Abstract
The aim of this study was to determine the remediation efficiency of petroleum-contaminated soil from an oilfield using different types of remediation treatments under laboratory conditions. Compared with unamended soil as the control treatment (T1), soil samples were amended with bulrush straw powder (T2), with biochar alone (T3) and in combination with nutrients (nitrogen and phosphorus) (T4). The remediation experiment was carried out for 8 weeks. The extent of hydrocarbon degradation was monitored gravimetrically, and the residual oil fractions were analyzed by gas chromatography. The characteristics of the polluted soil (water-holding capacity and nutrients) were improved significantly by biochar addition (p < 0.05). The total microbial count increased significantly in the treatment containing biochar and added nutrients (t = 23.429, p = 0.002). The degradation of total petroleum hydrocarbons (TPH) and the main hydrocarbon fractions was higher in T3 and T4, especially in T4, than in T1 and T2. The intensities of the n-alkane fraction, C27–C29 steranes and C33–C35 homohopanes were efficiently decreased in T4 compared to the other treatments. According to the results, petroleum-contaminated soil can be remediated efficiently by adding biochar and nutrients simultaneously, and this combination of remediation was superior to that observed with added bulrush straw powder.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Petroleum hydrocarbons consist of complex mixtures containing hundreds to thousands of compounds. Petroleum hydrocarbons can be released to soil by oil spillage, transportation and seeping, leading to serious and persistent soil pollution (Douglas et al. 2012). The quality of petroleum-contaminated soil declines significantly as the compactness of the soil increases, and the water-holding capacity, oxygen and nutrients, especially nitrogen, decrease. The highly toxic and mutagenic characteristics of petroleum compounds and their epoxides are harmful to microbes and higher organisms (Masakorala et al. 2013).
Among the various strategies adopted to decontaminate petroleum hydrocarbons from soil, bioremediation is considered cost-effective and environmentally friendly (Sanscartier et al. 2009). Biostimulation by adding biochar, bulking agents, nutrients, surfactants and other materials can accelerate petroleum degradation in soil (Bushnaf et al. 2011; Li et al. 2016). Physicochemical properties can be used to evaluate the usability and health of soil during the remediation processes. Different types of amendments can improve the quality of polluted soil, increase air/water retention and enhance enzyme activities (García-Delgado et al. 2015). Biochar is a solid elemental carbonaceous material that has recalcitrant carbon structures, which contribute to its long-term carbon storage and climate change mitigation abilities in comparison with the use of other bulking agents (Lehmann et al. 2006). Biochar has greater pore spaces, surface area and functional groups, which improve the physiochemical characteristics of soil, such as the soil structure, water-holding capacity, cation exchange capacity and nutrients (Lehmann et al. 2011; Tang et al. 2013). The addition of biochar to soil can improve soil fertility and the uptake and retention of organic contaminants and has been suggested as a means to help biomass waste management (Marchal et al. 2013). Biochar serves as a potential sorbent for the removal of petroleum hydrocarbons by influencing the cell numbers and activities of soil microorganisms and the transport and biodegradation of contaminants (Meynet et al. 2014).
Nutrients are critical factors in microbial growth and in changes in community structures. The amendment of petroleum-contaminated soil with nutrients could additionally accelerate microbial activities and increase species diversity (Karamalidis et al. 2010). Microorganisms play a key role in the degradation of hydrocarbon compounds. The combined action of added nutrients could stimulate the intrinsic hydrocarbon degraders in soil and enhance the degradation of petroleum hydrocarbon contaminants (Dadrasnia and Agamuthu 2013).
Although many reports have investigated the degradation of petroleum hydrocarbons, limited studies have attempted to investigate the change in soil properties and the decomposition of different types of petroleum compounds (Douglas et al. 2012; Dindar et al. 2013). The objective of the present study was to assess the differing effects of bulrush straw powder, biochar alone and added nutrients on the remediation of petroleum-contaminated soil. The powder and biochar were both produced from the same material, bulrush straw. We wanted to compare the use of the powder and biochar in this type of remediation. Furthermore, the soil properties and degradation extent of specific hydrocarbon fractions were investigated.
Materials and Methods
The petroleum-contaminated soil used in this study was collected from the top 20-cm layer in the Liaohe Oilfield, China. The soil was sieved through a 2-mm mesh after air-drying. The physicochemical and microbial characteristics of the soil employed in the experiments are presented in Table 1. The TPH content of the soil was 9.62 g kg−1 (54.78% saturated hydrocarbons, 16.84% aromatic hydrocarbons and 27.44% polar fractions).
Bulrush straw was dried at 60°C for 24 h, powdered and sieved to obtain a 0.4-mm fraction. Biochar was produced from dried bulrush straw via pyrolysis for 3 h at 300°C under limited oxygen in a controlled atmosphere furnace (Tang et al. 2013). The biochar was ground and passed through a 0.4-mm sieve before use.
Degradation assays were carried out in identical plastic trays (12.5-cm length, 12.5-cm width, and 11-cm height) in four treatments: T1, control treatment (nothing added to the soil); T2, 5% (w/w) bulrush straw powder added to the soil; T3, 5% (w/w) bulrush straw biochar added to the soil; and T4, biochar added to the soil in combination with (NH4)2SO4 and K2HPO4 as nitrogen and phosphorus sources to achieve a C׃N׃P molar ratio of 100׃10׃1. The nutrient sources were added to treatment 4 (T4) every two weeks. Each treatment had three replicates. The remediation experiment was carried out for 8 weeks. Once a week, the soil in the different treatments was turned over.
The pH was measured in 1׃2.5 soil/distilled water suspensions. The water-holding capacity of the soil was measured by soaking 20 g of soil in water for 2 h to form saturated soil and then draining the water for 10 h (Yi et al. 2016). The organic carbon, total nitrogen and total phosphorus contents in the soil were determined according to the Walkley–Black method, Kjeldahl procedure and perchloric acid digestion and sodium carbonate fusion method, respectively.
The TPH content was determined by extracting 10 g of soil with methylene chloride three times. The extract was concentrated on a rotary evaporator, and the amount of residual TPH was then determined gravimetrically (Mukherjee and Bordoloi 2011). The residual TPH was fractionated by silica gel column chromatography into saturated, aromatic and polar fractions. The different fractions were evaporated, and their corresponding amounts were calculated gravimetrically. Then, the saturated and aromatic fractions in crude oil were analyzed by gas chromatography–mass spectrometry (GC–MS) on an Agilent 7890 A gas chromatograph fitted with an HP-5MS column (30 m × 250 × 0.25 µm) and coupled to an Agilent 5975 C mass spectrometer. The column temperature was linearly programmed to increase from 35 to 300°C at 5°C min−1. The mass spectrometric data were acquired at 70 eV in electron ionization mode. The components were identified based on comparison of their total mass spectra against the NIST 08.L database.
Microorganisms were extracted from 10 g of soil using an autoclaved solution (90 mL of NaCl, 0.9%; 1 mL of Na5P3O10, 10.4%; and 0.1 mL of Tween 80.2%) (Salminen et al. 2004). The extracted solution was gradually diluted to 10−2 to 10−6 CFU mL−1. The microorganisms were cultured on agar plates at 30°C for 48 h. The total number of microorganisms was determined by serial dilutions. All the determinations were made in triplicate. The results are expressed as colony-forming units (CFU) per gram of dry soil.
Statistical analysis of the results was carried out using Student’s t test in SPSS 16.0. The data are shown as the average values and standard deviations of three replicates. The variance and significant differences among the various treatments were analyzed, and the tests were considered statistically significant if p < 0.05.
Results and Discussion
Over the course of bioremediation, changes in the physicochemical characteristics of the oil-contaminated soil were monitored by periodically measuring the pH; water-holding capacity; and organic carbon, nitrogen and phosphorus levels (Table 2). At the end of the restoration, the pH in the different treatments was not significantly lower than the pH of the initial soil. After bioremediation, the water-holding capacity of the soil in T2, T3 and T4 increased significantly (t = 5.60, p < 0.05). Added bulrush straw powder and biochar can improve soil compactness and absorb water more readily. Appropriate amendments can greatly improve the soil structure, O2 concentration and water-holding capacity, which, as essential parameters for TPH degradation, in turn lead to greater microbial populations and a high TPH degradation efficiency (Lehmann et al. 2011; Guerin 2015).
The levels of nitrogen and phosphorus were higher in the different treatments of bioremediated soil than in T1. Soil fertility and the C/N ratio were affected by the addition of biochar. The nutrient status of the soil was much better in T3 and T4 than in the other treatments, which illustrates the role of biochar in ion absorption, exchange and retention. Biochar affects the retention of nutrient cations, which can in turn bind nutrient anions such as phosphates and nitrates (Quilliam et al. 2012; Takaya et al. 2016).
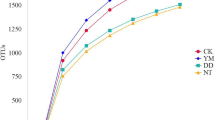
The total microbial count was used to assess the variability of the microbe abundance in the soil samples. The microbial CFU were estimated at the end of the study. A statistically significant difference was observed between the total bacterial numbers in the different treatments in week 8. The addition of biochar and nutrients resulted in greater increases in the microbial communities, with the highest cell count of 1.26 × 108 CFU per gram of dry soil. The bacterial counts in T3 and T2 reached 3.43 × 107 and 2.25 × 106 CFU g−1, respectively (Fig. 1). The cell numbers were significantly increased compared to the cell counts in the initial soil samples from the different treatments (t = 2.902, p = 0.02). Importantly, the addition of biochar in combination with added nutrients greatly increased the number of microorganisms. The pore spaces and high surface area of biochar provides shelter for microbes (Meynet et al. 2014). The structure of biochar allows this material to adsorb available nutrients, which benefit microbial growth, reproduction and metabolism (García-Delgado et al. 2015). Adding nutrients results in greater nutrient retention on the biochar surface and pore spaces (their surrounding) and in more abundant microbial communities. The availability of hydrocarbons increases simultaneously, and the degradation rate of TPH increases as well.
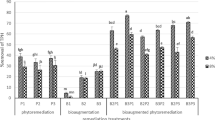
In the biodegradation study, the final efficacy of TPH degradation reached 39.51%, 46.92% and 51.35% in T2, T3 and T4, respectively (Fig. 2). The TPH level in T1 was reduced to approximately 28.2%. The degradation of TPH in T2 was insignificant (t = 4.37, p = 0.12) compared to the control soil. Biostimulation by biochar alone and in combination with added nutrients resulted in a significantly greater reduction of TPH values compared to T1, which ultimately achieved a much lower proportion of the starting level. Notably, simultaneously adding biochar and nutrient biostimulation significantly promoted the degradation efficiency. In terms of the bioremediation efficiency, the biostimulation in T4 was significantly different from that in all other treatments except T3 over the course of the entire experiment, which confirms the advantage of adding biochar and nutrients with specific bioremediation abilities to such types of petroleum-contaminated soil to achieve a high degradation rate. These experiments demonstrate that biochar addition increases the degree and efficiency of microbial degradation by reducing the toxicity of petroleum hydrocarbons to microorganisms in oil-contaminated soils (Cao et al. 2016). Additionally, biostimulation from added nutrients increases the microbial community, thereby increasing the TPH degradation rate (Gojgic-Cvijovic et al. 2012). After the more bioavailable hydrocarbons are degraded, recalcitrant residues and accumulated toxic metabolites remain, slowing the degradation efficiency with the passage of time (Mukherjee and Bordoloi 2011).
At the end of the treatment period, the saturated fraction in T2–T4 was reduced by 58.46%, 70.92% and 80.82%, respectively (Fig. 2). The degradation of saturated hydrocarbons was efficiently promoted by adding biochar as well as in combination with added nutrients, and the biodegradation efficiency increased by 29.41% and 39.31%, respectively, compared to T1. The pore spaces in the biochar can improve the water-holding capacity, air retention and nutrient absorption, which are benefit for microbial survival and saturated hydrocarbon degradation (Bushnaf et al. 2011).
The aromatic degradation in the biochar treatment was significantly higher than the reduction of these compounds in the control treatment and the treatment with added bulrush straw powder. The final degradation efficiency of the aromatic fraction was 44.57% and 50.62% in T3 and T4, and the aromatic fractions were reduced by 22.55% and 34.06% in T1 and T2, respectively (Fig. 2). Studies on the bioremediation of petroleum-contaminated soil by biochar revealed significant degradation of aromatic fractions (Qin et al. 2013).
The degradation rates of the polar fractions in all treatments were significantly lower than those of the saturated and aromatic fractions in the same treatment. The polar fractions were reduced by 21.37% in T4, followed by T3 (17.12%), T2 (9.75%) and T1 (6.11%) (Fig. 2). The polar fractions are a complex mixture of hydrophobic components, which are more resistant to microbial degradation (Yanto and Tachibana 2014).
To further understand the biodegradation differences between the four treatments, different hydrocarbon fractions were analyzed by GC–MS at the end of the experiments (Fig. 3). Compared to T1, the abundances and areas of some substances in T2 and T3 were decreased, such as the compounds C13, C17 and C22. GC–MS analysis confirmed that stimulation by biochar and in combination with the added nutrients was more effective in the degradation of most hydrocarbon fractions. A substantial decrease in the heavy fraction (i.e., C22, C27 and C31) in T4 compared to other treatments is apparent. As the most biodegradable hydrocarbon component, n-alkanes contribute significantly to the biodegradation potential of polluted soil compared to other fractions (Gojgic-Cvijovic et al. 2012). This observed decrease in hydrocarbon fractions may be explained by a previous study in which the presence of biochar and nutrients (N and P) affected the microbial community and activities and enhanced the biodegradation of n-alkanes in soil (Steinbeiss et al. 2009; Bushnaf et al. 2011).
To further understand the presence and intensity of steranes and terpanes, which are regarded as biomarkers and used in the identification of pollutant sources, the GC–MS abundances at m/z 218 and 191 were analyzed, respectively (Gallotta and Christensen 2012; Gojgic-Cvijovic et al. 2012). Figures 4 and 5 show the differences in the biodegradation of steranes and terpanes in all treatments. Regarding the presence of steranes (m/z 218) in T2, T3 and T4, the degradation efficiency of C27, C28 and C29 decreased successively. The distribution of C27, C28 and C29 steranes across all the treatments appeared as a “V” shape, and the abundances of C27 and C29 steranes were greater than the C28 sterane series. The degradation rates of steranes were reduced in the following order T4 > T3 > T2 > T1. The addition of biochar and nutrients was beneficial to sterane degradation.
The pentacyclic C29–C35 terpanes were significantly more abundant in the residual oils of all the treatments. The biodegradation rates of pentacyclic terpanes were the lowest, except homohopanes C33–C35, which might be due to their lower bioavailability (Bost et al. 2001). The treatment with added biochar and nutrients had the most appropriate degradation efficiency of terpanes. Steranes and terpanes were resistant to biodegradation and expected to be attacked by microbes after n-alkanes (Gojgic-Cvijovic et al. 2012). Biochar can provide shelter for microbes and increase air, water and nutrient retention, which aid microbial growth and the degradation of cyclic compounds (García-Delgado et al. 2015).
The present study demonstrated the feasibility of improving and remediating petroleum-contaminated soil by adding biochar and nutrients. Biochar amendment can improve the soil compactness, water-holding capacity, oxygen and nutrients and provide shelter for microbial growth and reproduction. The application of biochar alone and in combination with nutrients efficiently degraded the TPH and different fractions of petroleum hydrocarbons compared to the degradation of the same compounds in the treatment with added bulrush straw powder, especially in the consortium consisting of biochar and added nutrients. The degradation of saturated hydrocarbons (especially the light fraction of alkanes) occurred at a significantly higher rate than for the aromatic and polar fractions of petroleum hydrocarbons, and the decrease in intensity of sterane and terpane biomarkers was lowest. The presence of pores and the high surface area of biochar contributed to microbial growth and the degradation of petroleum hydrocarbons, which was superior to that observed with added bulrush straw powder. Added nutrients increased the concentration of available nutrients for microbial metabolism and accelerated degradation.
References
Bost FD, Frontera-Suau R, McDonald TJ, Peters KE, Morris PJ (2001) Aerobic biodegradation of hopanes and norhopanes in Venezuelan crude oils. Org Geochem 32:105–114. doi:10.1016/S0146-6380(00)00147-9
Bushnaf KM, Puricelli S, Saponaro S, Werner D (2011) Effect of biochar on the fate of volatile petroleum hydrocarbons in an aerobic sandy soil. J Contam Hydrol 126:208–215. doi:10.1016/j.jconhyd.2011.08.008
Cao Y, Yang B, Song Z, Wang H, He F, Han X (2016) Wheat straw biochar amendments on the removal of polycyclic aromatic hydrocarbons (PAhs) in contaminated soil. Ecotoxicol Environ Saf 130:248–255. doi:10.1016/j.ecoenv.2016.04.033
Dadrasnia A, Agamuthu P (2013) Dynamics of diesel fuel degradation in contaminated soil using organic wastes. Int J Environ Sci Technol 10:769–778. doi:10.1007/s13762-013-0224-1
Dindar E, Şağban FOT, Başkaya HS (2013) Bioremediation of petroleum-contaminated soil. J Bio Environ Sci 7:39–47
Douglas GS, Hardenstine JH, Liu B, Uhler AD (2012) Laboratory and field verification of a method to estimate the extent of petroleum biodegradation in soil. Environ Sci Technol 46:8279–8287. doi:10.1021/es203976a
Gallotta FDC, Christensen JH (2012) Source identification of petroleum hydrocarbons in soil and sediments from Iguaçu River watershed, Paraná, Brazil using the CHEMSIC method (CHEMometric analysis of selected Ion chromatograms). J Chromatogr A 1235:149–158. doi:10.1016/j.chroma.2012.02.041
García-Delgado C, Alfaro-Barta I, Eymar E (2015) Combination of biochar amendment and mycoremediation for polycyclic aromatic hydrocarbons immobilization and biodegradation in creosote-contaminated soil. J Hazard Mater 285:259–266. doi:10.1016/j.jhazmat.2014.12.002
Gojgic-Cvijovic GD, Milic JS, Solevic TM, Beskoski VP, Ilic MV, Djokic LS, Narancic TM, Vrvic MM (2012) Biodegradation of petroleum sludge and petroleum polluted soil by a bacterial consortium: a laboratory study. Biodegradation 23:1–14. doi:10.1007/s10532-011-9481-1
Guerin TF (2015) A safe, efficient and cost effective process for removing petroleum hydrocarbons from a highly heterogeneous and relatively inaccessible shoreline. J Environ Manag 162:190–198. doi:10.1016/j.jenvman.2015.07.016
Karamalidis AK, Evangelou AC, Karabika E, Koukkou AI, Drainas C, Voudrias EA (2010) Laboratory scale bioremediation of petroleum-contaminated soil by indigenous microorganisms and added Pseudomonas aeruginosa strain Spet. Bioresour Technol 101:6545–6552. doi:10.1016/j.biortech.2010.03.055
Lehmann J, Gaunt J, Rondon M (2006) Bio-char sequestration in terrestrial ecosystems – a review. Mitig Adapt Strategy Glob Change 11:395–419. doi:10.1007/s11027-005-9006-5
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effects on soil biota: a review. Soil Biol Biochem 43:1812–1836. doi:10.1016/j.soilbio.2011.04.022
Li X, Tong D, Allinson G, Jia C, Gong Z, Liu W (2016) Adsorption of pyrene onto the agricultural by-product: corncob. Bull Environ Contam Toxicol 96:113–119. doi:10.1007/s00128-015-1687-1
Marchal G, Smith KEC, Rein A, Winding A, Wollensen de Jonge L, Trapp S, Karlson UG (2013) Impact of activated carbon, biochar and compost on the desorption and mineralization of phenanthrene in soil. Environ Pollut 181:200–210. doi:10.1016/j.envpol.2013.06.026
Masakorala K, Yao J, Chandankere R, Yuan H, Liu H, Yu C, Cai M (2013) Effects of petroleum hydrocarbon contaminated soil on germination, metabolism and early growth of green gram, Vigna radiata L. Bull Environ Contam Toxicol 91:224–230. doi:10.1007/s00128-013-1042-3
Meynet P, Moliterni E, Davenport RJ, Sloan WT, Camacho JV, Werner D (2014) Predicting the effects of biochar on volatile petroleum hydrocarbon biodegradation and emanation from soil: a bacterial community finger-print analysis inferred modelling approach. Soil Biol Biochem 68:20–30. doi:10.1016/j.soilbio.2013.09.015
Mukherjee AK, Bordoloi NK (2011) Bioremediation and reclamation of soil contaminated with petroleum oil hydrocarbons by exogenously seeded bacterial consortium: a pilot-scale study. Environ Sci Pollut Res Int 18:471–478. doi:10.1007/s11356-010-0391-2
Qin G, Gong D, Fan MY (2013) Bioremediation of petroleum-contaminated soil by biostimulation amended with biochar. Int Biodeterior Biodegradation 85:150–155. doi:10.1016/j.ibiod.2013.07.004
Quilliam RS, Marsden KA, Gertler C, Rousk J, DeLuca TH, Jones DL (2012) Nutrient dynamics, microbial growth and weed emergence in biochar amended soil are influenced by time since application and reapplication rate. Agr Ecosyst Environ 158:192–199. doi:10.1016/j.agee.2012.06.011
Salminen JM, Tuomi PM, Suortti AM, Jørgensen KS (2004) Potential for aerobic and anaerobic biodegradation of petroleum hydrocarbons in boreal subsurface. Biodegradation 15:29–39. doi:10.1023/B:BIOD.0000009954.21526.e8
Sanscartier D, Reimer K, Koch I, Laing T, Zeeb B (2009) An investigation of the ability of a 14C-labeled hydrocarbon mineralization test to predict bioremediation of soils contaminated with petroleum hydrocarbons. Biorem J 13:92–101. doi:10.1080/10889860902902057
Steinbeiss S, Gleixner G, Antonietti M (2009) Effect of biochar amendment on soil carbon balance and soil microbial activity. Soil Biol Biochem 41:1301–1310. doi:10.1016/j.soilbio.2009.03.016
Takaya CA, Fletcher LA, Singh S, Okwuosa UC, Ross AB (2016) Recovery of phosphate with chemically modified biochars. J Environ Chem Eng 4:1156–1165. doi:10.1016/j.jece.2016.01.011
Tang J, Zhu W, Kookana R, Katayama A (2013) Characteristics of biochar and its application in remediation of contaminated soil. J Biosci Bioeng 116:653–659. doi:10.1016/j.jbiosc.2013.05.035
Yanto DHY, Tachibana S (2014) Potential of fungal co-culturing for accelerated biodegradation of petroleum hydrocarbons in soil. J Hazard Mater 278:454–463. doi:10.1016/j.jhazmat.2014.06.039
Yi YM, Park S, Munster C, Kim G, Sung K (2016) Changes in ecological properties of petroleum oil-contaminated soil after low-temperature thermal desorption treatment. Water Air Soil Pollut 227:1–10. doi:10.1007/s11270-016-2804-4
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 41571464), Program of Liaoning Education Department (No. LR2015035) and Environmental Science and Engineering Innovation Team Programme of Liaoning Shihua University ([2014]-11).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Y., Li, F., Rong, X. et al. Remediation of Petroleum-contaminated Soil Using Bulrush Straw Powder, Biochar and Nutrients. Bull Environ Contam Toxicol 98, 690–697 (2017). https://doi.org/10.1007/s00128-017-2064-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-017-2064-z