and parasympathetic branches of the ANS (see Chap. 2) produce an orderly increase and decrease in heart rate. The variations in heart rate produced SNS and PNS occur at different speeds or frequencies. Breathing air into the lungs temporarily shuts off the influence of the PNS on heart rate, and thus heart rate increases. When air is breathed out of the lungs, the parasympathetic influence occurs again and heart rate decreases. This oscillation in heart rate as a result of respiration is known as respiratory sinus arrhythmia. When we are experiencing acute stress, the SNS-driven response increases heart rate, but once the stress is over, the homeostatic increase in the PNS should rapidly bring heart rate to its normal slower rhythm. However, chronic stress, which is often exacerbated by negative thoughts and emotions, can overstimulate the SNS and render the PNS less effective in countering the SNS.
Furthermore, these autonomic branches are mediated by the sinoatrial (SA) node and atrioventicular (AV) nodes of the heart, also known as “pacemakers” in the heart. The SA node initiates an electrical signal that begins the cycle of the heart’s pumping. This signal passes through the AV node that spreads the current through the ventricles. As noted above, there are factors, such as diaphragmatic breathing that affect specific rhythms in the heart. Other factors that increase the component rhythm of the heart include baroreceptors, which are pressure sensors in the arteries, thermal regulation, and emotional reactivity, such as anxious thinking (Moss & Shaffer, 2009). Thus, higher heart rate variability is indicative of optimal support between the two branches of the ANS, and is associated with physiological resiliency, behavioral flexibility, and increased capacity to adapt well to stress (Beauchaine, 2001). When the ANS is not working well together, the heart shows less stability in resting rate and more difficulty responding to changing body needs.
Current evidence supports the notion that an individual’s “resonant frequency,” when heart rate variability is at its greatest, can be measured by biofeedback instruments (Moss & Shaffer, 2009; Mueller, n.d.). The overarching goal of HRV biofeedback is to help facilitate physical and emotional well-being or coherence by learning to self-regulate emotional experience (McCraty, 2008) through (1) physical and emotional relaxation, (2) cognitive restructuring to reduce anxious thoughts and negative emotions, and (3) controlled, smooth, effortless diaphragmatic breathing (Moss & Shaffer, 2009). HRV biofeedback typically uses either an electrocardiogram (EKG) with sensors placed on a participant’s wrists and torso to detect the electrical signal produced by the heart, or a photoplethysmogrpah (PPG) uses sensors placed on a finger (or in some cases the earlobe) to detect, amplify, and display real-time Interbeat Interval (IBI) so the user can visualize fluctuations in his or her pulse rate. Relaxed, diaphragmatic breathing helps facilitate having respiration and heart rate co-vary to produce a dominant HRV spike of around 0.1 Hz. Striefel (2008) has addressed the ethical issues of competence, informed consent, and home practice when using HRV biofeedback.
Biofeedback Precautions
Several adverse reactions can occur as a result of using biofeedback. The practitioner should be aware of the unfavorable conditions that may be produced or potentially exacerbated by its use (see Chap. 9, this volume; see also Schwartz and Andrasik, 2003). We briefly review several of these issues.
First is the case of patients taking medication for any purpose. Some patients may consider biofeedback a replacement for medication and prematurely or mistakenly stop taking medication they have received for some other purpose. Therefore, it is necessary to question patients closely regarding their medication history and also be willing to work closely with their physicians. The most dramatic example of this occurrence is diabetic patients who are taking insulin. In these cases, inducing relaxation may diminish the need for insulin, and the normal dosage that the patient had been taking may now precipitate a hypoglycemic coma. Changes in blood pressure as a result of efficacious biofeedback treatment are also an area of valid concern for patients taking medication for hyper- or hypotension and should be coordinated with his or her physician. In addition, seizures have occurred in patients undergoing biofeedback treatment for epilepsy.
Other problems may arise related to improper training of the patient by the therapist. An example might be treatment of bruxism with unilateral placement of EMG sensors, producing dislocation of the jaw through imbalance of the muscles. Muscle imbalance is also a potential precaution in the treatment of torticollis with biofeedback. All physical symptoms need to be evaluated medically prior to biofeedback treatment so that the cause of the symptom is not mistakenly ignored. For instance, headaches may result from injury, stroke, tumors, or other causes that require medical attention prior to biofeedback considerations.
Practitioners may also want to exercise caution in the selection of patients. For example, individuals experiencing psychosis, including hallucinations or delusions, and dissociative disorders are considered poor candidates for biofeedback training because they may misinterpret what is occurring, and they may have additional difficulty in controlling their cognitions or physiology.
Role of the Therapist and Other Factors
From the information covered thus far in this chapter, a reasonable conclusion may be that the major element in biofeedback is the instrument technology. However, it is how the instruments are used in the therapeutic relationship that determines the outcome. In fact, the clinician–patient dyad is by far the most important element in this form of therapy. More than many types of therapies, biofeedback requires that the therapist becomes in essence an effective coach and the patient becomes engaged, and motivated to learn to improve his or her performance, to practice between sessions and to transfer these skills to daily life. As noted, the relationship with the therapist is the foundation of the learning process.
A decisive factor in biofeedback success appears to be the extent to which cognitive restructuring helps patients recognize the ways in which the mind and body interact. Again, this occurrence is dependent on the patient’s relationship with his or her clinician to facilitate the process. Within this context, biofeedback may be considered an adjunct to a more traditional psychotherapeutic relationship. It is comparable to hypnosis, relaxation therapy, and so on, in that it is a tool used to treat a symptom complex, but only within the context of the total therapeutic relationship and well thought out treatment plan. Thus, clinicians may be thought of as theatrical directors: They set the stage for change to occur by educating, giving useful hints, pointers, feedback, and encouragement. The actual change that occurs is the result of the patient’s efforts. In this way the therapist becomes an instructor or coach who engages the patient’s own abilities to help himself or herself and to do so with increasing probabilities of success. Our original diagram of the biofeedback encounter (Fig. 14.1) may now be modified as indicated in Fig. 14.3.
Thus, the clinician receives information regarding the patient’s functioning from both the instrument and the patient, then provides information to the patient, allowing the patient better to use data acquired from the instrument. Again, the clinician, although not directly responsible for therapeutic change, plays an important role in the biofeedback loop. His or her empathetic skills, clinical demeanor, and effectiveness as a health educator interact with other factors, such as office setting, room temperature, and type of equipment, to affect the outcome of clinical feedback. It would be naive to believe that personality factors do not affect biofeedback skills acquisition. As noted in Chap. 6, individuals bring various strengths, vulnerabilities, and reservations to the clinical encounter. Also, as noted in Chap. 8, issues of mastery, self-control, and self-efficacy are quite germane to the biofeedback paradigm. Bandura (1982a, 1982b, 1997) has noted, however, that the perception of self-efficacy is even more salient than the degree of manifest self-efficacy. Once again, the clinician’s function is paramount in assisting the patient to recognize meaningful acquisition in lieu of possible propensities for self-debasement, catastrophic ideation, or general pessimism.
In their reviews of the utilization and delivery of biofeedback within research and clinical applications, Striefel (2007, 2008), Shellenberger and Green (1986), and Schwartz & Andrasik (2003) discuss some of the most common errors, in addition to ways of ensuring competence. The following errors have particular relevance to the use of biofeedback in the treatment of excessive stress:
-
1.
Failure of the clinician to receive proper training and experience in the use of applied biofeedback equipment and modalities.
-
2.
Failure of the inexperienced clinician to ask for and use prudent supervision.
-
3.
Failure to provide the patient with the appropriate number of training sessions. In biofeedback, which represents a form of learning, individual differences account for a large preponderance of the variation. In other words, no consistent rule governs the rate of skills acquisition. Nevertheless, it is apparent that most people cannot acquire useful biofeedback skills in only two or three sessions. Moreover, recall that 40–80 sessions may be required when using EEG biofeedback to treat ADHD.
-
4.
Failure to provide the patient with homework exercises that reinforce and extend the skills acquired within the office or laboratory setting. Again, one of the primary goals of biofeedback is to generalize the response to settings outside of the office.
-
5.
Failure of the clinician to recognize and facilitate the social–psychological and clinical aspects of biofeedback. Some clinicians erroneously believe that biofeedback is immune to the usual clinical variables that affect other aspects of clinical psychology and psychiatry. Therefore, it is often useful to conceptualize some forms of biofeedback as biofeedback-assisted psychotherapy.
-
6.
Failure of the clinician to recognize that the patient’s formation of a sense of control or self-efficacy serves as one of the most relevant clinical aspects or powerful therapeutic forces within the biofeedback paradigm.
-
7.
Failure of the clinician to allow the patient ample time to adapt or habituate to the physiological assessment process. Biofeedback, in addition to being a therapeutic intervention, is also an exercise in physiological assessment. In any such paradigm, the participant must be allowed to adapt to the novel stimuli represented by the biofeedback training environment. Adaptation needs to occur within every training session.
-
8.
Failure to take baseline measurements relevant to the biofeedback variables to be trained. In a sense, it is useful to consider the patient as the control within a single-subject research design and to structure the clinical paradigm with that in mind.
-
9.
Failure of the clinician to train the clinical patient to mastery as opposed to initial skills acquisition. In other words, too many patients are prematurely terminated by biofeedback clinicians who lose sight of the need to have patients overlearn the acquired skills in self-regulation.
The Past and Future of Biofeedback
Similar to the previous editions of this text, we remain optimistic about the clinical utility and applications of various forms of biofeedback, although there seems to be some perception that its prominence in complementary and alternative medicine may have waned somewhat in recent years. However, publications such as Applied Psychophysiology and Biofeedback, Journal of Evidence-Based Complementary & Alternative Medicine and the Journal of Neurotherapy attest to the continued interest in biofeedback. There are also some recent reports that biofeedback devices (e.g., EEG, GSR) may interface with the video gaming industry, with exercise training, and with performance enhancement and recovery in athletes. For example, there are assisted telemetry systems that allow a person to workout, ride, shoot, or actually compete, and have data transmitted to monitors or coaches who are located distally (http://Heartmath.com; http://www.Thoughttechnology.com). It is relevant to note, however, that the field of biofeedback as a clinical technology has not always been universally accepted by clinicians and researchers. Past criticisms and scrutiny have focused on the soundness and rigor of some of the research design and methodology (Schwartz & Andrasik, 2003), as well as the apparently exaggerated claims of applied applications and successes made by some practitioners. For a good review of the epistemological issues that affect the conduct of inquiry as it pertains to the investigation of the clinical efficacy of biofeedback, the reader is referred to Shellenberger and Green (1986, 1987) and Lehrer et al. (1994). The increased technological sophistication and implementation of virtual reality devices appears to be a vibrant opportunity to enhance and expand the applications of biofeedback.
There are several professional organizations, such as the Association of Biofeedback and Psychophysiology (AAPB) and the International Society for Neurofeedback and Research (ISNR), that allow interested professionals to advance their understanding and use of biofeedback, applied psychophysiology and applied neuroscience. They promote education and research, as well as ways to help improve peoples’ life functioning and well-being (AAPB, 2011; ISNR, 2011). Professionals who meet education and training standards can seek certification in general biofeedback, neurofeedback, and pelvic muscle dysfunction biofeedback through the Biofeedback Certification Institute of America (BCIA; http://www.bcia.org).
Summary
This chapter has explored biofeedback, the creation and clinical utilization of psychophysiological feedback loops for the purpose of treating excessive stress and/or its target-organ effects. Let us review the main points:
-
1.
Biofeedback gives the recipient access to learning paradigms that involve physiological functions not previously accessible to conscious alteration.
-
2.
Biofeedback can be used directly to modify the stress response itself, through the elicitation of the relaxation response or the alteration of target-organ activity.
-
3.
EMG, temperature, EEG, which is also referred to as neurofeedback, and EDR biofeedback are commonly used forms of clinical biofeedback, but the use of Heart Rate Variability (HRV) has blossomed in the past decade.
-
4.
The clinician’s impact on the biofeedback paradigm can mean the difference between clinical success or failure. For this reason, the clinician should receive training in not only clinical psychophysiology but also the fundamentals of counseling or clinical psychology.
-
5.
In understanding the process of therapeutic effect, one of the most important aspects of clinical biofeedback may be the creation of the perception of self-efficacy, as discussed by Bandura (1997) and supported by Rokicki and associates (1997) in the use of EMG biofeedback specifically.
-
6.
For precautions that should be followed in using biofeedback, refer to Chap. 9.
-
7.
Useful reviews of clinical biofeedback are found in Schwartz and Andrasik (2003), Shellenberger and Green (1986), and Basmajian (1989).
References
Allen, K. D., & Shriver, M. D. (1997). Enhanced performance feedback to strengthen biofeedback treatment outcome with childhood migraine. Headache, 37(3), 169–173.
Angelakis, E., Stathopoulou, S., Frymiare, J. L., Green, D. L., Lubar, J. F., & Kounios, J. (2007). EEG neurofeedback: A brief overview and an example of peak alpha frequency training for cognitive enhancement in the elderly. The Clinical Neuropsychologist, 21(1), 110–129.
Angoules, A. G., Balakatounis, K. C., Panagiotopoulou, K. A., Mavrogenis, A. F., Mitsiokapa, E. A., & Papagelopoulos, P. J. (2008). Orthopedics, 31(10), 980–984.
Arns, M., de Ridder, S., Strehl, U., Breteler, M., & Coenen, A. (2009). Efficacy of neurofeedback treatment in ADHD: The effects of inattention, impulsivity and hyperactivity: A meta-analysis. Clinical EEG and Neuroscience, 40(3), 180–189.
Association for Applied Psychophysiology & Biofeedback. (2011). About AAPB. Retrieved from: http://www.aapb.org/i4a/pages/index.cfm?pageid=3285
Baehr, E., Rosenfeld, J. P., & Baehr, R. (1997). The clinical use of an alpha asymmetry protocol in the neurofeedback treatment of depression: Two case studies. Journal of Neurotherapy, 2(3), 10–23.
Bandura, A. (1982a). Self-efficacy mechanism in human agency. The American Psychologist, 37, 122–147.
Bandura, A. (1982b). The self and mechanisms of agency. In J. Suls (Ed.), Psychological perspectives on the self (pp. 3–39). Hillsdale, NJ: Erlbaum.
Bandura, A. (1997). Self-efficacy: The exercise of control. New York, NY: Freeman.
Basmajian, J. V. (1963). Control and training of individual motor units. Science, 141, 440–441.
Basmajian, J. (1967). Muscles alive. Their function revealed by electromyography (2nd ed.). Baltimore, MD: Williams & Wilkins.
Basmajian, J. V. (1989). Biofeedback: Principles and practice for clinicians (3rd ed.). Baltimore, Maryland: Williams & Wilkins
Beauchaine, T. P. (2001). Vagal tone, development, and Gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology, 13, 183–214.
Biondi, D. M. (2005). Physical treatments for headache: A structured review. Headache: The Journal of Head and Face Pain, 45(6), 738–746.
Biondi, M., & Picardi, A. (1998). Temporomandibular joint pain–dysfunction syndrome and bruxism: Etiopathogenesis and treatment from a psychosomatic integrative viewpoint. In G. A. Fava & H. Freyberger (Eds.), Handbook of psychosomatic medicine (pp. 469–490). Madision, CT: International Universities Press.
Bisson, E., Contant, B., Sveistrup, H., & Lojoie, Y. (2007). Functional balance and dual-task reaction times in older adults are improved by virtual reality and biofeedback training. Cyberpsychology and Behavior, 10(1), 16–23.
Blanchard, E. B., Eisele, G., Vollmer, A., Payne, A., Gordon, M., Gornish, P., & Gilmore, L. (1996). Controlled evaluation of thermal biofeedback in treatment of elevated blood pressure in unmedicated mild hypertension. Biofeedback and Self-Regulation, 21(2), 167–190.
Blanchard, E. B., & Epstein, L. H. (1978). A biofeedback primer. Reading, MA: Addison-Wesley.
Brown, B. (1977). Stress and the art of biofeedback. New York, NY: Harper & Row.
Butler, R. N., Maby, J. I., Montela, J. M., & Young, G. P. (1999). Urinary incontinence: Primary care therapies for the older woman. Geriatrics, 54(11), 31–34, 39–40, 43–44.
Calkins, S. D., Graziano, P. A., & Keane, S. P. (2007). Cardiac vagal regulation differentiates among children at risk for behavior problems. Biological Psychology, 74(2), 144–153.
Caro, X. J., & Winter, E. F. (2011). EEG biofeedback treatment improves certain attention and somatic symptoms in fibromyalgia: A pilot study. Applied Psychophysiology and Biofeedback, 36(3), 193–200.
Chabot, R. J., & Serfontein, G. (1996). Quantitative electroencephalographic profiles in children with attention deficit disorder. Biological Psychiatry, 40, 951–963.
Chiarioni, G., & Whitehead, W. E. (2008). The role of biofeedback in the treatment of gastrointestinal disorders. Nature Reviews Gastroenterology & Hepatology, 5, 371–382.
Crider, A. B., & Glaros, A. G. (1999). A meta-analysis of EMG biofeedback treatment of temporomandibular disorders. Journal of Orofacial Pain, 13(1), 29–37.
Dannecker, C., Wolf, V., Raab, R., Hepp, H., & Anthuber, C. (2005). EMG-biofeedback assisted pelvic floor muscle training is an effective therapy of stress urinary or mixed incontinence: A 7-year experience with 390 patients. Archives of Gynecology and Obstetrics, 273, 93–97.
Davis, P. A., & Sime, W. E. (2005). Toward a psychophysiology of performance: Sport psychology principles dealing with anxiety. International Journal of Stress Management, 12(4), 363–378.
DiCara, L. V., & Miller, N. E. (1968a). Changes in heart rate instrumentally learned by curarized rats as avoidance responses. Journal of Comparative Physiology and Psychology, 65, 8–12.
DiCara, L. V., & Miller, N. E. (1968b). Instrumental learning of systolic blood pressure responses by curarized rats: Dissociation of cardiac and vascular changes. Psychosomatic Medicine, 30, 489–494.
DiCara, L. V., & Miller, N. E. (1968c). Instrumental learning of vasomotor responses by rats: Learning to respond differently in the two ears. Science, 159, 1485.
Diego, M. A., Field, T., Sanders, C., & Hernandez-Reif, M. (2004). Massage therapy of moderate and light pressure and vibrator effects on EEG and heart rate. International Journal of Neuroscience, 114, 31–45.
Doğan-Aslan, M., Nakipoğlu-Yüzer, G. F., Doğan, A., Karabay, İ., & Ӧzgirgin, N. (2010). The effect of electromyographic biofeedback treatment in improving upper extremity functioning of patients with hemiplegic stroke. Journal of Stroke and Cerebrovascular Diseases, 21(3), 187–193.
Donaldson, S., Donaldson, M., & Snelling, L. (2003). SEMG Evaluations: An overview. Applied Psychophysiology and Biofeedback, 28(2), 121–127.
Druzbicki, M., Kwolek, A., Depa, A., & Przysada, G. (2010). The use of a treadmill with biofeedback function in assessment of relearning walking skills in post-stroke hemiplegic pateients – A preliminary report. Neuologia i Neurochirurgia Polska, 44(6), 567–573.
El-Sayed, E., Larsson, J. O., Persson, H. E., & Rydelius, P. A. (2002). Altered cortical activity in children with attention-deficit/hyperactivity disorder during attentional load task. Journal of the American Academy of Child and Adolescent Psychiatry, 41, 811–819.
Everly, G. S., Jr., & Sobelman, S. H. (1987). The assessment of the human stress response: Neurological, biochemical, and psychological foundations. New York, NY: American Management Systems Press.
Field, T. (2009). Complementary and alternative therapies research. Washington, DC: American Psychological Association.
Foster, P. S. (2004). Use of the Calmset 3 Biofeedback/Relaxation System in the assessment and treatment of chronic nocturnal bruxism. Applied Psychophysiology and Biofeedback, 29(2), 141–147.
Frazier, T. W. (1966). Avoidance conditioning of heart rate in humans. Psychophysiology, 3, 188–202.
Friedman, B. H. (2007). An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biological Psychology, 74, 185–199.
Gatchel, R., & Price, K. (1979). Biofeedback: An introduction and historical overview. In R. Gatchel & K. Price (Eds.), Clinical applications of biofeedback: Appraisal and status. Elmsford, NY: Pergamon Press.
Gevensleben, H., Moll, G. H., & Heinrich, H. (2010). Neurofeedback training in children with ADHD: Behavioral and neurophysiological effects. Zeitschrift für Kinder – und Jugendpsychiatrie und psychotherapie, 38(6), 409–419.
Gevirtz, R. (2006). Applied psychophysiology/biofeedback in primary care medicine. Biofeedback, 34(4), 145–147.
Gevirtz, R., & Dalenberg, C. (2008). Heart rate biofeedback in the treatment of trauma symptoms. Biofeedback, 36(1), 22–23.
Gevirtz, R. N., & Lehrer, P. (2003). Resonant frequency heart rate biofeedback. In M. S. Schwartz & F. Andrasik (Eds.), Biofeedback: A practitioner’s guide (pp. 245–250). New York, NY: Guilford.
Glick, R. M., & Greco, C. M. (2010). Biofeedback and primary care. Primary Care: Clinics in Office Practice, 37(1), 91–103.
Gorini, A., Pallavincini, F., Algeri, D., Repetto, C., Gaggioli, A., & Giuseppe, R. (2010). Virtual reality in the treatment of generalized anxiety disorders. Annual Review of CyberTherapy and Telemedicine, 8, 31–35.
Graham, C., Cook, M. R., Cohen, H. D., Gerkovich, M. M., Phelps, J. W., & Fotopoulous, S. S. (1986). Effects of variation in physical effort on frontalis EMG activity. Biofeedback and Self-Regulation, 11(2), 135–141.
Green, E., & Green, A. (1977). Beyond biofeedback. San Francisco, CA: Delta.
Hall, J. E. (2011). Guyton and Hall textbook of medical physiology (12th ed.). Philadelphia, PA: Saunders Elsevier.
Hallman, D. M., Olsson, E. M., von Schéele, B., Melin, L., & Lyskov, E. (2011). Effects of heart rate variability biofeedback in subjects with stress-related chronic neck pain: A pilot study. Applied Psychophysiology and Biofeedback, 36(2), 71–80.
Herman, C., Blanchard, E. B., & Flor, H. (1997). Biofeedback treatment for pediatric migraine: Prediction of treatment outcome. Journal of Consulting and Clinical Psychology, 65(4), 611–616.
Hermens, H. J., Freriks, B., Disselhorst-Klug, C., & Rau, G. (2000). Development of recommendations for SEMG sensors and senor placement procedures. Journal of Electromyography and Kinesiology, 10, 361–374.
Holroyd, K. A., & Penzien, D. B. (1994). Psychosocial interventions in the management of recurrent headache disorders: I. Overview and effectiveness. Behavioral Medicine, 20(2), 53–63.
Holtmann, M., & Stadler, C. (2006). Electroencephalographic biofeedback for the treatment of attention-deficit hyperactivity disorder in childhood and adolescence. Expert Review of Neurotherapeutics, 6(4), 533–540.
Huang-Storms, L., Bodenhamer-Davis, E., Davis, R., & Dunn, J. (2007). QEEG-guided neurofeedback for children with histories of abuse and neglect: Neurodevelopmental rationale and pilot study. Journal of Neurotherapy, 10(4), 3–16.
Iellamo, F., Lagramant, J. M., Pigozzi, F., Spataro, A., Norbiato, G., Lucini, D., & Pagani, M. (2002). Conversion from vagal to sympathetic predominance with strenuous training in high-performance world class athletes. Circulation, 105, 2719–2724.
Imamura, M., Abrams, P., Bain, C., Buckley, B., Cardozo, L., Cody, J., & Vale, L. (2010). Systematic review and economic modelling of the effectiveness and cost-effectiveness of non-surgical treatments for women with stress urinary incontinence. Health Technology Assessment, 14(40), whole volume.
Ingjaldsson, J. T., Laberg, J. C., & Thayer, J. F. (2003). Reduced heart rate variability in chronic alcohol abuse: Relationship with negative mood, chronic thought suppression, and compulsive drinking. Biological Psychiatry, 54, 1427–1436.
International Society for Neurofeedback & Research. (2011). About ISNR. Retreived from: http://www.isnr.org/about-isnr/about-isnr.cfm
Isler, W. C. (2006). Collaborative approaches in primary care. Biofeedback, 34(4), 151–154.
Jacobs, G. D., Benson, H., & Friedman, R. (1996). Topographic EEG mapping of the relaxation response. Biofeedback and Self-Regulation, 21(2), 121–129.
Jadidi, F., Castrillon, E., & Svensson, P. (2008). Effect of conditioning electrical stimuli on temporalis electromyographic activity during sleep. Journal of Oral Rehabilitation, 35(3), 171–183.
James, L. C., & Folen, R. A. (1996). EEG biofeedback as a treatment for chronic fatigue syndrome: A controlled case report. Behavioral Medicine, 22(2), 77–81.
Jones, G. E., & Evans, P. A. (1981). Effectiveness of frontalis feedback training in producing general body relaxation. Biological Psychology, 12(4), 313–320.
Kaiser, D. A. (2006). What is quantitative EEG. Journal of Neurotherapy, 10(4), 37–52.
Kamiya, J. (1969). Operant control of the EEG alpha rhythm and some of its reported effects on consciousness. In C. T. Tart (Ed.), Altered states of consciousness (pp. 507–517). New York: Wiley.
Karavidas, M. K., Lehrer, P. M., Vaschillo, E., Vaschillo, B., Marin, H., Buyske, S., & Hassett, A. (2007). Preliminary results of an open label study of heart rate variability biofeedback for the treatment of major depression. Applied Psychophysiology and Biofeedback, 32, 19–30.
Karavidas, M. K., Tsai, P.-S., Yucha, C., McGrady, A., & Lehrer, P. M. (2006). Thermal biofeedback for primary Raynaud’s phenomenon: A review of the literature. Applied Psychophysiology and Biofeedback, 31(3), 203–216.
Kayiran, S., Dursun, E., Dursun, N., Ermutlu, N., & Karamürsel, S. (2010). Neurofeedback intervention in fibromyalgia syndrome: A randomized, controlled, rater blind clinical trial. Applied Psychopysiology and Biofeedback, 35(4), 293–302.
Khumar, S. S., Kaur, P., & Kaur, J. (1992). A study of therapeutic effect of GSR biofeed-back on mild hypertension. Journal of the Indian Academy of Applied Psychology, 18(1–2), 23–28.
Kimble, G. A. (1961). Hilgard and Marquis’ conditioning and learning. New York: Appleton–Century–Crofts.
Kleen, M., & Reitsma, B. (2011). Appliance of heart rate variability biofeedback in Acceptance and Commitment Therapy: A pilot study. Journal of Neurotherapy: Investigations in Neuromodulation, Neurofeedback and Applied Neuroscience, 15(2), 170–181.
Lande, R. G., Williams, L. B., Francis, J. L., Cragnani, C., & Morin, M. L. (2010). Efficacy of biofeedback for post-traumatic stress disorder. Complementary Therapies in Medicine, 18, 256–259.
Landy, S. (2004). Migraine throughout the life cycle: Treatment through the ages. Neurology, 62(5 suppl2)S2-S8
Lehrer, P. M., Carr, R., Sargunaraj, D., & Woolfolk, R. L. (1994). Stress management techniques: Are they all equivalent, or do they have specific effects? Biofeedback and Self-Regulation, 19(4), 353–401.
Lehrer, P., & Vaschillo, E. (2008). The future of heart rate variability biofeedback. Biofeedback, 36(1), 11–14.
Lehrer, P., Vaschillo, E., Lu, S.-E., Eckberg, D., Vaschillo, B., Scardella, A., & Habib, R. (2006). Heart rate variability biofeedback: Effects of age on heart rate variability, baroreflex gain, and asthma. Chest, 129(2), 278–284.
Levy, B. R., Slade, M. D., Kunkel, S. R., & Kasl, S. V. (2002). Longevity increased by positive self-perceptions of aging. Journal of Personality and Social Psychology, 83(2), 261–270.
Linden, W., & Moseley, J. V. (2006). The efficacy of behavioral treatments for hypertension. Applied Psychophysiology and Biofeedback, 31(1), 51–63.
Lindmark, S., Burén, J., & Eriksson, J. W. (2006). Insulin resistance, endocrine function and adipokines in type 2 diabetes patients at different glycaemic levels: Potential impact for glucotoxicity in vivo. Clinical Endocrinology, 65, 301–309.
Lindsley, D. B., & Sassaman, W. (1938). Autonomic activity and brain potentials associated with “voluntary” control of pilomotors. Journal of Neurophysiology, 1, 342–349.
Loo, S. K., & Barkley, R. A. (2005). Clinical utility of EEG in attention deficit hyperactivity disorder. Applied Neuropsychology, 12(2), 64–76.
Lubar, J. F. (1991). Discourse on the development of EEG diagnostics and biofeedback treatment for attention-deficit/hyperactivity disorders. Biofeedback and Self-Regulation, 16, 201–225.
Lubar, J. F. (1995). Neurofeedback for the management of attention-deficit/hyperactivity disorders. In M. Schwartz and Associates (Eds.), Biofeedback: A practitioner’s guide (2nd ed., pp. 493–522). New York: Guilford.
Lubar, J. F., & Deering, W. M. (1981). Behavioral approaches to neurology. New York: Academic Press.
Lubar, J. F., & Shouse, M. N. (1976). EEG and behavioral changes in a hyperkinetic child concurrent with training of the sensorimotor rhythm (SMR): A preliminary report. Biofeedback and Self-Regulation, 3, 293–306.
Luria, A. R. (1958). The mind of a mnemonist (L. Solotaroff, trans.). New York: Basic Books.
Lynch, D. J., McGrady, A. V., Nagel, R. W., & Wahl, E. F. (2007). The patient-physician relationship and medical utilization. The Primary Care Companion to the Journal of Clinical Psychiatry, 9(4), 266–270.
Manzi, V., Castagna, C., Padua, E., Lombardo, M., D’Ottavio, S., Massaro, … Iellamo, F. (2009). Dose–response relationship of autonomic nervous system responses to individualized training impulse in marathon runners. American Journal of Physiology-Heart and Circulatory Physiology, 296(6), H1733-H1740
Mariela, S. C., Matt, D. A., & Burish, T. G. (1992). Comparison of frontalis, multiple muscle site, and reactive muscle site feedback in reducing arousal under stressful and nonstressful conditions. Medical Psychotherapy: An International Journal, 5, 133–148.
Maunder, R. G., Nolan, R. P., Hunter, J. J., Lancee, W. J., Steinhart, A. H., & Greenberg, G. R. (2012). Relationship between social support and autonomic function during a stress protocol in ulcerative colitis patients in remission. Inflammatory Bowel Diseases, 18(4), 732–742.
McClure, C. (1959). Cardiac arrest through volition. California Medicine, 90, 440–448.
McCraty, R. (2008). From depletion to renewal: Positive emotions and heart rhythm coherence feedback. Biofeedback, 36(1), 30–34.
McDowell, B. J., Engberg, S., Sereika, S., Donovan, N., Jubeck, M. E., Weber, E., & Engberg, R. (1999). Effectiveness of behavioral therapy to treat incontinence in homebound older adults. Journal of the American Geriatric Society, 47(3), 309–318.
McGinnis, R. A., McGrady, A., Cox, S. A., & Grower-Dowling, K. A. (2005). Biofeedback-assisted relaxation in Type 2 diabetes. Diabetes Care, 28(9), 2145–2149.
McGrady, A., Graham, G., & Bailey, B. (1996). Biofeedback-assisted relaxation in insulin-dependent diabetes: A replication and extension study. Annals of Behavioral Medicine, 18(3), 185–189.
McGrath, P. J. (1999). Clinical psychology issues in migraine headaches. Canadian Journal of the Neurological Sciences, 26(Suppl. 3), S33–S36.
McKee, M. G. (2008). Biofeedback: An overview in the context of heart-brain medicine. Cleveland Clinic Journal of Medicine, 75(Suppl. 2), S31–S34.
Meany, J., McNamara, M., Burks, V., Berger, T. W., & Sayle, D. M. (1988). Psychological treatment of an asthmatic patient in crisis: Dreams, biofeedback, and pain behavior modification. Journal of Asthma, 25(3), 141–151.
Miller, N. E. (1969). Learning of visceral and glandular responses. Science, 163, 434–445.
Miller, R. M., & Chang, M. W. (1999). Advances in the management of dysphagia caused by stroke. Physical Medicine and Rehabilitation Clinics of North America, 10(4), 925–941.
Miller, N. E., & Dworkin, B. R. (1974). Visceral learning: Recent difficulties with curarized rats and significant problems for human research. In P., A. Obrist, A. H. Black, J. Brener, & L. V. DiCara (Eds.), Cardiovascular psychophysiology (pp. 313–331). Chicago: Aldine.
Monastra, V. J. (2005). Electroencephalographic biofeedback (neurotherapy) as a treatment for attention deficit hyperactivity disorder: Rationale and empirical foundation. Child and Adolescent Psychiatric Clinics of North America, 14, 55–82.
Mosastra, V. J. (2003). Clinical applications of electroencephalographic biofeedback. In M. S. Schwartz, F. Andrasik (Eds.), Biofeedback: A practitioner’s guide (pp.438-463). New York: Guilford.
Moser, D. K., Dracup, K., Woo, M. A., & Stevenson, L. W. (1997). Voluntary control of vascular tone by using skin-temperature biofeedback-relaxation in patients with advanced heart failure. Alternative Therapies in Health and Medicine, 3(1), 51–59.
Moss, D. (2008). Special Issue: The emergent science and practice of heart rate variability biofeedback. Biofeedback, 36(1), 1–4.
Moss, D. & Shaffer, F. (2009). Heart rate variability training. Retreived from: http://www.bfe.org/articles/hrv.pdf
Mueller, H. (n.d.). Heart rate variability biofeedback. Learning to change heart rhythms. Retreived from: http://www.drmueller-healthpsychology.com/heart_rate_variability.html
Nagai, Y., Cavanna, A., & Critchley, H. D. (2009). Influence of sympathetic autonomic arousal on tics: Implications for a therapeutic behavioral intervention for Tourette syndrome. Journal of Psychosomatic Research, 67(6), 599–605.
Nagai, Y., Critchley, H. D., Rothwell, J. C., Duncan, J. S., & Trimble, M. R. (2009). Changes in cortical potential associated with modulation of peripheral sympathetic activity in patients with epilepsy. Psychosomatic Medicine, 71(1), 84–92.
Nagai, Y., Goldstein, L. H., Fenwick, P. B. C., & Trimble, M. R. (2004). Clinical efficacy of galvanic skin response biofeedback training in reducing seizures in adult epilepsy: A preliminary randomized controlled study. Epilepsy & Behavior, 5, 216–223.
Nestoriuc, Y., & Martin, A. (2007). Efficacy of biofeedback for migraine: A meta-analysis. Pain, 128, 111–127.
Nestoriuc, Y., Martin, A., Rief, W., & Andrasik, F. (2008). Biofeedback treatment for headache disorders: A comprehensive efficacy review. Applied Psychophysiology and Biofeedback, 33, 125–140.
Neuman, N., Strehl, U., & Birbaumer, N. (2003). A primer of electroencephalographic instrumentation. In M. S. Schwartz & F. Andrasik (Eds.), Biofeedback: A practitioner’s guide (3rd ed., pp. 88–102). New York: Guilford Press.
Ogden, E., & Shock, N. (1939). Voluntary hypercirculation. American Journal of the Medical Sciences, 98, 329–342.
Packard, R. C., & Ham, L. P. (1996). EEG biofeedback in the treatment of Lyme disease: A case study. Journal of Neurotherapy, 1(3), 22–31.
Palermo, T. M., Eccleston, C., Lewandowski, A. S., Williams, A. C., & Morley, S. (2010). Randomized controlled trials of psychological therapies for management of chronic pain in childhood and adolescents: An updated meta-analytic review. Pain, 148(3), 387–397.
Pallavicini, F., Algeri, D., Repetto, C., Gorini, A., & Riva, G. (2009). Biofeedback, virtual reality and mobile phones in the treatment of generalized anxiety disorder (GAD): A phase-2 controlled clinical trial. Journal of CyberTherapy & Rehabilitation, 2(4), 315–327.
Palmer, L. S. (2010). Biofeedback in the management of urinary continence in children. Current Urology Reports, 11(2), 122–127.
Patel, C., & Marmot, M. (1988). Can general practitioners use training in relaxation and management of stress to reduce mild hypertension? British Medical Journal, 296, 21–24.
Peek, C. J. (2003). A primer of biofeedback instrumentation. In M. S. Schwartz, F. Andrasik (Eds.), Biofeedback: A practitioner’s guide (pp.43-87). New York:Guilford
Peniston, E. G., & Kulkosky, P. J. (1999). Neurofeedback in the treatment of addictive disorders. In J. R. Evans (Ed.), Introduction to quantitative EEG and neurofeedback (pp. 157–179). San Diego: Academic Press.
Rechlin, T., Weis, M., Spitzer, A., & Kaschka, W. P. (1994). Are affective disorders associated with alterations of heart rate variability? Journal of Affective Disorders, 32, 271–275.
Reid, G. J., & McGrath, P. J. (1996). Psychological treatments for migraine. Biomedical Pharmacotherapy, 50(2), 58–63.
Repetto, C., Gorini, A., Algeri, D., Vigna, C., Gaggioli, A., & Riva, G. (2009). The use of biofeedback in clinical virtual reality: The intrepid project. Studies in Health Technology and Informatics, 144, 128–132.
Rice, B. I. (2007). Clinical benefits of training patients to voluntarily increase peripheral blood flow: The warm feet intervention. The Diabetes Educator, 33(3), 442–454.
Richards, L., & Pohl, P. (1999). Therapeutic interventions to improve upper extremity recovery and function. Clinical Geriatric Medicine, 15(4), 819–832.
Richardson, I., & Palmer, L. S. (2009). Successful treatment for giggle incontinence with biofeedback. Journal of Urology, 182(4 Suppl), 2062–2066.
Rokicki, L. A., Holroyd, K. A., France, C. R., Lipchik, G. L., France, J. L., & Kvaal, S. A. (1997). Change mechanisms associated with combined relaxation/EMG biofeedback training for chronic tension headache. Applied Psychophysiology and Biofeedback, 22(1), 21–41.
Rubin, L. R. (1977). Reanimation of the paralyzed face. St. Louis, MD: Mosby.
Ryan, M., & Gevirtz, R. (2004). Biofeedback-based psychophysiological treatment in a primary care setting: An initial feasibility study. Applied Psychophysiology and Biofeedback, 29(2), 79–93.
Scharff, L., Marcus, D. A., & Masek, B. J. (2002). A controlled study of minimal-contact thermal biofeedback treatment in children with migraine. Journal of Pediatric Psychology, 27(2), 109–119.
Schneider, C. J. (1989). A brief history of biofeedback. Biofeedback, 17(1), 4–7.
Schwartz, M. S. (1995). Biofeedback: A practitioner’s guide (2nd ed.). New York: Guilford.
Schwartz, M. S., & Andrasik, F. (2003). Biofeedback: A practitioner’s guide (3rd ed.). New York: Guilford.
Schwartz, M. S., & Montgomery, D. D. (2003). Entering the field and assuring competence. In M. Schwartz & F. Andrasik (Eds.), Biofeedback: A practitoner’s guide (3rd ed., pp. 20–26). New York: Guilford.
Seifert, A. R., & Lubar, J. F. (1975). Reduction of epileptic seizures through EEG biofeedback training. Biological Psychology, 3, 157–184.
Sharpley, C. F., & Rogers, H. (1984). A meta-analysis of frontal EMG levels with biofeedback and alternative procedures. Biofeedback and Self-Regulation, 9, 385–393.
Shearn, D. W. (1962). Operant conditioning of heart rate. Science, 137, 530–531.
Shellenberger, R., & Green, J. (1986). From the ghost in the box to successful biofeedback training. Greeley, CO: Health Psychology.
Shellenberger, R., & Green, J. (1987). Specific effects of biofeedback versus biofeedback-assisted self-regulation training. Biofeedback and Self-Regulation, 12(3), 185–209.
Sokhadze, T. M., Cannon, R. L., & Trudeau, D. L. (2008). EEG biofeedback as a treatment for substance use disorders: Review, rating of efficacy, and recommendations for further research. Applied Psychophysiology and Biofeedback, 33(1), 1–28.
Staples, J. K., Abdel Atti, J. A., & Gordon, J. S. (2011). Mind-body skills groups for posttraumatic stress disorder and depression symptoms in Palestinian children and adolescents in Gaza. International Journal of Stress Management, 18(3), 246–262.
Sterman, M. B. (1973). Neurophysiological and clinical studies in an epileptic following sensorimotor EEG biofeedback training: Some effects on epilepsy. In L. Birk (Ed.), Biofeedback: Behavioral medicine. New York: Grune & Stratton.
Sterman, M. B., & Egner, T. (2006). Foundation and practice of neurofeedback for the treatment of epilepsy. Applied Psychophysiology and Biofeedback, 31(1), 21–35.
Sterman, M. B., & Friar, L. (1972). Suppression of seizures in an epileptic following sensorimotor EEG feedback training. Electroencephalography and Clinical Neurophysiology, 33, 89–95.
Stoyva, J. M., & Budzynski, T. H. (1974). Cultivated low-arousal: An anti-stress response? In L. DiCara (Ed.), Recent advances in limbic and autonomic nervous systems research (pp. 369–394). New York: Plenum Press.
Stoyva, J. M., & Budzynski, T. H. (1993). Biofeedback methods in the treatment of anxiety and stress disorders. In P. Lehrer & R. Woolfolk (Eds.), Principles and practice of stress management (2nd ed., pp. 263–300). New York: Guilford.
Striefel, S. (2007). Ethical and legal electromyography. Biofeedback, 35, 3–7.
Striefel, S. (2008). Ethical issues in heart rate variability biofeedback. Biofeedback, 36, 5–8.
Surmeli, T., & Ertem, A. (2009). QEEG guided neurofeedback therapy in personality disorders: 13 case studies. Clinical EEG and Neuroscience, 40(1), 5–10.
Tan, G., Dao, T. K., Sutherland, R. J., & Gevirtz, R. (2011). Heart rate variability (HRV) and posttraumatic stress disorder (PTSD): A pilot study. Applied Psychophysiology and Biofeedback, 36(1), 27–35.
Tsai, Y.-S., Lai, F.-C., Chen, S.-R., & Jeng, C. (2011). The influence of physical activity level on heart rate variability among asthmatic adults. Journal of Clinical Nursing, 20(1–2), 111–118.
Tyson, P. D. (1996). Biodesensitization: biofeedback-controlled systematic desensitization of the stress response to infant crying. Biofeedback & Self Regulation, 21(3), 273–290.
Varoqui, D., Froger, J., Pélissier, J.-Y., & Bardy, B. G. (2011). Effect of coordination biofeedback on (re)learning preferred postural patterns in post-stroke patients. Motor Control, 15, 187–205.
Verbunt, M., & Bartneck, C. (2010). Sensing senses: Tactile feedback for the prevention of decubitus ulcers. Applied Psychophysiology and Biofeedback, 35, 243–250.
Walker, J. E. (2011). QEEG-guided neurofeedback for recurrent migraine headaches. Clinical EEG and Neuroscience, 42(1), 59–61.
Weatherall, M. (1999). Biofeedback or pelvic floor muscle exercises for female genuine stress incontinence: A meta-analysis of trials identified in a systematic review. BJU International, 83, 1015–1016.
Wiederhold, B. K., & Rizzo, A. (2005). Virtual reality and applied psychophysiology. Applied Psychophysiology and Biofeedback, 30(3), 183–185.
Wong, Y. M. (2008). Early-generation biofeedback instruments and modern computers. Biofeedback, 36(3), 116–117.
Wood, D. P., Wiederhold, B. K., & Spira, J. (2010). Lessons learned from 350 virtual-reality sessions with warriors diagnosed with combat-related posttraumatic stress disorder. Cyberpsychology, Behavior, and Social Networking, 13(1), 3–11.
Acknowledgements
We would like to thank Russ Hibler, PhD, ABPP, Affiliate Faculty Loyola University Maryland, for his early review of this chapter.
Author information
Authors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Everly, G.S., Lating, J.M. (2013). Biofeedback in the Treatment of the Stress Response. In: A Clinical Guide to the Treatment of the Human Stress Response. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-5538-7_14
Download citation
DOI: https://doi.org/10.1007/978-1-4614-5538-7_14
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-5537-0
Online ISBN: 978-1-4614-5538-7
eBook Packages: Behavioral ScienceBehavioral Science and Psychology (R0)