Abstract
Seeds of several palms are reserve-rich, and have a complex and poorly understood mobilization. The massive endosperm and embryo of Acrocomia aculeata Lodd. ex Mart. possess large amounts of proteins, polysaccharides, and lipids. We evaluated cell features related to reserve mobilization during germination and initial development of seedlings of A. aculeata. Samples of the haustorium (the cotyledonary blade) at different developmental stages and of the entire digestion zone of the endosperm were processed by standard methods for ultrastructural evaluation. The haustorium reserve mobilization begins when germination starts, and proteins are the first to be metabolized, followed by polysaccharides and lipids. Haustorial cells present lipid bodies associated with glyoxysomes and protein vacuoles, which are involved in lipid mobilization. The digestion zone of the endosperm comprises cell layers adjacent to the haustorium, in which reserve mobilization begins after the protrusion of the cotyledonary petiole. The endosperm reserve mobilization is similar to observed in the haustorium, firstly proteins, polysaccharides, and lipids in the end. The abundant carbohydrates in the cell walls of endosperm are hydrolyzed, and the cells lose integrity, while digestion products accumulate around the haustorium. The sinuosities observed in the plasma membrane and the organelles predominating in the epidermal cells of the haustorium are consistent with absorption and transitory storage of reserves, and there is no evidence of the secretion of enzymes that can act in the mobilization of endosperm reserves. Therefore, the endosperm has a storage and self-degradation function, and the products of hydrolysis are transported towards the haustorium via the apoplastic route.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Palm seeds have many peculiarities associated with the nature of their reserve substances and the structure of the embryo. In general, the seeds of Arecaceae species have a bulky and rigid endosperm with abundant lipid, protein, and carbohydrate reserves (DeMason 1988; Sekhar and DeMason 1988). Reserve carbohydrates, especially mannans, are mostly stored in the thick cell walls (Buckeridge 2010).
The embryo of palms is immersed in the endosperm and located near the seed coat, separated from it only by the few layers of the micropylar endosperm (for more details, see Fig. 1 of Mazzottini-dos-Santos et al. 2018). This embryo is linear and with two distinct regions: the proximal region, adjacent to the micropylar endosperm, which corresponds to the cotyledonary petiole where the microscopic embryo axis is inserted, and the distal region, towards the middle of the seed, which is the cotyledonary blade, called haustorium in palm embryos (DeMason and Thomson 1981; Sekhar and DeMason 1988; Moura et al. 2010; Baskin and Baskin 2014; Mazzottini-dos-Santos et al. 2015). The seedling development occurs by the growth of the embryo axis concomitantly with a pronounced increase of the haustorium, which acts in the mobilization of endosperm reserves (DeMason 1984; Alang et al. 1988; Sugimuma and Murakami 1990; Mazzottini-dos-Santos et al. 2017).
Germination and development of palm seedlings are complex processes that can vary with the environment, constituting adaptive strategies (Orozco-Segovia et al. 2003; Henderson 2006; Baskin and Baskin 2014). However, ultrastructural studies on seed reserve mobilization in Arecaceae have been restricted to Phoenix dactylifera L. (DeMason 1985; DeMason et al. 1985) and Washingtonia filifera (Linden ex André) H.Wendl. (DeMason 1988; Sekhar and DeMason 1988).
The functions of the haustorium and endosperm during the mobilization of palm seed reserves are not fully elucidated, especially because the site of synthesis of endosperm-activating enzymes has not yet been identified. Similarly, the particularities of lipid mobilization in palm seeds, especially in the haustorium, as well as the transport of the products of hydrolysis, are little known. Based on structural analyses, Oo and Stumpf (1983), Sugimuma and Murakami (1990), and Verdeil and Hocher (2002) pointed out that the haustorium may be responsible for synthesizing hydrolytic enzymes that act on the endosperm. On the other hand, the endosperm has been indicated to have a certain degree of autonomy in the digestion and mobilization of its reserves. Sekhar and DeMason (1990), Zienkiewicz et al. (2014), and Mazzottini-dos-Santos et al. (2017) suggested that protein bodies of endosperm cells possess hydrolytic enzymes, similar to the aleurone layer of amylaceous seeds (Bewley et al. 2013). These observations provide evidence that protein bodies are related to lipid and mannan mobilization. However, the mechanisms by which protein bodies act on these reserves, as well as the way by which hydrolysis products are transported from the endosperm to the haustorium, are still unknown for palms.
Arecaceae comprises about 2700 species, many of which are of high ecological and economic importance notably associated with the high oil content of their fruits and seeds (Dransfield et al. 2008; Oliveira et al. 2013; Mazzottini-dos-Santos et al. 2015). Acrocomia aculeata Lodd. ex Mart. (the macaw palm) is one of the Arecaceae species that is known for its potential as a source for biodiesel production (Pires et al. 2013), which makes the species of particular interest to studies on germination and seedling establishment. The species has caught attention because it is widely distributed in tropical regions, especially in the Cerrado biome (Dransfield et al. 2008; Lorenzi et al. 2010), and has dormant and lipid-rich seeds (Moura et al. 2010; Pires et al. 2013). In this biome, A. aculeata seeds are usually dispersed in summer between November and February (Mazzottini-dos-Santos et al. 2017). Seeds of A. aculeata are orthodox, i.e. tolerant to desiccation and low temperatures. Their structure was first described by Moura et al. (2010), and several aspects of its germination, especially the search for strategies to overcome dormancy, have been studied in the last decade (Ribeiro et al. 2011, 2012, 2015). Seed reserve mobilization has only recently been emphasized in publications on this species, referring to biochemical (Bicalho et al. 2016) and histochemical (Mazzottini-dos-Santos et al. 2017) issues. These authors revealed the sequence of reserve mobilization—proteins, polysaccharides, and lipids—and its relevance to the seedling establishment in A. aculeata. However, no ultrastructural studies could be found that elucidate how cell machinery is organized in the endosperm and haustorium, which is indispensable data for understanding the dynamics of reserve mobilization.
According to Baskin and Baskin (2014), there still is a significant gap in knowledge of the germination of palm diaspores. We agreed with the authors and performed unprecedented ultrastructural studies during seed germination and seedling establishment in A. aculeata, to address the following questions: (1) Which cell structures are involved in the mobilization of seed reserves? (2) What are the peculiarities observed in the mobilization of embryo and endosperm reserves? (3) Are cytological characteristics indicative of the specific function of the haustorium and endosperm in the mobilization of seed reserves? (4) How are hydrolyzed products transported from the endosperm reserves to the haustorium?
2 Materials and methods
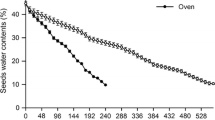
Between November (2014) and February (2015), about 200 ripe and freshly dispersed fruits of A. aculeata were collected on the ground, from native specimens in the municipality of Montes Claros, Minas Gerais, Brazil (16°42′34″ S; 43°52′48″ W), in an anthropized area used as pasture and previously occupied by cerrado stricto sensu vegetation. The fruits were stored in open boxes at room temperature for three to 4 months, until the water content (assessed by the oven method at 105 °C, as suggested by Ribeiro et al. 2011), initially almost 22%, reached 6%, value which allows the extraction of the seeds from the pyrene with minimal damage. Seeds were extracted using a bench vise, disinfected with 6% sodium hypochlorite, washed three times in water, and stored in plastic bags for 30 days under ambient conditions (Mazzottini-dos-Santos et al. 2017).
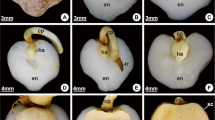
Before the experiments, the viability of the seed lot was evaluated by embryo culture in five replicates of ten embryos (Ribeiro et al. 2011), and 92% of the seeds proved to be viable. The disinfection was repeated, and the seeds were imbibed in a 0.4% H2O2 solution for 10 days with daily changes of solution. To promote the overcoming of dormancy, the seed operculum was removed manually with the aid of a utility knife, under aseptic conditions in a laminar flow chamber (Neves et al. 2013). The seeds were sown in polyethylene containers covered by lids, containing 120 mL of sterilized vermiculite moistened with a 0.4% H2O2 solution (Mazzottini-dos-Santos et al. 2017). Germination and seedling development were evaluated in a germination chamber (Nova Ética, BOD 411/FPD, Brazil) at 30 °C in the dark for 150 days. The germination conclusion was evidenced by the elongation of about 2 mm of the cotyledonary petiole and occurred circa 5 days after operculum removal (Ribeiro et al. 2011, 2015; Mazzottini-dos-Santos et al. 2017). Seven phases were sampled (ten samples in each phase) according to morphological changes during development: (1) dry seed (6% water content); (2) imbibed seed (after 10 days of immersion in 0.4% H2O2 solution, until 22% water content); (3) seedling with initial elongation of the cotyledonary petiole; (4) seedling with the emission of the taproot; (5) seedling with the emission of the first leaf sheath; (6) seedling with the emission of the second leaf sheath; and (7) seedling with the emission of the eophyll. Figure 1 illustrates the morphological traits observed in each phase (adapted from Mazzottini-dos-Santos et al. 2017).
Diagrams of seeds and seedlings of Acrocomia aculeata throughout development (phases I–VII), adapted from Mazzottini-dos-Santos et al. (2017). Longitudinal sections of the seed show the proportion of the seed coat, endosperm, and embryo (a, b) or seedling (c–g). a Phase I, dry seed with the linear embryo. b Phase II, imbibed seed. c Phase III, germinated seed, evidenced by the protrusion and elongation of the cotyledonary petiole. d–g Phases IV–VII, seedlings showing an evident digestion zone in the endosperm adjacent to the haustorium (in grey, indicated by black arrows); note the increase in the size of the haustorium while the endosperm is consumed. d Phase IV, seedling with elongated cotyledonary petiole and protruded taproot. e Phase V, seedling with first leaf sheath wrapped by ligule. f Phase VI, seedling with second leaf sheath emitted. g Phase VII, seedling with the first eophyll. cp, cotyledonary petiole; en, endosperm; eo, eophyll; ha, haustorium; li, ligule; s1, first leaf sheath; s2, second leaf sheath; sc, seed coat; tr, taproot
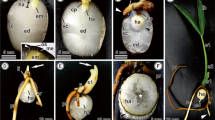
Samples were removed from the peripheral region of the haustorium at each studied phase. In the endosperm, the digestion zone of the haustorium was sampled only at phase IV because this is the first phase that shows this layer and is identical to subsequent phases (see Fig. 2). Samples were fixed in Karnovsky solution (Karnovsky 1965) for 24 h, dehydrated in an ethanol series, and post-fixed in 1% osmium tetroxide in 0.1 M phosphate buffer, pH 7.2 (Roland 1978). Ultrathin sections were contrasted with uranyl acetate and lead citrate and examined using a Tecnai G2-12-Spirit transmission electron microscope (Philips/FEI Company, Eindhoven, Netherlands) at 80 kV.
Diagram and detail of seeds of Acrocomia aculeata in transversal section, adapted from Mazzottini-dos-Santos et al. (2017). a Haustorium with invaginations in the protoderm, surrounded by the endosperm (grey area); the endosperm digestion zone is adjacent to the haustorium as marked by the dotted rectangle. b Digestion zone of the endosperm with five distinct regions: an outermost region with hard endosperm (right side); an intermediate region where degradation of the protein bodies occurs, followed by the region where the cytoplasm is degraded; an innermost region where there is cell depletion, followed by the region with collapsed cells around the haustorium (left side). Note the gradient of change in protein bodies from the outer to the inner regions in the seed: dense protein bodies → granular appearance → reduced amount of protein reserves → vacuoles with protein remnants. cc, region of collapsed cells; cd, region of cytoplasm degradation; de, region of cell depletion; en, endosperm; ha, haustorium; he, hard endosperm; pd, region of protein bodies degradation; pr, protoderm; ps, procambial strand
3 Results
Ultrastructure of haustorium cells
– In phase I (Fig. 1a), the cells of the protoderm of the haustorium possess dense protoplast, thin walls, except for the outer periclinal face (Fig. 3a, b), and primary pit fields with plasmodesmata connecting these cells to each other and to neighbouring cells; the cytoplasm is full of reserves and has several lipid bodies positioned around the protein bodies (Fig. 3a, b). During imbibition (phase II, Fig. 1b), protein mobilization begins in the haustorium, as evidenced by vacuoles containing protein remnants (Fig. 3c, d). After protrusion of the cotyledonary petiole (phase III, Fig. 1c), the epidermal cells of the haustorium show an organelle-rich cytoplasm with several mitochondria, poorly differentiated plastids (with prolamellar bodies), and glyoxysomes (Fig. 4a, c). Lipid bodies are less numerous than that from the previous stage, many of which are associated with small vacuoles containing protein remnants (Fig. 4d).
Protoderm of the haustorium of Acrocomia aculeata in phases I (a, b) and II (c, d). a Cell with outer periclinal face thicker than the others; this cell has dense cytoplasm containing several protein and lipid bodies. b Cells in paradermal section, presenting a dense protoplast on which predominates protein and lipid bodies. c Cell containing vacuoles with protein and numerous intact lipid bodies. d Central region of the cell showing the nucleus with an evident nucleolus; note a vacuole containing protein remnants, indicating the beginning of protein mobilization during imbibition, and some plastids (white arrows). cw, cell wall; lb, lipid body; no, nucleolus; nu, nucleus; pb, protein body; pr, protein remnants; st, starch; va, vacuole with protein
Epidermal cell of the haustorium of Acrocomia aculeata in phase III. a Epidermis surface showing cells with dense protoplast, highlighting nucleus and lipid bodies. b Detail of cell with many plastids, glyoxysomes, mitochondria, and small vacuoles. c Portion of cytoplasm showing plastids with dense stroma and scarce inner membrane system; glyoxysomes and mitochondria with well-developed cristae complete the representative organelles. Notice sinuosities of the plasma membrane. d Vacuoles with remnants of proteins involving lipid bodies. Black arrows indicate points of lipid bodies incorporation into vacuoles. gl, glyoxysome; lb, lipid body; mi, mitochondria; nu, nucleus; pl, plastid; va, vacuole
The cytoplasm of the epidermal cells of the haustorium (phase IV, Fig. 1d) remains rich in organelles (Fig. 5a), which are markedly more differentiated and more numerous than in the previous phase. There is a notable proliferation of endoplasmic reticulum, usually associated with ribosomes adjacent to the plasma membrane (Fig. 5b). In this region of the cell, small vesicles (Fig. 5b), sinuosities of the plasma membrane, and granular material in the periplasmic space are also present (Fig. 5c). Plastids with an evident internal membrane system accumulate starch grains (Fig. 5c). There is a proliferation of organelles, especially mitochondria and plastids, while small vacuoles contain remnants of proteins (Fig. 5a, c, d) and lipid droplets (Fig. 5d). Glyoxysomes with a granular matrix occur in the cytoplasm, usually positioned close to the protein vacuoles and lipid bodies (Fig. 5e).
Epidermal cells of the haustorium of Acrocomia aculeata in phase IV. a Cell with dense cytoplasm and increased number of organelles. b–e) Periphery of the epidermal cell. b Concentration of endoplasmic reticulum and vesicles close to the plasma membrane. c Accumulated substances in the periplasmic space, produced by sinuosities of the plasma membrane (white arrows); note an amyloplast with starch grain. d Central region of the cell near the nucleus; note the numerous mitochondria, plastids, and small vacuoles containing lipid droplets (black arrows). e Periphery of two epidermal cells, highlighting plasmodesma, the sinuosity of the plasma membrane (white arrow), and a glyoxysome with granular matrix near the vacuole with protein. cw, cell wall; er, endoplasmic reticulum; gl, glyoxysome; lb, lipid body; mi, mitochondria; nu, nucleus; pd, plasmodesma; pl, plastid; pr, protein remnants; st, starch; va, vacuole; vs, vesicle
In phase V (Fig. 1e), products resulting from endosperm digestion adjacent to the epidermis of the haustorium accumulate (Fig. 6a, b). Epidermal cells have a sinuous plasma membrane, and large amounts of substances can be observed in the periplasmic space (Fig. 6b). There is a remarkable reduction of lipid reserve in the cytoplasm in this phase, while the lipid bodies are associated with protein vacuoles (Fig. 6a). With the growth of the haustorium, a layer of cells subjacent to the epidermis accumulates a large quantity of starch (Fig. 6c), while in the inner region, aerenchyma of schizogenous origin is formed (Fig. 6d). The aerenchyma cells are bulky, vacuolated, and may possess small drops of lipids inside their vacuoles; small starch grains are present in the plastids (Fig. 6d).
Haustorium of Acrocomia aculeata in phases V (a–d), VI (e–f), and VII (g–h). a Epidermal cell with a remarkable reduction in the number of lipid bodies (asterisk: endosperm residues); note vacuoles associated with lipid bodies (black arrows) and a high number of glyoxysomes. b Periphery of the epidermal cell coated with endosperm residues (asterisk); note that substances are accumulating in the periplasmic space, and there is prominent sinuosity of the plasma membrane (white arrows). c Subepidermal cells with starch accumulation. d Inner layer of the haustorium showing large intercellular spaces and cells with bulky vacuoles containing lipids and starch grains in the cytoplasm. e–f Epidermal cells with spaces in the cell wall (arrowheads) and conspicuous accumulation of lipids in the cytoplasm. g Cell wall with remarkable layers of different electron density and sinuosity of the plasma membrane (white arrows). h Inner layer of the haustorium showing a cell with remnants of starch grains. cw, cell wall; er, endoplasmic reticulum; gl, glyoxysome; is, intercellular space; lb, lipid body; lp, lipids; mi, mitochondria; no, nucleolus; nu, nucleus; st, starch; va, vacuole
In phase VI (Fig. 1f), during which the mobilization of endosperm reserves is quite pronounced, spaces form in the walls of the outer periclinal face of epidermal cells (Fig. 6e) and in the anticlinal faces (Fig. 6f), indicating the loosening of cell walls. There is a conspicuous accumulation of lipids in the cells of the epidermis in this phase, and vacuoles exhibit denser content (Fig. 6f). In phase VII (Fig. 1g), cell walls show layers with varied electron densities, and the plasma membrane is notably sinuous (Fig. 6g). The aerenchyma cells have no reserves in this phase, except for a few small remnants of starch grains; the cells lose the integrity of their membrane and take on an empty appearance (Fig. 6h).
Ultrastructure of endosperm cells
– The endosperm exhibits cells with thick walls and dense cytoplasm, with a small population of organelles, but abundant lipid and protein bodies (Fig. 7a). Changes are observed in the cells of the digestion zone during the mobilization of reserves, which begins adjacent to the haustorium, and establishes a gradient towards the outer portion of the endosperm (Fig. 2a, b). Proteins are the first substances to be mobilized, a process identified by reduced electron density of the protein bodies, which are flanked by intact lipid bodies (Fig. 7a, d). The fusion of the protein bodies forms a single central protein body containing proteins and several crystalloid inclusions (Figs. 2b and 7a). Mitochondria with a dense matrix are present in the cytoplasm (Fig. 7b). The vacuoles exhibit a continuous reduction in protein reserve and inclusions, and their content acquires a flocculated appearance (Fig. 7d, e). There is the diffusion of electron-dense substances of the periplasmic space through the cell wall (Fig. 7a–e). The dissolution of the cell wall is identified by increased electron density, which starts at the innermost layer (Fig. 7f, g). After mobilization of the proteins, there may be a rupture of vacuolar membranes in cells of the digestion zone near the haustorium, culminating in the loss of cell integrity (Fig. 7g) or the retention of a single central vacuole, which may contain remnants of substances (Fig. 7h). The cell wall is partially collapsed after lipid mobilization; the nucleus and the organelles are degraded, and membrane residues are accumulated in the peripheral cytoplasm (Fig. 7h). At the end of cytoplasm degradation, cells with collapsed walls are present around the haustorium (Fig. 2b) and substances, especially lipids, accumulate in intercellular spaces (compare Fig. 7d with Fig. 7h).
Digestion zone of the endosperm of Acrocomia aculeata in phase IV. a–d Cell located in the intermediate region of the digestion zone (see Fig. 2b, region of degradation of protein bodies—pd). a Cell with prominent electron-dense content in the cytoplasm adjacent to the cell wall (white arrows), which indicates the beginning of mobilization of substances by the wall; note the cytoplasm with mitochondria, numerous lipid bodies and a single bulky protein body at the center of the cell with a granular appearance. b–c Periphery of the cell highlighting mitochondria and variation of electron density of the wall (white arrows), indicating mobilization of the substances of the cell wall. d Cell exhibiting a vacuole produced by the digestion of a protein body; note reduced amount of proteins in the vacuole and the contents in the intercellular space. e–g Cells of the digestion zone near the haustorium (see Fig. 2b, region of cytoplasm degradation—cd). e Cell showing a vacuole with protein remnants, surrounded by intact lipid bodies; note the variation in the electron density of cell wall (white arrows). f Periphery of a cell, highlighting the dissolution zone of the cell wall. g Cell in advanced stage of dissolution of the cell wall (white arrow). h Cell located in the inner region of the digestion zone of the endosperm (see Fig. 2b, region of cell depletion—de) showing the wall of two adjacent cells, and lipid droplets (black arrows) into the intercellular space; a large vacuole with remnants of proteins can be seen, and remaining membranes in the thin cytoplasm are found in the rectangle highlighted. cw, cell wall; dz, dissolution zone; fm, fibrillar material; is, intercellular space; lb, lipid body; mi, mitochondria; nu, nucleus; pb, protein body; pr, protein remnants; va, vacuole
4 Discussion
Mobilization of reserves in haustorium and endosperm cells
– Although the beginning of the mobilization of reserves and the activity of distinct cell structures occur at different times in the haustorium and endosperm of A. aculeata, the dynamics of these processes are similar in both. The ultrastructural analysis confirmed that reserve proteins are the first to be mobilized, followed by carbohydrates and lipids. Similar data were recorded, both in histochemical and biochemical studies, for the embryo and endosperm of P. dactylifera (DeMason 1985; DeMason et al. 1985), W. filifera (DeMason 1988), Butia capitata Becc. (Oliveira et al. 2013) and A. aculeata (Bicalho et al. 2016; Mazzottini-dos-Santos et al. 2017). In general, the protein reserve of the embryo is initially important as a source of energy for starting germination, in addition to supplying amino acids for the biosynthesis of new structural proteins or enzymes; on the other hand, the lipid reserve is essential during seedling growth (Tan-Wilson and Wilson 2012; Bewley et al. 2013). Mobilization of haustorium reserves in A. aculeata involves remarkable activity on the part of protein bodies and glyoxysomes, whereas in the endosperm, there is relevant participation of the protein bodies. The proliferation of mitochondria and glyoxysomes in the epidermis of the haustorium is apparent only after the elongation of the cotyledonary petiole, which breaks the seed coat and marks the conclusion of germination, thus explaining the late lipid mobilization. Glyoxysomes also occur in the epidermis of the haustorium of P. dactylifera and W. filifera, where they were always associated with lipid bodies (DeMason 1985, 1988). Oo and Stumpf (1983), Alang et al. (1988), Hayashi et al. (2001), and Graham (2008) detected specific glyoxysome enzymes (isocitrate liase and malate synthetase) in the haustorium during the development of Elaeis guineensis Jacq. According to these authors, such enzymes are involved with β-oxidation of fatty acids and the glyoxylate cycle.
Lipid mobilization in the haustorium and endosperm of A. aculeata involves the direct participation of protein bodies. After the mobilization of reserve proteins during seedling development, protein bodies assume the function of lytic vacuoles with the internalization of lipid bodies. This type of interaction between structures involved in seed reserve is described here, at the subcellular level, for the first time for palms. The internalization of lipid bodies into vacuoles with protein, including enzymes, has been reported for the cotyledon of Arabidopsis Heynh. (Poxleitner et al. 2006) and Olea europaea L. (Zienkiewicz et al. 2014). According to Poxleitner et al. (2006) and Zienkiewicz et al. (2014), the interaction between lipid bodies and the membrane of protein vacuoles in the species they studied is mediated by caleosins, which are proteins that contribute to the breakdown of the phospholipid layer of lipid bodies. Previous analyses with A. aculeata determined that the protein bodies store lipases (Mazzottini-dos-Santos et al. 2017), which suggests that this internalization facilitates the degradation of triacylglycerols (Huang 1996; Hayashi et al. 2001; Poxleitner et al. 2006).
After mobilization of the reserve proteins in the endosperm of A. aculeata, the fusion of vacuoles occurs. This process is common in many species during germination. It produces vacuoles with lytic properties (Poxleitner et al. 2006) of various origins: by fusion of vacuoles containing preformed hydrolytic enzymes (Bewley and Black 1994); through activation of stored enzymes (Jiang et al. 2001); or by the synthesis of hydrolytic enzymes that are sent to protein vacuoles by vesicles of dictyosomes or the endoplasmic reticulum (Herman and Larkins 1999). In the endosperm cells of A. aculeata, the inconspicuous occurrence of dictyosomes and endoplasmic reticulum, and the early mobilization of protein, associated with intensification of the degradation of the other reserves during seedling growth, suggest that hydrolytic enzymes are rapidly activated, but not synthesized. This feature is considered to be more energetically efficient since synthesizing enzymes would require more time and energy expenditure during seedling growth (Buckeridge 2010). Jiang et al. (2001) described vacuoles that have the double function of storage organelle and lytic vacuole, with them expressing one function during seed development and the other during maturation. According to the authors, the portioning of storage and lytic roles in the same vacuole may provide a rapid source of enzymes to initiate the degradation processes during the beginning of germination.
Cytological and functional traits of the haustorium and endosperm
– The ultrastructural evaluation revealed that the haustorium of A. aculeata is highly specialized for absorption. However, the haustorium is initially a reserve organ; the presence of cell structures such as protein and lipid bodies, also recognized by Mazzottini-dos-Santos et al. (2017), denotes this function. Embryos of orthodox seeds typically possess large amounts of reserves, notably lipids and proteins (Panza et al. 2004; Moura et al. 2010), as is the case for some palms such as E. guineensis (Alang et al. 1988), W. filifera (DeMason 1988), P. dactylifera (Sekhar and DeMason 1988) and B. capitata (Oliveira et al. 2013), as well as A. aculeata. During the seedling development of A. aculeata, there is a temporary storage of starch in the haustorium concomitant with lipid degradation, a process that has also been identified through biochemical (Bicalho et al. 2016) and histochemical (Mazzottini-dos-Santos et al. 2017) evaluations. Similar data were obtained for P. dactylifera (DeMason 1985), W. filifera (DeMason 1988) and B. capitata (Oliveira et al. 2013; Dias et al. 2018). The presence of glyoxysomes in the haustorium, which have been widely reported here, is related to the accumulation of carbohydrates from lipid degradation. This process occurs through gluconeogenesis (Alang et al. 1988; Graham 2008), so the occurrence of glyoxysomes in haustorial cells indicates how substances are converted in the haustorium of A. aculeata, as previously suggested by Mazzottini-dos-Santos et al. (2017).
Based on the present results, there is no cytological evidence that the haustorium functions in secreting enzymes that act on the endosperm. The endoplasmic reticulum, associated with ribosomes, may be involved in the synthesis of new enzymes that are essential for the degradation of starch and lipids, which are temporarily accumulated in the haustorium, a route also confirmed by the histochemical methods employed by Mazzottini-dos-Santos et al. (2017) during reserve mobilization in the endosperm of A. aculeata. In haustorial cells, we found no evidence for the secretion of enzymes that would act on the endosperm; on the contrary, the sinuosities observed in the plasma membrane and the temporary accumulation of reserves are all evidence that the haustorium acts in absorbing endosperm reserves, but without interfering in their digestion.
Data reported here also confirm that during the mobilization of reserves, the endosperm cells of A. aculeata do not possess a population of organelles that enables them to synthesize new enzymes. Still, the involvement of protein bodies in the mobilization of reserves is evident. Due to cell characteristics, Moura et al. (2010) suggested that the endosperm of A. aculeata functions only as a reserve tissue. The absence of endoplasmic reticulum, dictyosomes, and ribosomes in endosperm cells of P. dactylifera was also related to the inability of this tissue to produce enzymes (DeMason et al. 1983). However, Sekhar and DeMason (1988) indicated specific embryo-endosperm proteins, which is consistent with the hypothesis that the endosperm stores hydrolytic enzymes, possibly in protein bodies. The same condition was observed for A. aculeata, but with protein bodies being converted into lytic vacuoles. Cell changes that occur in the endosperm after germination, such as the diffusion of substances through the cell wall, the decrease in reserves of the protein bodies and the beginning of the dissolution of the cell wall in the periplasmic space, are all evidence that hydrolytic enzymes are stored in the endosperm tissue itself. Histochemical analyses of the endosperm of seeds of A. aculeata demonstrated that protein bodies are sites of lipases, which are transferred to the cytoplasm and act on the surface of lipid bodies (Mazzottini-dos-Santos et al. 2017), similar to what occurs during germination of O. europaea (Zienkiewicz et al. 2014). Endo-β-mannanase and α-galactosidase, enzymes involved in the hydrolysis of galactomannans, occur in the endosperm of dry seeds of A. aculeata and have high activity in the portion of endosperm adjacent to the haustorium (Bicalho et al. 2016; Mazzottini-dos-Santos et al. 2017). Several enzymes were diagnosed in the endosperm of P. dactylifera, among which the site of α-galactosidase was identified in protein bodies by immunocytochemistry (DeMason et al. 1985; Sekhar and DeMason 1990). In the endosperm of P. dactylifera and W. filifera, acid phosphatase, an enzyme involved in the mobilization of carbohydrates and phosphates, is also stored in protein bodies (DeMason et al. 1989), reinforcing our interpretation.
Transfer of reserve breakdown products
– Endosperm reserves are hydrolyzed and the products transported to the haustorium where they can be stored as transient starch, after which they are mobilized to the growing vegetative axis. In the endosperm of A. aculeata, these substances are transported via an apoplastic route through the cell wall, as suggested by our observations. On the other hand, there is evidence of symplastic transport through numerous plasmodesmata in the haustorium, as also registered here. Reduction in electron density and the formation of spaces in the walls of haustorial cells, as well as the accumulation of substances in the periplasmic space and the sinuosities of the plasma membrane, seem to indicate the route of absorption of endosperm products. It can be inferred that invaginations of the plasma membrane, also verified in P. dactylifera by DeMason (1985), are related to the transport of solutes from the apoplast to the symplast, with the latter being translocated via plasmodesmata. However, the associations of these cell changes were not described for the other palms that have been studied; thus, the absorption of endosperm products by the haustorium is not well understood yet. We believe that space formation, associated with changes in the electron density of the walls of the epidermal cells of the haustorium, may be related to increased porosity and loosening of the wall, which facilitates the direct absorption of substances of the endosperm, such as lipids. When mobilization of endosperm reserves intensifies, the present results reveal that there is a concentration of substances, especially lipids, on the surface of the haustorium—the same substances that are identified in epidermal cells of the haustorium in later phases. The pressure exerted by the expansion of the haustorium against the endosperm seems to explain the passage of lipids through the barrier imposed by the cell wall. Previous studies have confirmed the accumulation of lipids in the haustorium using histochemical analyses, suggesting that lipid absorption is facilitated by the pressure exerted between the growing haustorium and the hard endosperm surrounding it (Mazzottini-dos-Santos et al. 2017). The pressure exerted by the protoplast as a facilitator for the passage of lipids through the cell wall was described by Paiva (2016) to explain the release of oils produced by secretory cells; without such pressure, lipids would not cross the hydrophilic cell wall. This model can be applied here to explain lipid absorption by the haustorium.
Plasmodesmata are inconspicuous in the cells of the endosperm of A. aculeata, as verified by Moura et al. (2010). The formation of electron-dense layers, which spread throughout the extension of the cell wall, shows the possible transport of substances between cells via apoplast. The evidence of apoplastic transport and the gradual changes in the matrix of the protein bodies indicate those substances could be products of hydrolysis and enzymes. The transport of enzymes from protein bodies to adjacent cells via the cell wall is congruent with previous observations of P. dactylifera, which, although possessing plasmodesmata in the thickened walls of the endosperm, also shows the dissemination of enzymes through the cell wall (Sekhar and DeMason 1990).
Detailed ultrastructural evaluation during the mobilization of reserves in A. aculeata seeds makes it possible to emphasize that, in both the endosperm and haustorium, mobilization of reserves begins with proteins, followed by carbohydrates and lipids. Protein bodies and glyoxysomes are the organelles involved with the mobilization of lipids in the haustorium, whereas only protein bodies perform this function in the endosperm. There is no cytological evidence that the haustorium secretes enzymes that act on the endosperm, and so it only functions in absorption and as an organ of transient reserve. The endosperm is a cell-storage structure, yet it also plays an important role during the mobilization of lipids and carbohydrates of cell walls, with autonomy regarding enzyme activity. The transport of substances between cells in the haustorium is symplastic, while in the endosperm, it occurs via apoplast.
References
Alang ZC, Moir GFJ, Jones LH (1988) Composition, degradation and utilization of endosperm during germination in the oil palm (Elaeis guineensis Jacq.). Ann Bot 61:261–268. https://doi.org/10.1093/oxfordjournals.aob.a087553
Baskin JM, Baskin CC (2014) What kind of seed dormancy might palms have? Seed Sci Res 24:17–22. https://doi.org/10.1017/S0960258513000342
Bewley JD, Black M (1994) Seeds: physiology of development and germination. Plenum Press, New York
Bewley JD, Bradford KJ, Hilhorst HWM, Nonogaki H (2013) Seeds: physiology of development, germination and dormancy. Springer, New York
Bicalho EM, Motoike SY, Borges EEL, Ataíde GM, Guimarães VM (2016) Enzyme activity and reserve mobilization during macaw palm (Acrocomia aculeata). Acta Bot Bras 30:437–444. https://doi.org/10.1590/0102-33062016abb0181
Buckeridge MS (2010) Seed cell wall storage polysaccharides: models to understand cell wall biosynthesis and degradation. Plant Physiol 154:1017–1023. https://doi.org/10.1104/pp.110.158642
DeMason DA (1984) Growth parameters in the cotyledon of date seedlings. Bot Gaz 145:176–183
DeMason DA (1985) Histochemical and ultrastructural changes in the haustorium of date (Phoenix dactylifera L.). Protoplasma 126:168–177
DeMason DA (1988) Seedling development in Washingtonia filifera (Arecaceae). Bot Gaz 149:45–56. https://doi.org/10.1086/337690
DeMason DA, Thomson WW (1981) Structure and ultrastructure of the cotyledon of date palm (Phoenix dactylifera L.). Bot Gaz 142:320–328. https://doi.org/10.1086/337230
DeMason DA, Sexton R, Grant Reid JS (1983) Structure, composition and physiological state of the endosperm of Phoenix dactylifera L. Ann Bot 52:71–80. https://doi.org/10.1093/oxfordjournals.aob.a086554
DeMason DA, Sexton R, Gorman M, Reid JSG (1985) Structure and biochemistry of endosperm breakdown in date palm (Phoenix dactylifera L.) seeds. Protoplasma 126:159–167
DeMason DA, Stillman JI, Ellmore GS (1989) Acid phosphatase localization in seedling tissues of the palms, Phoenix dactylifera and Washingtonia filifera, and its relevance to controls of germination. Can J Bot 67:1103–1110. https://doi.org/10.1139/b89-144
Dias DS, Ribeiro LM, Lopes PSN, Melo GA, Muller M, Munné-Bosch S (2018) Haustorium-endosperm relationships and the integration between developmental pathways during reserve mobilization in Butia capitata (Arecaceae). Ann Bot 122:267–277. https://doi.org/10.1093/aob/mcy065
Dransfield J, Uhl NW, Asmussen CBA, Baker WJ, Harley MM, Lewis CE (2008) Genera palmarum: the evolution and classification of palms. Kew Publishing, Kew
Graham IA (2008) Seed storage oil mobilization. Annu Rev Plant Biol 59:115–142. https://doi.org/10.1146/annurev.arplant.59.032607.092938
Hayashi Y, Hayashi M, Hayashi H, Hara-Nishimura I, Nishimura M (2001) Direct interaction between glyoxysomes and lipid bodies in cotyledons of the Arabidopsis thaliana ped1 mutant. Protoplasma 218:83–94. https://doi.org/10.1007/BF01288364
Henderson F (2006) Morphology and anatomy of palm seedlings. Bot Rev 72:273–329. https://doi.org/10.1663/0006-8101(2006)72%5b273:MAAOPS%5d2.0.CO;2
Herman EM, Larkins BA (1999) Protein storage bodies and vacuoles. Plant Cell 11:601–613. https://doi.org/10.1105/tpc.11.4.601
Huang AHC (1996) Oleosins and oil bodies in seeds and other organs. Plant Physiol 110:1055–1061. https://doi.org/10.1104/pp.110.4.1055
Jiang L, Phillips TE, Hamm CA, Drozdowicz YM, Rea PA, Maeshima M, Rogers SW, Rogers JC (2001) The protein storage vacuole: a unique compound organelle. J Cell Biol 155:991–1002. https://doi.org/10.1083/jcb.200107012
Karnovsky MJ (1965) A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J Cell Biol 27:137–138
Lorenzi H, Noblick L, Kahn F, Ferreira E (2010) Flora Brasileira: Arecaceae (palmeiras). Instituto Plantarum, Nova Odessa
Mazzottini-dos-Santos HC, Ribeiro LM, Mercadante-Simões MO, Sant’Anna-Santos BF (2015) Ontogenesis of the pseudomonomerous fruit of Acrocomia aculeata (Arecaceae): a new approach to the development of pyrenarium fruits. Trees 29:199–214. https://doi.org/10.1007/s00468-014-1104-0
Mazzottini-dos-Santos HC, Ribeiro LM, Oliveira DMT (2017) Roles of the haustorium and endosperm during the development of seedlings of Acrocomia aculeata (Arecaceae): dynamics of reserve mobilization and accumulation. Protoplasma 254:1563–1578. https://doi.org/10.1007/s00709-016-1048-x
Mazzottini-dos-Santos HC, Ribeiro LM, Oliveira DMT (2018) Structural changes in the micropylar region and overcoming dormancy in Cerrado palms seeds. Trees 32:1415–1428. https://doi.org/10.1007/s00468-018-1723-y
Moura EF, Ventrella MC, Motoike SY (2010) Anatomy, histochemistry and ultrastructure of seed and somatic embryo of Acrocomia aculeata (Arecaceae). Sci Agr 67:375–495. https://doi.org/10.1590/S0103-90162010000400004
Neves SC, Ribeiro LM, Cunha IRG, Pimenta MAS, Mercadante-Simões MO, Lopes PSN (2013) Diaspore structure and germination ecophysiology of the babassu palm (Attalea vitrivir). Flora 208:68–78. https://doi.org/10.1016/j.flora.2012.12.007
Oliveira NCC, Lopes PSN, Ribeiro LM, Mercadante-Simões MO, Oliveira LAA, Silvério FO (2013) Seed structure, germination, and reserve mobilization in Butia capitata (Arecaceae). Trees 27:1633–1645. https://doi.org/10.1007/s00468-013-0910-0
Oo KC, Stumpf PK (1983) Some enzymic activities in the germinating oil palm (Elaeis guineensis) seedling. Plant Physiol 73:1028–1032. https://doi.org/10.1104/pp.73.4.1028
Orozco-Segovia A, Batis AI, Roja-Aréchiga M, Mendoza A (2003) Seed biology of palms: a review. Palms 47:79–94
Paiva EAS (2016) How do secretory products cross the plant cell wall to be released? A new hypothesis involving cyclic mechanical actions of the protoplast. Ann Bot 117:533–540. https://doi.org/10.1093/aob/mcw012
Panza V, Láinez V, Maldonado S (2004) Seed structure and histochemistry in the palm Euterpe edulis. Bot J Linn Soc 145:445–453. https://doi.org/10.1111/j.1095-8339.2004.00293.x
Pires TP, Souza ES, Kuki KN, Motoike SY (2013) Ecophysiological traits of the macaw palm: a contribution towards the domestication of a novel oil crop. Ind Crop Prod 44:200–210. https://doi.org/10.1016/j.indcrop.2012.09.029
Poxleitner M, Rogers SW, Samuels AL, Browse J, Rogers JC (2006) A role of caleosin in degradation of oil-body storage lipids during seed germination. Plant J 47:917–933. https://doi.org/10.1111/j.1365-313X.2006.02845.x
Ribeiro LM, Souza PP, Rodrigues-Junior AG, Oliveira TGS, Garcia QS (2011) Overcoming dormancy in macaw palm diaspores, a tropical species with potential for use as biofuel. Seed Sci Technol 39:303–317. https://doi.org/10.15258/sst.2011.39.2.04
Ribeiro LM, Oliveira DMT, Garcia QS (2012) Structural evaluations of zygotic embryos and seedlings of the macaw palm (Acrocomia aculeata, Arecaceae). Trees 26:851–863. https://doi.org/10.1007/s00468-011-0659-2
Ribeiro LM, Garcia QS, Müller M, Munné-Bosch S (2015) Tissue-specific hormonal profiling during dormancy release in macaw palm seeds. Physiol Plant 153:627–642. https://doi.org/10.1111/ppl.12269
Roland AM (1978) General preparations and staining of thin sections. In: Hall JL (ed) Electron Microscopy and cytochemistry of plant cells. Elsevier, New York, pp 1–62
Sekhar KNC, DeMason DA (1988) Quantitative ultrastructure and protein composition of date palm (Phoenix dactylifera) seeds: a comparative study of endosperm vs. embryo. Am J Bot 75:323–329. https://doi.org/10.1002/j.1537-2197.1988.tb13446.x
Sekhar KNC, DeMason DA (1990) Identification and immunocytochemical localization de α-galactosidase in resting and germinated date palm (Phoenix dactylifera L.) seeds. Planta 181:53–61. https://doi.org/10.1007/BF00202324
Sugimuma Y, Murakami T (1990) Structure and function of the haustorium in germinating coconut palm seed. Jpn Agr Res Q 24:1–14
Tan-Wilson AL, Wilson K (2012) Mobilization of seed protein reserves. Physiol Plantarum 145:140–153. https://doi.org/10.1111/j.1399-3054.2011.01535.x
Verdeil JL, Hocher V (2002) Digestion and absorption of food in plants: a plant stomach. Trends Plant Sci 7:280–281. https://doi.org/10.1016/S1360-1385(02)02269-0
Zienkiewicz A, Zienkiewicz K, Rejón JD, Alché JD, Castro AJ, Rodríguez-García MI (2014) Olive seed protein bodies store degrading enzymes involved in mobilization of oil bodies. J Exp Bot 65:103–115. https://doi.org/10.1093/jxb/ert355
Acknowledgements
The authors thank Centro de Microscopia of the Universidade Federal de Minas Gerais, Brazil (http://www.microscopia.ufmg.br), for processing samples, use of the equipment, and obtaining images; and Maria Olívia Mercadante Simões for providing equipment and support in several stages of this work.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil (CAPES, Finance Code 001), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG, Brazil, process CRA-APQ-01335-13, and doctoral scholarship of H.C.M.S.), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil, processes 305686/2018-6, 305638/2018-1 and 302216/2018-9, respectively, for research productivity grants of D.M.T.O., E.A.S.P. and L.M.R.).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by HCMS. All authors performed data analysis and participated in writing the manuscript. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mazzottini-dos-Santos, H.C., Ribeiro, L.M., Oliveira, D.M.T. et al. Ultrastructural aspects of metabolic pathways and translocation routes during mobilization of seed reserves in Acrocomia aculeata (Arecaceae). Braz. J. Bot 43, 589–600 (2020). https://doi.org/10.1007/s40415-020-00622-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-020-00622-7