Abstract
Mobilization of the major reserves within seed storage tissues occurs following the completion of germination to provide nutrients for the growing seedling until it becomes autotrophic. Starch, hemicelluloses, triacylglycerols (oils), and proteins are mobilized by distinct suites of enzymes, many of which are transcribed and synthesized de novo. Starch and proteins are converted to sugars and amino acids within the starch granules and protein storage vacuoles, respectively, before these catabolites are moved into the cytosol; hemicelluloses are released from cell wall polymers by specific hydrolases. Oils, in contrast, require the additional participation of two non-storage organelles within the cell, one of which, the glyoxysome, is formed de novo to accommodate the enzymes required for the catabolism of fatty acids. The final carbon product of reserve catabolism is sucrose that is translocated to the growing tissues, with proteins also yielding transportable amino acids. Regulation of starch mobilization from the endosperms of cereals, which is hormonally controlled, is well understood; in contrast, while the participation of hormones in hemicellulose mobilization in dicot endosperms is known, their role in the hydrolysis of the major cotyledon reserves is uncertain.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Seedling Growth Patterns
The first sign that germination has been completed is usually the appearance of the radicle through the surrounding structures, followed by an increase in its length and fresh weight. In many seeds the radicle penetrates the surrounding structures as soon as elongation of the hypocotyl/transition zone (Sect. 4.6.1) commences, but in others (e.g., faba or broad bean, and other beans) there is considerable growth before the testa is ruptured. In Arabidopsis, there is initially rupture of the testa, with the intact micropylar end of the endosperm being pushed through it by expansion of the embryo, and then this confining structure is ruptured to release the radicle. There are some seeds, however, from which the hypocotyl is the first structure to emerge; this occurs in some members of the Bromeliaceae, Palmae, Chenopodiaceae, Onagraceae, Saxifragaceae, and Typhaceae. In some grains (e.g., rice and barnyard grass) germinated under anoxic or hypoxic conditions coleoptile growth precedes that of the radicle (Fig. 4.14); such an unusual germination pattern is also sometimes found in wheat when grains begin to sprout on the parent plant (Fig. 2.18b).
The first hairs to be formed in the seedling following germination are in the collet region, at the junction (transition zone) between the hypocotyl and the radicle (Fig. 4.19e, f). These collet hairs have also been termed “hypocotyl hairs” or “collar rhizoids.” They are important to initially anchor the seedling in its substrate (e.g., soil), to facilitate the development of geotropism and to aid in water uptake until the root hairs develop. Collet hairs arise synchronously from every epidermal cell (trichoblast) in the collet region, whereas the later-forming root hairs arise successively from alternate root epidermal cells.
Seedlings can be conveniently divided into two types on the basis of the fate of their cotyledons following germination (Table 5.1): (1) epigeal, in which the cotyledons are raised out of the soil by extension of the hypocotyl and often become foliate and photosynthetic (Fig. 5.1a, b), and (2) hypogeal, in which the hypocotyl remains short and compact, and the cotyledons stay beneath the soil. The epicotyl expands to raise the first true leaves out of the soil (Fig. 5.2). The terms “epigeal (epigeous) germination” and “hypogeal (hypogeous) germination” are sometimes used, especially with respect to field emergence, but such use is incorrect because the phenomena relate to seedling growth, not to germination per se.
The epigeal type of seedling growth in (a) Phaseolus bean. The cotyledons swell only a little and turn green; they are shed when their storage reserves are depleted. (b) Castor bean in which the cotyledons expand and become green and photosynthetic after their reserves are mobilized, and remain so until the first true leaves open; then the cotyledons shrivel and fall off. Both of the above are dicots. (c) Onion. The single cotyledon of this monocot emerges above the soil but remains embedded in the endosperm, acting as an haustorium through which the hydrolyzed storage products are imported into the growing seedling. It degenerates after the reserves are depleted. Growth of the aerial parts of the seedling is from the basal plumule. (d) Peperomia peruviana, in which one haustorial cotyledon remains embedded in the seed endosperm below soil level, and the other emerges above and becomes green. Not drawn to scale
The hypogeal type of seedling growth as shown by (a) the dicot faba, or broad, bean and (b) the monocot maize. The two bean cotyledons remain below the ground and shrivel during depletion of their reserves, eventually degenerating completely. The single cotyledon (scutellum) of maize and other cereals remains below the soil surface; in some species (e.g., wild oat) it may grow into the starchy endosperm and aid in absorption of the products of storage reserve mobilization. After depletion of the reserves the scutellum degenerates. Not drawn to scale
In endospermous seeds showing the epigeal mode of seedling growth, e.g., castor bean, the endosperm may be carried above ground by the cotyledons as they utilize its food stores. In onion, another epigeal type, the absorptive tip of the single cotyledon may remain embedded in the degrading endosperm, while the rest of the cotyledon turns green. The cotyledon in monocots may become highly specialized for absorption; in the Gramineae, for example, it is modified to form the scutellum, which may become extended as an absorptive haustorium (Sect. 5.5.2). Highly developed haustorial cotyledons are found in the Palmae. When the small embryo of the date palm commences growth, the cotyledon tip enlarges to form an umbrella-shaped body buried within the endosperm, from which it absorbs the hydrolyzed reserves. Likewise, the absorptive cotyledon of the coconut enlarges to invade the endosperm.
An interesting pattern of growth is shown by some Peperomia spp. (Fig. 5.1d) and in asparagus (both dicots), in which one cotyledon emerges from the seed, and the other remains as an absorptive organ buried within the endosperm of the seed which remains in the soil: “semi-epigeal.” It has been suggested that the monocotyledonous condition evolved from this pattern of emergence, although this remains a matter for conjecture. The monocot onion seedling is a variation on the epigeal mode (Fig. 5.1c), where the single cotyledon remains within the storage tissue after its emergence from the soil.
5.2 Mobilization of Stored Reserves
The major mobilization of the polymeric food reserves present within the storage tissues of the seed commences after radicle protrusion, i.e., it is a post-germinative event. Some mobilization of these reserves can occur, often in the axis and a limited (e.g., micropylar) region of the endosperm before germination is completed; here the reserves are generally present in minor amounts, although the products of their hydrolysis might be important to support germination and early seedling establishment.
As the reserves contained within the storage tissues are mobilized, they are converted into forms that are readily transportable to the sites where they are required (usually the most rapidly metabolizing and growing organs of the seedling) for the support of energy-producing and synthetic events. Reliance on the stored reserves diminishes as the seedling emerges above the soil and becomes photosynthetically active (i.e., autotrophic). For the purpose of clarity this chapter is divided into sections, each of which covers the mobilization of one major type of storage reserve. It must be remembered, however, that storage organs usually contain substantial quantities of two or more major reserves (Table 1.2) and that hydrolysis and utilization of these occurs concurrently.
5.3 Stored Oligosaccharide Catabolism
While there are no low-molecular-weight storage triacylglycerols or proteins in seeds, the raffinose-family oligosaccharides (RFOs, Sect. 1.3.1) represent such a storage product for carbohydrates in the embryos and storage tissues of many species of monocots and dicots, and gymnosperms. These are oligomers of sucrose and galactose (Gal) (Fig. 5.3), the most commonly present being raffinose (galactosyl-sucrose) and stachyose (digalactosyl-sucrose). Removal of the Gal units requires the enzyme α-galactosidase, and hydrolysis of sucrose is by invertase. The released Gal is presumably converted to glucose following phosphorylation, conversion to UDP-Gal and epimerization to UDP-glucose. Relatively little research has been conducted on α-galactosidases or invertases during germination. There are reports that both soluble and vacuole-associated invertases are present in the embryo and storage tissues during and following germination, with an increase in their transcripts, synthesis or activity particularly during early seedling growth; but correlations of activity with RFO or sucrose mobilization are weak or absent.
Sucrose and raffinose-family oligosaccharides (RFOs) from mono- to tri-galactosyl sucrose (raffinose to verbascose). Shown are the enzymes required to break the links between the galactose units and between galactose and sucrose (α-galactosidase), and to convert sucrose to glucose and fructose (invertase)
α-Galactosidase is generally synthesized during development and is present in mature dry seeds, along with RFOs. This raises the question as to where the enzyme is sequestered when the seed is developing to prevent it from hydrolyzing its substrates. In the seeds of tomato, some legumes including soybean and pea, and date palm, α-galactosidase and RFOs are spatially separated, and in the dry seed the enzyme is present in protein storage vacuoles (PSVs), while the oligosaccharides lie within the cytoplasm. How the enzyme and substrate come together during and following germination is explained in a model for RFO hydrolysis in the pea seed (Fig. 5.4). Here, during germination, there is initially hydrolysis by an acidic α-galactosidase of RFOs imported from the cytoplasm into the intact PSV, the enzyme being synthesized and subsequently sequestered therein as the seed is developing. Following germination there is the expression of genes for, and the synthesis of, an alkaline α-galactosidase, which is located in the cytoplasm, and this also acts to hydrolyze the RFOs therein, in concert with that in the PSVs. Germinating pea seeds contain anabolic enzymes of the RFO pathway, as well as the catabolic ones, although activities of the latter predominate. Under stress conditions, however, when germination is slowed or impeded, temporary resynthesis of RFOs may occur.
Proposed model for the hydrolysis of RFOs in pea seeds during and following germination. During germination (top panel) RFOs are imported into the protein storage vacuole (PSV) from the cytoplasm and hydrolyzed to galactose (Gal) and sucrose (Suc) by an acidic α-galactosidase (α-Gal), the activity of which is initiated by acidification of the vacuole, presumably by H+ ion pumps in the membrane. The sugars are released from the PSV and utilized by the germinating seed. Following germination (lower panel) there is de novo synthesis of a cytoplasmic alkaline α-galactosidase to provide for further hydrolysis of the RFOs. After Blöchl et al. (2008)
The importance of RFOs as an early source of sugars to produce energy during germination has been questioned, at least in some species, e.g., inhibition of α-galactosidase activity in imbibed soybeans does not delay their germination, nor does the addition of sucrose or Gal improve it in the absence of enzyme activity. In contrast, impairment of RFO breakdown during germination of pea seeds considerably delays its completion. α-Galactosidase also plays a role in the mobilization of Gal-containing cell wall hemicelluloses, e.g., galactomannans, following germination (Sect. 5.6.2).
5.4 Pathways of Starch Catabolism
There are two catabolic pathways of starch: one hydrolytic and the other phosphorolytic.
The amylose and amylopectin in the native starch granule are first hydrolyzed by α-amylase, an endohydrolase that breaks the α(1 → 4) glycosidic links between the glucose residues randomly throughout the chains. The released oligosaccharides are further hydrolyzed by α-amylase (or with the cooperation of α-glucosidase—see below) until glucose and maltose are produced.
Multiple forms of this enzyme occur in germinated seeds of many species. Wheat, for example, contains over 20 α-amylase isoenzymes that fall into two groups separated by isoelectric focusing on the basis of their specific isoelectric point (pI, the pH at which a protein loses its electrical charge). Similarly, two groups of α-amylases occur in grains of other cereals, such as rice and barley; in the latter HvAMY1 (Hordeum vulgare α-amylase 1) has a stronger affinity for linear malto-oligosaccharides, whereas HvAMY2 plays a larger role in initial starch degradation. But α-amylases cannot hydrolyze the α(1 → 6) branch points of amylopectin, and hence highly branched cores of glucose units, called limit dextrins, are produced.
The small branches must be released by enzymes specific for the α(1 → 6) link (debranching enzyme, limit dextrinase) before being hydrolyzed to the monomer.
Another amylase, β-amylase, is an exohydrolase that cannot hydrolyze native starch granules; rather it cleaves away successive maltose units from the nonreducing end of large oligomers released by prior α-amylolytic attack. Again, amylopectin cannot be completely hydrolyzed, and the involvement of a debranching enzyme is essential. The importance of β-amylase in the mobilization of starch in cereals has been questioned, for some barley cultivars completely lack this enzyme yet grow into normal seedlings.
The disaccharide maltose, produced by α- and β-amylase action, is converted by α-glucosidase (maltase) to two glucose molecules. This enzyme can also cleave glucose from low-molecular-weight malto-oligosaccharides. There are several different α-glucosidases in the endosperm of a particular cereal, although the possibility exists that instead of being hydrolyzed there, maltose is transported instead, or also, into the growing embryo via the scutellum for cleavage to glucose therein.
Starch phosphorylase releases glucose-l-phosphate (Glc-1-P) by incorporating a phosphate moiety, rather than water, across the α(1 → 4) linkage between the penultimate and last glucose at the nonreducing end of the polysaccharide chain. Complete phosphorolysis of amylose by this exohydrolase is theoretically possible, and amylopectin can be degraded to within two or three glucose residues of an α(1 → 6) branch linkage; it is more likely to act upon polymeric chains released by α-amylase, however. The enzyme cannot attack starch granules, which first must be partly degraded by other enzymes.
To what extent this pathway of starch degradation occurs in seeds is unclear, although it is important in the mobilization of temporary starch in the plastids of leaves, and in potato tubers. It is unlikely to operate efficiently in the cereal endosperm because the storage cells are nonliving, and hence, there is no means to provide for the constant supply of required Pi. However, there is pronounced phosphorylase activity in the cotyledons of some germinated legumes during starch mobilization, e.g., pea.
5.4.1 Synthesis of Sucrose
The products of starch (and triacylglycerol) catabolism eventually are transported as sucrose into the growing root and shoot of the seedling. Glc-l-P released by phosphorolysis can be used directly as a substrate for sucrose synthesis, but glucose released by amylolysis first must be phosphorylated to glucose-6-phosphate (Glc-6-P) and then isomerized to Glc-l-P. This combines with a uridine nucleotide (UTP) to yield pyrophosphate (PPi) and the nucleotide sugar uridine diphosphoglucose (UDPGlc), which in turn transfers glucose to free fructose or to fructose-6-phosphate.
It is generally accepted that this latter reaction is the predominant, if not the only one involved in sucrose synthesis, whereas the sucrose synthase is important for sucrose catabolism. The phosphate moiety is cleaved from sucrose-6-P by sucrose phosphatase. In the seedling tissues, sucrose can be hydrolyzed to free glucose and fructose by invertase (β-fructofuranosidase, sucrase), or converted to UDPGlc and fructose by sucrose synthase.
5.5 Mobilization of Stored Starch in Cereal Grains
Although studied in all agronomically important cereals, much is known about starch mobilization and its control in germinated barley, in part because of the central role of this process in the production of malt for beer production.
An initial event is the release of cell wall-degrading enzymes, β-glucanases, from the scutellum into an intermediate layer of crushed cells (Fig. 1.1) that lies between the scutellar epithelium and the starchy endosperm. The digestion of this layer facilitates the release and passage of α-amylase from the scutellum into the starch-storing cells to commence digestion of this reserve. The initial production of this enzyme invariably occurs in the region of the scutellum, in the epithelial layer of this organ (e.g., rice), or the entire scutellum (e.g., sorghum), or in the few aleurone layer cells that penetrate the peripheral regions of the scutellum (e.g., barley). Later the enzyme is usually synthesized within the aleurone layer, the only living cells in the storage tissue, which lie to the outside of the mature cereal endosperm (in barley it is three cell-layers thick, in maize and wheat only one, and rice one to several), and is then secreted into the starchy endosperm. Thus, although α-amylase synthesized in, and released from the scutellum is important during the early stages of starch mobilization, e.g., in barley, wheat, rye and oat, most of the later hydrolysis is effected by enzyme from the aleurone layer. In rice, synthesis of α-amylase in the scutellum precedes that in the aleurone layer and is at least as important for starch hydrolysis; in maize, the scutellum is persistently a major source of the enzyme.
5.5.1 Synthesis and Release of α-Amylase and Other Hydrolases from the Aleurone Layer
Although the hydrolysis of starch by amylases is central to its mobilization, there is also collaborative activity of other enzymes to aid in the synthesis and movement of the major enzyme, α-amylase, from the living cells of the scutellum and aleurone layer to the nonliving starch-storing endosperm cells, and in the breakdown of the starch granule. Synthesis of α-amylase in the scutellum and aleurone layer requires the transcription of several genes for this hydrolase, and their subsequent translation. The hormonal regulation of this is considered in Sect. 5.5.3. In the aleurone layer cells of barley, about 60% of the newly synthesized protein is α-amylase, and therefore, a supply of amino acids is required to sustain this high level of production. This is achieved by the hydrolysis of stored proteins (mostly globulins, but also albumins and minor amounts of prolamin) present in the protein storage vacuoles (PSVs) of the mature aleurone layer cells (Fig. 5.5a, b). Aleurone layer cells, and those of the scutellum, are also rich in triacylglycerols that are sequestered in oil bodies (Fig. 5.5b), and these are mobilized by lipases, with the resultant fatty acids being converted to sugars (Sect. 5.7) as a source of energy for synthetic events, and for the synthesis of membrane lipids. Phytase is also required for mobilization of the phytin-containing globoid.
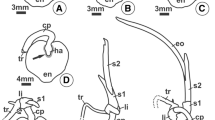
(a) Light micrograph of mature aleurone layer cells of a barley grain, showing the presence of protein storage vacuoles (also called aleurone grains, AG), which are not stained for protein, containing dark regions (phytin globoids, G). Cell wall (W), nucleus (N), outer seed coat (SC). (b) Electron micrograph of a protein storage vacuole of a barley aleurone layer cell containing protein (dark areas) and a globoid (G), with surrounding oil bodies (S). Cell wall (W), mitochondrion (M), microbody (glyoxysome, MB), involved in stored oil utilization. From Jones (1969)
A variety of enzymes involved in the mobilization of the carbohydrate reserves in the starchy endosperm are also synthesized in the aleurone layer, some facilitating the release of α-amylase, and others accompanying it to ensure the hydrolysis of starch and other stored reserves. These include limit dextrinase and α-glucosidase (maltase) to effect breakdown of the starch to glucose (Sect. 5.4) and enzymes to hydrolyze the starchy endosperm cell walls (e.g., β-1 → 3, 1 → 4 glucanases to hydrolyze the mixed-linkage glucans that make up about 75% of these walls in barley). In wheat the starchy endosperm cell walls are rich in arabinoxylans; rice and maize endosperm cell walls are also hemicellulose- as opposed to glucan-rich. To degrade these arabinoxylan-rich walls, and also those of the aleurone layer cells of barley and other cereals, pentosanases such as β-xylanase and α-arabinofuranosidase are synthesized and released from the aleurone layer, thus facilitating the passage of the hydrolases from this region to the starchy endosperm (there are no plasmodesmatal connections between the aleurone layer and the starchy endosperm), and through the cells of this storage tissue. Other enzymes synthesized in the aleurone layer and released into the starchy endosperm include endo- and exo-peptidases (Sect. 5.8) to hydrolyze the predominantly prolamin storage proteins, and phosphatases and nucleases for dephosphorylation of macromolecules and hydrolysis of remnant nucleic acids.
An exception to the above pattern of synthesis and release is shown by β-amylase, at least in barley, rye, rice, sorghum and wheat grains. This enzyme is synthesized in the starchy endosperm during its development, is present in the mature dry grain as up to 1% of total protein therein, and becomes bound to proteins on the periphery of the starch granules, and perhaps to other endosperm proteins, during maturation drying. It is activated when released by selective protein-cleaving hydrolases synthesized and released from the aleurone layer, or by reduction of disulfide bonds by which it is attached to other proteins. Maize grains do not accumulate β-amylase during their development, however; rather this is de novo synthesized in the aleurone layer following germination.
5.5.2 Starch Breakdown and the Fate of the Products of Hydrolysis
During synthesis of starch to form the granule there are abundant channels formed through this structure, extending from pores on the surface to the interior. When there is mobilization of the starch these channels become widened and the pores become deeply pitted before the surface of the granule has been degraded (Fig. 5.6a); these are the paths by which α-amylase, and presumably other hydrolases, penetrate into the granule as the starch is hydrolyzed. To further aid access of hydrolases to the starch, the membrane surrounding the amyloplast, the organelle in which the starch granules are synthesized, disintegrates; this may occur during drying of the mature endosperm, and/or upon subsequent imbibition of the grain. The products of starch degradation, glucose, maltose and small malto-oligosaccharides, along with the hydrolytic products of proteins and cell walls, are taken up into the scutellum for modification and transport into the growing embryo. In some cereal grains (e.g., those of the oat family, but not of barley or wheat), the scutellum elongates into the endosperm as digestion proceeds, thus presenting a much-increased surface area for absorption of the hydrolytic products into the growing embryo. The cells of the epithelial layer of the scutellum elongate and separate to form finger-like projections into the starchy endosperm (Fig. 5.6b, c). They are metabolically very active, with many mitochondria present, and there are numerous transporters in the plasma membrane of these cells, for sugar, amino acid and peptide uptake (Sect. 5.8.2.1). Within the scutellum reside the enzymes for the hydrolysis of di- or oligomeric sugars to glucose, and those for the synthesis of sucrose; there is a vascular conducting system that is continuous from the scutellum into the growing embryo through which this sugar is transported. In rice, when there is an excess of sugar flowing into the scutellum, it is temporarily converted to starch, in granules, in the cells around the vascular tissues. This is then hydrolyzed, converted back to sucrose, and loaded into the phloem by sucrose transporters for distribution in the embryo. A temporary deposition of starch also occurs in the micropylar region of the endosperm of some dicot seeds (e.g., celery and tomato) when stored oils and proteins in this region are mobilized during germination.
(a) Two scanning electron micrographs at low and high magnification that show the degradation of starch granules during hydrolysis in the endosperm of wheat. Scale bar left 10 μm, right 1 μm. From Dronzek et al. (1972). Courtesy of American Association of Cereal Chemists. (b) Scanning electron micrograph of the epithelial layer of cells of the extended scutellum of wild oat to show the extent of their expansion, increasing the surface area over which reserve breakdown products are absorbed from the starchy endosperm into the embryo. Courtesy of J. Sargent and M. Negbi. (c) Light micrographs that show the swelling and extension of the epithelial cells of the scutellum of wheat grains into the depleted cells of the intermediate layer (right) and the beginning of their separation, prior to major reserve mobilization. Protein storage vacuoles (pb) are present but are being depleted, forming smaller (black arrowheads) or empty vesicles (v) as the proteins are hydrolyzed to provide amino acids for the synthesis of hydrolases released into the starchy endosperm. Large white arrow: starch granule. From Swift and O’Brien (1972). Courtesy of CSIRO Publishing
Malting is a manipulated variation of the mobilization of starch in barley grains to produce the maximum amount of fermentable sugars for the brewing of beer and distilling of liquors. Successful malting requires considerable technological and biological understanding of reserve mobilization, garnered from centuries of research and experience; the steps involved are described only very superficially here. Imbibition (steeping) of the grain under tightly controlled conditions of hydration and temperature is necessary to initiate the synthesis of enzymes in the scutellum and aleurone layer while limiting root growth (chitting) to prevent utilization and hence loss of sugars. Barley grains are then transferred to germination beds on which humidification and temperature are again tightly controlled; during this stage there is the major synthesis of hydrolytic enzymes to mobilize cell walls (mixed-linkage glucanases) and storage proteins (proteinases) within the starchy endosperm, thus allowing easier access to the starch of the necessary degrading enzymes. β-Amylase is also activated. α-Amylase is synthesized in the aleurone layer and released into the starchy endosperm, but at this stage its ability to break down starch is restricted: only about 10% of the starch is hydrolyzed during malting. In some malting procedures the hormone gibberellin (GA) may be sprayed onto the grain during germination to enhance enzyme production in the aleurone layer, although germination of the grain during steeping is important to initiate hormone synthesis and release from the scutellum (Sect. 5.5.3). The next stage is kilning, in which the grain is heated from the 16°C germination temperature to about 60°C, and then briefly higher to 82°C for lager malts or 100°C for ale malts. Water is driven from the grain, to about 5%, the malt increases in color (the longer the kilning, the darker the malt), and heat-labile enzymes such as the glucanases, proteases and β-amylase are destroyed; α-amylase is heat resistant. The final result of malting is a grain with a friable endosperm that can be crushed; it has an altered composition of cell walls and proteins, starch that has undergone only limited degradation, and a high amount of α-amylase that has been released from the aleurone layer into the modified starchy endosperm. This malted product is then sold in the dry state and utilized by the brewers and distillers; mashing of the malt under appropriate conditions results in the release of sugars from the starch by α-amylase; their fermentation by yeast produces the required ethanol for beverages.
5.5.3 Hormonal Control of Starch Mobilization
There has been a large amount of research on the regulation of synthesis of starch-degrading enzymes, particularly of α-amylases in barley grains, for which Himalaya has been the cultivar of choice. Its advantages are that it is hull-less, the aleurone layer can be readily isolated from the starchy endosperm, and the former has an almost absolute requirement for GA to induce hydrolytic enzyme synthesis. While this is a good model system in which to understand hormonal regulation, many barley cultivars, and those of other cereals, do not respond so clearly to applied hormone, perhaps because the mature grains already contain considerable amounts of GAs that were imported from the parent plant during their development, and/or because there is considerable and rapid hormone synthesis and distribution from the embryo upon imbibition. Applied GA may speed up enzyme production, however, an advantage in the barley malting industry.
The aleurone layer is essentially a secretory tissue that responds to a hormone signal (GA) released from the embryo (scutellum) (Fig. 5.7). This hormone induces a number of profound changes in the metabolism of the aleurone layer, which lead to the synthesis and secretion of α-amylase and other enzymes (Sect. 5.5.1) to effect mobilization of the contents of the starchy endosperm. When GA is applied to isolated aleurone layers of barley there is the induction of transcripts for α-amylase within 2–3 h, which is followed by its translation to produce the enzyme (Fig. 5.8). At the time of its maximum synthesis, about 25% of the transcripts in the aleurone layer are for this enzyme.
A generalized diagram of a barley grain to show the relationship between embryo and aleurone layer in effecting the hydrolysis of starch by α- and β-amylase. Gibberellin (GA) is synthesized in the embryo and released from the scutellum (A). When it reaches the aleurone layer (B) it stimulates the synthesis of α-amylase that diffuses into the starchy endosperm (C) where it initiates the hydrolysis of starch (F). Activation of β-amylase in the starchy endosperm (E) follows the release of proteases de novo synthesized in the aleurone layer (D); this enzyme hydrolyzes starch polymers (F) released initially by α-amylase. The products of starch hydrolysis, mostly glucose and maltose, are absorbed by the scutellum (G), converted to sucrose and distributed to the growing seedling
(a) Increase with time in the amount of translatable transcripts for barley α-amylase in isolated aleurone layers incubated in water (−GA) or gibberellin (+GA). The mRNA for the enzyme was extracted from the aleurone layers at the various times indicated and its quantity measured as that supporting the synthesis of α-amylase in vitro. (b) Synthesis of the enzyme in vivo in response to GA. U: units of α-amylase activity. After Higgins et al. (1976). (c) Increase in GA-induced transcripts for GA-MYB precedes those for α-amylase, as part of the progression of events detailed in Fig. 5.10. After Gubler et al. (1995). Copyright American Society of Plant Biologists
While α-amylase is discussed here as if it were a single enzyme, in fact in barley and wheat it consists of several different isoforms (posttranscriptional/translational variants from the same gene) and isozymes (forms encoded by different genes of a multi-gene family) separable from each other on the basis of their isoelectric points (pI). In wheat and barley most are members of either the low pI (4.5–5.5, AMY1) or high pI (5.9–6.9, AMY2) group, each being encoded on different chromosomes; fewer variants are present in maize, sorghum, rice or oats and most are in the low pI category. In barley there are ten α-amylase genes expressed in the aleurone layer, of which six are for the high pI forms; these genes are transcribed more and earlier in response to GA. It is likely that the different enzymes have different affinities to bind to granules and the released polymers during starch degradation due to variations in the structure of their carbohydrate-binding sites.
Because the de novo synthesis of α-amylase requires a ready supply of amino acids (Sect. 5.5.1), an early response to GA is the hydrolysis of storage proteins within the aleurone layer. Proteases are present within the PSVs of mature dry and early-imbibed cells, but they have little or no activity because the internal pH of this organelle is above optimal for these enzymes. A decline in pH from approx. 7 to 5 or less is then achieved by GA-induced active pumping of H+ ions into the PSVs through their surrounding membrane (Fig. 5.9); this activates the proteases, thus resulting in the hydrolysis of the storage proteins and the release of amino acids to the protein-synthesizing complex in the cytoplasm. Other hydrolases, e.g., lipases, nucleases and phosphatases also are activated.
A model for changes that occur in protein storage vacuoles (PSVs) of the barley aleurone layer following exposure to GA. (a) The inactive enzymes, and their substrates, in the mature aleurone layer cell (and those exposed to the inhibitor ABA) are depicted for convenience as being in separate vacuoles; in vivo they are together in the same PSVs. (b) The hormone is perceived at the plasma membrane (PM) surface of the cell, and cytosolic signals including Ca2+ and its binding protein calmodulin (CaM) promote coalescence of the PSVs, acidification of the vacuole lumen (the decline in pH being due to an influx of H+ ions), activation of the hydrolytic enzymes and release of the degradation products into the cytosol. This model is based on studies using isolated protoplasts obtained by enzymatic removal of the cell walls of the aleurone layer. From Bethke et al. (1998). With permission of Oxford Univ. Press
The signalling pathway for GA (the active form being GA1), from its reception at the surface of the aleurone layer cell to the transcription of the α-amylase genes, is shown in Fig. 5.10. GA1 may be first detected by an appropriate receptor complex in the plasma membrane of the cell (step 1), which initiates two separate signal transduction pathways (step 2). Of these, the Ca2+-independent pathway leads to the transcription of the genes for α-amylase (steps 3–10) and other GA-induced hydrolases, while the other promotes a two- to threefold increase in steady-state cytosolic Ca2+ concentrations (steps 11, 12), perhaps by its import from the apoplast; this cation is important for enzyme activation (see later).
Diagrammatic representation of the induction of α-amylase synthesis in a barley aleurone layer cell by GA (GA1). A calcium-independent pathway (steps 1–11) induces the transcription of α-amylase (and other hydrolases) whereas activation and secretion of this enzyme requires a calcium-dependent pathway (step 12). The steps are explained in the text. From Taiz, L. and Zeiger, E., Plant Physiology, 5th Edition. Sinaur Associates, Sunderland, Mass., with permission
In the Ca2+-independent pathway GA1 binds to a soluble GA receptor protein (GID1) in the nucleus (step 3), which causes a change in its configuration, facilitating its binding to a DELLA-GRAS protein complex (depicted in blue and yellow, respectively, in step 4). An F-box protein (F-box proteins contain an F-box domain of amino acids that encourages protein–protein interactions) enters the nucleus and binds to this complex, and this allows for the addition of several ubiquitin molecules (ubiquitination) to the GRAS protein (step 5). The DELLA-GRAS proteins incorporated into GID1 (step 4) come from the upstream promoter region of a gene for GA-MYB, where they form a repressor-protein complex preventing its transcription. DELLA proteins (so named because they all contain the conserved amino acid sequence DELLA: aspartic acid, glutamic acid, leucine, leucine, alanine) have been identified in many plant tissues as negative regulators of GA responses, by blocking the promoter region of GA-responsive genes. Their removal is necessary for GA to induce the promoter. This occurs when the ubiquinated DELLA-GRAS protein is targeted and degraded by a specific set of proteases present within a hydrolytic proteasome complex within the nucleus (step 6).
Thus, with the repressor protein complex removed, the GA-MYB gene can now be transcribed (step 7) (Fig. 5.8c) and its mRNA migrates to the protein synthesizing complexes in the cytosol where the GA-MYB transcription factor is translated, followed by its import into the nucleus. It now binds to a specific GA-response element (GARE) in the promoter region of α-amylase gene (step 8) and other GA-induced genes to effect their transcription (step 9). The transcripts leave the nucleus and are translated on endoplasmic-reticulum-associated polysomes; the resultant proteins enter into the lumen of this rough endoplasmic reticulum (RER) and are transported through the endomembrane system via the default pathway to the Golgi (step 10). As this occurs the enzyme proteins may undergo posttranslational modifications. The enzymes are packaged into secretory vesicles (step 11) that migrate to the plasma membrane, with which they fuse to release the enzymes from the aleurone layer cell. As noted in Sect. 5.5.1 the walls of the aleurone layer cells can impair the movement of α-amylase into the starchy endosperm; GA-induced synthesis and secretion of degrading pentosanases results in the formation of channels in the intervening walls, and their eventual total digestion.
The Ca2+-dependent signal transduction pathway (step 12) plays an important role in the activation of α-amylase; the enzyme is a Ca2+-containing metalloprotein that must bind this cation while in the lumen of the ER or Golgi in order to be active when secreted. A GA-stimulated Ca2+-calmodulin (CaM)-dependent pathway also plays a role in ensuring secretion of the enzyme. In addition there are complex signal-transduction pathways involving Ca2+-sensors such as CaM, and Ca2+-activated protein kinases, as part of a second messenger complex coupled to hormone induction. These also play a role in the regulation of GA-induced cellular changes.
While the main focus of this section has been on the positive influence of GA on the induction of synthesis of α-amylase, this is suppressed in the presence of abscisic acid (ABA). For example, the acidification of the PSVs is prevented by ABA (Fig. 5.9a), and there is a suppression of transcription of genes for α-amylase and other hydrolases. Both hormones are synthesized by cereal embryos and diffuse to the aleurone layer; hence, it is likely that a balance in influence of the two is important in regulating the extent of hydrolase production and secretion. Whether or not such a balance influences α-amylase synthesis in the scutellum is unclear. In the aleurone layers of both barley and wheat there are numerous changes in transcript production under the influence of ABA of GA. Many more genes are up-regulated by ABA than are down-regulated, the number of the former being more or less equal to the number up-regulated by GA; but a larger number are down-regulated by ABA. This points to a complex interaction between these antagonists at the genome-, and consequently the cellular/metabolic-level.
The activity of ABA in barley aleurone layer cells involves synthesis of an ABA-induced protein kinase (PKABA1), which acts as a suppressor at some point along the signal transduction pathway for GA. Hence, as a result of this cross-talk at the intersection of the two hormonal pathways, there is inhibition of GA-induced expression of the genes for low and high pI α-amylases and the proteases responsible for mobilization of the storage proteins in the starchy endosperm, as simplified below:

In reality, this interaction is considerably more complex, and involves several proteins including transcription factors that are negative regulators of GA signalling, and regulatory proteins such as those of the 14-3-3 class (a conserved family of proteins that bind to diverse signalling proteins, including kinases and transmembrane receptors). Information obtained from Arabidopsis indicates that PKABA1 itself needs to be phosphorylated to be active. This key phosphorylation event is performed by a kinase involved in ABA signal transduction, such as SNF1-related protein kinase 2 (SnRK2). However, this kinase is suppressed by a phosphatase, such as ABI1 and ABI2, in the absence of ABA. This suppression of the kinase by the phosphatase is eliminated upon the perception of ABA by an ABA receptor, such as PYR1 (PYRABACTIN RESISTANCE1), because the receptor protein inactivates the phosphatase and activates the SnRK2 and PKABA1. This scheme is also very important in the regulation of germination by ABA (Sect. 6.6.1.1).
5.5.4 Programmed Cell Death of the Aleurone Layer and Other Tissues
Upon completion of mobilization of the reserves from the starchy endosperm of cereal grains the aleurone layer undergoes PCD, as does the scutellum. As a result there is autolysis of their cells from which nutrients are mobilized and transferred to the growing embryo. PCD of the aleurone layer commences in the cells nearest to the embryo and then extends to the more proximal ones. The demise of the aleurone layer is stimulated by GA, but is considerably delayed or prevented by ABA. Nitric oxide (NO), which is synthesized from NO −2 in the apoplast of aleurone layer cells, can also delay the onset of PCD by acting as an antioxidant.
Cell death is as a result of oxidative stress, which is stimulated by GA in two ways: (1) It promotes the breakdown of oils stored in the aleurone layer and during their conversion to sugars. β-Oxidation of the fatty acids (Sect. 5.7) in the glyoxysome releases hydrogen peroxide, a reactive oxygen species (ROS) (Sect. 8.4.1) that inflicts damage on macromolecules. Additional ROS are produced in the mitochondria. (2) It suppresses the expression of genes for enzymes that are able to defend the cells against the ROS attack, e.g., superoxide dismutase, catalase and ascorbate peroxidase. Death of the cells occurs when they become highly vacuolated accompanied by a loss of plasma membrane integrity; this results in loss of turgor and cytoplasmic collapse. In contrast, ABA maintains or promotes high expression of the genes for the defensive enzymes, prevents the hydrolysis of stored oils and stimulates mitochondria to minimize ROS production. In the aleurone layers of barley and wheat, in the presence of GA there is also an accumulation of nucleus-located nucleases late during PCD; DNA is not degraded in GA-insensitive mutants (wheat) or when ABA is present (barley).
PCD is a common phenomenon in plants, involved in events from cell differentiation to senescence. With respect to seed initiation and development it is operative, for example, during megaspore determination in the embryo sac, in the release of pollen from the anthers, in controlling the death of the nucellus and the suspensor during embryogenesis. It also causes the loss of metabolic integrity of the cells of the starchy endosperm of cereals so that it is nonliving at maturity, of the endosperms of castor bean and tomato as they become depleted of reserves following germination, and likewise in the expended cotyledons of germinated dicot seeds.
The regulation of PCD in the developing cereal endosperm is tightly controlled, so that there is the completion of at least most of the synthesis of the storage reserves before entry into the cell death program. This appears to be influenced by the hormones ABA and ethylene, the former delaying the program, and the latter accelerating it; therefore, progression of PCD could be regulated by ABA through its effect on ethylene synthesis. Why the aleurone layer, the only region of the endosperm that remains living in the mature grain, is immune from ethylene-induced PCD during late development is unknown; this hormone does not appear to play a role in its post-germination PCD either.
See also Sect. 5.8.3 for information on PCD in reserve tissues of dicots.
5.6 Mobilization of Stored Carbohydrate Reserves in Dicots
In contrast to the large amount of research on triacylglycerol (TAG) mobilization in dicots (Sect. 5.7), there have been relatively few studies on starch utilization, and mostly in legumes. In non-endospermic legumes the endosperm is broken down as a source of nutrients during seed development, being either residual in (e.g., soybean), or absent from (e.g., peas, Phaseolus bean) the mature seed; the cotyledons assume the role as the major storage organ. These may contain predominantly starch or TAGs. Endospermic legumes of the tribe Trifolieae retain a substantial endosperm at maturity (e.g., fenugreek, carob, guar) and it becomes the site of stored carbohydrate reserves, as hemicelluloses, whereas the cotyledons are the site of storage proteins.
The regulation of dicot reserve mobilization is discussed as a separate Sect. 5.10 following individual accounts of the mobilization of each of the reserves because there is only a limited amount of information on any one of them.
5.6.1 Starch-Storing Non-endospermic Legumes
Hydrolysis of starch reserves in the cotyledons commences after germination is completed. Their depletion in pea cotyledons is biphasic, an initial slow rate, during which starch phosphorylase is the dominant hydrolase, being followed by a more rapid starch loss as activity of amylases (probably both α- and β-) increases (Fig. 5.11a, b). To what extent the phosphorylase can attack the native starch granule is unknown; a role in the degradation of soluble glucans released by amylolytic attack, achieved by the relatively low amount of amylase activity at this time, is more likely. Mobilization is aided by disintegration of the amyloplast membrane, exposing the starch to cytosolic enzymes, which appear to include a limit dextrinase and the phosphorylase. Conversion of released malto-oligosaccharides by the latter enzyme to Glc-1-P likely occurs in the cytosol, and this in turn is converted to sucrose and exported to the growing seedling; free sugars and dextrins do not accumulate in the cotyledons (Fig. 5.11a). In studies of amylolysis of starch in chickpea, mung bean and Phaseolus bean seeds, increases in α-amylase activity in the cotyledons have been reported following germination. Starch mobilization in black gram seeds is purported to involve the import of the starch granules into lytic vacuoles, formed from protein storage vacuoles, for hydrolysis (Sect. 5.8.3).
(a) Changes in the amount of starch and dextrin (●), oligosaccharides (○), free sugars (▲), and extracted protein (Ñ) in the cotyledons of pea, cv. Early Alaska. (b) Changes in starch phosphorylase (○) and amylase (●) activities. After Juliano and Varner (1969)
5.6.2 Hemicellulose-Storing Endospermic Legumes
In many species of the Trifolieae a well-developed endosperm, with thick-walled cells containing the storage carbohydrate galactomannan, lies between the seed coat and the cotyledons. In fenugreek extensive deposition of this hemicellulose polymer to the inside of the primary walls during seed development results in the gradual occlusion of the living contents until in the mature seed the cells are dead (Sect. 3.2.2). The outermost region of the endosperm is the aleurone layer that is made up of a unilayer of living thin-walled cells devoid of galactomannan (Fig. 5.12a). In most endospermic legumes, however, the endosperm cell walls do not completely occlude the cytoplasm, and all cells have living contents at maturity (e.g., Chinese senna, Fig. 1.4).
Light micrographs of the outer region of a fenugreek seed in the lateral endosperm region: (a) During germination and before mobilization of the endosperm reserves. The three-layered seed coat (SC), a small part of the cotyledon (C), and the endosperm layer (A and E) are shown. The aleurone layer (A) is the outer living single-cell layer of the endosperm, the rest (E) being composed of large cells with thin primary walls to the inside of which is deposited the dark-staining galactomannan secondary cell wall that appears to completely fill the cell. (b) Following germination, when the galactomannan-rich cell walls in the endosperm are being dissolved. The dissolution zones (clear regions in the endosperm) begin at the aleurone layer, the source of hydrolytic enzymes, and spread toward the cotyledons. (c) The endosperm is almost depleted and only a remnant remains between the seed coat and the cotyledon. The aleurone layer is still present, but will soon disintegrate. Starch granules (stained blue) are present in the cells of the cotyledon. Courtesy of J.S.G. Reid, Univ. Stirling. For original micrographs see Reid, J. S. G. 1971. Planta 100, 131–142
In fenugreek seeds, and perhaps those of other endospermic legumes also, the endosperm plays a role in addition to that as a carbohydrate storage reserve. The high affinity of galactomannans for water (when imbibed, many become mucilaginous) allows the endosperm to regulate the water balance of the embryo during germination; this may be important to plants in their native habitat, since many members of the tribe Trifolieae have their origins in the dry regions of the eastern Mediterranean.
After emergence of the radicle, the galactomannan in the endosperm begins to be mobilized. There is a wave of hydrolysis in the fenugreek seed commencing close to the aleurone layer and moving toward the cotyledons (Fig. 5.12b) until the reserves are depleted (Fig. 5.12c). This is due to the synthesis and release from this layer of three critical enzymes: α-galactosidase, β-mannosidase (exo-β-mannanase), and endo-β-mannanase. α-Galactosidase is an exopolysaccharidase that cleaves the α-(1 → 6) link between the unit Gal side chains and the Man backbone.

Endo-β-mannanase is an endoenzyme that hydrolyzes oligomers of Man (tetramers or larger) to mannobiose or mannotriose, and β-mannosidase then converts these to Man. The latter enzyme might also act as an exo-mannopolysaccharidase and hydrolyze single Man residues from the oligomannan chain. Mannan breakdown by phosphorolysis appears not to occur.
The released Gal and Man are absorbed by the cotyledons, Gal by passive diffusion, but Man requires active uptake utilizing a carrier-specific component. Neither sugar accumulates in the cotyledons, but instead they are metabolized further, perhaps by initially being phosphorylated to Gal-1-P and Man-6-P. If not used directly for energy metabolism, they are transformed to sucrose and then to starch, which is remobilized when the sucrose content of the cotyledons falls after its transport to the axis. This sequestering of sugars as a large polymer is a convenient strategy for the removal of potentially osmotically damaging monomers, and for the retention of useful metabolites. Not surprisingly, an increase in α-amylase activity within the cotyledons coincides with starch hydrolysis. A summary of the events involved in galactomannan breakdown in endospermic legumes is shown in Fig. 5.13. However, not all the enzymes required for the conversion of Man to sucrose and starch have been located within the cotyledons, although it is reasonable to assume that they are there.
Flow diagram to illustrate the potential fate of the products of galactomannan mobilization in endospermic legumes. Enzymes: (1) α-galactosidase; (2) endo-β-mannanase and β-mannosidase; (3) galactokinase; (4) hexose phosphate uridyl transferase (a group of three enzymes that convert Gal-1-P + UTP ® UDPGal ® UDPGlc ® Glc-1-P + UTP); (5) mannokinase; (6) phosphomannomutase; (7) phosphomannoisomerase; (8) sucrose-6-P synthetase; (9) sucrose phosphatase; (10) C2 epimerase; (11) phosphoglucomutase; (12) sucrose synthase or sucrose-6-P synthase (see Sect. 5.4.1); (13) see Sect. 3.2.1; (14) see Sects. 5.4, 5.4.1. Gal, galactose; Man, mannose; Glc, glucose; Fru, fructose
Arabinogalactans are present in the thickened cell walls of lupin cotyledons, composed of β(1 → 4)-linked Gal residues with α(1 → 5)-arabinose side chains; these hemicelluloses are degraded at the same time as the stored reserves (proteins and oils) within the cells are mobilized. During cell wall utilization there is a transient increase in starch in the cotyledons, as in fenugreek, presumably because cell wall mobilization outstrips the ability of the cotyledons to export sucrose, the final product of arabinose and Gal conversion, to the growing axis. Cotyledons of seeds of the tropical legume tree Hymenaea courbaril contain xyloglucans in their cell walls as storage polysaccharides. During their mobilization there is an increase in xyloglucan hydrolases, free sugars, and a transient increase in starch in the cotyledons when transport of the sugars is slower than the rate of their production.
5.6.3 Hemicellulose-Containing Seeds Other than Legumes
A number of nonleguminous plants also store mannans, although few have received much attention as far as mobilization of their reserves is concerned. The role of hormones in the mobilization of cell wall galactomannans in tomato and lettuce seeds is detailed in Sect. 5.10.1.
Hydrolysis of polysaccharides in the endosperm of date palm (89% Man deposited in the secondary walls; much of the rest is cellulose) occurs when a haustorial projection from the hypogeal seedling (Sect. 5.1) grows into it. This results in preformed hydrolytic enzymes being released from protein storage vacuoles in the endosperm, which come into contact with the wall following loss of outer membrane integrity. The galactomannan is degraded to its constituent monomers, which are absorbed by the haustorium and transported to the growing axis; there they are converted to sucrose.
Mobilization of galactomannans from the cell walls of the lettuce seed endosperm commences when endo-β-mannanase activity increases within the endosperm itself, immediately after germination is completed. α-Galactosidase is present as a constitutive enzyme within the endosperm. The products of hydrolysis diffuse to the cotyledons, and small oligomannans are cleaved further by β-mannosidase located in their cell walls; the resultant Man residues are taken up by the cotyledon cells (summarized in Fig. 5.30). The breakdown of galactomannans within the endosperm of tomato also requires the synthesis of two isozymes of endo-β-mannanase, one in the micropylar endosperm, which is involved in the completion of germination (Sect. 4.6.1), and one in the lateral endosperm that mobilizes the cell walls following germination.
While the walls of the hard endosperm in seeds such as of coffee are very thick, and may account for over 50% of the cell volume, there is still some cytoplasm present in the mature cells, and they are capable of producing the appropriate hydrolases; there is no peripheral aleurone layer. Initially in the imbibed coffee seed there is the synthesis and expression of transcripts for endo-β-mannanase and β-mannosidase in the micropylar region of the endosperm during germination, presumably to facilitate radicle emergence, and later endo-β-mannanase in particular increases in the lateral endosperm as this major area of mannan reserves is mobilized. Following germination the cotyledons remain embedded in the endosperm until it is depleted and emerged above the soil, thus allowing for continued import of cell-wall-derived sugars into the growing seedling.
Some seeds store hemicelluloses other than mannans, and degrade them as a carbohydrate source following germination. The cell walls of nasturtium cotyledons contain “amyloids” that stain with iodine in a starch-like reaction. However, the walls are composed of (galacto)xyloglucans; these are degraded initially by xyloglucan endotransglycosylase and β-galactosidase to form oligomers, which are then converted to free monosaccharides by α-xylosidase and β-glucosidase; of these, the first three enzymes increase in activity in the cotyledons following germination, but the glucosidase is present in the dry seed and remains constant in activity following imbibition.
5.7 Stored Triacylglycerol Mobilization
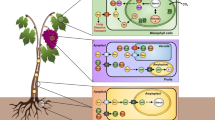
Seed oil- (TAG) catabolism, like that of its synthesis involves many enzymes located in several organelles within the storage cell. An overview diagram is shown in Fig. 5.14. Initial TAG hydrolysis (lipolysis) is by lipases, enzymes that catalyze the three-stage hydrolytic cleavage of the fatty acid ester bonds, ultimately to yield glycerol and free fatty acids (FFAs). The latter enter the peroxisome (often called the glyoxysome in seeds) for conversion to oxaloacetic acid (OAA), which then passes into the mitochondrion, and finally into the cytosol for conversion to sucrose, the sugar that is transported from the storage cotyledons to the growing regions of the seedling, or from the storage endosperm to the cotyledons and thence throughout the seedling.
Generalized schematic of triacylglycerol (TAG) mobilization in reserve tissues of seeds following germination. The TAGs in the oil body are hydrolyzed to free fatty acids (FFA) and glycerol (Gly), through the diacylglycerol (DAG) and monoacylglycerol (MAG) forms, possibly by the sequential action of several lipases (steps 1–3). Gly is converted to dihydroxyacetone phosphate (DHAP) by glycerol kinase (4) and Gly-3-P dehydrogenase (5). FFAs are transported to the glyoxysome and activated to acyl-CoAs (6) and enter the β-oxidation spiral (7). The acetyl-CoA product is converted to organic acids by the glyoxylate cycle and subsequent steps result in products such as oxaloacetic acid (OAA) in the mitochondrion (Mit) and the cytosol (8), which along with DHAP is converted by gluconeogenesis (9) to sucrose (Suc)
In more detail (Fig. 5.15), the FFAs released by lipases (step 1) are utilized in oxidation reactions in the glyoxysome to yield compounds containing fewer carbon atoms. The predominant oxidation pathway is β-oxidation, in which the FFA is first esterified with coenzyme A (CoA) in a reaction requiring ATP, and then, by a series of steps involving the successive removal of two carbon atoms this acyl CoA is broken down to acetyl CoA (steps 2–6). This requires that the enzymes in each step of β-oxidation sequentially accept substrates that are progressively 2C shorter in length; thus they either have multiple isoforms with different chain-length specificities, or they have broad substrate specificity.
Detailed pathways of TAG catabolism and sucrose synthesis. Enzymes: (1) lipases, e.g., SDP1; (2) fatty acid thiokinase; (3) acyl CoA dehydrogenase; (4) enoyl CoA hydratase (crotonase); (5) β-hydroxyacyl CoA dehydrogenase; (6) β-ketoacyl thiolase; (7) citrate synthase; (8) aconitase*; (9) isocitrate lyase; (10) malate synthase; (11) malate dehydrogenase**; (12) catalase; (13) succinate dehydrogenase; (14) fumarase; (15) malate dehydrogenase; (16) phosphoenolpyruvate carboxykinase; (17) enolase; (18) phosphoglycerate mutase; (19) phosphoglycerate kinase; (20) glyceraldehyde-3-phosphate dehydrogenase; (21) aldolase; (22) fructose-1,6-bisphosphatase; (23) phosphohexoisomerase; (24) phosphoglucomutase; (25) UDPG1c pyrophosphorylase; (26) sucrose synthase or sucrose-6-P synthase and sucrose phosphatase. (i) Glycerol kinase; (ii) α-glycerol phosphate oxidoreductase. Substrates: TAG, triacylglycerol; MAG, monoacylglycerol; Gly, glycerol; FFA, free fatty acid; PEP, phosphoenolpyruvate; 2PGA, 2-phosphoglyceric acid; 3PGA, 3-phosphoglyceric acid; DPGA, 1,3-diphosphoglyceric acid; G3P, glyceraldehyde-3-phosphate; FruDP, fructose-1,6-bisphosphate; Fru-6-P, fructose-6-phosphate; Glc-6-P, glucose-6-phosphate; Fig. 5.15 (continued) Glc-1-P, glucose-1-phosphate; UDPG1c, uridine diphosphoglucose; α-Gly P, α-glycerol phosphate; DHAP, dihydroxyacetone phosphate. Coenzymes and energy suppliers: FAD/(H), flavin adenine dinucleotide/(reduced); NAD/(H), nicotinamide adenine dinucleotide/(reduced); GTP, guanosine triphosphate; ATP, adenosine triphosphate; UTP, uridine triphosphate; GDP, guanosine diphosphate; ADP, adenosine diphosphate; AMP, adenosine monophosphate; CoA, coenzyme A. *located in the cytosol; ** present in the glyoxysome and cytosol, but in the latter location is part of the glyoxylate cycle
Saturated fatty acids with an even number of carbon atoms yield only acetyl CoA. Chains containing an odd number of carbon atoms, if completely degraded by β-oxidation, will yield the two-carbon acetyl moieties (acetyl CoA) and one three-carbon propionyl moiety (propionyl CoA, CH3CH2CO-S-CoA). This, in turn, can be degraded in a multistep process to acetyl CoA. The acetyl moiety may be completely oxidized in the citric acid cycle to CO2 and H2O or utilized initially via the glyoxylate cycle for carbohydrate synthesis (steps 8–11). This latter process is the most important during seedling establishment.
The oxidation of unsaturated fatty acids (e.g., oleic acid; 18:1Δ9cis) is by the same general pathways, although some extra steps are required. The double bonds of naturally occurring unsaturated fatty acids may be in the cis configuration, which is a block to β-oxidation. Thus, for step 4 to occur, they must be in the trans position. Hence, in a reaction involving at least four enzymes (three isomerases and a reductase) the FFA is converted to its oxidizable form:

which is the normal substrate for the next enzyme in the β-oxidation pathway, enoyl CoA hydratase (step 4). Polyunsaturated fatty acids containing two or more double bonds (e.g., linoleic acid, 18:2; linolenic acid, 18:3) cannot be degraded simply by β-oxidation either, but the appropriate enzymes (2,3 enoyl CoA isomerase, 3-OH acyl CoA epimerase and 2,4 dienoyl CoA reductase) that are required for the continuation of β-oxidation are present within the glyoxysome. For β-oxidation of ricinoleic acid (12-OH 18:1), the C8-intermediate (2-hydroxy 8:0) fatty acid requires conversion by an α-hydroxy acid oxidase and oxidative decarboxylation to circumvent the metabolic barrier caused by the hydroxyl group. The heptanoyl CoA so formed can be catabolized further by β-oxidation. Analyses of over 7,000 plant species has revealed that there are hundreds of different minor fatty acids in seed oils, some of which are family-, genus-, or even species-specific; their variation in structure and substitutions is extensive, but presumably for each there is an appropriate enzyme or enzymes in the glyoxysome to ensure that they are efficiently catabolized.
A by-product of β-oxidation is hydrogen peroxide (H2O2), a reactive oxygen species (ROS) that is damaging to macromolecules such as proteins and nucleic acids. This is broken down in the glyoxysome to water and molecular oxygen by catalase (step 12). In addition, there is a glyoxysome-membrane-bound H2O2-eliminating set of enzymes.
Directly coupled to the β-oxidation pathway is the glyoxylate cycle, which takes the acetyl CoA and, in a series of enzymatic reactions, links this to the glycolytic pathway, which then operates to produce hexose. The key enzymes for forging this link are malate synthase (MLS) and isocitrate lyase (ICL), which are unique to the glyoxylate cycle. Acetyl CoA is first converted to citrate (in the same manner as initiates its entry into the citric acid cycle: step 7), then to isocitrate, which is cleaved to produce succinate and glyoxylate. Another acetyl CoA is incorporated into the cycle (step 10) and is condensed with glyoxylate by MLS to yield malate. With each turn of the cycle one molecule of succinate is released (step 9) and is converted to oxaloacetate by citric acid enzymes in the mitochondria (steps 13–15), and then into the glycolysis pathway as phosphoenolpyruvate (step 16) for conversion to sucrose. For simplicity, the location of all glyoxylate cycle enzymes in Fig. 5.15 is depicted as being within the glyoxysome; however, two of the five enzymes involved, aconitase (step 8) and malate dehydrogenase (step 11) are present in the cytosol (although the recycling of NADH to NAD occurs within the organelle). This requires that there be present in the glyoxysome membrane efficient shuttling mechanisms so that intermediates in the cycle can readily pass into the cytosol and back again.
An important enzyme in the completion of gluconeogenesis is a pyrophosphatase (V-H+PPase), which is located in the vacuolar membrane and converts cytosolic pyrophosphate produced during steps 22 and 25 to phosphate (PPi to Pi). A mutant (fugu5) of Arabidopsis lacking this enzyme exhibits poor seedling establishment due to a decrease in sucrose synthesis in the cotyledons, where the oil is stored, although it can complete glyoxysome-related steps beyond β-oxidation. The cytosolic accumulation of PPi likely results in a feedback reaction suppressing steps 22 and 25, thus interfering with the completion of gluconeogenesis and resulting in a poorer supply of sucrose essential for seedling growth.
Glycerol, produced when the TAG is stripped of its fatty acids, enters the glycolytic pathway after its phosphorylation by glycerol kinase in the cytosol and is oxidized in the mitochondrion to the triose phosphate dihydroxyacetone phosphate. This is released into the cytosol and after conversion to glyceraldehyde-3-phosphate (G-3-P) is condensed by aldolase to another G-3-P in the reversal of glycolysis to yield hexose units (step 21), and ultimately sucrose (step 26). Alternatively, the triose phosphates may be converted to pyruvate and then oxidized through the citric acid cycle in the mitochondrion.
5.7.1 Mobilization of TAGs from Oil Bodies
While more research has been conducted on mobilization in the cotyledons and endosperms of oil-storing dicot seeds, the pattern of TAG utilization is similar in many ways in cereal grains (where the oil is mostly in the scutellum) and in gymnosperm megagametophytes.
Lipases are usually only detected following germination and are located in the oil body membrane as well as that of glyoxysomes; the close association between these organelles (Sect. 5.7.2) is consistent with an interaction between them to ensure lipolysis and the transfer from the former to the latter of FFAs for further modifications. Several lipases, with different pH optima, have been identified in oil-storing seeds, and their genes cloned, but in many instances their importance in the release of FFAs from TAGs has not been established. However, the Arabidopsis mutant sdp1 (sugar-dependent1) is defective in a lipase associated with the oil body membrane, and has considerably less ability to mobilize TAGs in the cotyledons, resulting in seedlings that exhibit poor growth compared to those of the wild type (Fig. 5.16a, b). The SDP1 enzyme likely initiates TAG mobilization in this species; because it shows preference for TAGs over DAGs or MAGs, other lipases may well be involved in completing lipolysis. The FFAs are imported into the glyoxysome by special membrane-associated transporters.
(a) Phenotypic differences between 5-day-old seedlings of wild-type Arabidopsis and a lipase-deficient mutant sdp1-1 (sugar-dependent1-1) showing poor growth of the latter. (b) Triacylglycerol (TAG) content of wild-type (WT) and mutant at 0 (blue bars) and 5 days (orange bars) from the start of imbibition; the failure to mobilize most of the storage oil results in poor growth of the seedling. After Quettier and Eastmond (2009). Courtesy of Elsevier
5.7.2 Role and Formation of the Glyoxysome
The overall process of conversion of FFA to glucose is termed gluconeogenesis, by definition the production of this sugar from a non-carbohydrate source. The FFAs are provided from the oil bodies, and subsequent steps require the participation of glyoxysomes and mitochondria, with the final synthesis of glucose; the subsequent formation of the transport sugar sucrose occurs in the cytosol. Consult Sect. 5.7 for details. Because of the metabolic collaboration between the three organelles it is not surprising to find them in juxtaposition within the cell (Fig. 5.17).
Electron micrograph of an oil-storing cell in the cotyledon of a dark-grown cucumber seedling. The glyoxysome (g) is in close proximity to the oil body (ob) and mitochondrion (m). The vacuole (v) and cell wall (cw) are also marked. Bar 0.5 μm. Courtesy of R.N. Trelease, Arizona State Univ. and R.T. Mullen, Univ. Guelph
Glyoxysomes are a special class of peroxisomes (previously referred to as microbodies) that contain all of the enzymes of the β-oxidation spiral, and also the glyoxylate cycle with the unique enzymes ICL and MLS. The glyoxylate cycle resembles the citric acid cycle, except that the decarboxylation steps between isocitrate and succinate are circumvented by the action of ICL, thus avoiding the loss of carbon as CO2. Like all peroxisomes they do not contain nuclear material or a protein-synthesizing complex; they are bounded by a single membrane and are slightly denser than mitochondria. Some are formed during mid- to late- stages of development in the oil-storing cells, and in mature seeds they are small, with a diameter of ∼0.2 μm and while they contain some of the component enzymes, these are insufficient in amount. By the time the glyoxysomes are fully active in the processing of FFAs in the germinated seed they have become 10–20 times larger and all of the enzymes are appropriately present; also, new organelles are formed. For this to be achieved there must be an import of new materials into their membranes, and of enzymes into the matrix.
A general, but simplified model for glyoxysome biogenesis follows. During seed development, in cells of oil-storage tissues, there is the formation of distensions in specific regions of the endoplasmic reticulum (peroxisomal ER, pER) where there is insertion of a certain subset of glyoxysomal membrane proteins, all of which are synthesized on free cytosolic polysomes (Fig. 5.18a). The resultant pre-glyoxysomal vesicles are stable in the mature dry seed, and following germination more are produced in a similar manner. Their enlargement to form mature glyoxysomes occurs by the addition (posttranslationally) of nascent matrix proteins, and other membrane proteins, followed by, or concomitant with, their fusion with other newly formed pre-glyoxysomal vesicles; fusion can also occur with preexisting mature glyoxysomes.
(a) Schematic representation of the steps involved in the biogenesis of glyoxysomes. Pre-glyoxysomal vesicles containing certain membrane proteins are budded off from a specific region of the ER (peroxisomal ER, pER). Select membrane proteins are synthesized in the cytosol on free polysomes and imported into the pER. Alternatively, membrane proteins may be imported into the ER at sites other than the pER (“general” ER) and migrate to this region. The released pre-glyoxysomal vesicles increase in size by fusion with preexisting immature glyoxysomes, or with daughter glyoxysomes formed by fission of mature glyoxysomes. As the glyoxysomes are increasing in size, matrix proteins and additional membrane proteins are imported from free polysomes within the cytosol. Based on information in Ma et al. (2011). Also see diagrams in Mullen, R.T. and Trelease, R.N., Biochim. Biophys. Acta 1763, 1655–1668 (2006). (b, inset) Model to explain glyoxysome proliferation. This occurs through sequential elongation, constriction and fission. The steps require the association of several proteins with the membrane at each stage, e.g., peroxins (PEX proteins) for elongation, and dynamin-related proteins (DRP-family proteins) for fission. After Kaur and Hu (2009). Courtesy of Elsevier
The mature glyoxysomes themselves may divide to form incomplete daughter glyoxysomes; this involves first their elongation (the membrane material for this being ER/pER-derived), then constriction in defined regions, followed by fission to form new organelles (Fig. 5.18b). As already indicated, there are two potential routes by which the necessary membrane proteins are added to the pre-glyoxysome as it grows. In both cases the proteins are synthesized on free polysomes within the cytosol: one route has them inserted directly into the pre- or daughter glyoxysome, and the other is for them to be inserted in the general ER and/or pER, from where nascent pre-glyoxysome vesicles bud and fuse with a daughter glyoxysome.
Similarly to the proteins incorporated into the glyoxysome membrane, those destined for the matrix or interior of the organelle are nuclear encoded, synthesized on free cytosolic ribosomes, and targeted posttranslationally. One of at least two types of conserved amino acid sequences is present on proteins destined for transport into the glyoxysome; these are the type 1 and type 2 peroxisomal targeting signals (PTSs). The PTS1 is most commonly present on matrix-bound proteins; it is located at the C-terminus of the protein as a terminal tripeptide, usually as a small-basic-large and hydrophobic amino acid motif, (e.g., serine-lysine-leucine: SKL). The PTS2 has a nonapeptide motif (arginine-valine-5 variable amino acids-histidine-phenylalanine: RV[X5]HF) near to the N-terminus, and is present on matrix proteins that are usually proteolytically processed after import into the organelle. As shown in the simplified model in Fig. 5.19, import of all matrix proteins can be divided into four stages: initial binding of the protein to be imported (termed the cargo protein) to its cognate receptor; transport and docking of the receptor-cargo complex at the glyoxysome membrane; translocation of this complex across the membrane into the matrix with release of the cargo; recycling of the receptor.
A model for the import of matrix proteins into the peroxisome/glyoxysome. (a) Following their synthesis on free cytoplasmic polysomes, matrix-destined (cargo) proteins with carboxy-terminal PTS1 or amino-terminal PTS2 sequences are recognized by their appropriate (cognate) PEX receptor proteins. (b) PEX5 can act as a co-receptor for PEX7 and (c) they travel to the glyoxysomal membrane where they are recognized by specific peroxisomal membrane-bound PEX docking proteins. (d) Two possible mechanisms of transfer of the cargo protein into the matrix are by a simple shuttle (left) or an extended shuttle (right) mechanism. For simplicity the docking proteins and PEX7-cargo protein are omitted from this step, although the latter follow the same pathway. Also not shown are the membrane-associated PEX proteins involved in the recycling of the receptor into the cytosol. Modified from diagrams in Laynton-Hogg et al. (2010). See also Ma et al. (2011)
Genes that are involved in peroxisome/glyoxysome biogenesis and maturation are called PEROXIN (PEX) genes, which are conserved in plants, animals and yeast; several PEX proteins play a role in the import of cargo proteins into glyoxysomes. The PTS regions in the proteins to be imported are recognized by receptor proteins in the cytosol (PEX5 or PEX7) (Fig. 5.19). These now combine and travel to the surface of the organelle. For a protein to enter into the glyoxysome matrix the receptors must first be recognized by docking proteins, which are a group of PEX proteins, the majority of which are called peroxisomal membrane proteins (PMPs) present on the glyoxysomal membrane outer surface. The next step is the translocation of the cargo protein across the membrane and its subsequent release into the matrix. This particular step is incompletely understood, but two types of mechanisms are proposed: (1) a simple shuttle mechanism operates allowing the receptor-cargo complex to pass through the membrane before the cargo is released and the receptor is returned to the cytosol; (2) the extended shuttle model proposes that the receptor protein remains in the membrane to form a pore through which other receptor-cargo proteins can pass, the cargo being released and the receptor recycled.
In species whose mode of seedling growth is epigeal the cotyledons turn green as they emerge from the soil into the light. During greening the glyoxysomes undergo a gradual loss of function as they are converted into peroxisomes (Fig. 5.20a); key FFA-catabolizing enzymes such as those of the glyoxylate cycle (ICL, MLS) and β-oxidation spiral decline within the organelle, while others, e.g., catalase and malate dehydrogenase, are retained and increase as new enzymes that are photorespiration-associated, e.g., hydroxypyruvate reductase (HPR), are imported (Fig. 5.20b). Coincidental with these changes in enzyme content during glyoxysome-peroxisome transformation is the cessation of expression of genes for FFA utilization, and an increase in expression of those necessary for peroxisome function. There is a brief period of overlap where enzymes for both functional activities are present. A number of specific PEX proteins play a role in the removal of the glyoxysomal enzymes, helping in their transport out of the evolving peroxisome so that they are destroyed in the cytosol, by the proteasome (a complex of proteases that degrade unneeded or damaged proteins).
(a) Representation of the changes occurring as the glyoxysome is transformed into a peroxisome in the cotyledon cells of Arabidopsis following germination. Representative enzymes of the glyoxysome: isocitrate lyase (ICL) and malate synthase (MLS), and of the peroxisome: hydroxypyruvate reductase (HPR). (b) Western blot showing the decline in ICL and MLS, and the increase in HPR as seedling development proceeds. There is an overlapping period of about 2 days when enzymes for both functions of the same organelle are present. Days signify time from imbibition of the seed. From Lingard et al. (2009). Courtesy of the National Academy of Sciences, USA
In oil-storing seeds where the cotyledons remain below ground, and in endosperms or megagametophytes that also become depleted of reserves, the glyoxysome degrades as the expended storage organ undergoes programmed cell death. While TAGs are present in the starchy endosperm of oat grains, it is not known if or how they are mobilized: the tissue in which they are stored is nonliving, there are no mitochondria or glyoxysomes, and the aleurone layer does not secrete lipases into it.
5.7.3 Utilization of the Products of TAG Catabolism
As with the products of starch and hemicellulose catabolism, those of TAG mobilization are a vital source of carbon and energy to support growth of the seedling. FFAs and glycerol are to a large extent converted to hexose, and finally to sucrose, by a sequence of reactions outlined in Fig. 5.15. Castor bean endosperms contain high amounts of sucrose-6-P synthase, sucrose phosphatase, and also sucrose synthase (Sect. 5.4.1). Here the major product of TAG mobilization is sucrose, which is taken up by active transport into the cotyledons. More than 80% of this sucrose is redistributed to the growing axis. If the embryonic axis is removed, there is temporary storage of sucrose in the endosperm, in vacuoles that develop as the storage products are degraded. Sucrose uptake by the cotyledons is thereby drastically reduced. Thus, as far as the growing seedling is concerned, removal or damage to the sink (axis) alters replenishment at the source (cotyledons).
The cotyledons of some seeds (e.g., pumpkin, watermelon, sunflower) can utilize acetyl CoA arising from β-oxidation of fatty acids for amino acid synthesis via partial reactions of the glyoxylate and citric acid cycle. The usual products are glycine, serine, glutamic acid, glutamine, and γ-amino butyric acid.
5.8 Storage Protein Mobilization
Hydrolysis of storage protein (polypeptides) in the protein storage vacuoles (PSVs) to their constituent amino acids requires a class of enzymes called proteases, some of which effect total hydrolysis whereas others produce small polypeptides that must be degraded further by peptidases. The proteases can be categorized as follows in relation to the manner in which they hydrolyze their substrates:
(1) Endopeptidases: these cleave internal peptide bonds within the protein to yield smaller polypeptides. These can be classified into four major groups: (a) serine endopeptidases, which have a serine in their active site where the peptide bond is broken; (b) cysteine endopeptidases, which have a cysteine in the active site; (c) aspartic endopeptidases, with two aspartates in the active site; and (d) metalloendopeptidases, which have a metal ion (usually Zn2+) in the active site.
(2) Aminopeptidases: these sequentially cleave the terminal amino acid from the free amino end of the polypeptide chain. There are multiple forms of these, located in the cytosol; they are active at neutral or slightly alkaline pHs.
(3) Carboxypeptidases: as (2), but single amino acids are sequentially hydrolyzed from the carboxyl end of the chain. There are multiple forms of these located within PSVs, and all contain serine in their active site; thus they are serine carboxypeptidases.
Both (2) and (3) are exopeptidases, and many are relatively nonspecific with respect to the amino acids that they cleave from the terminus of a polypeptide.

The liberated amino acids may be reutilized for protein synthesis or be deaminated to provide carbon skeletons for respiratory oxidation or conversion to other metabolites. Ammonia is produced by deamination, but this is prevented from reaching toxic concentrations by fixation into glutamine and asparagine, two commonly transported forms of amino acid.
5.8.1 Protein Mobilization During Germination
Whereas there are alternative sources of sugars (as oligosaccharides) to those from starch, hemicelluloses or TAGs for the embryo to utilize during germination (Sect. 5.3), such is not the case for amino acids, which are not present in sufficient amounts in the mature dry seed to sustain protein synthesis until the time of radicle emergence and subsequent post-germinative storage protein mobilization. Hence, there is germination and post-germination mobilization of proteins, which are mediated by different enzymes in different parts of the seed. This has been studied extensively in vetch and buckwheat seeds, although it is likely to represent the general pattern of proteolysis in leguminous and nonleguminous seeds; less detailed studies on cucumber, rapeseed, soybean, and Phaseolus bean support this. During germination the storage globulin proteins in the PSVs of both the axis and cotyledons are subjected to proteolysis, and the amino acids are reutilized to make more proteins in the same regions (Fig. 5.21). The enzymes involved in the mobilization of the proteins during germination are stored within the PSVs and are present in the dry seed, being activated following imbibition. By the time seedling growth is underway, the proteins in the axis are depleted and the source of amino acids for protein synthesis is now exclusively those exported from the cotyledons, although some are retained there for use in the synthesis of proteases and other hydrolases necessary for mobilization of the starch or oil reserves, for example. The post-germination synthesis of new proteases is essential for protein utilization in the cotyledons.
Comparisons of the patterns of storage protein mobilization in the axis and cotyledons of a dicot seed during and following germination. During germination the released amino acids from storage proteins in the axis and cotyledons, by the action of proteases already present in the dry seed, are reutilized for protein synthesis within the same region. Following germination, when the protein reserves in the axis have been depleted, the sole source of amino acids for seedling growth is the cotyledons; most amino acids are transported therefrom to the growing regions, although some are retained for the synthesis of proteases and other hydrolases required for the mobilization of the major storage reserves. Based on information in Müntz et al. (2001)
In vetch seeds the proteases to mobilize the globulins in the PSVs of the axis are synthesized during late embryogenesis; why they do not hydrolyze the storage proteins at that time is unknown, but it could be because the enzymes are in an inactive form, at an incorrect pH, or their substrate has to undergo some structural changes to permit them to be effective. Five different cysteine proteases are present in the axes of germinating seeds, located in the PSVs, and one additional type in the cotyledons, which appears not to be in this storage organelle. The mRNAs for at least three of the proteases are known to be present in the developing axes and cotyledons of the maturing embryo, and remain there during subsequent imbibition, to be replaced with transcripts for the same or different proteases as germination and seedling growth proceed.
5.8.2 Protein Mobilization Following Germination of Cereals
Reserve proteins are present in two separate regions of the cereal grain: in the aleurone layer and starchy endosperm (Sect. 1.3.3), with a minor amount being present in the scutellum and axis, which may be hydrolyzed to supply amino acids for protein synthesis during germination and early seedling growth prior to mobilization of the major endosperm reserves.
Proteases in the aleurone layer are discussed in Sect. 5.5.3 with respect to their role in providing amino acids for the synthesis of key enzymes for starch mobilization. Those necessary for the hydrolysis of the major protein reserves stored in the PSVs in the starchy endosperm are synthesized and secreted there from the aleurone layer, which also releases malic acid, acidifying the storage cells to about pH 5, thus optimizing the conditions for enzyme activity. During maturation drying of the endosperm the membranes of the PSVs tend to lose their integrity, aiding in the exposure of the storage proteins to their hydrolases upon subsequent rehydration.
The number of proteases involved in the mobilization of starchy endosperm proteins is generally large. In maize kernels at least 15 different endopeptidase activities are detectable during the first 6 days after the start of imbibition (DAI). Four groups of enzymes have been identified, based on the time of their appearance. Group I is present in the dry seed; it contains two metalloendopeptidases that decline in activity soon after imbibition. They appear not to be involved in the initial mobilization of zein, the major storage protein. Group II endopeptidases increase following germination and reach peak activity after 3 DAI. These are SH-(cysteine) endopeptidases and have a high affinity for γ-zein, the form of storage protein that is located peripherally in the PSVs, and thus is the first to be subjected to proteolysis. Group III enzymes achieve maximum activity at 5 DAI and are mostly cysteine endopeptidases that cleave α-zein, the form located internally within the PSVs. Group IV enzymes increase in activity only after 3 DAI, and their specificity is for α-zein. They are unable to hydrolyze γ-zein, but by the time they are present in the endosperm this form of zein is likely to have been completely mobilized. The site of synthesis of the endopeptidases that hydrolyze zein is either the scutellum or the aleurone layer. In addition to these groups of endopeptidases, it is likely that there are also several amino- and carboxy-peptidases involved in completing proteolysis, as well as the oligopeptide-degrading peptidases.
Consistent with these observations on maize, a general pattern of protein hydrolysis in both cereals and dicots seems to be emerging in that metalloendopeptidases are present first, then a series of cysteine-endopeptidases, followed by the terminal-acting (amino- and carboxy-) peptidases and the enzymes that hydrolyze the resultant oligopeptides, the peptidases. The different substrate specificities of the enzymes, as they arise, could account for the order in which storage proteins and their component forms are mobilized.
An even greater complexity of proteases occurs in germinated barley grains, where there are 42 distinct enzymes, the majority (27) being cysteine endopeptidases, along with serine endopeptidases (8), aspartic endopeptidases (4) and metalloendopeptidases (3). In barley, but more so in other cereals such as wheat, proteolysis of the starchy endosperm storage proteins is aided by their reduction by thioredoxin.
Thioredoxins (Trx) are small (12 kDa) oxido-reductive enzymes that contain a dithiol-disulfide active site (–HS SH– ↔ –S–S–). In plants there are six well-defined types that reside in different cell compartments and function in an array of metabolic events. Trxh is located in the cytosol and following imbibition of the wheat grain reduces the redox-active disulfide groups of gliadin and glutenin storage proteins to the sulfhydryl state (Fig. 5.22); this increases both the solubility of the proteins and their susceptibility to proteolysis. These changes are also accompanied by activation of thiocalasin, a Ca2+-dependent serine protease, which is then able to hydrolyze the reduced storage proteins. Thioredoxin may also play an indirect role in activating other hydrolytic enzymes in cereal grains, including some involved in starch hydrolysis such as α-amylase and a debranching enzyme, pullulanase. This is achieved by inactivating protein inhibitors (Sect. 5.8.4) of these enzymes, such as α-amylase/subtilisin inhibitor, which is not functional in its thioredoxin-reduced state. Whether or not thioredoxin plays a similar role in enhancing protein mobilization in dicot seeds is not known, but there is evidence that it can act as a reductant of protease inhibitors and 2S storage proteins. In addition to its role in post-germinative reserve mobilization, Trx positively influences germination through unidentified mechanisms; suppression of Trx gene expression prevents precocious germination (Sect. 2.4.2).
Regulation of storage protein mobilization and enzyme inhibitor activity in wheat endosperms by thioredoxin (Trx). Reduction of Trx requires the transfer of reducing power from NADPH + H+. The reduced form of Trx in turn reduces the storage protein, rendering it available for proteolytic cleavage. In the case of the enzyme inhibitor α-amylase/subtilisin the reduction of the disulfide bonds in its constituent cysteine residues to unlinked cystines renders it inactive. red, reduced; ox, oxidized
5.8.2.1 Uptake of Amino Acids and Peptides into the Embryo
Proteolytic activity within the starchy endosperm of cereal grains results in the production of amino acids, dipeptides, and a number of small oligopeptides. These soluble products are rapidly absorbed by the embryo, via the scutellum, with di- or tri-peptides being taken up more efficiently than free amino acids. Although this uptake of peptides does not appear to involve or require their hydrolysis, they are eventually cleaved by peptidases within the scutellum, and only free amino acids accumulate to any extent in the growing embryo. Active uptake mechanisms within the plasma membrane of the scutellum can distinguish between peptides and amino acids. Isolated scutella of barley, for example, can import the dipeptide alanine-phenyalanine several days sooner than alanine alone (Fig. 5.23a). The capacity of the scutellum to transport the dipeptide is acquired very early following imbibition, at the time when the whole grain is still germinating. During germination there is the synthesis of a plasma-membrane-associated transport protein, HvPTR1, in the scutellum from newly formed transcripts (Fig. 5.23b). HvPTR1 is a peptide transporter, which has a broad specificity for peptides with 2–4 amino acids and broad tolerance of their amino acid composition. In the presence of amino acids its transport capacity is inhibited, whereas in the presence of glucose it is enhanced. Such interactions between hydrolysis products and transporters may be important in balancing the flux of nitrogen and carbon into the embryo during and following germination. The inactivation of HvPTR1 by the presence of amino acids is the result of posttranslational phosphorylation of the serine residues in this protein.
(a) Development of the capacity for the transport of dipeptides and amino acids into barley scutella isolated from the grain at different times following the start of imbibition. Scutella were isolated at the times indicated and imbibed on a medium containing the dipeptide Ala-[14C]Phe (blue) or amino acid [14C]Ala (orange) for 10 min to determine their uptake. (b) Increase in the expression of the HvPtr1 gene for a membrane peptide transporter; the northern blot shows an increase in transcripts present in scutella dissected following imbibition of the barley grain from 12 to 96 h. From West et al. (1998). Courtesy of Wiley
A number of transporters have been identified in cereal grain scutella, several of which are synthesized de novo before or at the time that protein mobilization commences within the endosperm. In wheat and barley scutella there are at least four transporters for amino acids alone: two are nonspecific, one is specific for proline, and another for basic amino acids. Maize and rice also possess multiple uptake systems, but with some differences in specificity. The efficiency with which scutella take up certain peptides, compared to amino acids, also varies among species.
5.8.3 Protein Mobilization Following Germination of Dicots
Mobilization of storage proteins during germination is discussed in Sect. 5.8.1; here post-germination utilization of the major reserves in the storage tissues is followed. The large polymeric storage proteins (11S legumins and 7S vicilins) tend to be insoluble in the PSVs in which they are stored. Initially they need to undergo limited proteolysis by endopeptidases at exposed positions on their surface to effect structural changes, thus rendering them more susceptible to further enzymic degradation (Fig. 5.24). Subsequent hydrolysis by endo- and carboxy-peptidases results in the production of small peptides and amino acids, which are then transferred from the PSVs into the cytosol by active transporters; the membranes of these organelles remain intact following maturation drying, unlike those in the cereal endosperm. After transport into the cytosol the small peptides are subjected to amino-, di- and tri-peptidases to yield free amino acids.
Depiction of the pathways for the degradation of the major storage proteins (legumins and vicilins) in the PSVs of legume seeds. Storage protein degradation initially requires their limited hydrolysis by specific endopeptidases (iEP1, iEP2) to make them more susceptible to proteolytic degradation. The partially modified proteins are now available for further hydrolysis by these and other endopeptidases (EPs), as well as serine carboxypeptidases (CPs) to small peptides and amino acids. These are transported across the PSV membrane into the cytosol by amino acid (AATs) and peptide (PTs) transporters; degradation of the small peptides to amino acids is completed by aminopeptidases (APs), tripeptidases (TPs), and dipeptidases (DPs). The PSVs transform into lytic vacuoles during and following protein hydrolysis. From Wilson (2006). With permission of CAB International
The identities of the initiating endopeptidases vary with the type of storage protein or its particular subunit, as demonstrated in soybean cotyledons. Mobilization of the α and α′ subunits of the vicilin β-conglycinin, which have a 93% amino acid sequence identity to each other, is initiated by a serine endopeptidase, Protease C1. This enzyme does not hydrolyze the smaller β-subunit of β-conglycinin, however, which in contrast to the α and α′ subunits lacks a segment of 179 amino acids from the N-terminal end, and likely contains the target site for the Protease C1. To initiate hydrolysis of the β subunit there is requirement for a cysteine endopeptidase, Protease C2, which hydrolyzes the products resulting from the activity of Protease C1. Papain-like cysteine proteases of the C2 type are commonly present in the cotyledons of different legume species that contain the same type of storage protein.
In soybean cotyledons, within the same PSVs as the globulin β-conglycinin are Kunitz- and Bowman-Birk trypsin inhibitors (Sect. 5.8.4); their degradation is initiated by a different cysteine endopeptidase, Protease K1. In mung bean, however, the Bowman-Birk type trypsin inhibitor is first hydrolyzed by a serine endopeptidase, Proteinase F.
The proteases involved in the mobilization of the storage proteins may be synthesized and sequestered in PSVs during seed development. There are several reasons why the proteases are inactive until after germination: (1) The pH inside of the vacuole is unfavorable for activity of the enzymes, and must be changed in order for them to become catalytic. In developing mung bean cotyledons, for example, there is the accumulation of both a Bowman-Birk type trypsin inhibitor and Proteinase F in the same PSVs. The enzyme is inactive during maturation and germination because the pH inside the vacuole is too high; following germination H+ ions are introduced into the PSVs by proton pumps, lowering the internal pH, resulting in enzyme activation. Soybean Protease C1 is also activated following germination by acidification of the PSVs in which it is present along with its target storage proteins at seed maturity. (2) The enzyme is incapable of hydrolyzing the storage protein until after it has been modified by a different protease. In mature and imbibed seeds of mung bean and barley the storage proteins and carboxypeptidase are present in the same PSVs. However, this enzyme, being an exopeptidase, does not have a substrate to act upon until after the storage protein has been modified by an endopeptidase (as in Fig. 5.24), which is synthesized following germination. (3) There is an inhibitor of the protease that must be inactivated to allow the enzyme to hydrolyze the storage protein. This is rare, but occurs in buckwheat seeds (Sect. 5.8.4).
The cellular changes that precede and accompany proteolysis have been studied most thoroughly in the cotyledons of mung bean, although what occurs there is likely representative of events in the storage tissues of many dicots. In mung bean the major storage protein is a vicilin, which comprises 70–80% of the total, and the major enzyme responsible for its hydrolysis is a cysteine endopeptidase, vicilin peptidohydrolase (Vpase), with some participation by a carboxypeptidase. Dry and early-imbibed cotyledons contain tubular ER (T-ER), which is dismantled approx. 12–14 h after the start of imbibition. Although, overall, there is a net loss of membrane, at the same time there is a proliferation of a new type of cisternal ER (C-ER), with ribosomes attached (Fig. 5.25a). The Vpase is synthesized de novo from newly produced transcripts on polysomes attached to the C-ER, and the enzyme is inserted into the ER lumen and packaged into vesicles. These are transported to the PSVs, and degradation of the vicilin therein commences only after the peptidohydrolase has been inserted (Fig. 5.25b). Initially the vicilin is cleaved from 50- to 63-kD components to 20- to 30-kD components, and these are then hydrolyzed more slowly. As protein digestion proceeds, the emptying PSVs fuse to form a large vacuole containing an array of hydrolytic enzymes, thus becoming a lytic vacuole (Fig. 5.25c). Digestion of cell contents by the enzymes in this vacuole is achieved when vesicles are internalized by an autophagic process in which a portion of the cytoplasm is engulfed and sealed off by the PSV membrane (Fig. 5.25d). Although mung bean exhibits epigeal seedling growth, the first true leaves expand as the cotyledons appear above the soil, and hence, the latter do not become photosynthetic. Their cells are completely depleted of contents due to lytic vacuole activity, the result of programmed cell death (PCD) (Sect. 5.5.4).
Representation of a storage-protein-storing cell from a mung bean cotyledon illustrating the changes undergone by the protein storage vesicles (PSVs) and ER during reserve hydrolysis and cell autolysis. (a) Starting 12–24 h from imbibition. Formation of cisternal endoplasmic reticulum (C-ER) with ribosomes attached. PSVs contain storage proteins (shaded). (b) Three to five days from start of imbibition. Vicilin peptidohydrolase is synthesized on polysomes attached to newly formed C-ER and inserted into the lumen. Dilations (D) of the cisternae form containing the enzyme; these break off as transport vesicles (TV) and carry the peptidohydrolase to the PSVs, with which they fuse (F). The proteinase commences hydrolysis of the vicilin. (c) As proteins are hydrolyzed the PSVs coalesce to form lytic vacuoles (LV). Other proteases and hydrolytic enzymes, e.g., ribonucleases, phospholipases, start to be inserted into the PSV after synthesis on the C-ER. (d) Autophagic vacuoles (AV) form, engulfing cell contents such as the C-ER and mitochondria (M). More PSVs fuse to form a large LV containing many autolytic enzymes. Organelles and cell structures other than the ER and PSVs are omitted for clarity. Cell contents not drawn to scale. Based on the studies of M.J. Chrispeels and co-workers, e.g., Herman, E.M., Baumgartner, B. and Chrispeels, M.J., Eur. J. Cell Biol. 24, 226–235 (1981)
A similar mode of hydrolysis occurs in black gram seeds. Here the PSV becomes a lytic vacuole by the import of cysteine proteases, and as the storage protein is being hydrolyzed autophagic bodies enveloping cell contents, such as mitochondria, are ingested, as part of PCD. An apparent additional feature is that α-amylase is synthesized and targeted to the PSV, and starch granules are imported into this organelle for breakdown as it morphs into a lytic vacuole.
A variation on this mode of PCD occurs in the castor bean endosperm. Here, following mobilization of the protein and lipid reserves, an inactive papain-type cysteine endopeptidase (CysEP) is synthesized by polysomes on the ER and released from there as ribosome-associated vesicles called ricinosomes. The final stage of degeneration of the endosperm cells is marked by destruction of the nucleus and DNA fragmentation, breakdown of lytic vacuoles releasing proteases and nucleases and causing acidification of the cytosol, and disruption of the ricinosomes releasing the CysEP that becomes activated (achieved by removal of an N-terminal propeptide and a C-terminal KDEL [lysine-asparagine-glutamine-leucine] sequence) to participate in the hydrolysis of the remaining proteins. Similar KDEL-tailed CysEPs are involved in a variety of tissues undergoing PCD; they are euphemistically termed “corpse-processing proteases,” and are known to be active for example in depleted hypogeous cotyledons of vetch seedlings and megagametophytes of white spruce seedlings, as well as in a variety of plant- and seed-developmental events, e.g., during nucellus degeneration in developing castor bean seeds. Ricinosomes are likely involved in the final destruction of the reserve storage cells in many seeds, complementing the activity of lytic vacuoles. Their presence in the remnant cells of the micropylar endosperm of the tomato seed following germination is shown in Fig. 5.26, as PCD proceeds.
Remnant cells of the micropylar endosperm of tomato, at 144 h after the start of imbibition, undergoing programmed cell death (PCD) following protrusion through it of the radicle. The depleted protein storage vacuoles have become lytic vacuoles (PSV/LV) that are autophagic and ingesting cytosol contents. Present in the cell are numerous ricinosomes (R) exhibiting a rough surface, the source of proteolytic enzymes released into the cytosol. The blebbing of the plasma membrane (PM, arrowed) away from the cell wall (CW) is an indication that PCD is proceeding. Also marked are mitochondria (M), nucleus (N) in which there is chromatin condensation (not visible), and endoplasmic reticulum (ER). Inset: the inclusion of autophagic vesicles (A) in depleted PSV/LVs. For more micrographs showing the progression of PCD in the tomato lateral and micropylar endosperm see DeBono, A.G. and Greenwood, J.S. Can. J. Bot. 84, 791–804 (2006). Micrographs courtesy of J.S. Greenwood, Univ. Guelph
5.8.4 Protease Inhibitors
Within both monocot and dicot seeds are proteins that specifically inhibit the action of proteases in animals and, to a lesser extent, in plants. There are several families of protease inhibitors, including the following: (1) Bowman-Birk inhibitors, which are small proteins (8–9 kDa) with many intra-chain disulfide bridges; they are common in legumes, and in the aleurone layer and embryo of rice, and bind to and inactivate serine proteases. They can account for up to 5% of total seed protein in some legumes, e.g., soybean. (2) Kunitz-protease (Kunitz-family trypsin) inhibitors, which target serine proteases. They are approx. 21 kDa in mass and are widely present in legume seeds, accounting usually for about 3–5% of seed protein (e.g., soybean, Acacia spp.), and also in the embryo and aleurone layer of cereal grains. A sub-family of these is the bifunctional cereal trypsin/α-amylase inhibitors, some of which conduct only the protease inhibition. (3) Phytocystatins are inhibitors of cysteine proteases (e.g., papain) and are part of a larger family that targets this protease type; they are widespread in legume seeds and cereal grains.
Several functions have been suggested for seed protease inhibitors: (1) Storage protein. Because they can constitute between 5 and 15% of total protein in the mature seed of some species of cereals and legumes, they may play a role as storage albumins, being more readily mobilized than the insoluble globulins or prolamins during and following germination. Inhibitor proteins are also generally rich in sulfur-containing amino acids. (2) Seed inhibitors generally do not inhibit proteases within the seed itself. Therefore, their role is more likely in the protection or dissuasion from predation by insects or other pathogens. Some inhibitors can inhibit proteolytic digestive enzymes of insect pests or the secreted proteases of invading fungi and other microorganisms; in addition there are α-amylase inhibitors, and those that are bifunctional for amylases and proteases, thus providing a broad range of protection. (3) There is scant evidence that inhibitors influence the activity of proteases within the seed itself. Sometimes, as in mung bean, following germination there is a decline in the inhibitor content in the storage cotyledons as protease activity increases, but the two phenomena are not causally related. The only exception would appear to occur in buckwheat seeds. These contain several serine protease inhibitors that can suppress bacterial, fungal and insect proteases (and have been used in transformation experiments to improve resistance of tobacco and potato plants to biotic stress), as well as one that interacts with the native seed enzyme. A metalloendopeptidase exists in the PSVs of dry buckwheat seeds, inactivated by being complexed with an inhibitor. This enzyme-inhibitor complex can be disrupted in vitro by divalent cations (Zn2+), allowing protease activity. It is suggested that during the early stages of storage protein hydrolysis in the germinated seed, Zn2+ is released from phytin as this is mobilized. The cation then binds to the inhibitor and inactivates it, allowing metalloprotease activity to increase, thus initiating the mobilization of the major legumin storage protein.
5.8.5 Utilization of Liberated Amino Acids in Dicot Seedlings
The major transported forms of amino acids from the storage organs into and throughout the growing seedlings are the amides, i.e., asparagine and/or glutamine. Hence, the amino acids liberated from storage proteins must be further metabolized, including the conversion of amino nitrogen to amido nitrogen. The synthesis of asparagine involves the donation of an amino group from glutamine in a reaction catalyzed by the ATP-dependent enzyme asparagine synthase (AS):
Glutamine itself is formed from glutamate:
and glutamine can donate its amino group to form glutamate from α-ketoglutarate using the enzyme GOGAT (glutamate synthase).
Alternatively, glutamate dehydrogenase (GDH) can add ammonia to α-ketoglutarate to yield glutamate in the presence of NADPH. Glutamate can also be synthesized by transamination of α-ketoglutarate utilizing the amino group of other amino acids.
The fates of the amino acids released from storage proteins in relation to their transport and subsequent utilization in the growing seedling are summarized in Fig. 5.27.
The fate of amino acids liberated by storage protein hydrolysis, with emphasis on the fate of glutamine and asparagine, the major transport amino acids within the vascular tissue of the growing seedling. Enzymes: (1) aminotransferase; (2) glutamine synthase; (3) asparagine synthase; (4) asparaginase; (5) GOGAT; (6) glutamate dehydrogenase; (7) deaminase. Reactions: (A) deamination of amino acids to yield NH3; (B) direct interconversions of amino acid skeletons (Pro to Glu), or direct transport and utilization of amino acids without interconversion. Compounds: Glu, Glutamic acid; Gln, glutamine; Asp, aspartic acid; Asn, asparagine; NH3, ammonia; Pro, proline (high in the amino acid pool of cereals when storage prolamins are broken down); α-KG, α-ketoglutaric acid. Solid lines show the path of N, and dashed lines the path of C in or from amino acids. Shown is the fate of amino acids in cotyledons and their transfer to the growing regions via the vascular transport stream. For proteins mobilized in the endosperm or megagametophyte there must be the transport of amino acids from this tissue to the growing embryo and into its vascular system. Based on Miflin et al. (1981)
In cotyledons of various legume seeds there is an increase in the activity of the enzymes involved in glutamate, glutamine, and asparagine synthesis at a time when the major protein reserves are being hydrolyzed. Hence, as amino acids are being liberated from the stored form they undergo the appropriate conversions to the readily transportable asparagine. GDH, GS, and AS are present only in low amounts in the cotyledons of mature dry cotton seeds but increase appreciably during the first 2 days from the start of imbibition (likely by de novo synthesis), peaking approximately at the time of, or just prior to the commencement of, protein mobilization. In this seed, as in the legumes, the major transport form of amino acid is asparagine. The pattern of amino acid metabolism in pea cotyledons is unusual in that a major transported form of amino acid, and the one that accumulates in the storage tissue is homoserine, which is synthesized from aspartate. Glutamine is another transport form of amino acid, but not asparagine to any great extent.
In the gymnosperm loblolly pine and other Pinus spp., arginine is the major component of the free amino acid pool in the megagametophyte as the arginine-rich protein reserves are being mobilized. This amino acid is also high in amount in the growing seedling, and thus may be exported to there from the reserve tissue without prior conversion. Arginase, which converts arginine to ornithine and urea in the urea cycle, increases in activity in the shoot region of the embryo coincidentally with the influx of its substrate.
The predominant form of transported nitrogen from the endosperm of castor bean is glutamine, although some of the amino acids released from the mobilized storage proteins, e.g., aspartate, glutamate, alanine, glycine, and serine, can be converted to sucrose and transported as the sugar. The amide nitrogen derived from the deamination of these gluconeogenic amino acids is probably used in the production of glutamine. By comparison, amino acids that are not gluconeogenic are probably transported unchanged to the growing seedling; some might undergo modifications of their carbon skeleton to form glutamate.
Amino acids from seeds whose major site of storage is the endosperm are taken up into the cotyledons by active transporters in the membranes of the outermost cells, e.g., glutamine in castor bean. In species where the cotyledons are the storage organs, these are connected to the growing regions by a network of vascular conducting tissue, and the products of hydrolysis are translocated to the axis largely within the phloem. Loading of amino acids into the translocation stream might be aided in some species, e.g., broad bean, by the presence of transfer cells that border the xylem and phloem; these are specialized cells with an increased surface area to aid the transport of solutes over short distances (Fig. 3.2d). In other species, e.g., mung bean, there are no transfer cells, but the parenchyma cells adjacent to the phloem have extensive evaginations of the plasmalemma to form fine tubules (plasmalemmasomes): these also serve to increase the surface area for transport.
The import of amino acids and peptides into the embryo of cereal grains is discussed in Sect. 5.8.2.1.
5.9 Phytin Mobilization
Phytic acid (myo-inositol hexaphosphate) is the major phosphate reserve in many seeds, and in its storage form is a mixed salt with such elements as K+, Mg2+ and Ca2+ (Sect. 1.3.4).
Phytase, a specific phosphatase, hydrolyzes the phytin to release phosphate, its associated cations, and myo-inositol. Breakdown of the phytin is rapid and complete following germination, for myo-inositol phosphate esters with fewer than six phosphate groups do not accumulate within seeds. The released myo-inositol may be used by the growing seedling for cell wall synthesis, since this compound is a known precursor of pentosyl and uronosyl sugar units normally associated with pectin and certain other cell wall polysaccharides. When the ions become separated from the phosphate is not clear, although it is likely that they are cleaved together from the myo-inositol ring, and then the ions are released, perhaps by exchange with H+, as the phosphate is utilized in cell metabolism.
There are two pathways by which the phosphates are removed from the phytin, the major difference being whether initially it is the phosphate in position 4 of the myo-inositol ring that is removed, or in position 3 (Fig. 5.28). In cereals such as rye, barley, rice and oat, the former pathway operates, with the next phosphate being removed from position 3; additionally, there may be less active phytases that operate via the alternative position-3 pathway. In legumes, phytase removes the phosphate from position 3 first, with the next one being removed from position 4; again there may be low-activity phytases that act via the alternative position-4 pathway. There are also minor variations in the steps following the first position-4 dephosphorylation, accomplished by phytases of low activity (Fig. 5.28). Many phytases and phosphatases (acidic and basic) have been reported in storage tissues of legumes and cereals, and it is evident that the role they play in phytin mobilization is variable.
Pathways by which phosphate is removed from phytin during its mobilization in cereal grains and legume seeds. From Ins (1,2,3,4,5,6)P6 (phytin) the phosphate is either removed from position 4 (left side) or position 3 (right side) of the myo-inositol ring, in cereals or legumes, respectively, and then sequentially from positions 3 or 4 to yield a common intermediate, the tetraphosphate Ins (1,2,5,6)P4. This is then converted to the P3, P2, and finally the P1 form, inositol phosphate, Ins(2)P, from which the phosphate in position 2 is cleaved to release free myo-inositol. Minor phytases also initially remove the first phosphate via an alternative pathway in cereals and legumes. Also, in both there is an alternative pathway for removing subsequent phosphates (dotted lines), but this is low in activity compared to the major pathway (solid lines). Inset is an illustration to show the removal of the first phosphate from position 4 on the myo-inositol ring. Ins, myo-inositol. Based on information in Greiner et al. (2002) and references therein
Maize is unusual among cereals in that the majority of phytin is stored in the scutellum, rather than the aleurone layer, although small amounts may also occur in the coleoptile and radicle. Two phytase genes (PHYTI and II) have been isolated from this species, and their transcripts increase during and following germination in the tissues where phytin is present, at the time of its mobilization. In mature dry barley aleurone layers the PSVs contain phytin globoids and also acid phosphatases with high phytase activity, which increase over several days following imbibition. It is possible that they are activated by the influx of H+ ions into the PSVs, in the same manner as the proteases stored therein (Sect. 5.5.3), although there is synthesis of a different phytase following germination. Phosphate and ions released from the aleurone layer are presumably absorbed by the scutellum and distributed in the growing seedling.
The pattern in the cotyledons and axes of dicots is such that there is an increase in activity of phytases (or phosphatases) as the phytin reserves decline. Usually phytase activity is low in, or absent from dry seeds, and increases following germination, coincidentally with the hydrolysis of storage proteins. The released phosphate is transported to the growing axis. Surprisingly, the germinated seed may retain its capacity to synthesize phytin, for when isolated embryos from mature castor bean seeds are incubated in phosphate solutions there is an increase in phytin, especially within the cotyledons. The site of phytin deposition is not known, however, but it is not within PSVs. Temporary storage of phosphorus as phytin might be a way of conserving this important metabolite during early seedling establishment.
Other forms of phosphate, such as lipid, protein, and nucleic acid phosphate occur in smaller amounts in seeds. Phospholipids associated with membranes and phosphoproteins are probably dephosphorylated during their hydrolysis (acid phosphatases may play a role here); this is frequently associated with destruction of the cell for recycling of its contents to the growing regions during programmed cell death. The free phosphate is translocated to the growing axis, but the lipid and protein moieties are catabolized in situ.
5.10 Control of Reserve Mobilization in Dicots
While there is considerable information on the control of mobilization of stored reserves in cereal grains, particularly with respect to the hormonal influences on hydrolytic enzyme production in the aleurone layer (Sect. 5.5.1), much less is known about the control mechanisms in dicot seeds. There are several reports that hormones stimulate reserve mobilization, but this is largely the result of enhancing germination and/or seedling growth, and is not related directly to the control of production of the enzymes involved. Removal of the axis also influences the mobilization of the stored reserves; this is discussed further is Sect. 5.10.2. Of course, if this occurs in nature, such damage will be fatal, but if after germination the exposed axis in the intact seed is subjected to abiotic stresses such as water deficit, salinity, or cold temperatures its growth will temporarily slow down or cease, influencing the mobilization of reserves in the storage tissues.
Mobilization of stored reserves is affected by two processes: enzyme formation and enzyme activity; hence, its regulation can be achieved by controlling either or both. However, it is not always possible to determine which is responsible simply by measuring the overall rates of loss of the reserves. Furthermore, measurements of the activity of extracted enzymes do not necessarily reflect their in vivo activity, and storage tissues that have the same extractable enzyme activity may be mobilizing reserves to quite different extents. Therefore, an understanding of the regulation of mobilization should include knowledge about rates of reserve utilization, rates of enzyme formation, and in vitro and in vivo enzyme activity.
5.10.1 Regulation in Endospermic Dicots
Structurally, the endospermic legume fenugreek is closest to the cereal grain in that there is an aleurone layer surrounding a nonliving storage endosperm in which reserve galactomannans are present as thickened cell walls. The enzymes endo-β-mannanase, β-mannosidase, and α-galactosidase are released from the aleurone layer to effect cell wall hydrolysis (Sect. 5.6.2). How mobilization of reserves within the fenugreek endosperm is controlled by the embryo is not clear, but it is assumed that at least some temporal control exists because the hydrolytic enzymes do not increase in activity, nor are they released from the aleurone layer, until after germination is completed. Abscisic acid (ABA) is present in the endosperms of intact seeds; if this hormone is leached out from isolated endosperms, then endo-β-mannanase activity increases. Reapplication of ABA to leached endosperms prevents any increase in enzyme activity. Diffusible saponin-like substances are also present in the fenugreek (and carob) endosperm, and these strongly inhibit any increase in α-galactosidase activity. These, and perhaps ABA, may play a role in limiting galactomannan hydrolysis until germination is completed, although it remains unknown how their inhibitory effects are overcome, and whether some role is played by the germinated embryo. There is no known requirement for a stimulatory hormone such as GA.
More is known about the regulation of cell wall-degrading enzymes in the endosperms of germinated lettuce and tomato seeds. In both species the major stored reserves are lipid and protein within the cotyledons, although the early post-germinative mobilization of the endosperm cell walls may provide sugars for early seedling growth. In the lettuce seed, there is mobilization of galactomannans in the endosperm cell walls by endo-β-mannanase following red-light- or GA-stimulated germination. The endosperm in the dry and imbibed seed contains ABA, which prevents the synthesis of this enzyme during germination. If the endosperm is isolated at this time and placed in a buffer solution, however, there is a strong increase in endo-β-mannanase activity (Fig. 5.29a) because the inhibitor is leached out of this tissue into the surrounding medium; reapplication of ABA to washed isolated endosperms prevents an increase in enzyme activity (Fig. 5.29b). This leaching cannot occur in the intact seed because it is prevented in the presence of the outer fruit coat. Therefore, another mechanism is required to overcome the inhibitory effect of ABA; this is controlled by the embryo, as summarized in Fig. 5.30. During germination there is the release of a stimulus from the axis, possibly GA and/or cytokinins (CK) that initially must be transported into the cotyledons. What then occurs in the cotyledons is unknown, but as the result of the passage of the stimulus from the axis through them there is the production of a positive signal that is released into the endosperm following germination, and there it represses the negative influence of ABA on enzyme production. Direct application of GA and/or CK to the endosperm does not induce endo-β-mannanase activity in the presence of ABA (Fig. 5.29b). Hydrolysis of the cell wall galactomannans produces small oligomannans from which the galactose side chain is removed by α-galactosidase in the endosperm; this enzyme increases somewhat in activity following germination, again influenced by the axis. The free galactose and oligomannans are taken up by the cotyledons, the latter being converted to mannose by β-mannosidase that is constitutively present in its cell walls, being synthesized and sequestered there during seed development.
(a) Increase in endo-β-mannanase activity in isolated endosperms of lettuce following their dissection after 4 h of germination and incubation in a citrate-phosphate buffered medium for up to 48 h. Initially there is a build-up of enzyme in the endosperm cells, followed by a decline after 12 h of incubation as it is released into the cell walls and surrounding medium. (b) Inhibition of endo-β-mannanase production by ABA (1 μM) in isolated endosperms dissected after 4 h of germination and placed for a further 26 h on buffered medium (●). Control endosperms –ABA incubated only on buffered medium produced high enzyme activity (∆). Enzyme production was repressed (▲) when ABA was added to control endosperms 10 h after dissection. Incubation of ABA (1 μM)-treated endosperms with GA (100 μM) or the cytokinin benzyladenine (BA, 50 μM), singly or together, did not result in any increase in endo-β-mannanase activity (dotted line). After Halmer and Bewley (1979)
Model to explain the regulation of the breakdown of galactomannan-rich cell walls in endosperms of germinated lettuce seeds. During germination a signal passes from the axis through the cotyledons and then to the endosperm where, following germination, it overcomes the negative effect of the endogenous inhibitor ABA on endo-β-mannanase synthesis. α-Galactosidase activity also increases and the products of cell wall-galactomannan degradation are taken up by the cotyledons. Small oligomannans are then hydrolyzed to mannose residues by β-mannosidase already present within the cotyledon cell walls. Mannose and galactose are likely quickly converted to glucose, and then to sucrose for transport and utilization within the growing seedling as an energy source until mobilization of the lipid reserves commences within the cotyledons. From Bewley (1997). Courtesy of Elsevier
5.10.2 Regulation in Non-endospermic Dicots
It is clear that mobilization is frequently influenced by the embryonic axis, i.e., the radicle/hypocotyl and plumule, or, where the endosperm is the storage tissue, by the embryo as a whole. This point has been established by means of surgical experiments in which the axis (or embryo) is excised at different times during germination and seedling growth. An illustration of the role of the axis is provided by a study on protein mobilization in the cotyledons of mung bean seeds. In the 6 days following the start of imbibition, the seed completes germination and seedling growth ensues. During this time, the amount of storage protein in the cotyledons falls by about 75%, a change that is accompanied by a rise in activity of extractable proteinase (vicilin peptidohydrolase) (Fig. 5.31a, b + axis). The enzymatic breakdown of stored protein leads at first to an accumulation of free amino acids in the cotyledons, but these decrease after about 3 days as they, or their products, are transported into the growing axis (Fig. 5.31c + axis). The pattern is quite different, however, in isolated, imbibed cotyledons, from which the axis was previously removed. Here, the rates of protein hydrolysis and the increase in peptidohydrolase activity are reduced by about 75%, and the amino acids that arise from the limited protein breakdown accumulate in the cotyledons (Fig. 5.31a–c-axis). Hence, the mung bean axis appears to regulate the breakdown of the proteins stored in the cotyledons, at least partially, by controlling the formation of peptidohydrolase.
Influence of the axis on protein mobilization in the cotyledons of mung bean seeds. The embryonic axis was removed from dry seeds and the cotyledons imbibed in moist sand (-axis, dotted line), or intact seeds were germinated in moist vermiculite (+ axis, solid line) in darkness. (a) Storage protein. (b) Vicilin peptidohydrolase activity. (c) Free amino acids. After Kern and Chrispeels (1978)
Similar effects of the axis on reserve mobilization and changes in enzyme activity in the cotyledons, or of the embryo on these events in endosperms or megagametophytes, have been observed in the seeds of quite a number of species. Some examples are in Table 5.2. But there are also some cases where removal of the axis has little or no effect on enzyme activity, and so the tissues containing the enzyme seem to be autonomous. To add to the complexity, even within the same species (e.g., pea) some researchers find effects of the axis while others do not! It is important to note, however, that a change in extractable enzyme activity in the absence of the axis does not necessarily reflect a change in activity within the storage tissue itself. Some enzymes are sensitive to product inhibition, and their activity ceases when the products of even limited reserve hydrolysis accumulate, a situation that would occur if exit of the breakdown products was impeded by the absence of the growing axis (see later for a discussion of source–sink effects). Thus, an enzyme could be inactive in vivo but active when extracted and assayed in vitro.
An interesting case is the cucumber seed, which illustrates the important regulatory role of the tissues enclosing its storage organs, the cotyledons. After germination of an intact seed, the triacylglycerols (TAGs) stored in the cotyledons rapidly become depleted, but if the axis is removed from a newly imbibed seed, there is very little mobilization of these reserves (Fig. 5.32a, curve a). Removal of the testa and the inner membrane around the axis-free cotyledons allows substantial TAG breakdown to proceed, however, indicating that these enclosing tissues normally inhibit mobilization (Fig. 5.32a, curve b). The inhibition is probably due to a limitation of oxygen entry, affecting both enzyme synthesis and the oxidation of fatty acids, for which molecular oxygen is needed. The coat is also important in intact seeds that have germinated normally. TAG breakdown in the cotyledons of these seeds does not begin when the axis elongates but only when the testa is pushed off as a result of its being wedged against a peg of tissue on the elongating hypocotyl (Fig. 5.32a, curve d, Fig. 5.32b). Moreover, the start of TAG mobilization is advanced when the testa is experimentally removed from newly germinated seeds (Fig. 5.32a, curve c). In cucumber, therefore, both the axis and the testa have important regulatory influences in the mobilization of TAGs by the cotyledons; mobilization declines when the axis is removed, but when the testa is removed, it increases. The testa also inhibits α-amylase formation and starch mobilization in pea and mung bean cotyledons. In the case of the latter it has been suggested that the testa acts by preventing the loss of a diffusible inhibitor that, in some way, is also nullified when the axis is present.
(a) Triacylglycerol (TAG) mobilization in cucumber cotyledons. Total TAG content was extracted and measured at daily intervals from cotyledons of seeds treated in the following ways: (a) Radicle/hypocotyl axis removed from the dry seed; testa still around the cotyledons. (b) As (a), but surrounding testa and membrane also removed. (c) Testa removed from freshly imbibed seed; radicle/hypocotyl axis intact. (d) Fully intact seed; axis present and testa intact from the start of imbibition. The appearance of the intact seed/seedling over the first 2.5 days is shown at the bottom left of the diagram: Stage i, dry seed; stage ii, radicle emergence; stage iii, testa displacement. The testa becomes displaced at day 2; TAG breakdown in the cotyledons then begins. Adapted from Slack et al. (1977). (b) Larger diagram to show the displacement of the testa in the cucumber seedling due to it becoming hinged on the peg protruding from the hypocotyl that elongates to bring the cotyledons above the ground (epigeal seedling growth)
There are few reports on the influence of ABA on reserve mobilization. In most examples its effect is indirect, in that the hormone slows down or inhibits germination or seedling growth, thus reducing the synthesis and activity of hydrolytic enzymes. In Arabidopsis seeds, when ABA is applied at concentrations that inhibit germination, there is also a considerable reduction in TAG mobilization in the embryo, by some 50–65%, although there is still limited expression of the genes that encode β-oxidation and glyoxylate cycle enzymes. This is attributed to the expression of the gene for the transcription factor ABI4 (ABSCISIC ACID INSENSITIVE 4), a component of the ABA response signalling network, making the embryo sensitive to the hormone. The endosperm, although only one-cell thick, and not regarded as an important storage tissue, does contain TAGs, but the ABI4 gene is not expressed therein, rendering it insensitive to ABA; hence, TAG mobilization is not impaired. Likewise, in the abi4 mutant, which lacks the gene for the transcription factor, mobilization in the embryo is not diminished by ABA. TAG hydrolysis in the endosperm of tobacco seed also appears to be ABA insensitive. To what extent endogenous ABA controls reserve mobilization during and following germination in these seeds remains to be determined, however.
5.10.2.1 Mode of Regulation by the Axis
So how does the axis control the mobilization of reserves in the storage tissue? Two possibilities have been considered to explain this: (1) Specific regulatory substances move from the axis to the storage organs or tissues where enzyme production occurs, i.e., a hormonal mechanism. This explanation invokes a system similar to the one in cereal grains, where the embryo regulates enzyme production in the aleurone layer through the action of gibberellins that it secretes (Sect. 5.5.3), and possibly in lettuce (Sect. 5.10.1). (2) The axis is a sink, drawing off the products of reserve mobilization from the cotyledons or endosperm, which would otherwise arrest continued enzymatic activity by feedback inhibition. Evidence for these two options is discussed below:
(1) Hormonal control by the axis. Most of the support for the possibility that hormones control the development of activity of the mobilizing enzymes comes from testing the effects of adding growth regulators to isolated storage tissues following their dissection from the seed. This approach attempts to answer the question, can these chemicals replace the influence of the axis? In many cases, applied hormones induce greater breakdown of reserves in isolated tissue and/or increase the activity of enzymes concerned with mobilization. Cytokinins, for example, cause increases in amylolytic and proteolytic activity of isolated chickpea cotyledons as well as mobilization of carbohydrate and protein (Fig. 5.33), and in the activity of certain proteolytic enzymes and protein hydrolysis in excised squash cotyledons. They also enhance the activities of isocitrate lyase (in the glyoxylate cycle; Sect. 5.7) in watermelon and sunflower cotyledons. Activities of enzymes of the glyoxylate cycle in hazel cotyledons, for β-oxidation of fatty acids and for hydrolysis of stored protein reserves in castor bean endosperm, are increased by GA, which also promotes α-amylase activity in excised pea cotyledons. Auxin is also effective in the latter, as it is in inducing the mobilization of xyloglucan-hydrolyzing enzymes in the excised cotyledons of Hymenaea courbaril seedlings. Most frequently, however, the effects of applied growth regulators are relatively small (and may be indirect: see below) even though sometimes comparable with the action of the axis (e.g., Fig. 5.33). Nowhere do they match the strong induction of enzyme production that is achieved by GA in cereal aleurone layer cells (Sect. 5.5.3).
Effect of cytokinin on amylolytic and proteolytic activity in cotyledons of chickpea. Activities are shown of cotyledons in the intact seed (●), of excised cotyledons on water (o), and of excised cotyledons that were treated so as to restore the normal concentration of endogenous, native cytokinins (♦). (a) Total carbohydrate. (b) Amylase activity. (c) Protein content. (d) Proteolytic activity. Based on Muñoz et al. (1990)
Other approaches include the use of hormone-synthesis or -transport inhibitors in intact seeds or embryos, or attempts to correlate hormone content with hydrolytic activity. There are some instances, also, where diffusates or extracts of embryos stimulate enzyme activity in isolated storage tissue, e.g., fructose-1,6-diphosphatase in castor bean endosperm and isocitrate lyase in megagametophytic tissue of Ponderosa pine. But in no case has it been shown convincingly that a known hormone, e.g., a cytokinin, auxin, or gibberellin, moves from the embryo or axis to the storage tissue in the intact seed and exerts a unique control in regulating mobilizing activity; this is in marked contrast to the situation in the cereal aleurone layer.
(2) The axis as a sink. Many enzymes are inhibited by the products of the reactions they catalyze. The effect can involve repression of enzyme synthesis or inhibition of the activity of already existing enzyme proteins, or both. Such feedback inhibition is important in the regulation of activity of the enzymes for reserve mobilization. Growth of the axis requires the products of reserve breakdown, to where they are transported; hence, they do not accumulate in the storage tissues. The continual withdrawal of these products into the axis could account for the rise and maintenance of activity of the mobilizing enzymes; i.e., the axis regulates enzyme activity simply by virtue of its action as a sink.
This mechanism seems, in several cases, to account for the beneficial effect of the axis on mobilization. In cucumber, for example, although removal of the axis does not hinder development of several lipolytic enzymes in the cotyledons, TAG breakdown itself is much curtailed; hence, the activity of the enzymes apparently stops in the absence of the axis. Reducing sugars and sucrose accumulate in the excised cotyledons as lipolytic activity slows down. Moreover, addition of sucrose to isolated cotyledons leads to an even greater inhibition of lipolysis. The fact that TAG breakdown proceeds in isolated cotyledons when the testa is removed might be thought to argue against regulation by a sink, since the normal sink—the growing axis—is missing. However, such isolated cotyledons enlarge, making additional cell wall material (e.g., cellulose) as they do so, presumably from the sugars resulting from TAG catabolism; in addition, synthesis of starch occurs. So even though the axial sink is absent, two other sinks, cellulose and starch synthesis, serve to drain off the products of TAG mobilization and, by preventing their accumulation, permit the activity of the lipolytic enzymes to continue. The activity of extracted proteolytic enzymes from the cotyledons is similarly unaffected by removal of the axis, but within the cotyledons protein hydrolysis itself is minimal. Accumulated amino acids, especially leucine and phenylalanine, as well as the dipeptide tryptophylphenylalanine, inhibit the activity of an aminopeptidase in the cotyledons, thus preventing the completion of protein degradation.
Thus, in the absence of a sink for the products of reserve mobilization, normally the growing axis, hydrolytic activity and processing of the catabolites of the polymeric reserves is prevented, as illustrated in Fig. 5.34 for cucumber seeds. A temporary sink may replace the axis as the cells of the cotyledons expand, resulting in some breakdown of storage reserves; this may account for the positive effect of applied hormones that enhance cell wall growth and extensibility in isolated cotyledons.
An illustration of a potential regulatory mechanism for the mobilization of storage reserves in cucumber cotyledons in the absence of the axis. When the growing axis (sink) is removed there is a build-up of hydrolytic products in the cotyledons (source): amino acids and peptides from storage proteins, and sugars from triacylglycerols (TAG) following gluconeogenesis. These may slow down or inhibit activity of some key enzymes in the catabolic pathways, either directly or as a result of lowered transcription of their genes. The reduced mobilization of the reserves could be due to one or more of: feedback (substrate-level) inhibition of specific enzymes in the catabolic pathway, perturbation of the signalling pathways that are the fine control for cellular and metabolic interactions, a change in osmotic potential that decreases enzyme synthesis, or due to an imbalance of the C:N ratio. From data of Davies and Chapman (1980) and Slack et al. (1977)
Useful Literature References
Section 5.3
Blöchl A, Peterbauer T, Hofmann J, Richter A (2008) Planta 228:99–110 (Mobilization of RFOs in pea seeds)
Mitsuhashi W, Sasaki S, Kanazawa A, YangY-Y, Kamiya Y, Toyomasu T (2004) Biosci Biotechnol Biochem 68:602–608 (Invertases in germinating Arabidopsis)
Section 5.5
Bethke PC, Swanson SJ, Hillmer S, Jones RL (1998) Ann Bot 82:399–412 (pH activation of enzymes in the PSVs of barley aleurone layer protoplasts)
Dronzek BL, Hwang P, Bushuk W (1972) Cereal Chem 49:232–239 (Visual degradation of starch granules in wheat endosperm)
Fath A, Bethke PC, Belligni MV, Spiegle YN, Jones RL (2001) New Phytol 151:99–107 (Programmed cell death in the aleurone layer)
Fincher GB (2011) In: Ullrich SE (ed) Barley: production, improvement and uses. Wiley-Blackwell, Chichester, pp 449–477 (Endosperm mobilization in barley)
Gubler F, Kalla R, Roberts JK, Jacobsen JV (1995) Plant Cell 7:1879–1891 (GA-MYB gene expression in barley aleurone layers)
Higgins TJV, Zwar JA, Jacobsen JV (1976) Nature 260:166–169 (GA-induced α-amylase synthesis in barley)
Jones RL (1969) Planta 85:359–375 (Structure of the barley aleurone layer)
Matsukura C-A, Saitoh T, Hirose T, Ohsugi R, Perata P, Yamaguchi J (2000) Plant Physiol 124:85–94 (Sugar uptake and conversion in the rice scutellum)
Schoonheim PJ, Pereira DDC, De Boer AH (2009) Plant Cell Environ 32:437–447 (Cross-talk between ABA and GA signalling: 14-3-3 proteins)
Schwarz P, Li Y (2011) In: Ullrich SE (ed) Barley: production, improvement and uses. Wiley-Blackwell, Chichester, pp 478–520 (Use of barley in malting and brewing)
Smith AM, Zeeman SC, Smith SM (2005) Annu Rev Plant Biol 56:73–98 (Starch degradation in seeds and vegetative tissues)
Swift JG, O’Brien TP (1972) Aust J Biol Sci 25:469–486 (Structure of the scutellum of germinating wheat)
Section 5.6
Buckeridge MS (2010) Plant Physiol 154:1017–1023 (Synthesis and mobilization of cell wall hemicelluloses)
Juliano BO, Varner JE (1969) Plant Physiol 44:886–892 (Starch breakdown in pea)
Section 5.7
Ferjani A, Segami S, Horiguchi G, Muto Y, Maeshima M, Tsukaya H (2011) Plant Cell 23:2895–2908 (PPi and the regulation of gluconeogenesis)
Graham IA (2008) Annu Rev Plant Biol 59:115–142 (Storage oil mobilization in seeds)
Kaur N, Hu J (2009) Curr Opin Plant Biol 12:781–788 (Peroxisome proliferation in plants, mammals and yeast)
Laynton-Hogg T, Warriner SL, Baker A (2010) Biol Cell 102:245–263 (Import of proteins into the glyoxysome matrix)
Lingard M, Monroe-Augustus M, Bartel B (2009) Proc Natl Acad Sci USA 106:4561–4566 (Transition from glyoxysome to peroxisome)
Ma C, Agrawal G, Subramani S (2011) J Cell Biol 193:7–16 (Synthesis and assembly of peroxisomes)
Quettier A-L, Eastmond PJ (2009) Plant Physiol Biochem 47:485–490 (Lipases and storage oil hydrolysis)
Sections 5.8, 5.9
Gietl C, Schmid M (2001) Naturwiss 88:49–58 (Ricinosomes and PCD)
Greiner R, Alminger ML, Carlsson N-G, Muzquiz M, Burbano C, Cuadrado C, Pedrosa MM, Goyoaga C (2002) J Agric Food Chem 50:6865–6870 (Pathways of phytin breakdown)
He F, Huang F, Wilson KA, Tan-Wilson A (2007) J Exp Bot 58:1059–1070 (Acidification of PSVs and activation of proteases in soybean cotyledons)
Li Y-C, Ren J-P, et al (2009) Mol Plant 2:430–441 (Thioredoxins in wheat grains)
Maugenest S, Martinez I, Godin B, Perez P, Lescure A-M (1999) Plant Mol Biol 39:503–514 (Phytase genes in maize embryos)
Miflin BJ, Wallsgrove RM, Lea PJ (1981) In: Current topics in cellular regulation, vol. 20. Academic Press, New York, pp 1–43 (Glutamine metabolism in plants)
Müntz K, Belozersky MA, Dunaevsky YE, Schlereth A, Tiedemann J (2001) J Exp Bot 52:1741–1752 (Proteases and reserve mobilization during and following germination)
Shewry PR (1999) In: Shewry PR, Casey R (eds) Seed proteins. Kluwer Academic, Dordrecht, pp 587–615 (Enzyme inhibitors of seeds; several other chapters also)
Tooyooka K, Okamoto T, Minamakawa T (2001) J Cell Biol 154:973–982 (Autophagy in black gram cotyledons)
West CE, Waterworth WM, Stephens SM, Smith CP, Bray CM (1998) Plant J 15:221–229 (Scutellum peptide transport)
Wilson KA (2006) In: Black M, Bewley JD, Halmer P (eds) Encyclopedia of seeds. Science, technology and uses. CABI, Wallingford, pp 672–674 (Mobilization of storage proteins in dicots)
Section 5.10
Bewley JD (1997) Trends Plant Sci 2:464–469 (Regulation of mobilization of endosperm cell walls)
Davies HV, Chapman JM (1980) Planta 149:288–291 (Feedback inhibition in cucumber)
Halmer P, Bewley JD (1979) Planta 144:333–340 (Control of lettuce endo-β-mannanase production by the embryo)
Kern R, Chrispeels MJ (1978) Plant Physiol 62:815–819 (Protein mobilization in mung bean)
Muñoz JL, Martin L, Nicolas G, Villalobos N (1990) Plant Physiol 93:1011–1016 (Cytokinin effects on reserve mobilization in chickpea)
Penfield S, Li Y, Gilday AD, Graham S, Graham IA (2006) Plant Cell 18:1887–1899 (ABA responsiveness and TAG mobilization)
Slack PT, Black M, Chapman JM (1977) J Exp Bot 28:569–577 (Testa effects on lipid mobilization in cucumber)
Author information
Authors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Bewley, J.D., Bradford, K.J., Hilhorst, H.W.M., Nonogaki, H. (2013). Mobilization of Stored Reserves. In: Seeds. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-4693-4_5
Download citation
DOI: https://doi.org/10.1007/978-1-4614-4693-4_5
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-4692-7
Online ISBN: 978-1-4614-4693-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)