Abstract
There are two general classes of effects that sound, and ultrasound in particular, can have on a fluid. First, very significant modifications to the nature of food and food ingredients can be due to the phenomena of bubble acoustics and cavitation. The applied sound oscillates bubbles in the fluid, creating intense forces at microscopic scales thus driving chemical changes. Second, the sound itself can cause the fluid to flow vigorously, both on a large scale and on a microscopic scale; furthermore, the sound can cause particles in the fluid to move relative to the fluid. These streaming phenomena can redistribute materials within food and food ingredients at both microscopic and macroscopic scales.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
13.1 Basic Mechanisms
13.1.1 Physical Effects of Sound on Fluids
There are two general classes of effects that sound, and ultrasound in particular, can have on a fluid. First, very significant modifications to the nature of food and food ingredients can be due to the phenomena of bubble acoustics and cavitation. The applied sound oscillates bubbles in the fluid, creating intense forces at microscopic scales thus driving chemical changes. Second, the sound itself can cause the fluid to flow vigorously, both on a large scale and on a microscopic scale; furthermore, the sound can cause particles in the fluid to move relative to the fluid. These streaming phenomena can redistribute materials within food and food ingredients at both microscopic and macroscopic scales.
13.1.2 Bubble Acoustics and Cavitation
13.1.2.1 Fundamentals of Bubble Acoustics
A bubble in the context of ultrasound and food processing is a gas surrounded by a liquid. For bubble-acoustic phenomena to occur the substance, surrounding the bubble should allow the gas in the bubble to easily expand and contract; a liquid around the bubble permits easy expansion and contraction because it can flow. The liquid surrounding the bubble might be extremely viscous or contain solid particles, but as long as it can flow, bubble-acoustic phenomena can occur.
Gases are compressible, and they are much more compressible than liquids. Bulk modulus is a property representing the resistance of a substance to volumetric compression. The bulk modulus of air is only about 1/10,000 that of water, so while an air bubble is easily compressed, the surrounding liquid is comparatively incompressible. On the other hand, liquids have much higher densities than gases; the density of water is about 800 times that of air. Thus, a gas bubble in liquid is effectively a spring attached to a mass. The “spring” is a spherical spring owing to the compressibility of the gas, and the mass is a spherical shell of liquid, but the same phenomenon occurs for any mass attached to a spring. There is a natural frequency with which the mass bounces on the spring in response to a disturbance.
The physics of bubble acoustics was first mathematically formulated nearly a century ago (Rayleigh, 1917). For the special case in which the amplitude with which the bubble’s radius oscillates is a small fraction of its radius, the natural frequency, f 0, was simply derived by Minnaert (1933) as
where f 0 is in hertz, Γ is the polytropic index of the gas, P 0 is the total ambient pressure that includes atmospheric pressure plus any additional constant pressure imposed on the bubble, ρ 0 is the density of the liquid, and R 0 is the equilibrium radius of the bubble. Since Γ and ρ 0 are properties of the gas and liquid, respectively, for a given applied pressure the natural frequency of the bubble is inversely proportional to the radius. For the case of an air bubble in water, \(f_{\underline 0 } \approx 3.3/R_0\) in SI units. For example, a bubble with 1 mm in radius has a natural frequency of roughly 3 kHz while a bubble with 1 μm in radius has a natural frequency of roughly 3 MHz. Since the great majority of bubbles of practical relevance in the food industry are millimeters in size or smaller, their natural frequencies are vibrations measured in kilohertz or megahertz, in other words, vibrations that are classified as sound or ultrasound.
As with any naturally vibrating system, if the system is forced to vibrate near its natural frequency, resonance will occur. Resonance in a bubble-acoustic system would occur if the bubble is forced to vibrate by applied sound. Resonance can lead to large-amplitude vibrations in the fluid over the immediate vicinity of the bubble and extreme conditions in the gas, which could ultimately drive some physical and chemical modifications of interest to food technologists.
It is important to note that Minnaert’s equation (13.1) neglects many factors that are significant in a food processing context. For example, chemical reactions and phase transformations as the bubble expands and contracts, the viscosity of the liquid, the gas–liquid surface tension, the speed of sound in the liquid, and influence of other bubbles and the containing walls have all been ignored in equation (13.1). A good review of many of these effects is given in Leighton (1994) and detailed analyses of other effects have been made elsewhere, for example, by Prosperetti (1977) on viscous and thermal effects, Vokurka (1986) on sound speed in liquid, and Manasseh et al. (2004) on the effect of other bubbles. Nevertheless, for practical applications, the fact that in Minnaert’s equation f 0 is proportional to 1/R 0 provides an excellent rule of thumb that food technologists can use to estimate the size of bubbles that would be affected by sound or the sound frequency needed to drive a process at a particular scale.
It is possible for the bubble to be encapsulated in a thin, solid shell, and as long as the shell has some elasticity, bubble-acoustic phenomena can still occur (de Jong et al., 2002).
13.1.2.2 Cavitation
Many foods ingredients do not naturally contain significant quantities of gas bubbles. Rather, as ultrasound is applied, gas bubbles are created and then go on to modify the substance. Cavitation is the phenomenon by which bubbles can be created by ultrasound. Leighton (1994) and Brennen (1995) provide a good general introduction to all types of cavitation.
Liquids can be turned into gases (vaporized) if the temperature is raised sufficiently – simply boiling water is the classic illustration of this. However, vaporization is also possible if the pressure is dropped sufficiently. Many engineering systems can vaporize liquids by pressure drops, particularly systems with blades such as pumps and propellers or venturi nozzles. If the pressure in the wake of a pump blade is low enough, the liquid in the low-pressure zone will effectively “boil,” forming bubbles on the blade analogous to the bubbles formed on the hot plate of a heated saucepan. This is called cavitation and has been studied extensively from the mid-twentieth century owing to its importance for turbomachinery and submarine noise (for reviews, see Blake and Gibson, 1987; Brennen, 1995, 2005; Leighton, 1994).
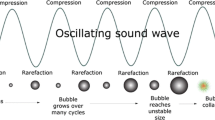
Sound propagation through a fluid is a wave with high pressure in the crests and low pressure in the troughs. In practical fluids, a few tiny bubbles, in the order of a micron in size or less, are always present and are referred to as cavitation nuclei (Brennen, 1995). During the low-pressure trough, liquid evaporates into the nuclei, enlarging them. The crest of each wave reverses this process, condensing the vapor in the bubbles back to liquid, and one might think that there would be no net effect. However, during the low-pressure trough, the bubble is expanded, so its surface area is larger than during the high-pressure crest when it is compressed. The process of evaporation thus benefits from a larger surface area than that of the competing process of condensation, and therefore, with the bubble grows slightly with each passing sound wave. This is called rectified diffusion (Brennen, 1995; Leighton, 1994).
Moreover, as the bubble grows, its radius gets closer to the Minnaert radius R 0 corresponding to the applied frequency f 0. As this happens, the bubble begins to resonate; the amplitude with which it expands and contracts gets larger, pumping vapor into the bubble at an accelerating rate until R 0 is passed. In general, the bubble population will eventually stabilize.
However, if the ultrasound power is high enough, the bubbles grow explosively. The amplitude of oscillation becomes extreme and unstable and usually the bubble collapses violently, fragmenting into a number of smaller bubbles. This is the analogue of the situation behind a cavitating pump blade, which is called ultrasonic cavitation (Brennen, 1995; Leighton, 1994). It can happen with the passage of only a single ultrasonic cycle of the appropriate frequency. Thresholds at which cavitation occurs have been measured and calculated for many conditions, but mostly for water and seawater rather than food ingredients. For example, 2 μm cavitation nuclei driven at 1 MHz will cavitate if the peak negative pressure is greater than 0.3 MPa, but at 5 MHz a peak pressure of 1 MPa would be required (Apfel and Holland, 1991).
13.1.2.3 Nonlinearity and Collapse
During cavitation the bubble expands and contracts through a spectacular amplitude; experimental imaging typically shows that cavitation bubbles can shrink to a diameter of only 1/10 of their maximum radius (see, e.g., Brenner et al., 2002; Brujan et al., 2001; Putterman and Weniger, 2000). Under these conditions, the assumption of small amplitude oscillation made by Minnaert (1933) is invalid and the behavior of the bubble is extremely nonlinear. The forces generated in the liquid are so high that they can make holes in metal surfaces nearby, which was one of the original engineering motivations for studying cavitation. As detailed in Section 3.2, the bubble collapse can create an intense micro-jet that can puncture plant cell walls, leading to food ingredient extraction.
Moreover, even a cursory attempt at calculating conditions inside the bubble quickly leads to the conclusion that conditions during cavitation collapse are so extreme that the ideal gas law is invalid. Clearly, if the bubble volume is being reduced by two to three orders of magnitude, temperatures during this extreme compression can potentially reach thousands of degrees. The gas breaks down, forming free radicals that diffuse into the liquid and form the basis of many of the chemical modifications to be discussed in Section 3.
13.1.3 Streaming Phenomena
13.1.3.1 General Streaming Phenomena
It is well known that a class of net fluid motions, both with and without particles, can be driven by oscillatory fluid waves (Riley, 2001). As any wave passes through a fluid it causes the fluid and any particles suspended in it to oscillate to and fro; after the passage of each wave, there is no net displacement of the fluid or particles according to linear theory. This is, of course, an exact model for experimental reality if the wave power is extremely low. In general, however, a rectification of the oscillatory motion is possible owing to nonlinear effects, giving a net drift of the fluid or particles within it.
The most general explanation for a net drift in fluid dynamics is that there is nonlinearity in the equations of fluid motion, and that this nonlinearity is quadratic (Batchelor, 1967). Hence, all such streaming motions will be proportional to the square of the wave amplitude and thus proportional to the wave power. Thus, the higher the power, the greater the net fluid motions, increasing in general linearly with power. The net motions are second-order effects. This means that although they vary with the square of the wave amplitude, their velocity is much weaker than the velocity with which the fluid oscillates as the waves pass. Furthermore, the quadratic nonlinearity is actually a velocity multiplied by the gradient in velocity with distance. Thus, in order for the net motion to be possible, there should be a gradient in the wave velocity with distance. The larger the gradient, the larger the local net motion.
The simplest example of a net motion relevant to ultrasound in the food industry is acoustic streaming. Acoustic streaming could be used to mix or stir food ingredients without any mechanical moving parts. It is induced by the dissipation that leads to a gradient in sound power with distance. It has often been termed the “quartz wind” (Eckart, 1948); another term is Eckart streaming.
Rayleigh (1883) analyzed the acoustic streaming induced by sound waves propagating between parallel plates. This is usually called Rayleigh streaming. An illustration of Eckart and Rayleigh streaming effects is given in Fig. 13.1. It can be seen that Rayleigh streaming forms flow cells with their boundaries aligned with the nodes and antinodes of a standing wave pattern, while Eckart streaming does not require boundaries or a standing wave. Appropriate design of food ingredient vessels could lead to one or the other type of streaming, depending on what kind of flow patterns are needed.
A particular type of streaming arises in the fluid-dynamical boundary layer very close to the boundary, causing a vortex in the boundary layer. This is called Schlichting streaming.
13.1.3.2 Sound Radiation Pressure on a Sphere
There is also a type of net motion where particles, droplets, or bubbles (a dispersed phase) dispersed in a fluid are made to move relative to the fluid (the continuous phase). This could be particularly useful in the food industry to separate a dispersed phase from a continuous phase. The existence of a velocity field with a non-zero gradient near the object makes a net motion possible. For simplicity the word “particle” will be used below to cover any possible dispersed-phase object: solid particles, droplets, or bubbles. The first analysis of this phenomenon was made by King (1934). King noted that particles could be made to migrate in either traveling or standing waves, but he calculated that the radiation force on a rigid sphere much smaller than the sound wavelength was an order of magnitude greater if the sphere was in a standing wave field rather than a traveling wave field. This finding means that practical ultrasonic separation equipment must be designed with the wavelength of the ultrasound in mind. Under these circumstances, the “radiation pressure” acting on an incompressible sphere, F r , was given by King (1934) as
where a is the particle radius, ρ 0 is the liquid density as before, κ is the wave number given by \(2\pi f/c\) where f is the frequency in hertz and c is the speed of sound, h is the distance of the particle from a node, A is the sound wave amplitude (the velocity potential amplitude in m2/s), and a relative-density factor D is given by
where ρ 1 is the particle density.
King’s “radiation pressure” F r is in fact a force since it has the dimensions of force and is usually called the direct radiation force (DRF). It can be seen straight away that the DRF is proportional to the square of the sound amplitude and hence proportional to the power. As noted in Section 1.3.1, this is a fundamental feature of all such net-motion phenomena. The factor D changes sign when \(\rho _0 /\rho _1 = 2.5\), i.e., when the specific gravity of the particle \(\rho _1 /\rho _0 = 0.4\). A particle with a specific gravity denser than 0.4 will be pushed from the nodes toward the antinodes, while a particle less dense than 0.4 will be pushed from the antinodes toward the nodes. In most practical situations of interest to the food industry, only bubbles would have a specific gravity less than 0.4, so the majority of particles or droplets would move toward the antinodes.
Yoshikawa and Kawashima (1955) extended King’s analysis to include compressible spheres; the result is similar to King’s, with the factor D being modified by a term that incorporates the compressibility. Gupta et al. (1995) proposed using the differences in DRF due to compressibility to segregate particles with different compressibility.
King (1934) analytically calculated the paths taken by particles as they moved to their target antinodes; Townsend et al. (2004) give a more recent example of a computation of particle paths.
13.1.3.3 Separation of Particles Much Smaller than the Wavelength and Larger than About a 100 μm
The time a particle takes to move toward the antinodes or nodes is relevant to many practical food industry applications, since this clearly affects whether ultrasonic separation will be feasible or not. Since the DRF changes sign on either side of the particle’s target antinode, it is a restoring force – like gravity acting on a pendulum bob. The period of this natural oscillation gives a convenient estimate of the time a particle would take to be separated, in the absence of viscosity. King (1934) showed that the inviscid migration time T i taken by a particle much smaller than the sound wavelength \((\kappa a < < 1)\) to reach its nearest antinode is estimated by
where h 0 is the particle’s initial distance from a node, \(K(\cos (2\pi h_0\, f/c))\) is the complete elliptic integral with argument \(\cos (2\pi h_0\, f/c)\) and \(u = \kappa A\), a relative-density factor d is given by
It is important to note that this estimate T i for an inviscid continuous phase is independent of particle size. For most typical applications, it predicts that particles initially uniformly distributed would arrive at the antinode, creating clear bands, in the order of a second. Numerous experiments since the 1920s have shown such estimates to be good.
However, as particles become smaller than roughly 100 μm, the drag force due to the continuous-phase viscosity begins to dominate. Viscous friction damps any tendency for the particle to overshoot its target. An alternative estimate of the migration time is necessary. Although King did not estimate it, a crude estimate is possible, by equating an average of the DRF with the Stokes drag (Batchelor, 1967) given by
where u is the speed the particle moves through the fluid and calculating the time T v taken to travel a quarter wavelength. This gives
Unlike in the inviscid relation, particle size a clearly matters in a viscous fluid, and virtually all food industry fluids will have significant viscosity.
The cubic relation between particle size a and the DRF means that for a given sound frequency and power, the DRF becomes dramatically weaker for smaller particles. This is significant for smaller particles where viscosity dominates. The cubic relation between frequency and DRF means the DRF becomes dramatically stronger with frequency and the separation speed faster. However, the attendant reduction in wavelength means the separation would occur over shorter lengths which would be of less practical use.
Finally, in practical devices there is also a lateral radiation force, which is due to variations in the acoustic field at right angles to the traveling wave (Spengler et al., 2003). This is two orders of magnitude weaker than the DRF but will tend to make particles clump within their target planes.
13.1.4 Acoustic Microstreaming
In Section 1.3.1 it was noted that steady streaming motions are possible if there is a gradient in the acoustic field, and that the larger the gradient, the larger the streaming velocity. In the vicinity of an acoustically oscillating microbubble, there is a significant change in the sound field over a very small distance, and hence the large gradient necessary for steady streaming is present in the small region around the microbubble. The resulting flows and shear forces, though often only a 100 μm in extent, can be very intense. This type of streaming flow, called acoustic microstreaming, was first experimentally noticed by Kolb and Nyborg (1956) and a convenient experimental review is given by Tho et al. (2007). Acoustic microstreaming can also be created around any small particle with a different acoustic impedance to the surrounding liquid, such as a solid grain in a liquid matrix, and indeed the separation effects noted in Section 1.3.2 could be considered as a manifestation of acoustic microstreaming. Nonetheless, the most powerful and varied microstreaming phenomena are those around cavitation bubbles, where they are called cavitation microstreaming. These intense small-scale flows could locally transport ingredients as well as breaking down food ingredient cells (Rooney, 1989; Ugarte-Romero et al., 2006).
13.2 Practical Ultrasonic Separation
Ultrasonic separation of food ingredients has obvious advantages over conventional methods like filtration and natural settling:
-
The acoustic forces are non-contact, in principle eliminating the need to clean and replace filters;
-
Acoustic separation is in principle quite rapid, apparently separating particles down to sub-micron size in seconds;
-
The acoustic forces are “gentle”: they do not involve large shearing forces that may damage delicate materials;
-
Removing the need for physical devices in the stream would further reduce shearing forces on the mixture, reduce the pressure head needed to pump liquid, and minimize clogging and consequent maintenance costs;
-
Acoustic separation could offer means of further segregating particles on the basis of their density and compressibility.
The first patent on acoustic separation of particles from liquid was filed by C.R. Holden in 1937, claiming mining industry applications. There have been a number of other patents, including Muralidhara et al. (1988) on the concurrent use of electric and acoustic fields and Gallego-Juarez et al. (1998) on the use of orthogonal ultrasonic fields of different frequencies in a gas.
Commercial devices using ultrasound for separation have been on the market for a few years. An example, used for the separation of live cells from a culture medium, is shown in Fig. 13.2.
This class of devices can only handle up to about 200 l/day, generally separating 95% of cells but falling sharply in efficiency if flow rates are pushed higher (Gorenflo et al., 2002). This is certainly suitable for biotechnology applications where there is high value in a small quantity of the product, usually pharmaceutical, and where conventional methods of filtration would kill or damage the delicate mammalian cells being cultured. However, such low flow rates would seem impractical for all but the most specialized food industry applications. Nonetheless, the Canadian/US/Austrian company SonoSep, founded in 1997, claims food processing as an application area. It licenses ultrasonic separation for bioengineering to the Netherlands company Applikon Biotechnology.
Ultrasonic separators have also been used in the photographic film industry for many years (Barbee and Brown, 1978). Although they are not commercial products, they are part of the plant equipment and were custom-made for fine bubble removal. In these industries, the need is to remove bubbles that have become entrained in film emulsion during its manufacture and would seriously affect the quality of photographic media were they not removed. As with the biotechnology application, the volume flow rates are quite small so that the use of ultrasonic separation is feasible at low power.
Attempts to develop laboratory precursors to industrial prototypes have also been made (e.g., Spengler and Jekel, 2000). Etrema Products in the USA developed an ultrasonic oil–water separator (Fig. 13.3) in 2000.
However, large-scale industrial applications of ultrasonic separation have been absent. The major stumbling block appears to be the bulk streaming phenomenon (Eckart and Rayleigh streaming) that tends to destroy the separation if power is ramped up, limiting the speed and scale of the application (Spengler et al., 2003). Complex baffle systems (e.g., Spengler and Jekel, 2000) have been proposed to overcome bulk streaming, but would clearly tend to eliminate some of the advantages noted above. A similar proposal is to limit the device size to a single quarter wavelength (e.g., Hawkes et al., 2002), with similar consequences.
An important new application is in microfluidics, where the separation of cells or large molecules like proteins may be achieved (Manasseh et al., 2006). Ultrasonic separation of cells in microdevices has been achieved (Nilsson et al., 2005) and clearly the flow rates in these applications are extremely small, making low power feasible. In principle, the bulk streaming problems of ultrasound should be absent at microscale, but the extremely large acoustic field gradients in microdevices can also lead to bulk streaming motions (Tho et al., 2006, 2007; Manasseh et al., 2005, 2006; see also Section 1.3.1).
13.3 Ultrasonic Extraction
13.3.1 Background Introduction
Ultrasonically assisted extraction of different vegetational materials has been studied since the 1950s. There were studies on extraction of chymosin from abomasa and aroma compounds from grape musts using ultrasound. It was reported that an ultrasonic extraction method could increase the recovery and purity of chymosin extracted from abomasum tissues (Kim and Zayas, 1991) and gave high extraction efficiency for aroma compounds in must and wine (Cocito et al., 1995). The use of ultrasound has been studied in assisting extraction of bioactive principles from herbs at laboratory and large scale (Vinatoru, 2001). The mechanism of ultrasonic extraction is based on the effect of sonication breaking vegetal cells and improving diffusion and osmotic processes (Vinatoru, 2001). This may result in an increase in the extraction efficiency as well as extraction rate. In addition, ultrasound has an effect on increasing the swelling of vegetal tissue; facilitating cell wall rupture and releasing intracellular components into water during sonication.
Horticultural production releases a large amount of waste that is found to contain a significant amount of nutritional components and dietary fiber, which are valuable substances. Extraction of substances/materials from horticultural products and waste could provide an additional income for producers and possibly reduce the cost of waste treatment. Conventional extraction is associated with many problems including high solvent consumption, long operating time, and low yield. A new extraction method using ultrasound could overcome these problems and also allow collection of functional ingredients in natural forms, which have more value in term of health benefits.
13.3.2 Extraction Mechanisms and Process Development
Extraction enhancement by ultrasound has been attributed to the propagation of ultrasound pressure waves and resulting cavitation phenomena as outlined in Section 1. High shear forces cause increased mass transfer of extractable materials (Jian-Bing et al., 2006). The implosion of cavitation bubbles generates macro-turbulence, high-velocity inter-particle collisions, and perturbation in micro-porous particles of the biomass which accelerates eddy and internal diffusion. Cavitation near liquid–solid interfaces directs a fast moving stream of liquid through the cavity at the surface (Blake and Gibson, 1987). Impingement by these micro-jets results in surface peeling, erosion, and particle breakdown. This effect provides exposure of new surfaces further increasing mass transfer (Fig. 13.4).
This phenomenon was confirmed by scanning electron microscopy of peppermint leaves and trichomes (leaf glands). After the leaves were ultrasonically treated for menthol extraction, microscopy found that there were two mechanisms involved in extraction: (a) the diffusion of product through the cuticle of peppermint glandular trichomes and (b) the exudation of the product from broken and damaged trichomes (Shotipruk et al., 2001).
Acceleration in solvent extraction kinetics and improved extraction yield of pyrethrine from pyrethrum was largely attributed to ultrasonics increasing the intra-particular diffusion of the solute, which is considered the rate-limiting step (Romdhane and Gourdon, 2002). If the substrate is dry, in aqueous extraction ultrasound may be used to facilitate swelling and hydration and cause enlargement of the cell wall pores (Vinatoru, 2001). Improved extraction performance was also attributed to diffusion through the plant cell walls, disruption, and washing out of the cell contents. Reduction in the size of vegetal material particles by ultrasonic disintegration will increase the number of cells directly exposed to extraction by solvent and ultrasonic cavitation (Vinatoru, 2001). Intensive ultra-sonication can also serve to reduce particle size in tomato juice (Food Science Australia, unpublished data).
Acoustic cavitation bubble collapse occurring at or in close vicinity to the surface of the plant membranes may cause microfractures (Vinatoru, 2001). The occurrence of microfracture by ultrasound was demonstrated in soybean flakes (Li et al., 2004). Cavitation at cell surfaces has the ability to punch holes through the cell wall as has been recently demonstrated with studies of bacterial cell sonication (Ugarte-Romero et al., 2006).
Variation in the extraction yield from different plant materials may result from structure, plasticity, or the compositional differences resulting in varying degrees of susceptibility to ultrasound shock waves and the likelihood that cavitation bubbles will contact with the plant surface causing micro-jetting (Li et al., 2004). Factors such as plant tissue turgor and the mobility of particles such as starch granules within the cell cytoplasm can be expected to influence ultrasound energy dispersion and extraction effectiveness (Zhang et al., 2005).
In their study on supercritical fluid extraction enhancement by ultrasound, Balachandran et al. (2006) were able to demonstrate that the effectiveness of ultrasound was gained by increasing superficial mass transfer and that effectiveness declined sharply after the readily accessible surface solute had been removed. However, by reducing the substrate particle size major gains in extraction efficiency and extraction time reduction could be achieved. Even in the supercritical environment ultrasound was demonstrated to inflict significant surface cell damage.
Solvent selection is usually based on achieving high molecular affinity between the solvent and solute. When ultrasound is also applied the cavitation will be affected by the physical properties of the solvent. Cavitation intensity decreases as vapor pressure and surface tension are increased. Li et al. (2004) demonstrated this phenomenon in soybean oil extraction where greater ultrasonic extraction was achieved by isopropanol compared with hexane, the latter having approximately fivefold higher vapor pressure.
13.3.3 Extraction Process for Functional Compounds
Ultrasonic extraction of industrial waste resulting from processing vegetable and plant material can be used to recover valuable components. Aqueous and combined aqueous/organic solvents are used for extraction. Ultrasound can be used to benefit both low-temperature and high-temperature extraction systems, regardless of solvent pH, ionic environment, surface tension, or surfactant processing aids.
Low-frequency ultrasound (16–100 kHz) can be used for the extraction of components/substances such as hydrophilic flavonoids (anthocyanins, tannins) and hydrophobic carotenoids (lycopene, beta-carotene, capsaicin, and lutein) from horticultural products such as carrot, ginger, tomato, grapes, olives, olive pomace, and capsicum and from their processing waste.
Preliminary study of ultrasonic extraction of carotenes in carrot waste showed that ultrasound enhanced the extraction yield in both organic solvent and water as shown in Table 13.1. The number of repeated extractions with fresh solvent or water provided additional benefit enhancing the yield of extracted material.
Extraction efficiency can be improved by using ultrasonic horn (sonotrodes) designed for the specific application. The type of sonotrode can increase solvent penetration, cavitation at surfaces, and thus removal of components and extraction efficiency. The organic load and nature of the material being processed will determine the type of sonotrode design. The design of a specific sonotrode will allow for greater penetration of the ultrasonic wave/cavitational energy, better coupling of energy to the product and improved energy efficiency resulting in greater extraction of components, solvent penetration, and removal of components from plant tissues. Focused sonotrodes with a frequency of 20–24 kHz gave better extraction yields than other frequencies in ultrasonic extraction of carotenes, whereas increasing power levels of sonication tended to increase the extraction yield (Tables 13.2 and 13.3).
13.3.4 Opportunities for Food Industry
A limited number of publications have included continuous ultrasonic process development and pilot-scale applications. The range of published extraction applications includes herbal, oil, protein, and bioactives from plant materials (e.g., flavones, polyphenolics), as summarized in Table 13.4.
Ultrasound has a unique capacity to both enhance extraction from substrates while simultaneously encapsulating the extracted substance with an encapsulant material in the extraction fluid by hydroxyl radical-initiated covalent bonding and microsphere formation. To successfully accomplish this, the encapsulating material should have a higher reductive potential than the material being extracted and be relatively more hydrophobic. Preferably, a mixed frequency ultrasound field is used, a relatively low frequency to facilitate extraction and a higher frequency under independent amplitude control to facilitate hydroxyl radical production for cross linking and microsphere formation. Proteins are suggested encapsulants as the sonochemistry and conditions favoring sphere development have previously been established. For scale up to industrial application treatment vessel geometries, frequency combinations, and frequency modulation to achieve the desired outcomes on a large scale need to be explored and optimized.
Potential exists for applying UAE for enhancing of aqueous extraction as an alternative to organic solvents. The presence of cavitation bubbles effectively renders the water more hydrophobic than its natural conditions. UAE can also enhance extraction of heat-sensitive bioactive and food components by enabling lower processing temperatures.
Sonochemical modification of bioactive compounds during the extraction process is possible which can be used to facilitate the extraction process or modify the extracted material in desirable ways, for example, either elimination or reduced use of enzyme in the commercial extraction processes for vegetable oils and grape juices (Ashokkumar et al., 2008; Jiménez et al., 2007).
There is a more detailed discussion of simultaneous extraction and component modification in Section 4.
State of the art in UAE can achieve improvements in extraction efficiency and extraction rate, which if realized on the industrial scale can achieve economic gains. Ultrasonic equipment engineering has developed to the extent that ultrasonic technology is sufficiently scalable to consider industrial-scale ultrasonic-aided extraction as a commercially viable option.
13.3.5 Separation of Extracted Components
Dispersed phases can in principle be separated in standing wave fields at Food Science Australia, the separation of emulsions and colloidal suspensions by ultrasound has been demonstrated (unpublished data). However, as the separation of the dispersed phase by ultrasound is dependent on the establishment of stable wave fields which necessitates different vessel geometries to those required for efficient extraction, it is unrealistic to expect separation to occur in the same processing chamber as the extraction process. Although the technologies for large scale acoustic separation are not as well developed as for extraction, it is nevertheless feasible that a practical acoustic separation technology could be developed to follow the extraction process. Acoustic separation technologies were discussed in more detail in Section 2 and were explained theoretically in Sections 1.3.2 and 1.3.3.
13.3.6 Industrial Extraction Application and Design
The use of ultrasound in food processing has been reviewed by Mason et al. (1996). Recently, the design of ultrasound processing equipment has advanced to provide industrially robust processing capability. Enabling design and operational features have included (a) automated frequency scanning to enable maximum power delivery during fluctuation of processing conditions; (b) non-vibrational flanges on sonotrodes for construction of high-intensity inline flow cells; and (c) construction of radial and hybrid sonotrodes to provide greater range in application design and product opportunities. Presently, 16 kW is the largest available single ultrasound flow cell, which can be configured in series or in parallel modules. Industrial ultrasound manufacturers within the last 2 years have promoted industrial processing capability for food extraction applications (Hielscher, 2007).
Several ultrasound reactor designs have been described by Chisti (2003) and Vinatoru (2001), the latter specifically for industrial extraction of plant tissue. These included (a) an ultrasonic horn (sonotrode) directly immersed into stirred bath or reactor; (b) a stirred reactor with ultrasound coupled to the vessel wall; and (c) recycling of product from the stirred reactor through an external ultrasonic flow cell. These configurations may provide both intermittent and continuous ultrasound exposure, from low intensity in a large volume reactor (0.01–0.1 W/cm3) to high intensity (1–10 W/cm3) in an external flow cell. Mixed frequency reactors have been shown to offer advantages with respect to process efficiency and energy distribution (Delgadino et al., 2002; Feng et al., 2002; Moholkar et al., 2000; Swamy and Narayana, 2001; Tatake and Pandit, 2002). Reactor geometries that are asymmetrical and polygons preferably with odd-numbered sides using swept frequencies are also reported to be more effective (Gogate et al., 2004; Puskas, W. (2008) retired RandD director for Branson Ultrasonics Inc. USA and Ney Ultrasonics Inc. USA, “personal communication”).
Modern ultrasonic systems include automated frequency scanning which adjusts operation of the system to the optimal frequency to ensure that maximum power is transmitted to the extraction vessel. The benefit of automated frequency scanning as opposed to a fixed frequency was demonstrated by Romdhane and Gourdan (2002), where the former achieved a 32% increase in pyrethrine extraction and a 30% increase in power delivered to the product.
Where it is not a disadvantage to extract oily materials as stable emulsions, ultrasound can be used to carry out aqueous extraction of oily materials into water with yields in the order of 50% (Food Science Australia, unpublished results). The presence of a dispersed phase contributes to the ultrasound wave attenuation. The active sonication region in a reactor is restricted to a zone located at the surface of the probe which favors treatment in flow-through reactor configurations.
The application of ultrasound at Food Science Australia has focused on the use of high-powered systems for extraction of bioactives. Principal targets have been polyphenols and carotenoids in both aqueous and solvent extraction systems. The ultrasound extraction trials have demonstrated improvements in extraction yield ranging from 6 to 35%, as summarized in Table 13.5. Results of ultrasonically treated Shiraz and Sangiovese grape marc showed 17 and 35% increase in phenolic compounds, respectively, higher recovery of these compounds was obtained from their respective seeds.
To improve extraction effectiveness the material to be extracted should be reduced to as smaller particle size as practical without denaturing the material to be extracted and commensurate with separation from the solvent post-extraction. If this is done very high yields and extraction rates are possible with ultrasonic augmentation of the extraction process (Balachandran et al., 2006).
The benefits of UAE for the food industry include (a) overall enhancement of extraction yield or rate; (b) enhancement of aqueous extraction processes which is of particular benefit where solvents cannot be used (juice concentrate processing); (c) providing the opportunity to use alternative (GRAS) solvents by improvement of their extraction performance; (d) enabling sourcing/substitution of cheaper raw product sources (variety) while maintaining bioactive levels; and (e) enhancing extraction of heat-sensitive components under conditions which would otherwise have low or unacceptable yields.
13.4 Simultaneous Extraction and Modification
13.4.1 Extraction and Molecular Weight Reduction of Polymeric Materials
The extraction of high-molecular weight biopolymers such as amylose from starch granules, pectin from cell wall materials, and chitin from seafood wastes and fungal sources can be facilitated by the application of ultrasound. However, experience suggests that there is a simultaneous reduction of molecular weight during this process.
While this has been seen as a problem it can also be an opportunity for novel applications of the extracted materials where high molecular weight is not desirable.
The challenge is how to achieve extraction and controllable molecular weight reduction. The mechanics of molecular weight reduction are likely to involve different mechanics to molecular weight reduction of polymers in solution where intense hydrodynamic flows between the synchronous expansion and contraction of cavitation bubbles stretch the polymers to the breaking point. In extraction one end of the polymer molecule is anchored in the substrate surface it is being extracted from and the other is in “solution.” Following the mechanism proposed for polymers in free solution it could be expected that cleavage at the surface will occur when the free length is long enough for the hydrodynamic flows to cavitation bubbles close to the surface generate sufficient force to snap the polymer from the surface. If this is the dominant mechanism, then for a given extraction and set of extraction conditions the molecular weight of the cleaved biopolymer would be expected to be in a relatively narrow size range. In our work with amylose extraction from rice starch granules it appears that this is the case. Other mechanisms for cleavage at the surface could be the impact of micro-jetting from cavitation bubbles at the surface at the point of attachment of the polymer to the surface. In this instance the cleaved polymer would be expected to have random molecular size, which has not been observed in our laboratory.
Chitin is the second most abundant biopolymer after cellulose, being the structural polymer found in crustacean, mollusc, and insect exoskeletons and fungal cell walls. It is built up from an acetylated amino glucan and is associated with magnesium and calcium phosphates and proteins. Extraction involves demineralization with hydrochloric acid followed by protein removal with sodium hydroxide. The functional value of the extracted chitosan is related to the polymer molecular weight and the degree of acetification. Ultrasound application during the acid demineralization of ground shrimp shell can reduce the process time but less mineral is extracted, more protein is extracted, and there is significant chitin loss from the shell particles into solution (Kjartansson et al. 2006) as low-molecular weight polymer fragments by a similar mechanism noted for the extraction of amylose from granular starch. In a process not dissimilar to ultrasonic cleaning the softer biopolymer material is removed from the hard mineral structure rather than vice versa until eventually everything ends up in solution or colloidal suspension.
However, sonication during the subsequent alkaline treatment following demineralization without ultrasound facilitates the removal of protein enabling lower residual protein contents and structurally transforms the chitin for more efficient subsequent processing. Sonication of extracted chitosan in solution results in molecular weight and solution viscosity reduction but does not reduce acetylation.
Ultrasonic molecular weight reduction of biopolymers in solution is related to the composition and structural configuration of the polymer. In homopolymers with a linear structure such as esterified cellulose, chitosan, or the starch polymer amylose the molecular weight is reduced by successive cleavages at the mid-point of the polymer molecule by the action of intense hydrodynamic flows between the synchronous expansion and contraction of cavitation bubbles stretching the polymers to breaking point. By contrast the other starch polymer amylopectin, while still a homopolymer, is highly branched and the cavitation-induced microstreaming cleaves the molecule in an apparently random manner. Other carbohydrate gum biopolymers such as pectin, alginate, carrageenan, guar, and locust bean are typically block copolymers built up from two or more different sugar monomers having varying degrees of esterification and branching and may be bound into a complex structure by ionic, hydrophobic, and hydrogen bonding and are cleaved in an unpredictable manner. Similarly proteins cleave unpredictably, being built from approximately 22 amino acid monomers; they are typically linear polymers but are folded into complex structures involving one or more polymer chains that are often stabilized by covalent, ionic, hydrophobic, and hydrogen bonds. Amino acids with sulfhydryl and phenolic residues can be modified by hydroxyl radicals generated by cavitation bubbles to form new covalent bonds between protein polymer chains. Radicals formed by the cleavage of biopolymers also have the potential to recombine into novel polymer structures.
13.4.2 Extraction and Modification of Antioxidant Capacity and/or Color
As noted elsewhere in this chapter anthocyanins are readily extracted by ultrasound but they are subject to modification by overprocessing, typically resulting in loss of color and antioxidative capacity. This can be countered by strategies managing the ultrasonic treatment intensity, reduction in particle size treated, and reducing the treatment exposure time. There is also the potential to regulate the amount of ultrasonic exposure to enhance the antioxidative capacity through the addition of –OH residues to the ring structures in the anthocyanin molecules.
In a recent study, Ashokkumar et al. (2008) have shown the potential use of high-frequency ultrasound for enhancing the antioxidant properties of phenolic compounds. While this work did not involve any extraction, the experimental results reported in this study clearly indicate that modification of the functionality of food ingredients is possible during the extraction process. Phenol was taken as a model compound. The reaction between phenol and hydroxyl radicals generated during acoustic cavitation resulted in the formation of hydroxylated phenols. The antioxidant activity of the sonicated phenol solution showed a significant antioxidant property (phenol alone did not shown any antioxidant activity). The results shown in Fig. 13.5 demonstrate that the antioxidant activity of hydroxylated phenols is higher than that of cyaniding 3-glucoside.
13.4.3 Extraction and Encapsulation
Lipophilic materials can be extracted into aqueous media by virtue of the increased hydrophobicity of the water induced by the presence of cavitation bubbles. When the material to be extracted is lipophilic and labile to oxidation, for example, a carotenoid pigment, it is desirable to protect the material against oxidation from either the oxidative radicals generated by cavitation or during subsequent handling. One strategy for protecting such materials is by encapsulation. Simultaneous extraction and encapsulation of lipophilic materials is possible with low-frequency ultrasound provided there is an appropriate protein, carbohydrate polymer, or surfactant present in the extraction solvent. Potentially the protein, carbohydrate polymer, or surfactant could originate in the substrate being extracted. Typically in this event the extracted lipophilic material unexpectedly forms a very stable emulsion.
References
Apfel, R. E., and Holland, C. K. (1991). Gauging the likelihood of cavitation from short-pulse, low-duty cycle ultrasound. Ultrasound in Medicine and Biology, 17, 179–185.
Ashokkumar, M., Sunartio, D., Kentish, S., Mawson, R., Simons, L., Vilkhu, K., and Versteeg, C. (2008). Modification of food ingredients by ultrasound to improve functionality: A preliminary study on a model system. Innovative Food Science and Emerging Technologies, 9, 155–160.
Balachandran, S., Kentish, E., Mawson, R., and Ashokkumar, M. (2006). Ultrasonic enhancement of the supercritical extraction from ginger. Ultrasonics Sonochemistry, 13, 471–479.
Batchelor, G. K. (1967). An introduction to fluid dynamics. Cambridge, MA, Cambridge University Press.
Barbee, E. H., and Brown, R. C. (1978). Sonic apparatus for removing gas from photographic emulsions. United States Patent 4,070,167.
Blake, J. R. and Gibson, D. C. (1987) Cavitation bubbles near boundaries. Annual Review of Fluid Mechanics, 19, 99–123.
Brenner, M. P., Hilgenfeldt, S., and Lohse, D. (2002). Single-bubble sonoluminescence. Review of Modern Physics, 74, 425–484.
Brennen, C. E. (1995). Cavitation and Bubble Dynamics. Oxford University Press, 245pp.
Brennen, C. E. (2005). Fundamentals of Multiphase Flow. Cambridge University Press, 407pp.
Brujan, E.-A., Nahen, K., Schmidt, P., and Vogel, A. (2001). Dynamics of laser-induced cavitation bubbles near an elastic boundary. Journal of Fluid Mechanics, 433, 283–314.
Chisti, Y. (2003). Sonobioreactors: Using ultrasound to enhance microbial productivity. Trends in Biotechnology, 21, 89–93.
Cocito, C., Gaetano, G., and Delfini, C. (1995). Rapid extraction of aroma compounds in must and wine by means of ultrasound. Food Chemistry, 52, 311–320.
de Jong, N., Bouakaz, A., and Frinking, P. (2002). Basic acoustic properties of microbubbles. Echocardiography, 19(3), 229–240.
Delgadino, A., Bonetto, J., and Lahey, T., Jr. (2002). The relationship between the method of acoustic excitation and the stability of single bubble sonoluminescence for various noble gasses. Chemical Engineering Communications, 189, 786–802.
Eckart, C. (1948). Vortices and streams caused by sound waves. Physical Review, 73(1), 68–76.
Feng, R., Zhao, Y., Zhu, C., and Mason, J. (2002). Enhancement of ultrasonic cavitation yield by multi-frequency sonication. Ultrasonics Sonochemistry, 9, 231–236.
Gogate, R., Mujumdar, S., Thampi, J., Wilhelm, M., and Pandit, B. (2004). Destruction of phenol using sonochemical reactors: Scale up aspects and comparison with conventional reactors. Separation and Purification Technology, 34, 25–34.
Gorenflo, V. M., Smith, L., Dedinsky, B., Persson, B., and Piret, J. L. (2002). Scale-up and optimizaton of an acoustic filter for 200 L/day perfusion of a CHO cell culture. Biotechnology and Bioengineering, 80(4), 438–444.
Gupta, S., Feke, D. L., and Manas-Zloczower, I. (1995). Fractionation of mixed particulate solids according to compressibility using ultrasonic standing wave fields. Chemical Engineering Science, 50(20), 3275–3284.
Hawkes, J. J., Coakley, W. T., Groschi, M., Benes, E., Armstrong, S. and Tasker, J. (2002). Single-half, wavelength ultrasonic particle filter; Predictions of the transfer matrix multilayer resonator model and experimental filtration results. Journal of the Acoustics Society of America, 111, 1259–1266.
Hielscher Gmhb, Berlin, Germany (2007). Ultrasound in the food industry. http://www.hielscher.com/ultrasonics/food_01.htm. Accessed 23 June, 2007.
Holden, C. R. (1937). Apparatus for the separation of solids in liquid suspension. United States Patent 2,071,260.
Jian-Bing, J., Xiang-hong, L., Mei-qiang, C., and Zhi-chao, X. (2006). Improvement of leaching process of Geniposide with ultrasound. Ultrasonics Sonochemistry, 13, 455–462.
Jiménez, A., Beltrán, G., and Uceda, M. (2007). High-power ultrasound in olive paste pretreatment. Effect on process yield and virgin olive oil characteristics. Ultrasonics Sonochemistry, 14, 725–731.
Gallego-Juarez, J. A., Riera- Franco de Sarabia, E., Rodriguez-Corral, G. Juarez, J. A. G., de Sarabia, E. R. F., and Corral, G. R. (1998). Multifrequency acoustic chamber for the agglomeration and separation of particles suspended in gaseous effluents. United States Patent 5,769,913.
Kim, S. M., and Zayas, J. F. (1991). Comparative quality characteristics of chymosin extracts obtained by ultrasound treatment. Journal of Food Science, 56(2), 406–410.
King, K. V. (1934). On the acoustic radiation pressure on spheres. Proceedings of the Royal Society of London, A., 147, 212–240
Kjartansson, G., Zivanovic, S., Kristbergsson, K., and Weiss, J. (2006). Sonication-assisted extraction of chitin from North Atlantic shrimps (Pandalus Borealis). Journal of Agricultural and food Chemistry, 54, 5894–5902.
Kolb, J., and Nyborg, W. (1956). Small-scale acoustic streaming in liquids. Journal of the Acoustical Society of America, 28, 1237–1242.
Leighton, T. G. (1994). The acoustic bubble. London, Academic.
Li, H., Pordesimo, L., and Weiss, J. (2004). High intensity ultrasound-assisted extraction of oil from soybeans. Food Research International, 37, 731–738.
Manasseh, R., Nikolovska, A., Ooi, A., and Yoshida, S. (2004). Anisotropy in the sound field generated by a bubble chain. Journal of Sound and Vibration, 278(4–5), 807–823.
Manasseh, R., Petkovic-Durana, K., Tho, P., Zhu, Y., and Ooi, A. (2005). Acoustic microstreaming applied to batch micromixing. SPIE International Symposium on Microelectronics, MEMS, and Nanotechnology, 11–15 December 2005, Brisbane, Australia.
Manasseh, R., Petkovic-Duran, K., Zhu, Y., and Ooi, A. (2006). Chaotic mixing using acoustic microstreaming for pathology screening diagnostics. 10th International Conference on Miniaturized Systems for Chemistry and Life Sciences, 5–9 November 2006, Tokyo, Japan.
Mason, J., Paniwynyk, L., and Lorimer, P. (1996). The use of ultrasound in food technology. Ultrasonics Sonochemistry, 3, S253–S260.
Minnaert, M. (1933). On musical air bubbles and the sound of running water. Philosophical Magazine, 16, 235–248.
Moholkar, S., Rekveld, S., and Warmoeskerken, G. (2000). Modeling of acoustic pressure fields and the distribution of the cavitation phenomena in a dual frequency sonic processor. Ultrasonics, 38, 666–670
Muralidhara, H. S., Parekh, B. K., and Nagabhusan, S. (1988). Solid-liquid separation process for fine particle suspensions by an electric and ultrasonic field. United States Patent 4,747,920.
Nilsson, M., Johansson, L., Lilliehorn, T., Lindvall, M., Piškur, J., Almqvist, M., Johansson, S., Laurell, T., and Nilsson, J (2005). Acoustic trapping of cells in a microfluidic format. 9th International Conference on Miniaturized Systems for Chemistry and Life Sciences, 9–13 October 2005, Boston, USA.
Prosperetti, A (1977) Thermal effects and damping mechanism in the forced radial oscillations of gas bubbles in liquids. Journal of the Acoustical Society of America, 61(1).
Putterman, S. J. and Weniger, K. R. (2000). Sonoluminescence how bubbles turn sound into light. Annual Review of Fluid Mechanics, 32, 445–476.
Rayleigh (Lord) (1883). On the circulation of air observed in Kundt’s tubes and some allied acoustical problems. Philosophical Transactions of the Royal Society London Series A, 175, 1–21.
Rayleigh (Lord) (1917). On the pressure developed in a liquid during the collapse of a spherical cavity. Philosophical Magazine, 34, 94–98.
Riley, N. (2001). Steady streaming. Annual Review of Fluid Mechanics, 33, 43–65.
Romdhane, M., and Gourdan, C. (2002). Investigation in solid-liquid extraction – influence of ultrasound. Chemical Engineering Journal, 87, 11–19.
Rooney, J. A. (1989). Shear as a mechanism for sonically induced biological effects. Journal of the Acoustical Society of America, 52, 1718–1724.
Shotipruk, A., Kaufman, B., and Wang, Y. (2001). Feasibility study of repeated harvesting of menthol from biologically viable Mentha piperata using ultrasonic extraction. Biotechnology Progress, 17, 924–928.
Spengler, J., Coakley, W. T., and Christensen, K. T. (2003). Microstreaming effects on particle concentration in an ultrasonic standing wave. AIChE Journal, 49(11), 2773–2782.
Spengler, J., and Jekel, M. (2000). Ultrasound conditioning of suspensions – studies of streaming influence on particle aggregation on a lab- and pilot-plant scale. Ultrasonics, 38, 624–628.
Swamy, M., and Narayana, L. (2001). Intensification of leaching process by dual-frequency ultrasound. Ultrasonics Sonochemistry, 8, 341–346.
Tatake, A., and Pandit, B (2002). Modelling and investigation into cavitation dynamics and cavitational yield: Influence of dual-frequency ultrasound sources. Chemical Engineering Science, 57, 4987–4995.
Tho, P., Manasseh, R., Zhu, Y., Metcalfe, G., and Ooi, A (2006) Method for microfluidic mixing and mixing device. Australian Patent Application 2005901760.
Tho, P., Manasseh, R., and Ooi, A. (2007) Cavitation microstreaming patterns in single and multiple bubble systems. Journal of the Fluid Mechanics, 576, 191–233.
Townsend, R. J., Hill, M., Harris, N. R., White, N. M. (2004). Modelling of particle paths passing through an ultrasonic standing wave. Ultrasonics, 42, 319–324.
Ugarte-Romero, E., Feng, H., Martin, E., Cadwallader, R., and Robinson, J. (2006). Innactivation of Echerichia coli in apple cider with power ultrasound. Journal of Food Science, 71, 102–109.
Vinatoru, M. (2001) An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrasonics Sonochemistry, 8, 303–313.
Vokurka, K. (1986) Comparison of Rayleigh’s, Herring’s and Gilmore’s models of gas bubbles. Acustica, 59, 214–219.
Yoshikawa, K., and Kawashima, Y (1955). Acoustic radiation pressure on a compressible sphere. Acustica, 5, 167–173.
Zhang, T., Niu, X., Eckhoff, R., and Feng, H. (2005). Sonication enhanced cornstarch separation. Starch-Starke, 57, 240–245.
Acknowledgments
The authors thank Yonggang Zhu at CSIRO, who investigated aspects of ultrasonic separation and Jenny Zho at CSIRO who investigated the sonication of swollen starch granules.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Vilkhu, K., Manasseh, R., Mawson, R., Ashokkumar, M. (2011). Ultrasonic Recovery and Modification of Food Ingredients. In: Feng, H., Barbosa-Canovas, G., Weiss, J. (eds) Ultrasound Technologies for Food and Bioprocessing. Food Engineering Series. Springer, New York, NY. https://doi.org/10.1007/978-1-4419-7472-3_13
Download citation
DOI: https://doi.org/10.1007/978-1-4419-7472-3_13
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4419-7471-6
Online ISBN: 978-1-4419-7472-3
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)