Abstract
Steroid hormones are endogenous chemicals controlling many endocrinology functions. Mass spectrometry technologies have been applied for analyses of steroid hormones as biomarkers in endocrinology and pathology diagnoses, doping drugs in athletes and racing horses, residuals in food safety concerns, and environmental pollutants in water and sediments. Both liquid chromatography mass spectrometry (LC-MS or LC-MS/MS) and gas chromatography mass spectrometry (GC-MS or GC-MS/MS) are broadly used in research, clinical, pharmaceutical industry, competition sports, food safety, and environmental testing laboratories. Sample preparation techniques, such as deconjugation and extraction, are critical procedures for isolating steroid hormone from sample matrices, including biological fluids, tissues, environmental water and sediments. Chemical derivatization modifies the physicochemical properties of steroid hormone molecules to improve their chromatographic performances and to enhance their sensitivities to mass detection. Chromatographic techniques such as HPLC, UPLC, and GC have direct impact on separation of analytes, MS interface, and analysis throughput. The method sensitivity and specificity of LC-MS and GC-MS depend largely on the analyte status, i.e., easiness of ionization, derivatization, sample matrix, and MS detection mode, e.g., ESI, APCI, APPI, MAILDI, or EI. LC-MS and GC-MS methodologies should be developed and validated following scientific and regulatory guidelines, and the steroid hormones analyses should be standardized.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Atmospheric Pressure Chemical Ionization
- Supercritical Fluid Chromatography
- Derivatization Reagent
- Helix Pomatia
- Sulfonyl Chloride
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
1.1 Steroid Hormones: Chemical Structures and Functions
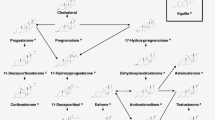
Steroid hormones are composed of 18–21 carbon atoms, which are bonded together by four fused rings: three cyclohexane rings (designated as rings A, B, and C) and one cyclopentane ring (as ring D), with an exception of estrogens’ ring A as a benzene ring. Hydroxyl and/or ketone groups are attached at C3, C11, C17, C20, or C21 positions, and methyl group(s) is attached at C18 and/or C19 positions of the steroid skeletons, as shown in Fig. 1. These molecules are neutral, unionized, nonvolatile, lipid soluble, and stable during extraction and analysis by mass spectrometry. Originated from cholesterol, the biosynthetic pathways of steroid hormones are well established [1–3]. The major biotransformation reactions for steroid hormones include oxidation, reduction, conjugations with glucuronic acid, sulfate, and glutathione [4, 5]. As most of the biotransformation pathways and metabolites of steroid hormones are clearly defined, structure elucidation or identification is not a major concern for steroid analysis [6]. However, since a large portion of endogenous steroid hormones exists as their glucuronide and sulfate conjugates, especially in urine, deconjugation of the steroid hormones may be needed before LC-MS/MS and GC-MS analysis [7, 8].
The major classes of steroid hormones: progestagens, mineralocorticoids, glucocorticoids, androgens, and estrogens. Enzymes, their cellular location, substrates, and products in human steroidogenesis. Boron WF, Boulpaep EL (2003) Medical Physiology: a cellular and molecular approach, p 1300, Elsevier/Saunders (reprinted with permission from Elsevier/Saunders)
According to bonding receptors and biological functions, steroid hormones are classified as progestagens (or progestogens, pregnancy hormones), mineralocorticoids (mineral retention hormones), glucocorticoids (glucose metabolism and inflammation hormones), androgens (male hormones), and estrogens (female hormones). Endogenous steroid hormones are chemical messengers regulating many life functions, such as controlling pregnancy, salt and water balance, metabolism, inflammation, immune functions, and development of sexual characteristics. Many serious health problems, diseases, and clinical syndromes are indicated by steroid hormones deviations from normal levels. Steroid hormone analysis plays important roles in health care, including disease diagnosis, food safety, and environmental protection. For example, endogenous steroid hormones are critical biomarkers for clinical diagnoses in endocrinology, physiology, and pathology; and steroid hormones are also broadly used as medicines for treatment of varieties of diseases, such as inflammation, malfunctions in immune systems, underdevelopment syndromes, and disorders in endocrine systems [2, 9]. On the other hand, steroid hormones are abused by some athletes [10] and racing horse owners [11] to enhance their sport performances. Steroid hormones are also found as hazardous residuals in eatable meats [12] and are monitored as environmental pollutants in water and sediments [13]. Therefore, development and application of specific, sensitive, and robust analytical methods and methodologies for steroid hormone analyses have significant impacts on clinical analyses, disease diagnoses and treatments, antidoping drug screening, food safety examination, and environmental quality monitoring. A large number of articles have been published on analyses of steroid hormones by mass spectrometry and other technologies, and progress and challenges of MS technologies and applications in steroid hormone analyses have been briefly reviewed (see Table 1).
1.2 Challenges to Analytical/Bioanalytical Technologies and Methodologies
Immunoassays (IA) and radioimmunoassays (RIA) are widely accepted and routinely applied for clinical and environmental analyses of steroid hormones, but they have limitations in specificity and accuracy. Therefore, more selective, accurate, and sensitive technologies such as LC-MS/MS and GC-MS/MS methodologies are needed to improve the IA and RIA testing techniques for steroid hormones and metabolites [4, 12, 14, 19]. LC-MS/MS and GC-MS methods are increasingly used for analyzing steroid hormones due to their high selectivity and sensitivity. The typical LC-MS/MS methods by direct injection [20, 21] and GC-MS methods [6] have limit of quantitation (LOQ) at ng/mL level, and a number of LC-MS/MS [2, 8, 22] and GC-MS/MS [23] methods are able to achieve LOQ at pg/mL levels when the steroid hormone samples are chemically derivatized before injection. Nevertheless, in many cases, clinical diagnostic tests need to determine steroid hormones at low pg/mL, and even fg/mL levels [4, 15, 18]. In order to improve the MS method sensitivity and sample preparation efficiency, many studies on sample preparation have been carried out, including deconjugation, extraction, and derivatization. Different chromatographic techniques and MS detection modes have also been investigated.
On the other hand, many LC-MS/MS and GC-MS methodologies and their applications to steroid hormone analyses have been developed for the purpose of scientific research or specific studies only, while they might not have been validated according to regulatory guidelines, and their results might not correlate with those from the widely accepted bioanalytical techniques, e.g., IA and RIA. Therefore, standardization of the LC-MS/MS and GC-MS technologies and methodologies, including facilities, instruments, reference standards, procedures, data system, etc., plays a critical role in transferring the LC-MS/MS and GC-MS technologies to daily testing procedures for clinical diagnosis, sports antidoping screening, food safety control, and analysis of environmental contaminations in water and sediments. Only until their specificity, accuracy, precision, calibration mode, sensitivity, and robustness are validated according to regulatory guidelines, and the test results are comparable or consistent with those obtained from the existing techniques such as IA and RIA, the LC-MS/MS and GC-MS/MS techniques may not be accepted as reliable clinical diagnostic methodologies for steroid hormone determination [18].
2 Sample Preparation
The major sample preparation procedures of steroid hormone analyses include extraction, deconjugation, and derivatization, as shown in Fig. 2. A number of examples of steroid hormone sample preparation are summarized in Table 2. Application of stable isotope labeled (e.g., deuterated or 13carbon labeled) steroid hormones as the internal standard or isotope dilution is a standard of practice during quantitative steroid hormone analyses by LC-MS/MS and GC-MS whenever possible. These isotope labeled internal standards are added into the samples before deconjugation or extraction procedures. They undergo the same deconjugation and/or extraction procedures and LC-MS/MS or GC-MS analysis as the steroid samples do. The efficiency of deconjugation and/or extraction, assay accuracy and precision are calibrated and calculated with the isotope internal standards, leading to more accurate, precise, and robust methods and results.
2.1 Deconjugation of Steroid Hormones
A large percentage of steroid hormones exist as glucuronide, sulfate, and glutathione conjugates in body fluids and tissues. These steroid conjugates may be analyzed directly by LC-MS/MS using either electrospray ionization (ESI) [5, 40–43] or atmospheric pressure chemical ionization (APCI) [44] mode. However, in many cases, the presence of glucuronide and sulfate conjugates in samples may reduce MS detection sensitivity and the total amount of a hormone (both unconjugated and conjugated) that can be determined by LC-MS/MS [8] or GC-MS [6] after the deconjugation and derivatization of the steroid hormones. Thus deconjugation of steroid hormone conjugates become a critical sample preparation procedure in LC-MS/MS and GC-MS analyses.
Both estrogen glucuronide and sulfate conjugates may be deconjugated with hydrochloric acid in methanol [45], but acid solvolysis is not a selective reaction, and the harsh condition may cause side reactions and by-products. An alternative deconjugation procedure applies β-glucuronidase from Escherichia coli to hydrolyze steroid glucuronide conjugates first, and then hydrolyzes steroid sulfate conjugates with sulfuric acid [20]. Steroid hormone glucuronide conjugates may be hydrolyzed with β-glucuronidase from different sources, e.g., Escherichia coli. [38, 46], limpets [47] or Helix pomatia [39]. An attention should be paid to the fact that β-glucuronidase from Escherichia coli does not affect the chemical structures of steroid hormones during deconjugation, while β-glucuronidase from Helix pomatia can convert 3β-hydroxy-5-ene steroids into 3-oxo-4-ene steroids, and change 3β-hydroxy-5α-reduced and 3β-hydroxy-5β-reduced steroids into 3-oxo-5α-reduced and 3-oxo-5β-reduced steroids, respectively, because Helix pomatia also contains other enzymes, including cholesterol oxidase, 3β-hydroxysteroid oxidoreductase/3-oxosteroid-4,5-eneisomerase, and 6-hydroxylase. When glucuronide conjugates of steroid hormones with 3-hydroxy-5-ene structure, e.g., progestagens and androgens, are deconjugated using β-glucuronidase from Helix pomatia, the hormone quantitation may not be accurate or representative [48]. However, β-glucuronidase/sulfatase from Helix pomatia are commonly used for hydrolyzing both glucuronide and sulfate conjugates simultaneously, especially for estrogens and metabolites, because the phenolic ring A of estrogens is not affected by those enzymes in Helix pomatia. The deconjugation of steroid glucuronide and sulfate conjugates is simplified as one step incubation of β-glucuronidase/sulfatase with steroid samples at 37 °C for 20 h or at 55 °C for 3 h [8, 28, 39].
2.2 Extraction of Steroid Hormones from Biological Matrices, Environmental Water and Sediments
The techniques used for extracting steroid hormones and metabolites include protein precipitation (PP) with an organic solvent, liquid–liquid extraction (LLE), and solid phase extraction (SPE). As shown in Fig. 2, selection of a sample extraction technique is based on the steroid hormone sample (unconjugated or conjugated), quantity, matrix and the objective of an analytical method or test. For example, if a method or test needs to analyze only the unconjugated steroid hormones in a small volume of serum, e.g., <1 mL, a small volume of acetonitrile can precipitate the proteins and extract steroids from the sample [25]. The procedure is very simple, but the acetonitrile extract contains more nonrelated components than the extract from LLE [22]. When a small volume of biological fluid is extracted by LLE with a solvent, whether the sample undergoes deconjugation and/or derivatization or not, the LLE extract is cleaner than PP extract, but may not be as clean as an extract from SPE. However, LLE may reduce the sample loss, experiment procedures and errors, and save time in comparison to SPE [8, 25, 28]. In contrast, if a larger amount of sample is available, e.g., 2–10 mL of urine or 1–10 L of environmental water, SPE is a better choice, because it concentrates the sample and minimizes the interferences from other materials, leading to a higher sensitivity and selectivity of the method or test [34, 37, 39]. The sample extraction throughput can be significantly enhanced by using automated 96-well SPE plates [31, 49].
2.3 Derivatization
Chemical derivatization is a standard procedure for GC-MS analysis of steroid hormones, because steroid hormones are not volatile to go through GC column [1, 6]. The major concerns of derivatization reagents for GC-MS analysis of steroid hormones are the completeness of the derivatization reaction and the volatility of hormone derivatives. The typical derivatization reagents for GC-MS samples are silylation reagents, e.g., N-methyl-N-trifluorotrimethyl acetamide (MSTFA, [37,39]) and N,O-bis(trimethylsilyl) trifluoroacetamide (BSTFA, [33]), which react with both alcoholic and phenolic hydroxy groups on steroid hormone molecules.
One of the major advantages of LC-MS/MS over GC-MS or GC-MS/MS is that steroid hormones may be analyzed directly by LC-MS/MS without derivatization procedures, which are time-consuming and tedious [22, 50–53]. However, a number of studies demonstrated that the chemically derivatized steroid hormones were significantly more sensitive to LC-MS/MS detection than the underivatized hormones, because the neutral molecules of estrogens and metabolites might not be effectively ionized under electrospray ionization (ESI) or atmospheric pressure chemical ionization (APCI) modes [4, 21, 25, 54, 55]. In order to enhance the steroid hormone molecules sensitivity for LC-MS/MS analysis at pg/mL level, chemical derivatization is an effective technique for analysis of steroid hormones and metabolites. A list of derivatization reagents and application examples for steroid hormone analyses by LC-MS/MS and GC-MS are presented in Table 3.
An ideal derivatization reagent is able to react with steroid hormones and metabolites selectively and quantitatively under mild conditions within a short time, and those hormone derivatives are stable and easily ionized during LC-MS/MS analysis. Based on their functional groups, the derivatization reagents used for LC-MS/MS analyses of steroid hormones and metabolites may be classified into seven major classes:
-
1.
Hydrazide, e.g., (carboxymethyl)trimethylammonium chloride hydrazide (Girard T reagent) [4, 56], and p-toluenesulfonhydrazide [36]; and hydroxylamine [25]
-
2.
Benzyl bromide, e.g., pentafluorobenzyl bromide [55, 59] and 4-nitrobenzyl bromide [60]
-
3.
Fluorobenzene or fluoropyridine, e.g., 2,4-dinitro-5-fluorobenzene analogues [54] and 2-fluoro-1-methyl-2-pyridinium p-toluenesulfonate [61]
-
4.
Sulfonyl chloride, e.g., dansyl chloride, 1,2-dimethylimidazole-4-chloride, and pyridine-3-sulfonyl chloride; 4-(1-H-pyrazol-1-yl)benzenesulfonyl chloride [62]
-
5.
Carboxylic acid N-hydroxysuccinimide ester, e.g., N-methyl-nicotinic acid N-hydroxysuccinimide ester [21]
-
6.
Carbonyl chloride and acetic anhydride, e.g., picolinoyl chloride [64]
-
7.
o-Phenylenediamine [67]
The first class of derivatization reagents, hydrazide reagents and hydroxylamine, react with most of the ketolic steroid hormones and metabolites (ketone group on C3, C17, or C20), i.e., androgens, progestagens, corticoids, and ketolic estrogens [4,25, 36, 56]. However, they are not suitable for steroid hormones without ketolic group(s), such as estradiol, estriol and their related metabolites. Hydroxylamine is a typical derivatization reagent for steroid hormone profiling, because it can react with all the ketolic hormones, and provide unique mass fragments for each steroid moiety during LC-MS/MS analysis. When these unique mass fragments or daughter ions are used for multiple reaction monitoring (MRM) quantitation, the method selectivity is much higher than those using a daughter ion from a derivatization reagent, e.g., m/z 171 for dansyl ion [25].
The second to seventh classes of reagents react with hydroxyl steroid hormones and metabolites, especially estrogens. Pentafluorobenzyl bromide estrogen derivatives, belonging to the second class, are sensitive to both ESI+ [1] and APCI− [55,61] modes, and usually have lower limits of quantitation (LOQ) values under APCI− mode than those of derivatives of dansyl chloride and 2-fluoro-1-methyl-pyridinium p-toluenesulfonate under ESI+ mode, because there were little interference from analogue compounds and the matrix background under APCI− mode. Nevertheless, the derivatization reaction of estrogens with pentafluorobenzyl bromide was ten times longer than the derivatization reaction with dansyl chloride (30 min vs. 3 min at 60 °C) [61]. A study by Higashi et al. indicated that the derivatization reactions of estrogens with 4-nitrobenzyl bromide (the second class), 2,4-dinitro-fluorobenzene (the third class) and 4-nitrobenzoyl chloride (the sixth class) were not as complete as the reaction with 4-nitrobenzene sulfonyl chloride (the fourth class) [60].
The fifth class, carboxylic acid N-hydroxysuccinimide ester, is also not as reactive as sulfonyl chloride, and its derivative is not as sensitive to LC-MS/MS either [21]. The sixth class of reagents, carbonyl chloride and carboxylic acid anhydride, can react with both phenolic and alcoholic hydroxyl groups of steroids. However, the selectivity, speed, and completeness of derivatization reactions of these reagents with steroids are not as good as those of sulfonyl chloride derivatization reagents [60, 64, 66]. Since the other four classes of derivatization reagents, sulfonyl chloride, benzyl bromide, carboxylic acid N-hydroxysuccinimide ester, and fluorobenzene, are able to selectively react with phenolic hydroxyl group of estrogens and metabolites, the carbonyl chloride and carboxylic acid anhydride reagents become less preferable for derivatizing estrogens and metabolites. The seventh class, o-phenylenediamine, is a specific derivatization reagent for estrogen o-quinones, potential carcinogens, such as estrone-2,3-quinone, estrone-3,4-quinone, estradiol-2,3-quinone, estradiol-3,4-quinone [67].
Works published so far suggest that sulfonyl chloride is a preferred reagent for derivatizing estrogens and their metabolites, due to its reaction completeness and selectivity. In addition, a sulfonyl chloride reagent containing a basic or preionized nitrogen atom, e.g., on dansyl, pyridine, imidazole, pyrazole, or piperizine ring, could significantly enhance the ionization of estrogen derivatives under ESI+ mode, and increase the detection sensitivity [4, 54, 62]. Dansyl chloride is a typical sulfonyl chloride reagent used for derivatizing estrogens and metabolites from varieties of matrices, such as river water [50, 61], mouse plasma and brain [27], human urine [28], breast tissue [26], and serum [8]. However, dansyl derivatives have a disadvantage that the common dansyl fragment m/z 171 from background of the derivatization reagent and from those derivatives may have negative impacts on the method selectivity and accuracy during LC-MS/MS analysis, especially for those isomers with the same molecular ions and fragments, because the elevated dansyl fragment background noise may reduce the analyte signal–noise ratio, and cross interfere quantitation of other analytes with the same fragments [8, 25, 28].
3 Comparison of LC-MS/MS, GC-MS, and Immunoassays
3.1 LC-MS/MS and UHPLC-MS/MS vs. GC-MS and GC-MS/MS
The bioanalytical methods developed in recent years focused more on LC-MS/MS and GC-MS/MS techniques, because the earlier studies demonstrated that LC-MS/MS and GC-MS/MS are significantly more sensitive in analyzing estrogens and metabolites than LC-MS and GC-MS [18, 68]. Analyzing both unconjugated and conjugated steroid hormones directly is the major advantage of LC-MS and LC-MS/MS over GC-MS and GC-MS/MS, because the sample preparation procedures of deconjugation and derivatization can be avoided [5, 7, 40–42, 44]. In complex samples, separation of steroid hormones and metabolites by LC or GC is still one of the major concerns, because many steroid hormones and metabolites have the same molecular weights and MS fragments, and they may interfere with each other if not separated. For example, 2-hydroxyestrone and 4-hydroxyestrone have same molecular weight, even after they are derivatized with MSTFA for GC-MS analysis [48] or with dansyl chloride or p-toluenesulfonhydrazide for LC-MS/MS analysis [8, 35]. If the derivatives are not separated, GC-MS or LC-MS/MS is unable to distinguish the derivatives of 2-hydroxyestrone from those of 4-hydroxyestrone, whether the silylated, dansylated, or hydrozone steroid fragments are used for quantitation.
An LC column with a length of 150 mm can separate up to 23 steroid hormones and metabolites [20]. A typical LC-MS/MS method developed by Xu et al. was able to separate 15 estrogens and metabolites using a 150 × 2 mm, 4 μm LC column, which had a run time of 100 min [8, 28]. The separation efficiency can be improved by using smaller particle size LC columns, e.g., 100 × 2.0 mm, 2.5 μm column [69] or 50 × 2.1 mm, 1.8 μm column [21], also leading to a significantly reduced run time (e.g., less than 30 min). Similarly, ultra high performance liquid chromatography (UHPLC) is able to significantly improve separation efficiency and to reduce run time [40, 70–72]. Two-dimensional (2D) LC-MS/MS with column-switching technique has been used for determination of unconjugated and conjugated estrogens in river water [51] and sediment [73], and this technique can significantly reduce the analysis time, and increase method separation capability and detection sensitivity. When estrogens and their derivatives of dansyl chloride and pentafluorobenzyl bromide are analyzed by HPLC, 2D-LC and UHPLC with ESI, APCI, APPI, and APCI/APPI MS modes, the UHPLC-ESI−-MS/MS significantly enhances sensitivities of the derivatives over the native estrogens, in the order UHPLC > 2D-LC > LC, and ESI > APPI > APCI = APCI/APPI [74]. On the other hand, the cleaner and more efficient supercritical fluid chromatography (SFC)-mass spectrometry was also used for analysis of estrogens and metabolites [75], but the SFC technology was less versatile and robust than LC, UHPLC, and GC.
Since the neutral molecules of steroid hormones and metabolites are not easily ionized under either APCI+/− or ESI+/− modes, LC-MS/MS is less sensitive when used directly in either APCI+/− or ESI+/− modes, with LOQs at ng/mL level as shown in Table 4 [20, 21, 52]. It has been observed that estrone, 16α-hydroxyestrone, 2-methoxyestrone, 4-methoxyestrone, and 2-hydroxy-3-methoxyestrone are sensitive to APCI+ mode, while 2-hydroxyestrone and 4-hydroxyestrone are sensitive to APCI− mode, and even more sensitive to ESI− mode [29]. Estrone, estradiol, estradiol, and estriol are sensitive to ESI− mode, and testosterone is sensitive to ESI+ mode [76, 77]. Similarly, estrone and estradiol are sensitive to APPI− mode, and testosterone is sensitive to APPI+ mode with LOQs in a range of 1.5–10 pg/mL [22], which are comparable with those steroid hormones and metabolites derivatized with hydroxylamine or dansyl chloride, and detected under ESI+ mode [2, 8].
As most steroid hormones and metabolites are already identified, high resolution MS technologies with higher selectivity, e.g., time of flight mass spectrometry (TOF-MS), Fourier transform ion cyclotron resonance mass spectrometry (FTICR-MS), Orbitrap-MS, MALDI, etc., may not have major advantages over triple quadrupole MS in quantitative analysis, because the triple quadrupole MS takes shorter data acquisition time, leading to a higher sensitivity. For example, LC-ESI−-MS/MS is 4–6 times more sensitive than LC-ESI−-TOF-MS in analysis of estrogens [76]. On the other hand, MS interface also plays an important role in steroid hormone analysis. When analyzed by nanospray ES-Q-TOF-MS, Girard P derivative of testosterone at 0.5 pg/μg (0.1 pg of sample) can be detected, while 10 pg of sample is needed for matrix-assisted laser desorption ionization (MALDI)-Q-TOF-MS analysis, because of significant background interferences from the MALDI matrix [58].
GC-MS is a matured technology in analyses of steroid hormones, because GC-MS interface and electron impact ionization (EI) MS mode are stable and easily standardized [1]. GC-MS is a very powerful tool for profiling steroid hormones in biological matrices, such as that more than 70 steroid hormones and metabolites can be separated and quantitated by a single GC-MS run, with LOQ in range of 0.1–10 ng/mL [6, 48]. The derivatization procedure may not be a challenge, because there are many derivatization reagents available, e.g., BSTFA and MSTFA, and the silylation reaction is straightforward and quantitative. In addition, the sensitivity of GC-MS can be improved from low ng/mL to 0.6 pg/mL LOQ level by GC-MS/MS technology [23]. That is why GC-MS is still broadly used today for analyses of steroid hormones in human plasma [32], urine [7, 37, 39], and environmental water [34, 45, 78, 79]. If both LC-MS/MS and GC-MS/MS methodologies require similar sample preparation procedures, e.g., deconjugation, extraction, and derivatization, and they provide the same or comparable sensitivity in steroid hormone analyses, the choice of an LC-MS/MS or a GC-MS/MS methodology may depend on the instrument availability and the cost of the testing.
3.2 LC-MS/MS and GC-MS vs. Immunoassays
IA and RIA are broadly used technologies in steroid hormone analyses by both clinical laboratories and environmental agencies, because they are sensitive, convenient, simple, rapid, and inexpensive. Their disadvantage is lack of selectivity or specificity [4, 14, 15]. Since selectivity or specificity of bioanalytical methodologies is essential for clinical diagnosis testing, a number of studies have been conducted to compare LC-MS/MS and GC-MS methodologies with immunoassays in steroid hormone analysis. A study for determining nine androgens and three estrogens in blood showed that LC-MS/MS, GC-MS, and RIA provided similar results, however, RIA resulted in significant higher values for all related hormones than LC-MS/MS and GC-MS, due to its cross interferences from analogue compounds [80]. Another study also indicated that direct RIA overestimated estrogen sulfate in plasma than GC-MS and LC-MS/MS [65]. Similar results were observed in analysis of estrogens by IA and RIA against LC-MS/MS as well [19]. All these studies suggest that LC-MS/MS and GC-MS are more selective or specific and accurate than RIA. Nevertheless, LC-MS/MS and GC-MS instruments require more sophisticated expertise to perform the bioanalytical testing, and to provide reliable interpretations of the results for clinical diagnoses.
4 Standardization of Analytical Procedures
Development of LC-MS/MS or GC-MS analytical methods is just the first step in bioanalysis. A number of additional procedures should also be established to make the whole bioanalytical platform including reference standards, methodologies and data systems in compliance with scientific and regulatory guidelines or requirements. Otherwise, the results from various techniques, e.g., IA, RIA, LC-MS/MS, and GC-MS, may not be comparable and acceptable for clinical diagnosis. As there are a large number of scientific publications and clinical applications on steroid hormone analyses, organizations like the Center for Disease Control and Prevention, Division of Laboratory Science of National Center for Environmental Health have attempted to standardize the clinical laboratory practice, analytical methodology and data management in steroid hormone analyses (CDC/NCEH/DLS, [81–83]). The National Institute of Standards and Technology (NIST) has made efforts on development of standard methods and reference materials for the determination of hormones in human serum [84–86]. A number of researchers also proposed a series of standardization procedures, including method development, validation, data interpretation and correlation between different bioanalytical techniques, and establishing international databases of human hormones [2, 14, 18]. In contrast to the clinical laboratories, EPA considers steroid hormones as environmental pollutants in water and sediments, and it has established an LC-MS/MS Method #539 following EPA Chemical QC Guidelines [77].
4.1 Guidelines for GLP, Reference Standards, and Analytical Methodology
Current Good Laboratory Practice from Food and Drug Administration (FDA GLP, 21CFR58) is a general guideline for pharmaceutical industry, and Good Laboratory Practice from Environmental Protection Agency (EPA GLP, 40CFR792) is a guideline for agriculture chemical industry, and environmental protection agencies and organizations. Both guidelines emphasize on compliance in personnel, facilities, articles, and documentation for animal and analytical experiments. In order to standardize the analytical methodology of steroid hormone analysis, the clinical and the environmental laboratories and facilities should follow FDA or EPA GLP guidelines. These guidelines include the following major requirements: (1) to train related analysts, (2) to calibrate and maintain instruments, (3) to characterize reference standard, (4) to conduct analytical testing following standard operation procedures (SOPs), (5) to record analytical procedures, protocols, reports, deviations, investigation, etc. Besides GLPs, guidelines for reference standards and analytical methodology are also important for steroid hormone analyses, as summarized in Table 5.
4.2 Reference Standards
The primary reference standards, including internal standards, of steroid hormones used for LC-MS/MS or GC-MS analyses in GLP laboratories should be obtained from authentic sources, e.g., US Pharmacopeia (USP) and NIST. Relative inexpensive secondary or working standards for daily testing may be used as alternatives of the primary standards, and they may be obtained from commercial sources. These working standards should be characterized against the primary reference standards using compendia or validated methods before being applied for GLP testing purposes. A reference standard program should be established in each laboratory, institute, or company to monitor the specification, quality, characterization, stability, storage, inventory, and replacement of those reference standards. A certificate of analysis should be issued after characterization of each standard batch, and it should include the information of manufacturer or source, date of manufacture, date of analysis, testing results (purity or strength, moisture, impurities, etc.), storage conditions, and expiration or retesting date. More detailed information on reference standard can be found in USP34-NF29 and International Organization for Standardization (ISO) Guides 30-35.
4.3 Standardization of Methodology
In general, the analytical methodology evaluates and defines bioanalytical/analytical procedures using a number of parameters, including selectivity or specificity, accuracy, precision, matrix effect, recovery, calibration model (linearity) and range, sensitivity (limit of quantitation—LOQ, and limit of detection—LOD), sample stability, ruggedness and robustness [87]. The USP and ICH guidelines listed in Table 4 focus on the role of analytical methodology in quality control and compliance of drug substances and drug products in pharmaceutical industry. The IUPAC, ISO, and NIST 1297 guidelines emphasize on the definition and evaluation of trueness and uncertainty in analytical methodology. The FDA guideline is the most direct guidance on bioanalysis for human and nonhuman (animal and biological) studies in pharmacology, toxicology, pharmacokinetics, and drug metabolism. Typical bioanalytical methodology guidelines and LC-MS/MS methods for steroid hormone analyses are shown in Table 6.
To standardize the steroid hormone analyses, standard operation procedures (SOPs) should be generated for all laboratory functions, e.g., facilities, instrument, reference standards, samples, procedures, data collection and processing, documentation, etc., following GLP, reference standard, and the methodology guidelines. All the laboratory activities should follow these SOPs. Furthermore, the experimental procedures or techniques, e.g., deconjugation, extraction (LLE or SPE), derivatization, isotope dilution, instrument setting (e.g., LC-MS/MS modes), etc., should also be standardized. It is a challenge to ask different laboratories meet the same criteria, e.g., sensitivity and precision, using different models of instruments, and to make the results comparable and reliable for clinical diagnosis, due to the significant differences in LC-MS/MS hardware (configuration, ionization modes, and parameter setting) and software (data acquisition speed and processing) from different vendors and different models.
Standardization of GC-MS methodology for steroid hormones is relatively straightforward, because most of the unconjugated steroid hormones have a hydroxyl group(s) at C3, C11, C17, or C21 position, which may be easily derivatized with a trimethylsilylation agent, e.g., MSTFA, and derivatives may be well separated by GC, and detected by MS using EI+ mode [6]. Similarly, standardization of LC-MS/MS methodology for estrogens and metabolites is also not very complicated, because all unconjugated estrogens and metabolites have phenolic hydroxyl group(s) at C3, and C2 or C4 positions, which may be derivatized with a sulfonyl chloride, e.g., dansyl chloride, and analyzed LC-MS/MS using ESI+ [8, 84, 88]. The other derivatization agents, e.g., hydrazide and hydroxylamine, may react only with those ketolic steroids, but will miss those nonketolic steroids [4, 25, 56]. If steroid hormones are analyzed without derivatization, they may need to be analyzed by LC-MS/MS using both positive and negative modes for different hormones, and the LOQ values of steroid hormones fall in a very broad range, because of the sensitivity variations of steroid hormones to different MS modes [22, 29, 77].
5 Applications of Mass Spectrometry in Steroid Hormones Analyses
5.1 Clinical Chemistry and Food Safety
Varieties of LC-MS/MS and GC-MS technologies and methodologies have been developed and applied for clinical chemistry diagnostic testing. For example, adrenal steroids, glucocorticoids, androgens, and estrogens are biomarker of many endocrinology diseases [2], pediatric development syndromes [9], and congenital adrenal hyperplasia [89]. Endogenous steroids are also related to prevalent cardiovascular disease in old men and women [90], and other age-related diseases [60]. Elevated estrogens and metabolites in plasma and serum of postmenopausal women are used as biomarkers for risk assessment of breast, ovary, and thyroid cancers, bone homeosis and osteoporosis in postmenopausal women [4, 15, 39, 91]. Androgens may be related to prostate cancer progression and treatment [31], and hyperandrogenism may cause polycystic ovary syndrome and androgen-secreting tumors [32].
In addition, steroid hormones in many kinds of biological fluids and tissues have been determined by LC-MS/MS and GC-MS technologies and methodologies. For example, LC-MS/MS has been utilized for monitoring (1) plasma corticosteroids and metabolites to evaluate their therapeutic and side effects as clinically used medicines [92], (2) estrogens in human cerebrospinal fluid [93] and peritoneal fluid [94], (3) urinary endogenous estrogen metabolites [95, 96], (4) estrogens in breast tissue [26], and (5) steroid hormones as residuals in edible matrices [12]. Polyphenol phytoestrogens in foods and human biological fluids are also measured by mass spectrometry technologies [97].
5.2 Antidoping Steroid Screen for Athletes and Racing Horses
Corticosteroids are used by some athletes and racing horses to enhance their performances. In order to prohibit drug doping, many sport organizations, e.g., International Olympic Committee, attempt to monitor the corticosteroids in urine of athletes. An example of earlier GC-MS method in steroid screen consists of procedures of deconjugation of glucuronide and sulfate, derivatization with MSTFA and GC-MS analysis [98]. The more recent LC-MS/MS methods separate the corticosteroids by LC, and analyze them with ESI-MS (either positive or negative mode) without derivatization [10, 99]. Similarly, corticosteroids in racing horse urine are monitored by LC-ESI-MS/MS as well [11].
5.3 Determination of Steroid Hormones as Environmental Pollutants
Steroid hormones are found as pollutants in drinking water, waste water, river and sediments. The major concerns of analytical methodologies for monitoring steroid hormones from environmental samples are extraction techniques from aqueous or solid matrices. Since sample volume or amount is not an issue in most cases, SPE is the method of choice. Both LC-MS/MS and GC-MS technologies are broadly applied for steroid analyses of environmental samples, such as LC-MS/MS analyses of steroid hormones in effluents of wastewater treatment plants [100] and estrogens in water [101, 102], and GC-MS analyses of steroid hormones in environmental water [34, 45, 78, 79]. A study by Grover and colleagues showed that GC-MS was the simplest technique in determination of steroid hormones in environmental water samples, but lack of sensitivity; LC-MS/MS was more sensitive than GC-MS, but susceptible to matrix interferences; and GC-MS/MS was the recommended technique, because it was more selective and sensitive than GC/MS and LC-MS/MS [103].
6 Summary
LC-MS/MS has similar sensitivities in analysis of steroid hormones as IA, RIA, and GC-MS/MS, but LC-MS/MS and GC-MS/MS have much higher selectivity or specificity. The steroid hormones from different sources or matrices should be extracted with LLE or SPE, depending on the sample volume and matrices. Isotope (as internal standard) dilution is a standard procedure for quantitative analysis of steroid hormone and metabolites. Deconjugation is required in order to determine the total steroid hormones, because the very low levels of unconjugated and conjugated steroid hormones in biomatrices may not be feasible to be analyzed at the same time by LC-MS/MS, GC-MS, or GC/MS/MS. Derivatization can enhance MS detection sensitivity for many steroid hormones and metabolites, while the derivatization reagents and procedures should be selected based on the techniques of LC-MS/MS, GC-MS, or GC/MS/MS, and their ionization modes. Depending on the objectives of the method applications, bioanalytical procedures should be developed and validated according to the scientific and regulatory guidelines, e.g., FDA, EPA, ISO, NIST, IUPAC, and then these procedures may be standardized following CDC/NCEH/DLS practices. LC-MS/MS is increasingly applied for steroid analysis in research, clinical, pharmaceutical, and food industries, sports and environmental testing, due to its selectivity, sensitivity, and versatility.
References
Shimada K, Mitamura K, Higashi T (2001) Gas chromatography and high-performance liquid chromatography of natural steroids. J Chromatogr A 935:141–172
Kushnir MM, Rockwood AL, Bergquist J (2010) Liquid chromatography–tandem mass spectrometry applications in endocrinology. Mass Spectrom Rev 29:480–502
Soldin SJ, Soldin OP (2009) Steroid hormone analysis by tandem mass spectrometry. Clin Chem 55:1061–1066
Blair IA (2010) Analysis of estrogens in serum from postmenopausal women: past, present and future. Steroids 75:297–306
Rathahao E, Page A, Jouanin I, Paris A, Debrauwer L (2004) Liquid chromatography coupled to negative electrospray/ion trap mass spectrometry for the identification of isomeric glutathione conjugates of catechol estrogens. Int J Mass spectrom 231:119–129
Moon J, Jung H, Moon M, Chung B, Choi M (2009) Heat-map visualization of gas chromatography-mass spectrometry based quantitative signatures on second steroids metabolism. J Am Soc Mass Spectrom 20:1626–1637
Gomes RL, Meredith W, Snape CE, Sephton MA (2009) Analysis of conjugated steroid androgens: deconjugation, derivatization and associated issues. J Pharm Biomed Anal 49:1133–1140
Xu X, Roman JM, Issaq HJ, Keefer LK, Veenstra TD, Ziegler RG (2007) Quantitative measurement of endogenous estrogens and estrogen metabolites in human serum by liquid chromatography-tandem mass spectrometry. Anal Chem 79:7813–7821
Rauh M (2010) Steroid measurement with LC–MS/MS. Application examples in pediatrics. J Steroid Biochem Mol Biol 121:520–527
Deventer K, Delbeke FT (2003) Validation of a quantitative screening method for corticosteroids by liquid chromatography tandem mass spectrometry. In: Shanzer W, Geyer H, Gotzmann A, Mareck U (eds) Recent advances in doping analysis, vol 11, Spert and Buch Strauβ, Köln, pp 23–31
Leung GNW, Chung EW, Ho ENM, Kwok WH, Leung DKK, Tang FPW, Wan TSM, Yu NH (2005) High throughput screening of corticosteroids and basic drugs in horse urine by liquid chromatography-tandem mass spectrometry. J Chromatogr B 825:47–56
Noppe H, Le Bizec B, Verheyden K, De Brabander HF (2008) Novel analytical methods for the determination of steroid hormones in edible matrices. Anal Chim Acta 611:1–16
Pacakova V, Loukotkova L, Bosakova Z, Stulik K (2009) Analysis for estrogens as environmental pollutants—a review. J Sep Sci 32:867–882
Stanczyk FZ, Clarke NJ (2010) Advantages and challenges of mass spectrometry assays for steroid hormones. J Steroid Biochem Mol Biol 121:491–495
Giese RW (2003) Measurement of endogenous estrogens: analytical challenges and recent advances. J Chromatogr A 1000:401–412
Higashi T (2006) Trace determination of steroids causing age-related diseases using LC/MS combined with detection-oriented derivatization. Chem Pharm Bull 54:1479–1485
Higashi T, Shimada K (2004) Derivatization of neutral steroids to enhance their detection characteristics in liquid chromatography–mass spectrometry. Anal Bioanal Chem 378:875–882
Stanczyk FZ, Lee JS, Santen RJ (2007) Standardization of steroid hormone assays: why, how, and when? Cancer Epidemiol Biomarkers Prev 16:1713–1719
Ziegler RG, Faupel-Badger JM, Sue L, Fuhrman BJ, Falk RT, Boyd-Morin J, Henderson MK, Hoover RN, Veenstra TD, Keefer LK, Xu X (2010) A new approach to measure estrogen exposure and metabolism in epidemiologic studies. J Steroid Biochem Mol Biol 121:538–545
Hauser B, Deschner T, Boesch C (2008) Development of a liquid chromatography–tandem mass spectrometry method for the determination of 23 endogenous steroids in small quantities of primate urine. J Chromatogr B 862:100–112
Yang W, Regnierb FE, Slivac D, Adame J (2008) Stable isotope-coded quaternization for comparative quantification of estrogen metabolites by high-performance liquid chromatography–electrospray ionization mass spectrometry. J Chromatogr B 870:233–240
Harwood DT, Handelsman DJ (2009) Development and validation of a sensitive liquid chromatography–tandem mass spectrometry assay to simultaneously measure androgens and estrogens in serum without derivatization. Clin Chim Acta 409:78–84
Santen RJ, Demers L, Ohorodnik S, Settlage J, Langecker P, Blanchettd D, Gosse PE, Wang S (2007) Superiority of gas chromatography/tandem mass spectrometry assay (GC/MS/MS) for estradiol for monitoring of aromatase inhibitor therapy. Steroids 72:666–671
Zhao M, Bakera SD, Yan X, Zhao Y, Wright WW, Zirkinb BR, Jarow JP (2004) Simultaneous determination of steroid composition of human testicular fluid using liquid chromatography tandem mass spectrometry. Steroids 69:721–726
Regal P, Vázquez BI, Franco CM, Cepeda A, Fente C (2009) Quantitative LC–MS/MS method for the sensitive and simultaneous determination of natural hormones in bovine serum. J Chromatogr B 877:2457–2464
Taioli E, Im A, Xu X, Veenstra TD, Ahrendt G, Garte S (2010) Comparison of estrogens and estrogen metabolites in human breast tissue and urine. Reprod Biol Endocrinol 8:93–99
Xia Y, Chang SW, Patel S, Bakhtiar R, Karanam B, Evans DC (2004) Trace level quantitation of deuterated 17β-estradiol and estrone in ovarietomized mouse plasma and brain using liquid chromatography/tandem mass spectrometry following dansylation reaction. Rapid Commun Mass Spectrom 18:1621–1628
Xu X, Veenstra TD, Fox SD, Roman JM, Issaq HJ, Falk R, Saavedra JE, Keefer LK, Ziegler RG (2005) Measuring fifteen endogenous estrogens simultaneously in human urine by high-performance liquid chromatography-mass spectrometry. Anal Chem 77:6646–6654
Hsu J, Chang Y, Chen T, Lin L, Liao P (2007) Evaluation of electrospray ionization and atmospheric pressure chemical ionization for simultaneous detection of estrone and its metabolites using high-performance liquid chromatography/tandem mass spectrometry. J Chromatogr B 860:49–56
Li Q, Lam MHW, Wu RSS, Jiang B (2010) Rapid magnetic-mediated solid-phase extraction and pre-concentration of selected endocrine disrupting chemicals in natural waters by poly(divinylbenzene-co-methacrylic acid) coated Fe3O4 core-shell magnetite microspheres for their liquid chromatography–tandem mass spectrometry determination. J Chromatogr A 1217:1219–1226
O’Brien Z, Post N, Brown M, Madan A, Coon T, Luo R, Kohout TA (2009) Validation and application of a liquid chromatography–tandem mass spectrometric method for the simultaneous determination of testosterone and dihydrotestosterone in rat prostatic tissue using a 96-well format. J Chromatogr B 877:3515–3521
Yokokawa A, Yamamoto K, Omori Y, Shibasaki H, Shinohara Y, Kasuya Y, Furuta T (2009) Simultaneous determination of androstenedione, 11β-hydroxyandrostenedione, and testosterone in human plasma by stable isotope dilution mass spectrometry. J Chromatogr B 877:621–626
Gomes RL, Avcioglu E, Scrimshaw MD, Lester JN (2004) Steroid estrogen determination in sediment and sewage sludge: a critique of sample preparation and chromatographic/mass spectrometry considerations, incorporating a case study in method development. Trends Anal Chem 23:737–744
Zuo Y, Zhang K, Lin Y (2007) Microwave-accelerated derivatization for the simultaneous gas chromatographic–mass spectrometric analysis of natural and synthetic estrogenic steroids. J Chromatogr A 1148:211–218
Xu X, Ziegler RG, Waterhouseb DJ, Saavedrac JE, Keefer LK (2002) Stable isotope dilution high-performance liquid chromatography–electrospray ionization mass spectrometry method for endogenous 2- and 4-hydroxyestrones in human urine. J Chromatogr B 780:315–330
Xu X, Keefer LK, Waterhouse DJ, Saavedra JE, Veenstra TD, Ziegler RG (2004) Measuring seven endogenous ketolic estrogens simultaneously in human urine by high-performance liquid chromatography-mass spectrometry. Anal Chem 76:5829–5836
Knust U, Strowitzki T, Spiegelhalder B, Bartsch H, Owen RW (2007) Optimization of an isotope dilution gas chromatography/mass spectrometry method for the detection of endogenous estrogen metabolites in urine samples. Rapid Commun Mass Spectrom 21:2245–2254
Moon J, Jung H, Moon M, Chung B, Choi M (2008) Inclusion complex-based solid-phase extraction of steroidal compounds with entrapped β-cycodextrin polymer. Steroids 73:1090–1097
Moon J, Kim K, Moon M, Chung B, Choi M (2011) A novel GC-MS method in urine estrogen analysis from postmenopausal women with osteoporosis. J Lipid Res 52:1595–1603
Kumar V, Nakada N, Yasojima M, Yamashita N, Johnson AC, Tanaka H (2009) Rapid determination of free and conjugated estrogen in different water matrices by liquid chromatography–tandem mass spectrometry. Chemosphere 77:1440–1446
Nguyen HP, Yang SH, Wigginton JG, Simpkins JW, Schug KA (2010) Retention behavior of estrogen metabolites on hydrophilic interaction chromatography stationary phases. J Sep Sci 33:793–802
Ramanathan R, Cao K, Cavalieri E, Gross ML (1998) Mass spectrometric methods for distinguishing structural isomers of glutathione conjugates of estrone and estradiol. J Am Soc Mass Spectrom 9:612–619
Reddy S, Iden CR, Brownawell BJ (2005) Analysis of steroid conjugates in sewage influent and effluent by liquid chromatography-tandem mass spectrometry. Anal Chem 77:7032–7038
Schlusener MP, Bester K (2005) Determination of steroid hormones, hormone conjugates and macrolide antibiotics in influents and effluents of sewage treatment plants utilizing high-performance liquid chromatography/tandem mass spectrometry with electrospray and atmospheric pressure chemical ionization. Rapid Commun Mass Spectrom 19:3269–3278
Liu Z, Kanjo Y, Mizutani S (2011) Removal of natural free estrogens and their conjugates in a municipal wastewater treatment plant. Clean – Soil, Air, Water 39:128–135
Hauser B, Mugisha L, Preis A, Deschner T (2011) LC–MS analysis of androgen metabolites in serum and urine from east African chimpanzees (Pan troglodytes schweinfurthii). Gen Comp Endocrinol 170:92–98
Tang PW, Law WC, Wan TSM (2001) Analysis of corticosteroids in equine urine by liquid chromatography-mass spectrometry. J Chromatogr B 754:229–244
Moon J, Ha Y, Moon M, Chung B, Choi M (2010) Systematic error in gas chromatography–mass spectrometry based quantitation of hydrolyzed urinary steroids. Cancer Epidemiol Biomarkers Prev 19:388–397
Zhang H, Henion J (1999) Quantitative and qualitative determination of estrogen sulfates in human urine by liquid chromatography/tandem mass spectrometry using 96-well technology. Anal Chem 71:3955–3964
Qin F, Zhao Y, Sawyer MB, Li X (2008) Hydrophilic interaction liquid chromatography-tandem mass spectrometry determination of estrogens conjugates in human urine. Anal Chem 80:3404–3411
Qin F, Zhao Y, Sawyer MB, Li X (2008) Column-switching reversed phase–hydrophilic interaction liquid chromatography/tandem mass spectrometry method for determination of free estrogens and their conjugates in river water. Anal Chim Acta 627:91–98
Tso J, Aga DS (2010) A systematic investigation to optimize simultaneous extraction and liquid chromatography tandem mass spectrometry analysis of estrogens and their conjugated metabolites in milk. J Chromatogr A 1217:4784–4795
Yan W, Zhao L, Feng Q, Wei Y, Lin J (2009) Simultaneous determination of ten estrogens and their metabolites in waters by improved two-step SPE followed by LC–MS. Chromatographia 69:621–628
Nishio T, Higashi T, Funaishi A, Tanaka J, Shimada K (2007) Develpment and application of electrospray-active derivatization reagents for hydroxysteroids. J Pharm Biomed Anal 44:786–795
Penning TM, Lee S, Jin Y, Gutierrez A, Blair IA (2010) Liquid chromatography-mass spectrometry (LC-MS) of teroid hormone metabolites and its applications. J Steroid Biochem Mol Biol 121:546–555
Johnson DW (2005) Ketosteroid profiling using Girard T derivatives and electrospray ionization tandem mass spectrometry: direct plasma analysis of androstenedione, 17-hydroxyprogesterone and cortisol. Rapid Commun Mass Spectrom 19:193–200
Hala D, Overturf MD, Petersen LH, Huggett DB (2011) Quantification of 2-hydrazinopyridine derivatized steroid hormones in fathead minnow (pimephales promelas) blood plasma using LC-ESI+/MS/MS. J Chromatogr B 879:591–598
Griffiths WJ, Liu S, Alvelius G, Sjovall J (2003) Derivatization for the characterisation of neutral oxosteroids by electrospray and matrix-assisted laser desporption/ionization tandem mass spectrometry: the Girard P derivatives. Rapid Commun Mass Spectrom 17:924–935
Arai S, Miyashiro Y, Shibata Y, Kashiwagi B, Tomaru Y, Kobayashi M, Watanabe Y, Honma S, Suzuki K (2010) New quantification method for estradioal in the prostatic tissues of nenign prostatic hyperplasia using liquid chromatogrophy-dandem mass spectromentry. Steroids 75:13–19
Higashi T, Takayama N, Kyutoku M, Shimada K, Kohb E, Namiki M (2006) Liquid chromatography–mass spectrometric assay of androstenediol in prostatic tissue: influence of androgen deprivation therapy on its level. Steroids 71:1007–1013
Lin Y, Chen C, Wang G (2007) Analysis of steroid estrogens in water using liquid chromatography/tandem mass spectrometry with chemical derivatizations. Rapid Commun Mass Spectrom 21:1973–1983
Xu L, Spink DC (2008) Analysis of steroidal estrogens as pyridine-3-sulfonyl derivatives by liquid chromatography electrospray tandem mass spectrometry. Anal Biochem 375:105–114
You J, Zhao H, Sun Z, Suo Y, Chen G (2009) 10-Ethyl-acridine-2-sulfonyl chloride: a new derivatization agent for enhancement of atmospheric pressure chemical ionization of estrogens in urine. Chromatographia 70:45–55
Yamashita K, Okuyama M, Watanabe Y, Honma S, Kobayashi S, Numazawa M (2007) Highly sensitive determination of estrone and estradiol in human serum by liquid chromatography-electrospray ionization tandem mass spectrometry. Steroids 72:819–827
Giton F, Caron P, Bérubé R, Bélanger A, Barbier O, Fiet J (2010) Plasma estrone sulfate assay in men: comparison of radioimmunoassay, mass spectrometry coupled to gas chromatography (GC–MS), and liquid chromatography-tandem mass spectrometry (LC-MS/MS). Clin Chim Acta 411:1208–1213
Mitamura K, Yatera M, Shimada K (2000) Studies on neurosteroids Part XIII. Characterization of catechol estrogens in rat brains using liquid chromatography-mass spectrometry-mass spectrometry. Analyst 125:811–814
Yamashita K, Masuda A, Hoshino Y, Komatsu S, Numazawa M (2010) Assay of labile estrogen o-quinones, potent carcinogenic molecular species, by high performance liquid chromatography–electrospray ionization tandem mass spectrometry with phenazine derivatization. J Steroid Biochem Mol Biol 119:141–148
Díaz-Cruz MS, López de Alda MJ, López R, Barceló D (2003) Determination of estrogens and progestogens by mass spectrometry techniques (GC/MS, LC/MS and LC/MS/MS). J Mass Spectrom 38:917–923
Cheng C, Hou J, Wang S, Xu B, Liu S, Yan Z (2011) Development and validation of an LC-MS/MS method for determination of fifteen estrogens and metabolites in human serum. In: Pittsburgh conference, Atlanta, GA, 18 March 2011
Nordstrom A, O’Maille G, Qin C, Siuzdak G (2006) Nonlinear data alignment for UPLC-MS and HPLC-MS based metabolomics: quantitative analysis of endogenous and exogenous metabolites in human serum. Anal Chem 78:3289–3295
Novakoca L, Matysova L, Solich P (2006) Advantages of application of UPLC in pharmaceutical analysis. Talanta 68:908–918
Zelena E, Dunn WB, Broadhurst D, Francis-McIntyre S, Carroll KM, Begley P, O’Hagan S, Knowles JD, Halsall A, Consortium H, Wilson ID, Kell DB (2009) Development of a robust and repeatable UPLC-MS method for the long-term metabolomic study of human serum. Anal Chem 81:1357–1364
Matějíček D (2011) On-line two-dimensional liquid chromatography–tandem mass spectrometric determination of estrogens in sediments. J Chromatogr A 1218:2292–2300
Lien G, Chen C, Wang G (2009) Comparison of electrospray ionization, atmospheric pressure chemical ionization and atmospheric pressure photoionization for determining estrogenic chemicals in water by liquid chromatography tandem mass spectrometry with chemical derivatizations. J Chromatogr A 1216:956–966
Xu X, Roman JM, Veenstra TD, Van Anda J, Ziegler RG, Issaq HJ (2006) Analysis of fifteen estrogen metabolites using packed column supercritical fluid chromatography-mass spectrometry. Anal Chem 78:1553–1558
Labadie P, Hill EM (2007) Analysis of estrogens in river sediments by liquid chromatography–electrospray ionisation mass spectrometry—comparison of tandem mass spectrometry and time-of-flight mass spectrometry. J Chromatogr A 1141:174–181
U.S. Environmental protection agency method 539: determination of hormones in drinking water by solid phase extraction (SPE) and liquid chromatography electrospray ionization tandem mass spectrometry (LC-MS/MS). EPA document No. 815-B-10-001, November 2010. http://www.epa.gov/drink/
Mouatassim-Souali A, Tamisier-Karolak S, Perdiz D, Cargouet M, Levi Y (2003) Validation of a quantitative assay using GC/MS for trace determination of free and conjugated estrogens in environmental water samples. J Sep Sci 26:105–111
Zhou Y, Zhou J, Xu Y, Zha J, Ma M, Wang Z (2009) An alternative method for the determination of estrogens in surface water and wastewater treatment plant effluent using pre-column trimethylsilyl derivatization and gas chromatography/mass spectrometry. Environ Monit Assess 158:35–49
Hsing AW, Stanczyk FZ, Bélanger A, Schroeder P, Chang L, Falk RT, Fears TR (2007) Reproducibility of serum sex steroid assays in men by RIA and mass spectrometry. Cancer Epidemiol Biomarkers Prev 16:1004–1008
Myers GL (2008) Introduction to standardization of laboratory results. Steroids 73:1293–1296
Rosner W, Vesper H (2008) CDC workshop report improving steroid hormone measurements in patient care and translation research. Steroids 7:1285
Vesper HW, Botelho JC, Shacklady C, Smith A, Myers GL (2008) CDC project on standardizing steroid hormone measurements. Steroids 73:1286–1292
Tai SS-C, Welch MJ (2005) Development and evaluation of a reference measurement procedure for the determination of estradiol-17β in human serum using ID-LC/MS/MS. Anal Chem 77:6359–6363
Tai SS-C, Xu B, Welch MJ, Phinney KW (2007) Development and evaluation of a candidate reference measurement procedure for the determination of testosterone in human serum using isotope dilution liquid chromatography/tandem mass spectrometry. Anal Bioanal Chem 388:1087–1094
Tai SS-C, Xu B, Welch MJ (2006) Development and evaluation of a candidate reference measurement procedure for the determination of progesterone in human serum using ID-LC/MS/MS. Anal Chem 78:6628–6633
Peters FT, Maurer HH (2002) Bioanalytical method validation and its implications for forensic and clinical toxicology—a review. Accred Qual Assur 7:441–449
Nelson RE, Grebe SK, O’Kane DJ, Singh RJ (2004) Liquid chromatography mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clin Chem 50:373–384
Lai C, Tsai C, Tsai F, Wu J, Lin W, Lee C (2002) Monitoring of congenital adrenal hyperplasia by microbore HPLC–electrospray ionization tandem mass spectrometry of dried blood spots. Clin Chem 48:354–356
Naessen T, Sjogren U, Bergquist J, Larsson M, Lind L, Kushnir MM (2010) Endogenous steroids measured by high-specificity liquid chromatography-tandem mass spectrometry and prevalent cardiovascular disease in 70-year-old men and women. J Clin Endocrinol Metab 95:1889–1897
Santen RJ, Lee JS, Wang S, Demers LM, Mauras N, Wang H et al (2008) Potential role of ultra-sensitive estradiol assays in estimating the risk of breast cancer and fractures. Steroids 73:1318–1321
Ionita IA, Akhlaghi F (2010) Quantification of unbound prednisolone, prednisone, cortisol and cortisone in human plasma by ultrafiltration and direct injection into liquid chromatography tandem mass spectrometry. Ann Clin Biochem 47:350–357
Nguyen HP, Li L, Gatson JW, Maass D, Wigginton JG, Simpkins JW, Schug KA (2011) Simultaneous quantification of four native estrogen hormones at trace levels in human cerebrospinal fluid using liquid chromatography–tandem mass spectrometry. J Pharm Biomed Anal 54:830–837
Xu X, Othman ER, Issaq HJ, Hornung D, Al-Hendy A, Veenstra TD (2008) Multiplexed quantitation of endogenous estrogens and estrogen metabolites in human peritoneal fluid. Electrophoresis 29:2706–2713
Nielen MWF, van Bennekom EO, Heskamp HH, van Rhijn JA, Bovee TFH, Hoogenboom LAP (2004) Bioassay-directed identification of estrogen residues in urine by liquid chromatography electrospray quadrupole time-of-flight mass spectrometry. Anal Chem 76:6600–6608
Xu X, Keefer LK, Ziegler RG, Veenstra TD (2007) A liquid chromatography–mass spectrometry method for the quantitative analysis of urinary endogenous estrogen metabolites. Nat Protoc 2:1350–1355
Wilkinson AP, Wahala K, Williamson G (2002) Identification and quantitation of polyphenol phytoestrogens in foods and human biological fluids. J Chromatogr B 777:93–109
Bowers LD (1997) Analytical advances in detection of performance-enhancing compounds. Clin Chem 43:1299–1304
Peng L, Farcase T, McGinley Identification of steroids in urine and plasma by LC/MS/MS using strata X and Gemini C18. Application note: TN-1026. www.phonomenex.com
Ingrand V, Herry G, Beausse J, de Roubin M (2003) Analysis of steroid hormones in effluents of wastewater treatment plants by liquid chromatography-tandem mass spectrometry. J Chromatogr A 1020:99–104
Habauzit D, Armentgaud J, Roig B, Chopineau J (2008) Determination of estrogen presence in water by SPR using estrogen receptor dimerization. Anal Bioanal Chem 390:873–883
Miége C, Bados P, Brosse C, Coquery M (2009) Method validation for the analysis of estrogens (including conjugated compounds) in aqueous matrices. Trends Anal Chem 28:237–244
Grover DP, Zhanga ZL, Readman JW, Zhou JL (2009) A comparison of three analytical techniques for the measurement of steroidal estrogens in environmental water samples. Talanta 78:1204–1210
Guidelines
International Conference on Harmonisation of Technical Requirements for Registrations of Pharmaceuticals for Human Use, ICH Harmonised Tripartite Guideline, Validation of Analytical Procedures: Text and Methodology, Q2(R1), Current Step 4 version, 2005, 13 pp. http://www.ich.org/products/guidelines/quality/article/quality-guidelines.html
U.S. Food and Drug Administration CFR—Code of Federal Regulations Title 21, Part 58—Good laboratory practice for nonclinical laboratory studies. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?CFRPart=58
U.S. Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM), May 2001, Guidance for industry—bioanalytical method validation. http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm064964.htm
The United States Pharmacopeia–National Formulary (USP–NF), USP34-NF29 S1. General Requirements/<11>USP Reference Standards, pp 38–40. Pharmacopeial Forum 35(6):1507
The United States Pharmacopeia–National Formulary (USP–NF), USP34-NF29 S1. General Information/<1225>Validation of Compendial Procedures, pp 779–782. Pharmacopeial Forum 35(2):444
U.S. Environmental protection agency CFR—Code of Federal Regulations Title 40—Protection of Environment PART 792—Good laboratory practice standards. http://www.access.gpo.gov/nara/cfr/waisidx_07/40cfr792_07.html
U.S. Environmental protection agency, Chemical QC guidelines. http://www.epa.gov/sam/qc-chem.htm
The National Institute of Standards and Technology, Analytical chemistry division, Development of Reference Methods and Reference Materials for the Determination of Hormones in Human Serum. http://www.nist.gov/mml/analytical/organic/hormonesinserum.cfm
The National Institute of Standards and Technology, Taylor BN, Kuyatt CE (1994) Guidelines for evaluating and expressing the uncertainty of NIST measurement results. NIST Technical Note 1297, pp 1–20. http://www.nist.gov/pml/pubs/tn1297/index.cfm
Thomson M, Ellison SLR, Wood R (2002) International union of pure and applied chemistry, harmonized guidelines for single laboratory validation of method of analysis. Pure Appl Chem74:835–855. http://www.iupac.org/objID/Article/pac7405x0835
International Organization of Standardization, Guide:
ISO Guide 30:1992 Terms and definitions used in connection with reference materials
ISO Guide 30:1992/Amd 1:2008 Revision of definitions for reference material and certified reference material
ISO Guide 31:2000 Reference materials—contents of certificates and labels
ISO Guide 32:1997 Calibration in analytical chemistry and use of certified reference materials
ISO Guide 33:2000 Uses of certified reference materials
ISO Guide 34:2009 General requirements for the competence of reference material producers
ISO Guide 35:2006 Reference materials—general and statistical principles for certification. http://www.iso.org/iso/search.htm?qt=guide+35&searchSubmit=Search&sort=rel&type=simple&published=on
International Organization of Standardization, Published:
ISO 5725-1:1994 Accuracy (trueness and precision) of measurement methods and results—Part 1: general principles and definitions
ISO 5725-2:1994 Accuracy (trueness and precision) of measurement methods and results—Part 2: basic method for the determination of repeatability and reproducibility of a standard measurement method
ISO 5725-3:1994 Accuracy (trueness and precision) of measurement methods and results—Part 3: intermediate measures of the precision of a standard measurement method
ISO 5725-4:1994 Accuracy (trueness and precision) of measurement methods and results—Part 4: basic methods for the determination of the trueness of a standard measurement method
ISO 5725-5:1998 Accuracy (trueness and precision) of measurement methods and results—Part 5: alternative methods for the determination of the precision of a standard measurement method
ISO 5725-6:1994 Accuracy (trueness and precision) of measurement methods and results—Part 6: use in practice of accuracy values. http://www.iso.org/iso/search.htm?qt=5725&published=on&active_tab=standards
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Yan, Z., Cheng, C., Liu, S. (2012). Applications of Mass Spectrometry in Analyses of Steroid Hormones. In: Xu, Q., Madden, T. (eds) LC-MS in Drug Bioanalysis. Springer, Boston, MA. https://doi.org/10.1007/978-1-4614-3828-1_10
Download citation
DOI: https://doi.org/10.1007/978-1-4614-3828-1_10
Published:
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4614-3827-4
Online ISBN: 978-1-4614-3828-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)