Abstract
Natural resources are continuously depleted globally, and accelerated climate change is a consequence of irresponsible human action. Planetary resources have to be better utilized not to threaten living ecosystems, the biodiversity and cause further land degradation. New nature-based and cost-effective materials are appearing for remediation purposes but need continued development since they require extra knowledge about structure-function relations. Within emerging circular economy new waste streams are detected which can serve as substrate for new valuable and smart materials and at the same time provide energy and even carbon removal. Biomass has been generated from both agriculture and forestry but lately also municipal solid waste has been recognized as resource in waste valorization. Waste can be converted to new products by hydrothermal processes that yield hydrochar and thermal pyrolysis processes to produce biochar; a multiuse carbon material. Ideal waste utilization processes have good energy yield at the same time as new materials are formed. Carbon removal can become a part of environmental societal solutions dealing with sustainable waste processing and application of new value-added products coming from development of new smart materials. Carbon removal efforts are currently supported through the voluntary market and the total value of global carbon markets grew by over 20% in 2020 – the fourth consecutive year of record growth. This chapter displays different waste streams and their suitability for thermal treatments to produce hydrochar or biochar for understanding of how the choice of feedstock together with optimization of thermal process parameters will give best smart products.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The rise of standard of living globally is virtuous but it’s always connected to increased consumption and demand for consumer goods followed by increased generation of municipal solid waste (MSW) [1]. Several competing technologies are available for treating this waste and their sustainability is becoming a prominent factor. In addition to ecological and societal sustainability technologies need to be economically feasible to be applied on larger scale. One important quality criterion for waste treatment is suitability for energy and fuel production. In Europe the main renewable energy source is wood, which represents over 60% of all non-conventional energy used in the EU-27 [2]. Lignocellulosic woody biomass constitutes arborous forestry residues and residues from the wood processing industry. The activated sludge process produces solids that have mostly been considered as a waste without any good reuse and sewage sludge from biogas reactors has created challenges for reuse [3]. Sludge is, however, a suitable biomass for fuel production. The advantage with the hydrothermal process (HGT) is that the biomass does not need drying pretreatment and requires less energy. The yield of solid product, hydrochar, is greater in lower temperature pyrolysis (200–300°C) [4].
From the prospect of climate change, there is a great demand for swift and efficient methods to capture and sequester carbon away from the atmosphere. It was very recently reported that production, use and storage of biochar are carbon negative, and if applied into practice an estimated sequestration of 0.3–2 Gt CO2 year−1 by 2050 could be achieved [5]. The most relevant technologies offering carbon removal from atmosphere are forestation, direct air carbon capture with utilization and storage, carbon sequestration into soil, and wooden building elements for biochar production. The carbon fees on the voluntary carbon markets range from 12 to 1,045 European euro per ton CO2 [5]. These carbon removal services by means of biochar are currently offered through full-bodied marketplaces that require wide-ranging certification, verification, and monitoring to add credibility and authenticity. Simultaneously biochar production is hopefully improving with increasing knowledge on feedstock usability, pyrolysis and in future more tailored applications to show that the biochar system is realistic to be applied at large scale [6].
Just as there are new usable waste streams, so are their potential uses that require more studies to become effective in environmental remediation, which is an actual field application of biochars [7]. Biochar production from lightly contaminated waste timber (WT) has been coined as a promising waste handling option for valorization of such residues into biochar sorbents that can be used for contaminant stabilization [8]. A challenge with wood waste is the presence of trace environmental pollutants that threaten the sustainable recycling of this waste. Impurities comprise adhesives, paints, trace metals fire retardants, waxes and plastics. Very recent studies have proposed thermochemical treatment of wood waste like gasification and pyrolysis that can give different new products, but also energy. Polycyclic aromatic hydrocarbons (PAHs), volatile organic compounds (VOCs), and trace metals are formed and/or emitted during thermochemical conversion. Their formation depends on both the operating conditions and the type of feedstock used. These pollutants may also appear in the resulting biochar [9, 10].
2 Biowaste Streams for Thermal Treatment
2.1 Composition of Agroforestry Waste (AFWs)
Timber logging generates large amounts of forestry waste residue. The global forests cover 4 billion ha, which is almost 30% of total land area, and on average 0.62 ha/capita [11]. Around 50% of this forest area is in developing countries [12]. The forest residue is typically stumps, branches, and leaves, and wood processing waste in form of logs and sawdust. The recovery of different residues depends on geography and related conditions, like type of tree species. For every cubic meter of logged material removed from the forest it has been estimated that a cubic meter of waste remains in the forest. The types of processing waste are bark removal and branch trimming (about 12% of this material arrives at mill facilities, slabs/blocks/further trimmings (about 34%) and sawdust constitutes about 12%. Waste comes also from kiln drying, shavings (about 6%), and sawdust/trimming (about 2%). On the scale woody biomass contributes around 4.6 Gt annually, from which 60% is used for energy, 20% is used as industrial “round wood,” and the remaining 20% is in the primary production pool remaining in the forest where it decays. A surprisingly large part, ~80% of forest tree mass is then lost as waste, and from that wood about 20% ends up in kiln-dried sawn product [13].
Agricultural biomass wastes and residues are mainly crop stalks, leaves, roots, fruit peels, and seed/nut shells. These residues are mainly discarded or burned although they are valuable supplies of feedstock material [13]. It imposes challenges to estimate the degree of produced crop biomass in relation to what is the “loss” in production, including harvesting and processing, and also in relation to what is considered as “waste” that again entails retail or consumer loss. The production of “food” seems to be measured as the edible parts of a crop (harvest index), which again is not taking into account non-edible biomass parts, that are crops or not. One example to stress this point is sugarcane that requires processing generating waste streams in addition to the primary biomass waste in harvesting.
Based on Food and Agricultural Organization [14] estimations, Russia, Indonesia, USA, Brazil, and China produce most AFWs and industrial wood wastes. The potential production of residues could be more than 700 Mt./p.a. This large loss is a resource that could be used as fuel source. Typically, in the developing countries they are main household fuel and major energy source as part of industrial energy consumption [15]. The composition of AFW greatly influences the performance of AFW conversion system. In developing countries, most of the biomass residues are left in the field to decompose or alternatively burned on the spot, resulting in major environmental impacts. In the urbanization process the demand for products increase and alternative sustainable energy sources and raw material supplies are in need. So far, the biomass wastes are not efficiently taken into reuse as material and source of energy. Even less activity has been devoted to develop “low-carbon” solutions for valorization.
Forest residue amounts are defined as 46% of total forest stock [16]. Globally Russian Federation, Indonesia, USA, and Brazil produce most the forest residue, 5,718, 2,221, 2078 and 1,613 million tons (Mt), respectively. China, Sweden, France, and Finland are the next largest producers of forest residues of which Sweden and Finland represent Nordic countries with large proportion of forests land cover.
2.2 Municipal Solid Waste
Municipal solid wood waste (MSWW) constitutes a quite low share of total MSW, but the relatively high volume and inadequate prospects for reuse are causing cities difficulties in treatment, selection, and transport of the waste with the goal to mitigate MSWW environmental impacts [17, 18]. The common solution has often been the incineration of MSWW to produce energy [18]. In the time of circular economy worldwide [19], alternative policies have become the norm to reduce the environmental impact of incineration and instead promote the reuse of this waste category and prolong its life cycle [17]. In the present situation incineration should only be used as last of options since wood waste entails great reuse potential, and by recycling MSWW many opportunities arise still including efficient energy recovery.
3 Hydrothermal Carbonization (HGT)
Biochar has long been a known way of carbonization of different types of biomasses and in recent years hydrothermal carbonization (HTC) has in parallel been developed as an alternative method of processing biomass for value-added products [20, 21]. The solid char product formed during HTC is called hydrochar to be distinguished from biochar which is formed in pyrolysis process in temperatures from 300–650°C [22]. During the HTC process, biomass is heated in an oxygen free environment in presence of subcritical water under autogenous 2–10 MPa pressure [23]. The HTC process has several benefits compared to pyrolysis, including a lower energy consumption and the generation of less emissions. It is especially suited for high moisture feedstocks with a high moisture content that produces lower amounts of solid material after drying, and that makes them inadequate sources for pyrolysis [23]. This gives possibilities for a variety of feedstocks to be used for the production of hydrochar when drying the feedstock is not necessary [24]. Another gain compared to biochar is that by HTC the char yield is larger and produced with lower amounts of energy. Since the feedstock does not need to be dried, and operating temperatures for HTC (200–300°C) are lower, the yield is greater with less energy compared to biochar pyrolysis [25]. The char biomass is activated in the presence of liquid heating up the process, which enables lower process temperature compared to biochar production. The heating of the biomass initiates hydrolysis, dehydration, decarboxylation, and aromatization that changes the physical structure of the biomass (Fig. 1) [26]. The hydrolysis is the primary reaction in HTC, and it has lower activation energy than the other reactions.
3.1 Feedstock Nature
The classification of feedstocks into wet and dry biomass can be done based on initial moisture content. Newly harvested biomass like sewage sludge, vegetable residues, algae, animal wastes, etc. often has high moisture content (>400%) and is then called “wet biomass.” Agricultural residues and some wood species have low moisture content (<30%) when they are harvested and are thus called “dry biomass” [24]. The wet biomass can be dried to become low moisture content feedstock with complementing drying techniques, but their downside is the high-energy requirement that will be economically costly.
Biomass is an excellent source for bioenergy [27], that basically is clean energy and HTC can be applied to large varieties of feedstock like lignocellulosic residues [28], animal wastes [29], agricultural residues, food wastes [30], municipal wastes [31], and wastewater treatment plants’ (WWTPs) activated sludge [32] that have detrimental effects on the quality of hydrochar. Overall, both hydrochar and biochar have their advantages and disadvantages. In future research biomass treatment can be combined with the hydrothermal carbonization process and pyrolysis process. The catalytic performance of the product materials needs to be further investigated [33].
Alternative fuels are sought to achieve required process temperatures in industrial processes as electricity becomes more expensive [34]. The gas phase obtained in hydrothermal carbonization can be subjected to a methanation stage, when the resulting methane could be used to fuel the process. Such process idea is depicted in Fig. 2. Such solution could be a step forward for integrated process according to circular economy principles. Moreover, the needed hydrogen for methanation should come from renewable sources.
Integration of HTC with methanation of gas phase [34]
Biomass has so far been utilized in many ways, but pyrolysis is a new way of dealing with biomass sustainably. The biological conversion has been applied with biogas reactors in fermentation and anaerobic digestion where the crucial step is what to do with the sludge to avoid it becoming a waste. Densified biomass like pelletization of forest residue has the challenge that it has mainly been used for heat production instead of channeling it into the biochar system (FAO) according to the principles of circular economy. The forms of biomass utilization are compared with pyrolysis and hydrothermal processes in Table 1.
4 Pyrolysis for Biochar Production
Biochar is a solid, carbon-rich product acquired from the pyrolysis process under an oxygen-limited atmosphere and high temperature [22]. The handling of all kinds of agriculture and forestry has become a priority. In the EU, about 23 Mt/p.a. of biomass (dry) is available as residual straw from cereals [16] whereas from example emerging economies like India, ca. 368 Mt/p.a. straw residue is available [35].
Biochar can be produced in different types of units and reactors to achieve the desired yield and quality. Reactors are similar, but the oxygen use, heating rate, and final temperature affect the quality and distribution of final products [36]. The thermal process for optimizing biochar yield is slow pyrolysis, which is conducted in 300–700°C in the absence of air producing bio-oil and biogas as by-products. Torrefaction is another process optimizing biochar yield, carried out at 200–300°C in the absence of air and it does not produce by-products. By prolonging the biochar residence time at ~400°C for more hours a higher yield and quality could be obtained [36].
4.1 Slow Pyrolysis, Temperature Regulation
In slow pyrolysis the temperature range is 300–600°C, with a long residence time (several hours to several days) and requires only low heating rate. It is generally believed that slow pyrolysis is the best pyrolysis method optimizing the biochar yield and structure [36, 37]. Zhang et al. [38] prepared three types of cow dung biochar under slow pyrolysis which revealed differences in morphology, surface area, pore structure, surface charge, and oxygen-containing functional groups where the biochar yield was 30–60% and the specific surface area <400 m2/g.
The temperature is an important parameter in the design of biochar that governs physicochemical properties of the pyrolyzed product. It affects the aromatic condensation and aromaticity of biochar. With increased pyrolysis temperature the liquefied aromatic ring structure in biochar increases, at the same time as the unstable nonaromatic ring structure decreases [39]. Along with aromaticity hemicellulose, cellulose, lignin, protein, polysaccharide, and other macromolecules decrease in the resulting biochar. This leads to lower polarity of the solid product, but also lower hydrophilicity of the surface, forming separated aromatic rings. Zhang et al. [39] found that the pyrolysis temperature played a significant role in the properties of biochar. The temperature correlated positively with the carbon content, ash content, pH, surface roughness, and conductivity. The (O + N)/C, O/C, and H/C ratios, however, correlated negatively with temperature [39]. The higher temperatures favor formation of crystal structures. Biochar produced in lower temperatures becomes acidic, polar with low aromaticity and hydrophobicity. With the increasing temperature functional acidic groups like –OH and –COOH decrease along with biochar yield. This leads to appearance of more alkaline functional groups, higher pH and ash when the biochar surface area increases as volatiles evaporate from the biomass.
4.2 Pyrolysis Atmosphere
In recent years the biochar synthesis has been refined and a number of different functional structures of biochar are better controlled through adjustment of synthesis parameters, not only pyrolysis time and pyrolysis temperature, but also by choice of biomass, and different pretreatment process. It is the gap between functional structures and mechanisms that needs to be bridged for achieving better results with biochar applications [40].
It is possible to optimize the pyrolysis process by changing the atmosphere in-situ activation for production of more potent biochars. This has been reported in several studies where the conventional N2 atmosphere has been changed, for instance, to CO2 as carrier gas [41, 42]. The activation with CO2 improved the aromatic surface properties of biochar in temperatures of 500, 600, and 700°C [43].
Using spent coffee ground (SCG) the authors Cho, Chang [44] found two key roles of CO2: the thermal cracking of VOCs appearing from the thermal degradation and the reaction of CO2 with VOCs. They concluded that the morphological modification was initiated after depleting VOCs by the thermal degradation of the SCG sample in CO2 atmosphere.
4.3 Co-Pyrolysis of Biomass with Activator/Dopant
When biochar is going to be used as a catalyst or adsorbent sufficient surface functionality is wanted providing more active sites for catalysis and adsorption of pollutants. High porosity and large surface areas are also advantageous for biochar utilized for storage of energy since they enable higher fluxes of mass transfer and active loading [45]. For biochar use, porosity and surface area of biochar and surface are critical and need to be properly assessed, or else desirable biochar features must be stimulated through suitable activation strategies (Table 2). Directly after the production process the biochar has low tendency to absorb or adsorb compounds. The surface area, pore size, pore volume, and the amount of pores present contribute to the reaction characteristics [56]. The produced biochars without proper activation contain (1) abundant intermolecular spaces as a result of bond breakage between the organic components (2) clogged pores that cause generation of tar (3) inadequate pore size reducing the distribution of surface area (4) condensate contaminants like, ashes, etc. that decrease the pore size and volume.
4.4 Activation by Chemical Agents
Chemical activation methods are generally one-step processes where the agents are added to biochar and subjected to further pyrolysis. An activated biochar is usually washed for removing excess chemical, and after that the surface is ready for adsorptive reactions depending on target use. Typical chemical activation is oxidation, sulfonation, and amination and agents used are H2O2, SO3H, ZnCl2, acids like HNO3, bases like KOH, NaOH. The activation agent is selected on the bases of the target use of biochar. Adsorbing negatively charged elements requires activation with bases imposing positive surface giving affinity to adsorbate. For adsorbing positively charged element, biochar is in turn activated with acid for improving adsorption of positively charged elements [57].
4.4.1 Activation by H2O2
H2O2 is a low-cost activation agent used at ambient temperature that splits into H2O and O2 and it is used as oxidizing agent because of the following reasons (1) low cost, (2) works at low temperature, (3) end products are H2O and O2. Biochar made from grape wood activated by H2O2 at 350°C has been shown to effectively adsorb the cyhalofop herbicide (35.4%) due to strong affinity of herbicide to biochar [58].
4.4.2 Activation by Metals
Metal ions are often applied as agents for catalysis [59]. Iron, cobalt, and other metallic biomass elements have been used in advanced oxidation systems [60]. Loading metal is thought as one of the operational ways for expanding the catalytic ability of biochar. When metal particles are dispersed in biochar, they can lower metal leaching. Biochar can prevent the aggregation of metal nanoparticles and thus offers many more accessible active sites. Compared to other catalysis supports, biochar has economical and efficiency advantages.
Zn-Co-layered double hydroxide (Zn-Co- LDH) nanostructures were incorporated with biochar through hydrothermal process [61]. After loading on biochar, the specific surface declined from 112.9 to 95.7 m2/g. At the same time gemifloxacin degradation efficiency was raised from 60.4% to 92.7%.
There are several techniques to analyze biochar properties. The surface chemistry can best be studied by Fourier transformation infrared spectroscopy, X-ray diffraction analysis, and X-ray photoelectron spectroscopy. Structural analysis is done by scanning electron microscopy and Brunauer-Emmett-Teller analysis. The elements in biochar can be revealed by energy-dispersive-X-ray spectroscopy. The acidity and basicity can be determined by temperature-programmed desorption using ammonia [46].
Usually before co-pyrolysis biomass is premixed by impregnation or mechanical mixing with an activator/dopant, and then pyrolyzed. Co-pyrolysis of biomass with activators/dopants such as KOH, Ca(OH)2, ZnCl2, MgCl2, FeCl3, chlorapatite, Fe(NO3)3, KMnO4, melamine, and urea has been explored. By using hazelnut shells as a raw material, Zhao et al. [62] undertook the chemical activation of ZnCl2 for co-pyrolysis and found that hazelnut shells were an effective material for producing a microporous structure. Liu et al. [63] pretreated straw with a Ca(OH)2 water solution, and then synthesized the calcium-rich biochar in-situ with black liquor as a precursor, whereby Ca(OH)2 was found to be a mesoporous forming agent in the synthesis process. With corn stalk as raw material and urea as nitrogen source, Li et al. [64] prepared a nitrogen-doped fractional porous biochar by in-situ co-pyrolysis. The addition of urea promoted the formation of biochar pores, and nitrogen atoms successfully became a part of the biochar skeleton. Biochar attained through the co-pyrolysis of biomass with activators/dopants has a larger surface area and more surface oxygen-containing functional groups in comparison with original biochar.
4.5 The Quality and Safety of the Produced Biochars
Waste material is an important resource in Circular economy [65]. The recycling and upgrading of waste require detailed evaluation of possible waste contaminants. In pyrolysis there is the need to assess them in the biochar product. In the pyrolysis process enrichment of metals takes place since most of them are not released by emissions [66]. Pyrolysis can release metals as a volatile metal mixture together with aerosols from the gases. Organic compounds like polyaromatic hydrocarbons (PAHs) can leak during pyrolysis as aromatic rings condensation, fused into PAH-like sheet assemblies [67]. The quantity of toxic elements in biochars has been examined [68]. PAHs are especially produced during incomplete combustion of biomass, and thus are integrally generated during biochar production. Due to their well-known toxicity and carcinogenic traits, they constitute an environmental risk.
The quality of biochars is an important issue especially in the commercial market where the biochar is intended for a spectrum of different uses. The European Biochar Certificate highlights the specific contaminant threshold levels in biochar for agricultural soil improvement [69]. The threshold levels are given as total content in the solid phase. Concerning contaminants, the bioavailable concentrations are crucial in accurate risk assessment and need some attention [70]. The heavy metal contents are dependent on both feedstock and process parameters of the performed pyrolysis [71]. The rise in pH supports lower heavy metal solubility [72]. The pH increase in biochar may not last due to leaching where soil pH might in the long term be reduced. Biochar application in agriculture requires thorough assessment of biochar quality since toxic organic contaminants of biochar may end up in the environment. European Biochar Certificate (EBC) values of the molar ratio of H/Corg <0.7 and O/Corg <0.4 does not ensure that biochar will not cause phytotoxicity [10].
5 Biochar for the Remediation of Contaminated Soil and Water
Soil amendment is the most common application of biochar and several studies have demonstrated the benefits of such practice [73, 74]. Biochar as an amendment in soil has been shown to improve soil properties such as soil C content, increase water holding capacity, and increase aggregate formation, stability [75] and plant growth [76]. It could also improve soil quality and fertility [77, 78]. The high porosity of biochar has taken advantage to immobilize heavy metals in soils [79], and in consequence reduce their uptake by plants [80], and remediate organic pollutant-contaminated soils [81].
5.1 Heavy Metals
Soil pollution poses a threat to human and environmental health as contamination can migrate into groundwater, or drain into other water bodies. But it may also get into the food chain and eventually reach humans [79]. Just in the USA more than 100,000 contaminated sites have been identified [82] and in Europe the estimate for the total number of potentially contaminated sites is 2.5 million [83]. Due to their high toxicity and health risks the remediation of heavy metal (HM)-contaminated soil has become a priority in the environmental agenda [84]. The use of biochar for the remediation of HM-contaminated soil has become a sustainable solution (Fig. 3). Biochar as a porous material has the capacity to bind HMs from soil and that way reduce the uptake of HMs by plants [80, 85, 86]. The raw materials and feedstock for biochar production together with the pyrolysis temperatures are the most important criteria affecting the binding capacity of biochar. The interactions between biochar and HMs in soil can be direct in precipitation, complexation, or electrostatic attraction. The interactions can also be relatively indirect depending on soil pH, minerals, or organic carbon [87]. Biochar adsorbs the metal ions from contaminated soils due to its stronger sorption sites and high affinity to metal ions [88].
Choudhary et al. [89] used biochar from pine needle litter to serve for Pb adsorption from contaminated water. The study showed that Pb adsorption increased significantly as pH and temperatures increased and desorption results were promising with a lead recovery of 90–93%. They concluded that biochars possess the potential for aqueous removal of other metal cations [89].
5.2 Phytoremediation and Related Microbes
The most recent studies have been testing biochar as a carrier of HM-reducing strains or HM-tolerant bacteria and its effects upon its addition into contaminated soil [90]. Cr contaminated soil is of great concern due to the high toxicity of Cr(IV). A recent study successfully immobilized a Cr(IV)-reducing strain into biochar to treat Cr-polluted soil. Soil properties improved with aggregate formation, organic carbon content, and cation exchange increased. The Cr(IV) was transformed into less toxic Cr(III) and that Cr-residue fraction increased by 63.38% compared with control. The aggregates also reduced the Cr absorption of Ryegrass from the root and enhanced its growth [91]. Biochar inoculation with a HM-tolerant strain was reported to significantly increase residual fraction of Cd and Cu leading to the decreased bioavailability of the metals in soil. The inoculated biochar enhanced soil enzyme activity and the soil microbial community recovered at the end of the incubation, showing improved soil function after metal stabilization [92].
It has been demonstrated that solubility of Pb and Cd decreased significantly with biochar produced from agriculture residues providing evidence that biochar decreased HM toxicity [93]. Soil properties improved; pH increased together with organic matter and nutrient content. Maize planted on the treated soil with biochar performed better compared to the control demonstrated as an increase in biomass [93].
The interaction among biochar, plants, and microbes might alter the HMs behavior in the soil [94]. The beneficial roles of biochar on plant growth and on the enhancement of microbial activity were likely to improve the phytoremediation efficiency of the hyperaccumulators [95]. Biochar incorporation does not decrease the total heavy metal content of the soil but it reduces the bioavailability and phytotoxicity of heavy metals. Therefore, phytostabilization of metal-contaminated soils can be enhanced by combining metal immobilizing plants with biochar [96]. Biochar was used in Cd soil contamination for cell immobilization of two cadmium resistant bacteria (CRB), Arthrobacter sp. and Micrococcus sp. [97]. Biochar-immobilized (BC) CRB were able to survive in cadmium-contaminated soil (Fig. 4). The inoculation of BC-Micrococcus sp. increased the root dry weight of C. laxum planted in cadmium-contaminated soil. Plants inoculated with either BC-Arthrobacter sp. or BC-Micrococcus sp. had the highest cadmium contents in the shoots and the roots. They concluded that C. laxum combined with BC-Arthrobacter sp. or BC-Micrococcus sp. inoculation achieved a high efficiency of cadmium phytoextraction in metal-contaminated soil.
Biochar of cassava stem (Manihot esculenta L. Crantz). HM Phytoremed with bacterial immobilization. Characteristics of (a) biochar, (b) BC-Arthrobacter sp., and (c) BC-Micrococcus sp. observed under SEM at ×10,000 magnification remove 5 [97]
5.3 Organic Pollutants
Biochar from biomass waste has successfully been used for remediation of organic pollutants [98]. Interactions of free radical-based chemical reactions and biochar-microbial communities are the most important mechanisms involved in the degradation of soil organic pollutants using biochar. Pollutants are preferentially adsorbed onto the surface and pores of the biochar in free radical-based chemical reactions. The radicals formed in advance oxidation process degrade the pollutants [73].
Colored contaminated water has been treated with different types of biochar with good results. An example is the recent study where engineered biochar was produced from food waste digestate to remove azo dye pollutant from water. Results were promising with a removal of >99% of the dye upon the addition of biochar (0.5 g/L) and peroxymonosulfate (1 mM) to wastewater. The removal efficiency was attributed to the catalytic sites in the biochar which could activate peroxymonosulfate to produce reactive oxygen species [99]. Biochar from wood apple fruit shell waste was used in the removal phenol and chlorophenols (4-CPh and 2,4-DCPh) from contaminated aqueous media [100]. The study revealed that this biochar was an effective adsorbent of these organic pollutants with pH and temperature being vital parameters to take into account for a rapid uptake and high sorption capacity: it could be used for the treatment of contaminant wastewater [100]. Efficient removal of Rhodamine B dye was achieved by a magnetic biochar produced from waste wood [101]. The adsorption process was governed by a chemical reaction and the adsorption process was a single-layer and heterogeneous surface adsorption. The equilibrium was established within 1 min, indicating an excellent adsorption efficiency making this magnetic biochar a prospect in wastewater treatment [101].
Modified biochars with active oxidation agents, e.g. persulfate, peroxymonosulfate, chlorine, iodine, etc., have been utilized to generate free radicals and improve the degradation of organic pollutants [73]. Biochar from waste lychee branches was together with persulfate used for the removal of bisphenol A (BPA) in soils [102]. This type of biochar activates persulfate to generate sulfate and hydroxyl radicals for BPA degradation. Liu et al. 2020 [102] concluded that the combination of biochar and persulfate could be used for in in-situ remediation of organic contaminated sites.
Herbicides are organic chemicals that may undesirably impact human, wildlife, beneficial plants and soil organisms. In a multifaceted experiment by Wu, Liu [103] different types of biochar (peanuts (BCP), chestnuts (BCC), bamboo (BCB), maize straw (BCM), and rice hull (BCR) were applied to soil to study their sorption capacity, degradation, and effect on the bioavailability of the herbicide oxyfluorfen. The most important results showed that the biochars exhibited different sorption capacities for oxyfluorfen in the following order: BCR > BCB > BCM > BCC > BCP and that the addition of biochar to soil reduced the bioavailability of oxyfluorfen. Oxyfluorfen degraded faster in BCR-amended soil compared to unamended soil, i.e., degradation increased by ~1.2-fold with addition of just 2% BCR. Interestingly, the adsorption capacity of amended soil for oxyfluorfen decreased with increased aging time, however, it was still higher on the amended soil compared to the unamended soil after 6 months. In conclusion, the study indicates that the introduction of biochar is an effective method to modify soil contaminated with oxyfluorfen and to decrease the risk of contamination [103]. Sugarcane top-derived biochar was added to different types of soils to evaluate the sorption capacity toward atrazine herbicide [104]. The sorption coefficients had a positive correlation with the amount of biochar added into soil. The study indicated, however, that as a result of adsorption the degradation of atrazine decreased and that it could be a method to prevent atrazine leaching into groundwater [104].
The combination of biochar and compost has proven to be effective in the remediation of organic pollutants, when both amendments improve soil quality and fertility. The application of compost and biochar amendment decreased the concentration and bioavailability of PHCs [105]. The addition of compost enhanced biodegradation, while biochar contributed to lock the hydrocarbons in contaminated soils [105]. Hussain, Khan [106] observed that the combination of biochar, compost, and immobilized microorganisms resulted in the highest PHCs removal from soil compared to the control or the treatments alone.
6 Toward Circular Economy: Recycled Waste for Biochar Production
Pyrolysis and hydrothermal carbonization of biomass have attracted attention as expedient waste management methods [107]. Biochar has been produced from several organic waste material such as guayule bagasse, cotton gin waste [108], coconut shell [109], empty fruit bunches [110], and rice husk [111]. Biochar pyrolysis has been reported for food waste digestate and food waste [99], straw from crops (corn, wheat and bulrush) [112, 113], pig manure [107], and wood waste materials.
When waste wood is used as heating energy it will release CO2 to the atmosphere, which is against current policies in Europe for reaching carbon neutrality. Furthermore, waste wood in landfills will create methane emission as product of the decomposition of lignocellulose compounds [114]. The construction and demolition industry produces waste wood that has become a viable source of biomass for the production of biochar. Countries including Taiwan have already implemented the reuse of waste wood as material and energy resource in carbon-negative policies, to reduce greenhouse gases (GHG) emissions [114]. Waste wood like wood shavings, waste timber, bark and pine needles litter has already successfully been produced [89, 109, 115]. The increase of the production of biochar from waste wood could become a global solution to reduce the landfills. It has been estimated that just in Finland wood waste accounts for 3,268,000 t from which 401,000 t comes from construction industry [116, 117].
Differently produced biochars have increasingly been used for soil amendment but also contributing to carbon sequestration. An interesting application is the introduction of biochar into building material like cement and concrete.
6.1 Novel Applications of Biochar
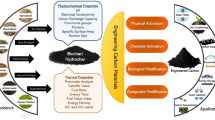
The use of biochar as soil amendment is the most common application of this material [78], however in recent years there has been several other useful and sustainable applications. Some of these examples are briefly mentioned in this chapter as in Fig. 5.
Pyrolysis presenting the three main products, biochar, gas, and bio-oil. Potential applications for the carbon sequestered in biochar including soil fertility, feed in livestock farming, incorporation to building material, organic fertilizer, and environmental remediation. Bio-oil can be used for heating, but especially for upgrading to fuel, chemicals and even deposited as geological sequestration
6.1.1 Carbon Sequestration for Carbon Neutrality
For achieving the goals of carbon (C) neutrality, municipalities need worldwide to apply negative emission technologies. The application of biochar as soil amendment represents a sustainable long-term carbon storage [118, 119] that has a mitigating influence on climate change and global warming. The conversion of waste wood into biochar has a positive effect on carbon sequestration. Around 50% of the carbon in waste wood is retained in biochar instead of being released into the atmosphere. In this procedure the organic carbon is moved to a slower carbon cycle reservoir (biochar) that can remain in soil even for centuries [120]. Urban demonstration areas using trees and biochars for C sequestration have been implemented in Helsinki, Finland to show that urban C sinks in public parks need to be visible and scientifically sound for reliable cost-effective verification of carbon sequestration. Valuable synergies emerged from co-creation of urban C sink parks between stakeholders (scientists, city officials, companies, and citizens) for increasing impact of biochar application [121].
6.1.2 Composting
The addition of biochar into compost (Fig. 5) could increase the aeration process as biochar is a porous material with low density [122]. Moreover, due to its high sorption capacity it could reduce the loss of nitrogen and immobilize HMs and organic pollutants present in the compost materials [123]. It has also been demonstrated that it could reduce the emission of GHGs [124]. Moreover, research has demonstrated that the combined application of biochar and compost to soils could increase both; their agronomic value and reduce nutrients losses [125].
6.1.3 Livestock
Significant benefits of biochar in feed (Fig. 5) to improve animal health have been reported [126]. In an extensive survey (>100 scientific publications) on biochar in animal feeding, positive effects on digestion, feed efficiency, toxin adsorption, blood values, and meat quality were widely observed. GHG emissions were also reduced when biochar was added to feed [126]. In Europe it has recently been approved to add biochar to livestock feed at 1–2% for reducing vet and medical costs [127]. Biochar has further been effective in animal bedding for odor control [128].
6.1.4 Concrete
Other biochar applications include the incorporation of biochar into the concrete mixture for building (Fig. 5), with positive results and as carbon sequestration opportunity. Akhtar and Sarmah [129] tested three different waste sources of biochar to replace cement content up to 1%. The study concluded that biochar has the potential to improve the concrete properties, e.g. flexural strength while replacing minor fractions of cement [129]. Biochar from residual biomass of a bio-ethanol industry in mixture with concrete improved the sound absorption coefficient [130]. Biochar-concrete mixtures have also shown an improved water tightness, mechanical strength and minimal internal damage to concrete micro-structure [131].
7 Conclusion
The utilization of waste biomass for pyrolysis opens up great avenues for valorization of it to hydrochar and biochar. These higher value products may find a wide environmental range of applications ranging from fertilizers to remediation tools and use in building material to support the transition toward carbon neutral societies. The future success of these materials requires good separation of waste and channeling of waste streams into economic valorization. This will further depend on the ability to tailor various biomasses for specific purposes in an economically feasible way. The removal of carbon from atmosphere through biochar systems and the growth of the voluntary carbon market will speed up new applications for these very stable carbon products helping to reach carbon neutrality.
References
Drews S, Antal M, van den Bergh JC (2018) Challenges in assessing public opinion on economic growth versus environment: considering European and US data. Ecol Econ 146:265–272
EUROSTAT (2019) Share of energy from renewable sources 2019 data. European Commission
Lu X et al (2021) Co-hydrothermal carbonization of sewage sludge with wood chip: fuel properties and heavy metal transformation behavior of hydrochars. Energy Fuel 35(19):15790–15801
Fang J et al (2018) Minireview of potential applications of hydrochar derived from hydrothermal carbonization of biomass. J Ind Eng Chem 57:15–21
Fawzy S et al (2021) Industrial biochar systems for atmospheric carbon removal: a review. Environ Chem Lett. https://doi.org/10.1007/s10311-021-01210-1
Kim Y et al (2022) Agricultural waste streams as resource in circular economy for biochar production towards carbon neutrality. Curr Opin Environ Sci Health. Accepted
Oleszczuk P et al (2014) Effect of pesticides on microorganisms, enzymatic activity and plant in biochar-amended soil. Geoderma 214:10–18
Sørmo E et al (2020) Waste timber pyrolysis in a medium-scale unit: emission budgets and biochar quality. Sci Total Environ 718:137335
Freddo A, Cai C, Reid BJ (2012) Environmental contextualisation of potential toxic elements and polycyclic aromatic hydrocarbons in biochar. Environ Pollut 171:18–24
Ruzickova J et al (2021) A comprehensive assessment of potential hazard caused by organic compounds in biochar for agricultural use. J Hazard Mater 403:123644
d’Annunzio R et al (2015) Projecting global forest area towards 2030. For Ecol Manag 352:124–133
van den Born GJ, van Minnen JG, Olivier JGJ, Ros JPM (2014) Integrated analysis of global biomass flows in search of the sustainable potential for bioenergy production. P.N.E.A. Agency, The Hague
Tripathi N et al (2019) Biomass waste utilisation in low-carbon products: harnessing a major potential resource. NPJ Clim Atmos Sci 2(1):1–10
Forestry Department FAO (2018) Global forest products facts and figures. Food and Agriculture Organization of the United Nations, p 20
Rafael S et al (2015) Impact of forest biomass residues to the energy supply chain on regional air quality. Sci Total Environ 505:640–648
Baruya P (2015) World forest and agricultural crop residue resources for cofiring. International Centre for Sustainable Carbon, London, p 66
Faraca G et al (2017) Environmental assessment of presence of impurity materials and chemical pollutants in wood waste meant for recycling. In: Abstract from SETAC Europe 27th Annual Meeting. Brussels, Belgium
Zhou H et al (2014) An overview of characteristics of municipal solid waste fuel in China: physical, chemical composition and heating value. Renew Sust Energ Rev 36:107–122
Winans K, Kendall A, Deng H (2017) The history and current applications of the circular economy concept. Renew Sust Energ Rev 68:825–833
Stemann J, Putschew A, Ziegler F (2013) Hydrothermal carbonization: process water characterization and effects of water recirculation. Bioresour Technol 143:139–146
Oliveira I, Blöhse D, Ramke H-G (2013) Hydrothermal carbonization of agricultural residues. Bioresour Technol 142:138–146
Lehmann J, Joseph S (2015) Biochar for environmental management: science, technology and implementation. Routledge
He C, Giannis A, Wang J-Y (2013) Conversion of sewage sludge to clean solid fuel using hydrothermal carbonization: Hydrochar fuel characteristics and combustion behavior. Appl Energy 111:257–266
Kambo HS, Dutta A (2015) A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew Sust Energ Rev 45:359–378
Takaya CA et al (2016) Phosphate and ammonium sorption capacity of biochar and hydrochar from different wastes. Chemosphere 145:518–527
Funke A, Ziegler F (2010) Hydrothermal carbonization of biomass: a summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod Biorefin 4(2):160–177
Azzaz AA et al (2020) Hydrochars production, characterization and application for wastewater treatment: a review. Renew Sust Energ Rev 127:109882
Li D-C, Jiang H (2017) The thermochemical conversion of non-lignocellulosic biomass to form biochar: a review on characterizations and mechanism elucidation. Bioresour Technol 246:57–68
Heilmann SM et al (2014) Phosphorus reclamation through hydrothermal carbonization of animal manures. Environ Sci Technol 48(17):10323–10329
Cantero-Tubilla B et al (2018) Characterization of the solid products from hydrothermal liquefaction of waste feedstocks from food and agricultural industries. J Supercrit Fluids 133:665–673
Bevan E, Fu J, Zheng Y (2020) Challenges and opportunities of hydrothermal carbonisation in the UK; case study in Chirnside. RSC Adv 10(52):31586–31610
Danso-Boateng E et al (2015) Hydrothermal carbonization of primary sewage sludge and synthetic faeces: effect of reaction temperature and time on filterability. Environ Prog Sustain Energy 34(5):1279–1290
He X et al (2021) Hydrothermal and pyrolytic conversion of biomasses into catalysts for advanced oxidation treatments. Adv Funct Mater 31(7):2006505
González-Arias J et al (2021) Hydrothermal carbonization of biomass and waste: a review. Environ Chem Lett. https://doi.org/10.1007/s10311-021-01311-x
Hiloidhari M, Das D, Baruah D (2014) Bioenergy potential from crop residue biomass in India. Renew Sust Energ Rev 32:504–512
Wang D et al (2020) Biochar production and applications in agro and forestry systems: a review. Sci Total Environ 723:137775
Wang L et al (2020) New trends in biochar pyrolysis and modification strategies: feedstock, pyrolysis conditions, sustainability concerns and implications for soil amendment. Soil Use Manag 36(3):358–386
Zhang P et al (2019) Characteristics of tetracycline adsorption by cow manure biochar prepared at different pyrolysis temperatures. Bioresour Technol 285:121348
Zhang X et al (2020) Effect of pyrolysis temperature and correlation analysis on the yield and physicochemical properties of crop residue biochar. Bioresour Technol 296:122318
Zhou X et al (2021) New notion of biochar: a review on the mechanism of biochar applications in advannced oxidation processes. Chem Eng J 416:129027
Sørmo E et al (2021) Stabilization of PFAS-contaminated soil with activated biochar. Sci Total Environ 763:144034
Kończak M et al (2020) Carbon dioxide as a carrier gas and mixed feedstock pyrolysis decreased toxicity of sewage sludge biochar. Sci Total Environ 723:137796
Godlewska P et al (2019) Adsorption capacity of phenanthrene and pyrene to engineered carbon-based adsorbents produced from sewage sludge or sewage sludge-biomass mixture in various gaseous conditions. Bioresour Technol 280:421–429
Cho S-T et al (2015) Genome analysis of Pseudomonas fluorescens PCL1751: a rhizobacterium that controls root diseases and alleviates salt stress for its plant host. PLoS One 10(10):e0140231
Liu W-J, Jiang H, Yu H-Q (2015) Development of biochar-based functional materials: toward a sustainable platform carbon material. Chem Rev 115(22):12251–12285
Balajii M, Niju S (2019) Biochar-derived heterogeneous catalysts for biodiesel production. Environ Chem Lett 17(4):1447–1469
Lokman IM, Rashid U, Taufiq-Yap YH (2015) Production of biodiesel from palm fatty acid distillate using sulfonated-glucose solid acid catalyst: characterization and optimization. Chin J Chem Eng 23(11):1857–1864
Dong T et al (2015) Two-step microalgal biodiesel production using acidic catalyst generated from pyrolysis-derived bio-char. Energy Convers Manag 105:1389–1396
Fadhil AB, Aziz AM, Al-Tamer MH (2016) Biodiesel production from Silybum marianum L. seed oil with high FFA content using sulfonated carbon catalyst for esterification and base catalyst for transesterification. Energy Convers Manag 108:255–265
Nuradila D, Ghani W, Alias AB (2017) Palm kernel shell-derived biochar and catalyst for biodiesel production. Malays J Anal Sci 21(1):197–203
Wang S et al (2017) A novel peat biochar supported catalyst for the transesterification reaction. Energy Convers Manag 139:89–96
Jung J-M et al (2017) Biodiesel synthesis using chicken manure biochar and waste cooking oil. Bioresour Technol 244:810–815
González ME et al (2017) Functionalization of biochar derived from lignocellulosic biomass using microwave technology for catalytic application in biodiesel production. Energy Convers Manag 137:165–173
Wang S et al (2017) Transesterification of vegetable oil on low cost and efficient meat and bone meal biochar catalysts. Energy Convers Manag 150:214–221
Ahmad J et al (2018) Synthesis of char-based acidic catalyst for methanolysis of waste cooking oil: an insight into a possible valorization pathway for the solid by-product of gasification. Energy Convers Manag 158:186–192
Anto S et al (2021) Activation strategies for biochar to use as an efficient catalyst in various applications. Fuel 285:119205
Tan X-F et al (2017) Biochar as potential sustainable precursors for activated carbon production: multiple applications in environmental protection and energy storage. Bioresour Technol 227:359–372
Gámiz B et al (2019) Understanding activation effects on low-temperature biochar for optimization of herbicide sorption. Agronomy 9(10):588
Luo H et al (2020) Recent progress on metal-organic frameworks based-and derived-photocatalysts for water splitting. Chem Eng J 383:123196
Cao X et al (2020) Preliminary study on the electrocatalytic performance of an iron biochar catalyst prepared from iron-enriched plants. J Environ Sci 88:81–89
Gholami P et al (2020) Photocatalytic degradation of gemifloxacin antibiotic using Zn-Co-LDH@ biochar nanocomposite. J Hazard Mater 382:121070
Zhao B et al (2017) Surface characteristics and potential ecological risk evaluation of heavy metals in the bio-char produced by co-pyrolysis from municipal sewage sludge and hazelnut shell with zinc chloride. Bioresour Technol 243:375–383
Liu X et al (2019) Black liquor-derived calcium-activated biochar for recovery of phosphate from aqueous solutions. Bioresour Technol 294:122198
Li Y et al (2019) Nitrogen-doped hierarchical porous biochar derived from corn stalks for phenol-enhanced adsorption. Energy Fuel 33(12):12459–12468
European Commission (2019) Report from the Commission to the European Parliament, the Council, the European Economic and Social Committee, and the Committee of the Regions, on the implementation of the Circular Economy Action Plan. European Commission, Brussels
Dong J et al (2015) Partitioning of heavy metals in municipal solid waste pyrolysis, gasification, and incineration. Energy Fuel 29(11):7516–7525
Kołtowski M, Oleszczuk P (2015) Toxicity of biochars after polycyclic aromatic hydrocarbons removal by thermal treatment. Ecol Eng 75:79–85
Hale SE et al (2012) Quantifying the total and bioavailable polycyclic aromatic hydrocarbons and dioxins in biochars. Environ Sci Technol 46(5):2830–2838
European Biochar Certificat (EBC) (2012) Summary of the EBC certificiation. https://www.european-biochar.org/en/ct/29-Summary-of-the-EBC-certificiation. Accessed 30 Oct 2021
Reichenberg F, Mayer P (2006) Two complementary sides of bioavailability: accessibility and chemical activity of organic contaminants in sediments and soils. Environ Toxicol Chem 25(5):1239–1245
Beesley L et al (2015) Biochar and heavy metals. In: Lehmann J, Joseph S (eds) Biochar for environmental management2nd edn. Tylor & Francis, pp 563–594
Devi P, Saroha AK (2014) Risk analysis of pyrolyzed biochar made from paper mill effluent treatment plant sludge for bioavailability and eco-toxicity of heavy metals. Bioresour Technol 162:308–315
Kamali M et al (2022) Biochar for soil applications-sustainability aspects, challenges and future prospects. Chem Eng J 428:131189
Zhu X et al (2017) Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: a review. Environ Pollut 227:98–115
Diatta AA et al (2020) Effects of biochar on soil fertility and crop productivity in arid regions: a review. Arab J Geosci 13(14):1–17
Rafique M et al (2020) Residual effects of biochar and phosphorus on growth and nutrient accumulation by maize (Zea mays L.) amended with microbes in texturally different soils. Chemosphere 238:124710
Alkharabsheh HM et al (2021) Biochar and its broad impacts in soil quality and fertility, nutrient leaching and crop productivity: a review. Agronomy 11(5):993
Zhanhua Z et al (2022) Organic amendments combined with biochar for improving soil and plant quality in a Torreya grandis plantation. J Soils Sediments. https://doi.org/10.1007/s11368-021-03127-2
Shakya A, Agarwal T (2020) Potential of biochar for the remediation of heavy metal contaminated soil. In: Biochar applications in agriculture and environment management. Springer, Berlin, pp 77–98
Seleiman MF et al (2020) Effects of ZnO nanoparticles and biochar of rice straw and cow manure on characteristics of contaminated soil and sunflower productivity, oil quality, and heavy metals uptake. Agronomy 10(6):790
Rao MA et al (2017) Biochar based remediation of water and soil contaminated by phenanthrene and pentachlorophenol. Chemosphere 186:193–201
Connell DW et al (2005) Basic concepts of environmental chemistry. CRC/Taylor & Francis
Van Liedekerke M et al (2014) Progress in the management of contaminated sites in Europe. EUR 26376. Publications Office of the European Union, Luxembourg, p 68
Selvi A et al (2019) Integrated remediation processes toward heavy metal removal/recovery from various environments-a review. Front Environ Sci 7:66
Bashir S et al (2018) Cadmium immobilization potential of rice straw-derived biochar, zeolite and rock phosphate: extraction techniques and adsorption mechanism. Bull Environ Contam Toxicol 100(5):727–732
Lahori AH et al (2017) Use of biochar as an amendment for remediation of heavy metal-contaminated soils: prospects and challenges. Pedosphere 27(6):991–1014
He L et al (2019) Remediation of heavy metal contaminated soils by biochar: mechanisms, potential risks and applications in China. Environ Pollut 252:846–855
Adnan M et al (2020) Coupling phosphate-solubilizing bacteria with phosphorus supplements improve maize phosphorus acquisition and growth under lime induced salinity stress. Plan Theory 9(7):900
Choudhary V et al (2020) Batch and continuous fixed-bed Lead removal using Himalayan pine needle biochar: isotherm and kinetic studies. ACS Omega 5(27):16366–16378
Yrjälä K, Lopez-Echartea E (2021) Structure and function of biochar in remediation and as carrier of microbes. In: Sarmah AK (ed) Biochar: fundamentals and applications in environmental science and remediation technologies. Elsevier
Chen Y et al (2021) Remediation of chromium-contaminated soil based on bacillus cereus WHX-1 immobilized on biochar: Cr(VI) transformation and functional microbial enrichment. Front Microbiol 12(408):641913
Tu C et al (2020) Biochar and bacteria inoculated biochar enhanced Cd and Cu immobilization and enzymatic activity in a polluted soil. Environ Int 137:105576
Alaboudi KA, Ahmed B, Brodie G (2019) Effect of biochar on Pb, Cd and Cr availability and maize growth in artificial contaminated soil. Ann Agric Sci 64(1):95–102
Kiran BR, Prasad MNV (2019) Biochar and rice husk ash assisted phytoremediation potentials of Ricinus communis L. for lead-spiked soils. Ecotoxicol Environ Saf 183:109574
Gong X et al (2019) Biochar facilitated the phytoremediation of cadmium contaminated sediments: metal behavior, plant toxicity, and microbial activity. Sci Total Environ 666:1126–1133
Edenborn S et al (2015) Influence of biochar application methods on the phytostabilization of a hydrophobic soil contaminated with lead and acid tar. J Environ Manag 150:226–234
Chuaphasuk C, Prapagdee B (2019) Effects of biochar-immobilized bacteria on phytoremediation of cadmium-polluted soil. Environ Sci Pollut Res 26(23):23679–23688
Abbas S et al (2021) Chapter 30 - alteration of plant physiology by the application of biochar for remediation of organic pollutants. In: Hasanuzzaman M, Prasad MNV (eds) Handbook of bioremediation. Academic Press, pp 475–492
Huang S et al (2020) Engineered biochar derived from food waste digestate for activation of peroxymonosulfate to remove organic pollutants. Waste Manag 107:211–218
Kumar NS et al (2021) Engineered biochar from wood apple shell waste for high-efficient removal of toxic phenolic compounds in wastewater. Sci Rep 11(1):2586
Zhang Y et al (2019) Synthesis of porous Fe/C bio-char adsorbent for rhodamine B from waste wood: characterization, kinetics and thermodynamics. PRO 7(3):150
Liu J et al (2020) Activation of persulfate with biochar for degradation of bisphenol A in soil. Chem Eng J 381:122637
Wu C et al (2019) Sorption, degradation and bioavailability of oxyfluorfen in biochar-amended soils. Sci Total Environ 658:87–94
Huang H et al (2018) Effects of biochar amendment on the sorption and degradation of atrazine in different soils. Soil Sediment Contam Int J 27(8):643–657
Cipullo S et al (2019) Linking bioavailability and toxicity changes of complex chemicals mixture to support decision making for remediation endpoint of contaminated soils. Sci Total Environ 650(Pt 2):2150–2163
Hussain F et al (2021) Soil conditioners improve rhizodegradation of aged petroleum hydrocarbons and enhance the growth of Lolium multiflorum. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-021-16149-7
Gascó G et al (2018) Biochars and hydrochars prepared by pyrolysis and hydrothermal carbonisation of pig manure. Waste Manag 79:395–403
Ndoun MC et al (2021) Adsorption of pharmaceuticals from aqueous solutions using biochar derived from cotton gin waste and guayule bagasse. Biochar 3(1):89–104
Silvani L et al (2019) Can biochar and designer biochar be used to remediate per- and polyfluorinated alkyl substances (PFAS) and lead and antimony contaminated soils? Sci Total Environ 694:133693
Yavari S et al (2017) Synthesis optimization of oil palm empty fruit bunch and rice husk biochars for removal of imazapic and imazapyr herbicides. J Environ Manag 193:201–210
Bielská L et al (2018) Sorption, bioavailability and ecotoxic effects of hydrophobic organic compounds in biochar amended soils. Sci Total Environ 624:78–86
Wang Y et al (2017) Remediation of petroleum-contaminated soil using bulrush straw powder, biochar and nutrients. Bull Environ Contam Toxicol 98(5):690–697
Cao Y et al (2016) Wheat straw biochar amendments on the removal of polycyclic aromatic hydrocarbons (PAHs) in contaminated soil. Ecotoxicol Environ Saf 130:248–255
Tsai W-T (2021) Carbon-negative policies by reusing waste wood as material and energy resources for mitigating greenhouse gas emissions in Taiwan. Atmos 12(9):1220
Hagemann N et al (2020) Wood-based activated biochar to eliminate organic micropollutants from biologically treated wastewater. Sci Total Environ 730:138417
Official Statistics of Finland (OSF) (2018) Waste statistics [e-publication]. Appendix table 1. Waste generation by industry, 2018, 1,000 tonnes
Official Statistics of Finland (OSF) (2018) Waste statistics [e-publication]. Appendix table 2. Waste treatment in 2018, 1,000 tonnes
Ma Y et al (2019) The global warming potential of straw-return can be reduced by application of straw-decomposing microbial inoculants and biochar in rice-wheat production systems. Environ Pollut 252(Pt A):835–845
Shen H et al (2020) Intense warming will significantly increase cropland ammonia volatilization threatening food security and ecosystem health. One Earth 3:126–134
Yargicoglu EN et al (2015) Physical and chemical characterization of waste wood derived biochars. Waste Manag 36:256–268
Tammeorg P et al (2021) Co-designing urban carbon sink parks: case carbon lane in Helsinki. Front Environ Sci 9:672468
Antonangelo JA, Sun X, Zhang H (2021) The roles of co-composted biochar (COMBI) in improving soil quality, crop productivity, and toxic metal amelioration. J Environ Manag 277:111443
Sanchez-Monedero MA et al (2018) Role of biochar as an additive in organic waste composting. Bioresour Technol 247:1155–1164
Guo X-X, Liu H-T, Zhang J (2020) The role of biochar in organic waste composting and soil improvement: a review. Waste Manag 102:884–899
Bong CPC et al (2021) Integrating compost and biochar towards sustainable soil management. Chem Eng Trans 86:1345–1350
Schmidt H-P et al (2019) The use of biochar in animal feeding. PeerJ 7:e7373
Redling A (2019) Why producing biochar from wood is more viable than ever for C&D recyclers. In: Construction & Demolition Recycling
O’Toole A et al (2016) Current and future applications for biochar. In: Biochar in European soils and agriculture. Routledge, pp 275–302
Akhtar A, Sarmah AK (2018) Novel biochar-concrete composites: manufacturing, characterization and evaluation of the mechanical properties. Sci Total Environ 616–617:408–416
Cuthbertson D et al (2019) Biochar from residual biomass as a concrete filler for improved thermal and acoustic properties. Biomass Bioenergy 120:77–83
Gupta S, Kua HW, Pang SD (2020) Effect of biochar on mechanical and permeability properties of concrete exposed to elevated temperature. Constr Build Mater 234:117338
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd
About this chapter
Cite this chapter
Yrjälä, K., Ramakrishnan, M., Zheng, H., Lopez-Echartea, E. (2022). Pyrolysis to Produce Hydrochar and Biochar Carbon Material for Carbon Removal and Sustainable Environmental Technology. In: Tanaka, S., Kurasaki, M., Morikawa, M., Kamiya, Y. (eds) Design of Materials and Technologies for Environmental Remediation. The Handbook of Environmental Chemistry, vol 115. Springer, Singapore. https://doi.org/10.1007/698_2022_845
Download citation
DOI: https://doi.org/10.1007/698_2022_845
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-5235-7
Online ISBN: 978-981-19-5236-4
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)