Abstract
The neurobiology of eating disorders (EDs) is largely unknown. However, brain imaging studies over the past decade have identified neurotransmitter alterations that could be part of dysfunctional behavior characteristics of EDs. In this chapter we focus on a specific behavioral construct, the brain reward system, and demonstrate a functional brain imaging approach toward identifying dopamine function in anorexia nervosa (AN). We demonstrate how human brain reward activation can be used in a translational approach to test whether computer models, based on basic science research, can predict expected in vivo reward system activation, and how such an approach can identify specific biologic alterations in a psychiatric population.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keyword
- Anorexia nervosa
- Brain Imaging
- Dopamine
- Food reward
- Learning signal
- Neurocomputational Modeling
- Neurotransmitter
- Reward processing
- Reward system
- Reinforcement
- Substance use
- Taste
- Temporal Difference Model
1 Introduction
Anorexia nervosa (AN) is a severe psychiatric disorder associated with food avoidance, severe emaciation, and the highest mortality rate among the psychiatric disorders (Sullivan 1995; APA 2000). Clinical evidence suggests that individuals with AN have disturbances in the processing of naturally rewarding stimuli, such as food. The processing of food reward is complex and modulated by cognitive, emotional, and biologic factors that involve learned behaviors and genetic predisposition. In the brain, cortical and subcortical networks integrate external food stimuli with the internal drive and motivation to eat, resulting in behavior activation and approach or avoidance of food. Brain regions implicated in this process include the insula, the basal ganglia (anteroventral and dorsal striatum, nucleus accumbens), the ventral tegmental area (VTA), the anterior cingulate, orbitofrontal, and mesial temporal cortex, as well as the hypothalamus. This network, called the reward system, processes food and other stimuli in order to “achieve an optimal stimulus response” (Cooper et al. 2003). The function of the reward system in patients with AN has been inadequately studied. Clinically, AN subjects are able to refrain from eating over prolonged periods of time and do not respond to mechanisms that would, in most people, stimulate the drive to eat and approach food. Recent studies in AN subjects suggest abnormal brain activity in striatal parts of the basal ganglia that are related to dopamine receptor function (Frank et al. 2005; Wagner et al. 2005). These brain regions have been associated with the processing of immediate and delayed rewards in control subjects, how much reward someone may expect from a stimulus in the future, as well as the amount of motivation a person has to approach a possible reward. In this chapter we describe the use of a model founded on neuroscience that is primarily based on brain dopamine neurotransmission and brain imaging, and we discuss how such an approach can be used to identify pathways directly linked to underlying neurobiologic abnormalities in AN. Specifically, computer models based on basic science research can identify specific neuronal brain function, and results can be used to validate fMRI results and identify disorder-specific abnormalities.
2 Possible Mechanisms Underlying Reward System Abnormalities in AN
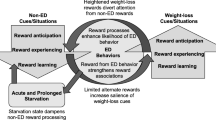
The motivation to eat and approach food is an important part of the reward pathway and is particularly disturbed in AN. AN women like sweet stimuli and do not deny having an appetite (Schweiger and Fichter 1997). Clinically, AN individuals frequently express some desire to eat and gain weight but usually continue to be drawn to the goal of weight loss and food restriction. Moreover, acting on the desire to eat is even more difficult, and thus AN individuals seem unable to direct their behavior and essential motor function toward necessary goals of weight gain. While the AN patient still has the ability to eat, move, experience taste, and even likes sweet stimuli more than controls (Drewnowski et al. 1987), she cannot activate herself sufficiently to eat adequately. This pattern is reminiscent of dopamine-deficient mice (Cannon and Palmiter 2003). These rodents like sweet sucrose or noncaloric artificial sweetener solution more than water, but show more infrequent initiation of drinking compared to controls. They are hypophagic (Zhou and Palmiter 1995) and will die of starvation even with readily available food in their cage, without motor impairment being the cause for the hypophagia. It was hypothesized that these mice have a reduced capacity to respond to rewarding stimuli [“lower basal and maximum response capacity” (Cannon and Bseikri 2004)]. Thus, dopamine-deficient mice may have a reduced motivation or capacity to obtain rewards and may not be able to sufficiently direct their behaviors toward the appropriate goals (Cannon and Bseikri 2004), such as food intake and maintenance of a normal body weight. AN, in fact, has been associated with reduced brain dopamine and abnormal dopamine receptor function in the anteroventral striatum (Kaye et al. 1999; Frank et al. 2005). Many cognitive prefrontal processes, such as fear of weight gain, cannot be addressed in the dopamine-deficient mouse model. However, low anteroventral striatal dopamine-related activity may be associated with less-afferent prefrontal input (Montague et al. 2004) and decreased motivation to approach food rewards. While speculative, it is possible that AN individuals possess a reduced drive to approach food stimuli in conjunction with overvalued ideas and desires for thinness and fear of weight gain. Together, such a constellation may lead to an imbalance of cortical and subcortical drives resulting in reduced food appetence and subsequent weight loss. Another function of the dopamine system is alerting the individual for novel stimuli and initiating learning from those experiences (Schultz 1998, 2002). It appears that the initial phase of food reduction and weight loss is associated with a sense of excitement (Bergh and Sodersten 1996); however, long-term or chronic anorexic subjects do not experience such happiness or excitement but are merely trying to avoid or ameliorate perceived aversive states, such as weight gain or feelings of being fat (APA 2000). This behavior seems to have aspects in common with substance use withdrawal. Koob and Le Moal (Koob and Le Moal 2005) describe a compulsive state that follows the intoxication of a drug addict characterized by withdrawal and negative affect, emotional pain, alexithymia, and dysphoria. Instead of the positive rewards experienced during the initial phase of drug use, plastic changes in the reward pathways create an antireward system that “drives aversive states” (Koob and Le Moal 2005). This negative antireward system has been associated with altered dopaminergic states. AN and drug users may lie on the opposite ends of the spectrum with increased dopamine receptor binding in AN but reduced dopamine receptors in the substance users (Volkow et al. 2004). AN subjects are chronically dysphoric, have difficulties expressing their emotions, and cannot enjoy things as much as others (Anderluh et al. 2003), and we believe that AN subjects might be stuck in an antireward system with reduced dopamine function, whereas such a state is only temporary in drug users before they self-medicate with another hit or drink. Interestingly, alcoholism (Kampov-Polevoy et al. 2003) and benzodiazepine use (Yasoshima and Yamamoto 2005) have also been associated with altered sweet taste response. In summary, there may be a combination of dopamine abnormalities, either trait or state related, and psychologic factors that control eating behavior and influence the motivational system.
3 Taste and Reward System Activation Research in AN
Taste by itself is a reward system activator, at least in part through dopamine activation in the brain. Taste receptors on the tongue project to the brain stem nucleus of the solitary tract (NST) and from there to the parabrachial nucleus (PBN). Then the taste pathway bifurcates to a thalamic/cortical tract and the hypothalamic/limbic pathway with projections to the amygdala and nucleus accumbens (Norgren et al. 2006; Hajnal et al. 2009). Thus, taste directly stimulates the dopamine pathway. Taste paradigms in AN can be applied as long as disorder-specific mechanisms are taken into account. Sweet taste perception is overall preserved in AN, but the hedonic experience of sweet taste is particularly biased by the fear of weight gain. Several studies have investigated taste perception in AN in the past. Some found in AN subjects reduced (Toth et al. 2004) or altered (Drewnowski et al. 1987) taste sensitivity while ill and after refeeding. Others found no disturbance in the ability to rate sweet perception (Di Costanzo et al. 1998) but found altered hedonic ratings compared to controls, where AN preferred sweeter stimuli (Sunday and Halmi 1990). Another group did not find such abnormalities (Simon et al. 1993). Exercising is a possible confound of hedonic taste perception studies as well, since more than 3 h of activity per week decreased preference ratings for high-sucrose and high-fat stimuli in controls (Crystal et al. 1995). This is important since women with AN frequently exercise. Hedonic taste response is heavily biased by the perception of calorie intake and the possibility of weight gain as a deterrent to enjoy food. In fact, one study found that sweet taste pleasantness ratings were preserved in a paradigm where AN did not swallow but spit out the sweet (sugar) taste stimulus (Eiber et al. 2002). In the same paradigm, hedonic ratings were reduced when subjects had to swallow the sugar stimulus, suggesting strong cognitive bias toward the high-caloric fluid. Taken together, there is limited evidence for taste perception alterations per se in AN, although the studies performed are few with small sample sizes. Potentially more important, the hedonic rating results tend to be biased by an overriding fear of weight gain.
In summary, taste response can be used in AN to test reward system abnormalities. However, the cognitive and emotional activation specific to AN has to be taken into account in the model.
4 Reward Pathway Function and Neurobiology
The ideal function of the brain reward system is to ensure that we make the best choices in response to environmental stimuli (Cooper et al. 2003) in order to support our long- and short-term goals. Natural rewarding food-related stimuli have been studied extensively (Saper et al. 2002). A large number of studies have also examined recreational drugs, alcohol, monetary reward, and other potentially rewarding stimuli (Martin-Soelch et al. 2001; Grigson 2002). The mechanisms of reward processing in the brain, though, are shared across stimuli (Grigson 2002). The main psychological components of this system are motivation, learning, and emotion or affect (Berridge and Robinson 2003). One line of research distinguished conscious “liking,” relating to an emotional response to stimuli, from “wanting” which relates to intrinsic motivation reflective of how much an individual is willing to work for a reward. For the concept of wanting, the term “incentive salience” of stimuli has been proposed (Berridge 1996; Berridge and Robinson 1998). An underlying principle of reward processing seems to be “the anticipation of outcomes of behavior in situations with varying degrees of uncertainty” (Schultz 2006). That means we frequently have to make choices in an environment with various types of stimuli with respect to which of those stimuli might fit best our desires and needs. A stimulus can have several rewarding effects for an organism (Wise 2002). The brain tries to assess and anticipate the outcome obtained from a stimulus, weighing between available stimuli in order to determine which should be approached based on the highest reward value. While there are genetically determined contingencies of reward, for instance the innate liking of sweet taste (Bartoshuk and Beauchamp 1994), learning, and conditioning, reward-predicting experiences are important for the development of personal preference and reward system activation (Schultz 2006). From our exposure to stimuli and positive or negative reward experience, we learn what to approach and what to avoid. While we develop representations in the brain of what we like and what we do not, new experiences update these representations continuously based on their reward value.
Various neurotransmitters and regions in the brain have been found to process rewarding stimuli. Corticostriatal–hypothalamic pathways have been implicated in the receipt of sensory stimuli and subsequent processing of those stimuli’s reward value, followed by behavioral activation in order to approach or avoid stimuli (Kelley et al. 2005). Dopamine pathways, mainly in the VTA, striatum, and nucleus accumbens, extending to the orbitofrontal cortex, may be involved in the approach and motivational aspects of related behavior (Kelley et al. 2005; Schultz 2006). Thus, dopamine activation may be associated with the “wanting” concept described by Berridge (Berridge and Robinson 2003). Opioid neurotransmission is localized closely to dopaminergic neurons in the ventral and dorsal striatum. Opioid activation in these areas, regulated by cholinergic interneurons, may mediate the pleasure that is associated with stimuli, and GABA neurotransmission into the anteroventral striatum may prevent excessive response to rewarding stimuli (Kelley et al. 2005). In addition, there appears to be a fine balance of opioid receptor-driven activation of dopamine and GABA neuronal activation of the reward system via glutamatergic neurons (Mansvelder 2005; O’Reilly et al. 2007).
The prefrontal cortex exerts voluntary control over behavior (Price 2005) and is an important input for the reward system (Wise 2005) in terms of deliberate control and bias toward behavior. It appears that afferent phasic dopamine input to the prefrontal cortex may be needed in order to modulate goals based on rewarding experiences (Montague et al. 2004). These dopamine pathways are influenced by various factors. Food restriction may increase dopamine receptor binding in the nucleus accumbens and caudate/putamen (Carr et al. 2003), and dopamine D2 receptors may regulate dopamine release (Cooper et al. 2003). Alterations in this mechanism may be particularly involved in substance use but also food-related disorders (Blum et al. 1995). In addition, nonpredictability is an important determinant for the activation of the anteroventral striatum dopamine system since unexpected stimuli elicit a greater response in functional dopamine transmission (Berns et al. 2001). Furthermore, serotonin pathways have been hypothesized to alter the mediation of brain reward response (Higgins and Fletcher 2003) with, for instance, serotonin 2C receptor activation attenuating drug-seeking behavior.
5 Reward Brain Imaging Studies and Anorexia Nervosa
Elements of the brain reward system have been identified through anatomic and electrophysiologic studies in nonhuman primates and neuroimaging studies in humans. Single-cell firing rates within the basal ganglia, VTA, nucleus accumbens – a part of the ventral striatum, amygdala, orbital–frontal and prefrontal cortex – are modulated by rewarding stimuli (Apicella et al. 1991; Hikosaka and Watanabe 2000; Schultz et al. 2000; Wise 2002). Neuroimaging studies using a variety of reward stimuli have corroborated the electrophysiology data. In particular, studies using monetary rewards have revealed patterns of reward-related activity in the amygdala, orbital–frontal and mesial prefrontal cortex, cingulate cortex, and portions of the striatum to which these limbic regions project (Delgado et al. 2000, 2003; Knutson et al. 2000; Breiter et al. 2001; May et al. 2004). The ventral striatum is involved in reward processing per se, while the dorsal striatum is involved in linking action to outcome (O’Doherty et al. 2004; Tricomi et al. 2004). Like the ventral striatum, more dorsal sectors of the striatum are also modulated by dopamine. However, these regions are also implicated in motor and cognitive control, specifically the learning of stimulus response associations. The caudate nucleus is activated by those tasks in which there exist both a perceived connection between action and outcome and some uncertainty about whether the action will lead to the desired outcome (Tricomi et al. 2004). O’Doherty and colleagues (O’Doherty et al. 2004) postulated that projections to the ventral striatum might be involved in reward prediction (“critic”) and instrumental learning, whereas projections to dorsal striatum (“actor”) might be involved in the modulation of stimulus response in order to choose the best behavioral response based on previous experience. Most recently, immediately rewarding processes have been related to ventral striatal activation, whereas delayed gratification may activate dorsal striatal brain response (Tanaka et al. 2004). This is important since, phenotypically, AN have a very high degree of self-control and are able to delay gratification in various aspects of life. Aside from delaying eating, they are extremely goal oriented and perfectionistic, they tend to work harder than peers, and they seem to prefer delaying reward over not meeting their immediate goals and standards. Abnormalities in the anteroventral and dorsal striatal reward system in AN could contribute to food restraint and the ability to refrain from commonly desired pleasurable experiences for long periods of time. Taken together, brain imaging in human controls show that testing reward pathway activation in vivo is possible, and that results obtained are consistent with the animal literature and single neuron-recording studies. It is debated whether brain imaging such as fMRI can identify pathways directly related to neurotransmitter function. Recent data provide evidence suggesting that this is in fact the case (see limitations).
Very few studies exist that specifically assessed reward system brain activation in AN. One study suggested dopamine abnormalities in AN after recovery (Frank et al. 2005), while functional magnetic resonance imaging studies in recovered AN also showed abnormalities in response to a monetary gain task (Wagner et al. 2007). Ill AN were recently shown to have increased ventral striatal response to pictures of underweight bodies. Taken together, the few studies available suggest reward system abnormalities in AN, but the neurobiologic underpinnings remain largely unknown.
6 Neurocomputational Modeling and Brain Imaging
The integration of animal models with human in vivo studies remains a crucial step in translational research. Computer models should be able to predict human behavior (based on the basic research findings) using specific tasks that apply the animal model in the human. That is, the computer model is used to test whether the brain imaging results gathered during the task application indeed reflect the brain activation obtained in the previously validated basic research. If this is the case, then one can make actual inferences on human neurobiologic brain processes related to disease.
6.1 Principals of the Temporal Difference Model
Behavioral models have been developed that mathematically describe aspects of reward under conditions that can be related to brain imaging results. This sets the stage for “Hypothesis-driven research” that “has been shown to be an excellent model for pursuing investigations in neuroscience” (Koslow 2005). Such neurocomputational models link psychological theories with underlying neural substrates. In this chapter, we describe the application of the temporal difference (TD) model (Schultz 2006) and will provide preliminary data.
Various models describe concepts of reinforcement learning (Sutton and Barto 1981), which both concerns the anticipatory control of actions and prediction of potential reward values (Worgotter and Porr 2005). The TD model has been intensively studied and applied to the neural reward system and is a valid model for studying reinforcement learning and dopamine neuronal pathway activity in the basal ganglia. It has been studied in vivo in relation to dopamine system activation in rodents using single-neuron recordings (Schultz 1998, 2002; Schultz et al. 2000) as well as in humans using brain imaging techniques (O’Doherty et al. 2003, 2004; Tanaka et al. 2004). This model (Sutton and Barto 1981; Worgotter and Porr 2005) integrates elements of the individual “value” of a reward and the prediction of its rewarding property in the future. Since rewards do not always occur and are not entirely predictable, a temporal difference error is introduced in the model that functions as a “learning signal.” This error is dependent on whether the predicted reward is consistent with the received reward or if there is a discrepancy between prediction and reality (error). Another element in the model is the discount factor, which takes into account how many times a reward has been received and how many times the subject has had the opportunity to learn from experience to derive predictions.
6.2 Temporal Difference Model Study Design
The study design that we use was originally described by O’Doherty et al. (2003). Each trial consists of the presentation of one of three arbitrary visual stimuli (see Fig. 1) followed 2 s later by 1 ml of a pleasant sweet taste (1 M sucrose) (CS+), a neutral tasteless solution [CSneut: 25 mM KCl, 2 mM NaHCO3, (Francis et al. 1999; Frank et al. 2003)], or no taste (CS−). The visual stimuli are presented on a gray background and are removed from the screen after 2 s to coincide with taste delivery. A fixation cross is then presented for the remainder of the trial (4 s). The allocation of each stimulus to a given trial type is counterbalanced across subjects. There is a total of 280 trials in the experiment, 100 each of CS+ and CS− and 80 of CSneut. The whole experiment lasts a total of ~28.5 min. The first ten CS+ stimuli presented are paired on each occasion with reward (sweet taste), in 20 out of 90 subsequent CS+ presentations, the reward is omitted (CS+ omit). Further, in 20 presentations of the CS−, a reward is unexpectedly delivered (CS-unexpreward). The CSneut condition is primarily included to provide a low valence rinse for the glucose taste during the experiment. The presentation order is randomized and delivered to subjects using E-Prime (Psychology Software Tools, Pittsburgh, PA).
Schematic tasks application, modeled after O’Doherty et al. (2003). Subjects learned to associate a specific fractal with a particular taste. In 20% of cases, there was a mismatch of fractal and expected taste (“Sucrose fractal” but no taste delivery; “Nothing fractal” but Sucrose delivery). A total of 280 trials are applied; every 2.1 s a brain scan is acquired
6.3 Data Analysis
Image processing is done with the SPM 5 software package (http://www.fil.ion.ucl.ac.uk/spm/doc/spmbib.html). We performed whole brain analyses and also used region of interest-based analyses that include the VTA, ventral striatum (VS), amygdala, orbitofrontal cortex, and cingulate cortex for percent signal change extraction. There is a primary and a secondary analysis pathway in the paradigm. The primary analysis pathway identifies brain region activation in response to the conditioned fractal or unconditioned sweet taste application as well as brain response when taste is expected by not received. Main effect results are obtained for condition and subject group, as well as time activity curve data that illustrate brain activation over time in response to the individual stimulus.
The secondary analysis pathway involves the mathematical models that try to bridge brain function and actual behavior. As described earlier, computational modeling tries to explain a real-world problem in a simplified model that then gets translated into a mathematical equation that can be used to correlate with brain imaging results. This serves the purpose of predicting brain response following a stimulus, and depending on factors such as learning rate and received reward, different brain regions are implicated. That is, the subject performs a specific task or Reward Paradigm and learns under which conditions reward occurs. The time course of reward experience is known by the experimenter. In the taste experiment, reward delivery or omission is part of the paradigm and is recorded by the stimulus-applying computer. Those reward receipt or loss experiences are assigned a reward value in the computational model, and those reward values are then correlated with the functional magnetic resonance imaging brain response.
7 Use of Neurocomputational Models and Testing of Alternate Models (Fig. 2)
On each trial, the predicted value (V) at any time (t) within a trial is calculated as a linear product of the weights (w i ) and the presence or absence of a CS stimulus at time t, coded in the stimulus representation vector x i (t):
Principals of the Neurocomputational Modeling Approach. A study participant in the functional magnetic resonance brain imaging (fMRI) scanner performs the taste task. The blood oxygen level-dependent (BOLD) brain response is measured. The fist brain activation analysis tests in the whole brain activations that relate to the schedule of reward application and omission, reward expectation, etc. In the second analysis pathway, we use the individual reward task schedule data for each participant and import those data into a computer algorithm that computes the expected brain response based on the animal literature. Those outcomes are then regressed with the human in vivo data in order to (1) test whether significant regression results indeed occur in the expected brain regions and (2) make group comparisons whether controls have a stronger or weaker activation pattern to a particular learning rate than the anorexia nervosa group
Learning occurs by updating the predicted value of each time point t in the trial by comparing the value at time t + 1 to that at time t, leading to a prediction error or δ(t):
where r(t) = reward at time t. The parameter γ is a discount factor, which determines the extent to which rewards arriving earlier are more important than rewards that arrive later on. Similar to O’Doherty’s study (O’Doherty et al. 2003), we set γ = 0.99. The weights w i are then updated on a trial-by-trial basis according to the correlation between prediction error and the stimulus representation:
where α = learning rate. We assign six time points to each trial and use each subject’s individual event history as input. On each trial, the CS (visual fractal) is delivered at time point 1, and the UCS reward (sweet taste stimulus) is delivered at time point 3. Like O’Doherty (O’Doherty et al. 2003), we use as learning rate of the model (α) two values for α: a lower learning rate (α = 0.2) and a higher learning rate (α = 0.7). In the original study, the lower learning rate best modeled brain activation in the ventral striatum and orbitofrontal cortex. The temporal difference error δ(t) is created for the CS and the positive and negative UCS for each time point, and an ideal activation curve is created and convolved with the hemodynamic response curve. That curve is then modeled to the functional magnetic resonance imaging data in order to generate brain regions that respond according to the model. We cannot predict whether restricting-type anorexia nervosa women have simply reduced brain response to the stimuli or if a different learning rate explains altered learning response. This is an empirical task during data analysis. Reward prediction error: The TD error is calculated from the difference between the actual reward r(t) and the temporal difference of the estimated value function V(t). We vary the discount factor γ as 0, 0.3, 0.6, 0.8, 0.9, and 0.99: small γ for immediate reward prediction and large γ for long future reward prediction. We then use either V(t) or δ(t) as the explanatory variable in a regression analysis in the SPM brain imaging analysis program for correlation with brain activity.
8 Preliminary Results
We recruited 15 ill AN and 17 CW for this study. Both groups were similar in age, but AN individuals had higher scores on the Eating Disorders Inventory 3, Beck Depression Inventory, and lower body mass indices (BMI; data not shown). Between-group pleasantness and sweetness ratings were similar for both the Sucrose and Artificial Saliva Solutions.
Within groups, the unexpected receipt or omission of the sucrose taste stimulus activated dopamine-related brain regions such as the striatum, VTA, insula, and cingulate cortex. The whole brain group comparison indicated that unexpected receipt of the sweet taste stimulus activated brain dopamine regions more in AN than in controls, and unexpected omission of that stimulus caused more of a negative response in AN compared to the CW. These results suggest that brain taste-reward pathways linked to central dopamine transmission are functional but are overactive in ill AN compared to CW when receiving the stimulus unexpectedly, or during the omission of the taste. This response may thus suggest an increased temporal difference response in AN compared to CW. Such an over-responsiveness is consistent with clinical observations in EDs of heightened fear to novel or unexpected food stimuli, which results in stimulus avoidance and behavioral inflexibility.
In support of our biologic finding, a recent report showed that ill AN are hyper-sensitive to both rewarding as well as punishing stimuli (Jappe et al. 2010).
Furthermore, we imported the subject-specific reward schedule data (reward schedule during the brain imaging procedures) into a computer simulation and generated preliminary data for the VTA and AVS. As expected, the regression of the outcome activation data regressed in both CW and AN individuals significantly not only with VTA and AVS but also the insula and parts of the orbitofrontal cortex. This suggests that this dopamine-proposed mechanism involves other higher order function brain regions.
9 Limitations of the Model
The use of model-based fMRI is still in its infancy in psychiatry and there is still a lot to be learned. Several questions are still debated. The exact relationship between blood oxygen level dependent (BOLD) and brain function is not clear, such as the relationship of BOLD to action or response for instance. Evidence suggests that BOLD may reflect local processing of incoming stimuli rather than neuronal outflow to other brain areas (Logothetis 2002). Another key issue here is whether the BOLD fMRI does, in fact, reflect the proposed dopamine brain activation. The TD model used in our studies is dopamine based (Schultz 2002) and O’Doherty adapted the model to the fMRI environment (O’Doherty et al. 2003). Another carefully designed study showed that the VTA also can reflect dopamine activation when using the appropriate tasks (D’Ardenne et al. 2008). While results suggest dopamine is responsible for the BOLD response, the mechanism for such a relationship is not clear. Recent studies, however, indicate that BOLD fMRI in fact does reflect dopamine activation. Knutson for instance proposed that dopamine nucleus accumbens activation would stimulate dopamine D1 receptors and activate the BOLD signal via membrane potential changes (Knutson and Gibbs 2007). In summary, while there is still uncertainty about the exact mechanisms that determine BOLD response, increasing evidence indicates that DA function directly relates to BOLD fMRI response.
Another issue with our underlying dopamine concept is the number of brain regions involved in the model. The classic TD model focuses on the VTA and AVS and does not take other areas into account. This is a rather reductionistic model considering that emotional and cognitive processes, like learning and associations, impact the reward system activation. In response, O’Reilly created an extension to that model that takes into account the amygdala, hypothalamus, and orbitofrontal cortex (O’Reilly et al. 2007; Hazy et al. 2010). We are currently adapting computer models such as the Pavlovian Primary Reward/learned Reward model (O’Reilly et al. 2007; Hazy et al. 2010) to our imaging studies.
10 Future Outlook
We are still at the beginning of this line of research. However, results are encouraging and support the notion that we can actually study neurotransmitter-related brain pathways and identify patterns that are disorder related. This is an important step forward in the use of brain imaging, linking specific neurobiology in a psychiatric population. Such neurobiologic alterations could be used to improve treatment. For instance, making treatment very predictable may help ameliorate the dopamine hyper-responsiveness in AN, and we might be able to target specific problem behaviors with medication moderating dopamine activity.
References
Anderluh MB, Tchanturia K et al (2003) Childhood obsessive–compulsive personality traits in adult women with eating disorders: defining a broader eating disorder phenotype. Am J Psychiatry 160(2):242–247
APA (2000) Diagnostic & statistical manual of mental disorders: DSM-IV-TR. American Psychiatric Association
Apicella P, Ljungberg T et al (1991) Responses to reward in, monkey dorsal and ventral striatum. Exp Brain Res 85(3):491–500
Bartoshuk LM, Beauchamp GK (1994) Chemical senses. Annu Rev Psychol 45:419–449
Bergh C, Sodersten P (1996) Anorexia nervosa, self-starvation and the reward of stress. Nat Med 2(1):21–22
Berns G, McClure S et al (2001) Predictability modulates human brain response to reward. J Neurosci 21(8):2793–2798
Berridge KC (1996) Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev 20(1):1–25
Berridge KC, Robinson TE (1998) What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev 28(3):309–369
Berridge KC, Robinson T (2003) Parsing reward. Trends Neurosci 26(9):507–513
Blum K, Sheridan PJ et al (1995) Dopamine D2 receptor gene variants: association and linkage studies in impulsive–addictive–compulsive behaviour. Pharmacogenetics 5(3):121–141
Breiter HC, Aharon I et al (2001) Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron 30(2):619–639
Cannon C, Bseikri M (2004) Is dopamine required for natural reward? Physiol Behav 81(5):741–748
Cannon CM, Palmiter RD (2003) Reward without dopamine. J Neurosci 23(34):10827–10831
Carr K, Tsimberg Y et al (2003) Evidence of increased dopamine receptor signaling in food-restricted rats. Neuroscience 119:1157–1167
Cooper J, Bloom F et al (2003) The biochemical basis of neuropharmacology. Oxford University Press, Oxford
Crystal S, Frye CA et al (1995) Taste preferences and sensory perceptions in female varsity swimmers. Appetite 24(1):25–36
D’Ardenne K, McClure SM et al (2008) BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science 319(5867):1264–1267
Delgado MR, Nystrom LE et al (2000) Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol 84:3072–3077
Delgado MR, Locke HM et al (2003) Dorsal striatum responses to reward and punishment: effects of valence and magnitude manipulations. Cogn Affect Behav Neurosci 3(1):27–38
Di Costanzo V, Rodde G et al (1998) Food preferences in anorectic girls at the beginning of therapy. Diabetes Metab 24(3):262–271
Drewnowski A, Halmi KA et al (1987) Taste and eating disorders. Am J Clin Nutr 46(3):442–450
Eiber R, Berlin I et al (2002) Hedonic response to sucrose solutions and the fear of weight gain in patients with eating disorders. Psychiatry Res 113:173–180
Francis S, Rolls ET et al (1999) The representation of pleasant touch in the brain and its relationship with taste and olfactory areas. Neuroreport 10(3):453–459
Frank G, Kaye W et al (2003) The evaluation of brain activity in response to taste stimuli – a pilot study and method for central taste activation as assessed by event related fMRI. J Neurosci Methods 131(1–2):99–105
Frank G, Bailer UF et al (2005) Increased dopamine D2/D3 receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [11C]raclopride. Biol Psychiatry 58(11):908–912
Grigson PS (2002) Like drugs for chocolate: separate rewards modulated by common mechanisms? Physiol Behav 76:389–395
Hajnal A, Norgren R et al (2009) Parabrachial coding of sapid sucrose: relevance to reward and obesity. Ann N Y Acad Sci 1170:347–364
Hazy TE, Frank MJ et al (2010) Neural mechanisms of acquired phasic dopamine responses in learning. Neurosci Biobehav Rev 34:701–720
Higgins GA, Fletcher PJ (2003) Serotonin and drug reward: focus on 5-HT2C receptors. Eur J Pharmacol 480(1–3):151–162
Hikosaka K, Watanabe M (2000) Delay activity of orbital and lateral prefrontal neurons of the monkey varying with different rewards. Cereb Cortex 10(3):263–271
Jappe LM, Frank GKW et al (2010) Heightened sensitivity to reward and punishment in anorexia nervosa. International Journal of Eating Disorders (Article first published online: 28 JUN)
Kampov-Polevoy AB, Ziedonis D et al (2003) Association between sweet preference and paternal history of alcoholism in psychiatric and substance abuse patients. Alcohol Clin Exp Res 27(12):1929–1936
Kaye WH, Frank GK et al (1999) Altered dopamine activity after recovery from restricting-type anorexia nervosa. Neuropsychopharmacology 21(4):503–506
Kelley AE, Baldo BA et al (2005) Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav 86(5):773–795
Knutson B, Gibbs SE (2007) Linking nucleus accumbens dopamine and blood oxygenation. Psychopharmacology (Berl) 191(3):813–822
Knutson B, Westdorp A et al (2000) FMRI visualisation of brain activity during a monetary incentive dealy task. Neuroimage 12:20–27
Koob GF, Le Moal M (2005) Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci 8(11):1442–1444
Koslow SH (2005) Discovery and integrative neuroscience. Clin EEG Neurosci 36(2):55–63
Logothetis NK (2002) The neural basis of the blood-oxygen-level-dependent functional magnetic resonance imaging signal. Philos Trans R Soc Lond B Biol Sci 357(1424):1003–1037
Mansvelder HD (2005) Yin and yang of VTA opioid signaling. Focus on “both kappa and mu opioid agonists inhibit glutamatergic input to ventral tegmental area neurons”. J Neurophysiol 93(6):3046–3047
Martin-Soelch C, Leenders KL et al (2001) Reward mechanisms in the brain and their role in dependence: evidence from neurophysiological and neuroimaging studies. Brain Res Brain Res Rev 36(2–3):139–149
May JC, Delgado MR et al (2004) Event-related functional magnetic resonance imaging of reward-related brain circuity in children and adolescents. Biol Psychiatry 55:359–366
Montague R, Hyman S et al (2004) Computational roles for dopamine in behavioural control. Nature 431:760–767
Norgren R, Hajnal A et al (2006) Gustatory reward and the nucleus accumbens. Physiol Behav 89(4):531–535
O’Doherty JP, Dayan P et al (2003) Temporal difference models and reward-related learning in the human brain. Neuron 38(2):329–337
O’Doherty J, Dayan P et al (2004) Dissocaible roles of ventral and dorsal striatum in instrumental conditioning. Science 304:452–454
O’Reilly RC, Frank MJ et al (2007) PVLV: the primary value and learned value Pavlovian learning algorithm. Behav Neurosci 121(1):31–49
Price JL (2005) Free will versus survival: brain systems that underlie intrinsic constraints on behavior. J Comp Neurol 493(1):132–139
Saper CB, Chou TC et al (2002) The need to feed: homeostatic and hedonic control of eating. Neuron 36(2):199–211
Schultz W (1998) Predictive reward signal of dopamine neurons. J Neurophysiol 80(1):1–27
Schultz W (2002) Getting formal with dopamine and reward. Neuron 36(2):241–263
Schultz W (2006) Behavioral theories and the neurophysiology of reward. Annu Rev Psychol 57:87–115
Schultz W, Tremblay L et al (2000) Reward processing in primate orbitofrontal cortex and basal ganglia. Cerebral Cortex 10(3):272–284
Schweiger U, Fichter M (1997) Eating disorders: clinical presentation, classification and etiologic models. In: Jimerson DC, Kaye WH (eds) Balliere’s clinical psychiatry. Balliere’s Tindall, London, pp 199–216
Simon Y, Bellisle F et al (1993) Taste responsiveness in anorexia nervosa. Br J Psychiatry 162:244–246
Sullivan PF (1995) Mortality in anorexia nervosa. Am J Psychiatry 152(7):1073–1074
Sunday SR, Halmi KA (1990) Taste perceptions and hedonics in eating disorders. Physiol Behav 48(5):587–594
Sutton RS, Barto AG (1981) Toward a modern theory of adaptive networks: expectation and prediction. Psychol Rev 88(2):135–170
Tanaka SC, Doya K et al (2004) Prediction of immediate and future rewards differentially recruits cortico-basal ganglia loops. Nat Neurosci 7(8):887–893
Toth E, Kondakor I et al (2004) Nonlinear and linear EEG complexity changes caused by gustatory stimuli in anorexia nervosa. Int J Psychophysiol 51(3):253–260
Tricomi EM, Delgado MR et al (2004) Modulation of caudate activity by action contingency. Neuron 41:281–292
Volkow ND, Fowler JS et al (2004) Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry 9(6):557–569
Wagner A, May C et al (2005) Reward-related neural responses in anorexia and bulimia nervosa after recovery using functional magnetic resonance imaging. Biol Psychiatry 57(S7):709
Wagner A, Aizenstein H et al (2007) Altered reward processing in women recovered from anorexia nervosa. Am J Psychiatry 164(12):1842–1849
Wise RA (2002) Brain reward circuitry: insights from unsensed incentives. Neuron 36(2):229–240
Wise RA (2005) Forebrain substrates of reward and motivation. J Comp Neurol 493(1):115–121
Worgotter F, Porr B (2005) Temporal sequence learning, prediction, and control: a review of different models and their relation to biological mechanisms. Neural Comput 17(2):245–319
Yasoshima Y, Yamamoto T (2005) Effects of midazolam on the expression of conditioned taste aversion in rats. Brain Res 1043(1–2):115–123
Zhou QY, Palmiter RD (1995) Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell 83(7):1197–1209
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Frank, G.K.W. (2010). Reward and Neurocomputational Processes. In: Adan, R., Kaye, W. (eds) Behavioral Neurobiology of Eating Disorders. Current Topics in Behavioral Neurosciences, vol 6. Springer, Berlin, Heidelberg. https://doi.org/10.1007/7854_2010_81
Download citation
DOI: https://doi.org/10.1007/7854_2010_81
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-15130-9
Online ISBN: 978-3-642-15131-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)