Abstract
The pursuit of environmental sustainability and the harnessing of renewable energy sources pose significant challenges, compelling researchers to explore innovative solutions. Carbon materials have emerged as crucial players in both energy, environmental, and agricultural applications, owing to their exceptional properties. Biomass waste, abundant and often overlooked, has captured attention as a promising precursor for the development of carbon-based products. This is particularly evident in the creation of biochar and hydrochar, whose characteristics are intricately shaped by production methods, source materials, and process conditions. These variables collectively influence their suitability for diverse purposes, ranging from energy storage and conversion to soil and water restoration, making them invaluable tools in sustainable agriculture and environmental conservation, as well as in the capture of greenhouse gases. The versatility of biomass-based activated carbon is further enhanced by the diverse array of feedstocks and activation pathways employed. This adaptability renders it suitable for a multitude of applications, creating a symbiotic relationship between resource abundance and functional efficacy. This comprehensive review aims to evaluate contemporary thermochemical methods for converting organic waste into high-value carbon materials. Moreover, it delves into strategies that augment the functionality of these materials, including activation processes and surface modifications. The review also illuminates recent advancements in the realms of energy, agriculture, and environmental research. It consolidates existing literature on physicochemical characteristics and techno-economic assessments of engineered carbon materials, providing a nuanced understanding of their potential impact. While exploring challenges, prospects, and future research directions, this review outlines the synthesis of carbon compounds from biomass. It emphasises the capacity to produce distinct chars with unique properties through various production methods, tailoring them to the specific requirements of diverse environmental applications.
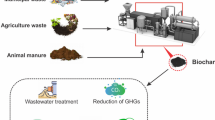
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organic waste as renewable material has an immense potential for energy recovery and various environmental applications. Organic waste is composed of discarded plant and animal residues including food waste, yard waste, agricultural waste, forest residue, animal manure, sludge, and processed organic waste from industries. Valorisation of organic waste into sustainable by-products through chemical, thermal, and biological processes has been greatly emphasised in the recent years (He et al. 2022). Apart from the conventional processes of composting and anaerobic digestion (AD), advanced biochemical conversion techniques specific to each type of organic waste which helps in the recovery of novel bio-based assets including pectin, enzymes, biopolymers, oligosaccharides, and relatively high intermediates like lactic and furfural acid are also being carried out. However, longer residence times, high waste processing costs, and limited flexibility in the process-products are some of the major constraints for adopting them on a commercial scale (Li et al. 2022).
Thermochemical conversion processes of organic waste which includes pyrolysis, gasification, combustion, and hydrothermal techniques are commended for their flexibility in process conditions for the recovery of by-products specific to variable applications (Yang et al. 2016). While pyrolysis is thermal degradation of waste in the absence of air at temperatures around 300–800 °C, gasification occurs at much higher temperature (700–1200 °C). A maximum temperature of 300 °C is required for the gradual heating process known as torrefaction to occur in an inert or oxygen-deficient atmosphere. Furthermore, hydrothermal conversion of biomass involves treating biomass with high-temperature water or steam at elevated pressures. The process is conducted under conditions typically ranging from 180 to 300 °C, utilising both temperature and pressure to facilitate the efficient conversion of biomass into desired end products (Sharma et al. 2020; Funke and Ziegler 2010). Hydrothermal processes utilise pressure (using steam) along with temperature the degradation of the material. The major by-products of all these thermal degradation processes are solid carbon materials, liquid oil, and syngas, whose composition and properties vary with respect to the technique and associated process parameters adopted (Tag et al. 2016).
As a major by-product, biochar is the traditional name given to solid by-product of slow/fast pyrolysis, torrefaction, and gasification (Yu et al. 2017; Zhou et al. 2018). Likewise, the solid carbon material derived from hydrothermal carbonisation of waste is referred to as hydrochar. Despite the fact that some research misinterprets the terms "biochar" and "hydrochar", under a blanket name of biochar (Liu et al. 2019), in the current review article we have adopted the widely accepted individual literature conventions for bio-/hydrochar definitions as stated above. The carbonisation mechanism, composition, and the surface characteristics of chars are influenced by the feedstock's inherent characteristics along with the process parameters including temperature, holding time, heating rate, reaction catalyst, etc., (Tag et al. 2016). For hydrothermal carbonisation, pressure and water-feed ratio are other determining factors. The major defining factors of the chars include their carbonaceous skeletal structure, surface area, surface functionality along with some nutrient content which drive their applicability in energy and environment related fields. Moreover, the physicochemical properties of chars can be engineered through process modifications or post-processing techniques to advance their utility in varied applications (Panahi et al. 2020a; b). A brief overview of the focussing areas of selected review articles on biochar and hydrochar is presented in Table 1.
Several publications have recently been published on storing energy of carbon materials obtained from biomass, mitigation of increasing contaminants, and their use in industrial applications (Zhu and Xu 2020) and application of chars as supercapacitors (Cuong et al. 2021; Rashidi et al. 2022). However, critical reviews encapsulating all the engineered carbon materials derived from organic wastes for energy and environmental applications are limited. Hence, the chief objective of this review paper is to accumulate the knowledge on engineered carbon materials derived from thermochemical conversion of organic wastes its further application in the field of energy and the environment. The article highlights the key advances in production and modification techniques of these carbon materials for specific applications. The article critically focusses on the advancements in the energy and environmental related applications of these carbonaceous materials and identifies potential research gaps. The sustainability of the char industry along with the main challenges towards the wide applications of chars in water, energy, and agricultural sectors has been deeply investigated. Finally, the article also summarises the available literature on techno-economic assessment of these materials and briefly discusses the challenges, opportunities, and future research needs in the industry.
Biochar and hydrochar: production and mechanism
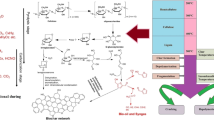
Biochar is a highly permeable carbonaceous granular by-product majorly derived from the pyrolysis of organic materials. Pyrolysis for biochar production can be categorised as slow, fast, or flash pyrolysis (Ighalo et al. 2022) process depending on the heating rate, pyrolysis temperature, pressure, and retention time. Specific methods of pyrolysis with their process parameters, and product compositions are presented in Table 2. The most common feedstocks for carbonisation are rice husk, rice straw, shells, fruit and vegetable waste, animal manure, sludge, woodchips, residue of crops, and sugarcane waste (Miandad et al. 2016). The primary components of biomass are cellulose, hemicellulose, and lignin, which are subjected to pyrolysis's depolymerisation, fragmentation, and cross-linking reactions (Lehmann et al. 2021). Cellulose is initially broken down into oligosaccharides through depolymerisation, after which D-glucopyranose is generated (Li et al. 2001). This D-glucopyranose then experiences an intramolecular rearrangement, resulting in the formation of levoglucosan. Levoglucosan is involved in the production of levoglucosenone via a dehydration step. This levoglucosenone can then follow several pathways, including dehydration, decarboxylation, aromatisation, and intramolecular condensation, to eventually yield a biochar product (Li et al. 2001). Levoglucosan can undergo a sequence of rearrangements and dehydration processes to give rise to hydroxymethyl furfural (HMF). This HMF can be either decomposed into bio-oil and syngas or undergo a series of polymerisation, aromatisation, and intramolecular condensation reactions to generate biochar. The specific pathways for cellulose decomposition during pyrolysis are depicted in Fig. 1a. The pyrolysis mechanism of hemicellulose is akin to that of cellulose. It begins with depolymerisation into oligosaccharides, followed by the breaking of glycosidic linkages in the xylan chain and rearrangement of the depolymerised molecules to yield 1,4-anhydro-D-xylopyranose (Shen et al. 2010). This 1,4-anhydro-D-xylopyranose can then undergo various reactions, including dehydration, decarboxylation, aromatisation, and intramolecular condensation, resulting in the production of biochar or its decomposition into lower molecular weight compounds like bio-oil and syngas (Liu et al. 2015a, b). The specific pathways for hemicellulose decomposition during pyrolysis are outlined in Fig. 1b.
Lignin decomposition majorly involves the breakage of β-O-4 lignin linkage resulting in the generation of several free radicals (Vanapalli et al. 2021a). The formation of decomposed compounds (majorly biochar) occurs through free radical interactions with protons from other species. Free radicals are created through the rupture of the β-O-4 lignin linkage. These free radicals have the ability to seize protons from other species possessing weak C–H or O–H bonds, leading to the creation of decomposed compounds (Yu et al. 2017). These radicals can be transferred to other molecules, initiating and propagating chain reactions. The chain reactions ultimately conclude when two radicals collide and combine to create stable compounds. However, it is important to note that observing these radicals during pyrolysis is a challenging task, making it difficult to determine the exact mechanism of lignin pyrolysis. The specific pathways for lignin decomposition during pyrolysis are illustrated in Fig. 1c.
Thermal degradation initiates during the drying stage, where the moisture in the biomass is evaporated, and only physical changes occur in the material, while the chemical composition is almost unchanged. The thermal response of the materials is evident at the pre-heating stage, and the chemical composition starts to transform. Hemicellulose, one of the unstable components of biomass, breaks down into carbon dioxide, carbon monoxide, a minute proportion of acetic acid, etc. The phase of solid dissociation occurs under the condition of hypoxia to produce carbon-rich biochar, and the liquid products produced contain acetic acid, wood tar, and methanol (Lin et al. 2022). The gaseous products contain CO2, CO, CH4, H2, etc.
The process of hydrochar formation differs slightly from that of pyrolysis biochar. Hydrothermal conversion of biomass (preferably wet biomass) for the recovery of solid hydrochar has been emerging as a noticeable technology for municipal organic waste management (He et al. 2022). Hydrothermal conversion process utilises distilled or deionised water as a stimulant under low temperature to convert feedstock within a range of residence time (few minutes to several hours) (Varsha et al. 2022). The difference between pyrolysis and hydrothermal carbonisation is presented in Table 3. The exact mechanism of hydrothermal carbonisation is still unknown and is very complicated. The literature suggests that factors including pH, process temperature, type of feedstock, residence time, pressure, and phenolic compounds majorly affect the quality, yield, and stability of hydrochar (Li et al. 2022). Numerous researchers reported that hydrothermal carbonisation is a multi-step chemical process. The first and foremost step is hydrolysis where water followed by dehydration, decarboxylation, polymerisation, and aromatisation (Cai et al. 2016a, b; Lyu et al. 2018). Hydrolysis is the first and foremost reaction where the larger component of the biomass reacts with water and convert into smaller components which in turn produces hydrochar. Usually, the components present in the biomass, viz., hemicelluloses, glucose, fats, and lipids, are partially stable and easily hydrolysed under lower temperature (150–180 °C) in comparison, high temperature (250 °C) is required to hydrolyse cellulose content in the biomass.
The mechanisms governing hydrochar formation from cellulose are presented in Fig. 2a. The extended cellulose chains undergo degradation into smaller molecules, specifically oligomers. These oligomers are subsequently converted into glucose, and a portion of the glucose undergoes isomerisation to form fructose. The products of hydrolysis then undergo a sequence of isomerisation, dehydration, and fragmentation reactions, giving rise to important intermediates such as 5-HMF or furfural, as well as the products derived from them (Promdej and Matsumura 2011). These intermediates engage in additional polymerisation and condensation reactions, along with reverse aldol condensation and intermolecular dehydration. This process results in the formation of hydrochar. Hydrochar derived from cellulose exhibits a polyaromatic structure characterised by polyfuranic rings. It features a hydrophobic core and a hydrophilic shell. Similar to cellulose, hemicellulose (xylan) initially undergoes hydrolysis into its monomers (xylose), followed by the generation of another crucial intermediate, furfural (Fig. 2b), as reported by several researchers. Furfural serves as the precursor for char formation through the process of polymerisation. The SEM (scanning electron microscope) spectra revealed that the surface of hydrochar derived from D-xylose was covered with microspheres, each having diameters ranging from 1 to 5 µm (Kang et al. 2012).
Most of the lignin fragments are challenging to dissolve and distribute within the aqueous phase when the hydrothermal carbonisation (HTC) temperature is not sufficiently high (for instance, below 377 °C at a water density of 954 kg/m3). However, a portion of the lignin can be dissolved in water at 200 °C (Kang et al. 2012). Because of its intricate structure and high molecular weight, the reaction mechanisms involving lignin are quite complex.
Figure 2c illustrates a streamlined process for the formation of hydrochar from lignin. In the first stage, the dissolved lignin experiences decomposition via hydrolysis and dealkylation in a uniform reaction. This process leads to the creation of phenolic products like syringols, guaiacols, catechols, and phenols (Wang et al. 2018a, b, c). Subsequently, these intermediates engage in a cross-linking reaction and re-polymerise to form phenolic char. Finally, most of lignin, which could not be dissolved in water, is converted into polyaromatic hydrochar (PH) via solid formation mechanisms similar to pyrolysis.
A comparative review of biochar and hydrochar in terms of physicochemical properties
Due to a lower level of dehydration in the hydrothermal carbonisation (HTC) process, hydrochar frequently exhibits a lower carbon content compared to biochar produced under conventional temperature ranges (Bargmann et al. 2013), especially when utilising crop residues as the feedstock biomass. In contrast to biochar, hydrochar demonstrates a notable reduction in ash content (Fang et al. 2018). Unlike biochar, which retains all the ash from the feedstock during HTC, hydrochar retains only a portion of it (Parshetti and Balasubramanian 2014). While all the ash in the feedstock is retained by biochar during HTC, only some of it is retained by hydrochar (Parshetti and Balasubramanian 2014). As a result, biochar is perhaps more alkaline than hydrochar. Biochar has a wider pore capacity and a higher surface area than hydrochar because of its higher production temperatures and the possibility of gas flow (in some situations) (Lehmann 2012). Because the breakdown products remain persistent on the hydrochar, it is less porous (has a smaller surface area). The qualities of hydrochar versus biochar could differ depending on the applications. For instance, it is preferable for most forms of hydrochar to have a reasonably low ash concentration when being utilised as fuel. The reduced ash percentage of hydrochar suggests that it may be a more acceptable precursor for activated carbon, even though large surface area and pore volumes are associated with increased sorption ability. Table 4 briefly depicts the comparison of physicochemical properties between biochar and hydrochar.
Engineering carbon materials from biochar and hydrochar
In regards of their several specific properties, like low cost, high performance, and lesser environmental burden, chars (biochar and hydrochar) have developed keen interest as effective alternatives to conventional carbon-rich materials (Li et al. 2019a, b; Liang et al. 2019; Mao et al. 2019; Zhang et al. 2019a, b, c, d, e). Typically, chars can be characterised by their surface properties such as negative surface functional groups and a porous skeletal structure. However, the surface area and surface functionalisation of pristine chars do not match up to the performance of commercial activated carbon materials (Jung et al. 2019; Luo et al. 2019). For instance, pristine biochar was observed to have very low contaminant adsorption capability from highly concentrated wastewater. Furthermore, it was very hard to segregate from water because of its small particle size and low density, which severely restricts its application (Tan et al. 2016). Similarly, the low porosity of hydrochar due to the deposition of persistent decomposition products on its surface needs to be compensated through further modifications to be efficient carbon materials in environmental remediation (Fang et al. 2018).
Given that specific surface area is a key parameter governing the overall performance of carbonaceous materials, chars need to be further enhanced to compensate for their lower surface area and exhibit comparable reactivity to conventional carbonaceous materials. Engineering carbon materials from these chars involve techniques including surface grafting of oxygen-containing functional groups, doping with hetero-atoms, physical, chemical, and biochemical activation, and incorporation into composites which is popularly referred to as char engineering (Sik et al. 2015; Zhang et al. 2020a, b, c). Figure 3 shows the different production and modification methods of engineered char materials.
Engineered char is a derivative of pristine char that has been modified physically, chemically, or biologically to improve its physicochemical, and biological properties (e.g. specific surface area, porosity, cation exchange capacity (CEC), surface functional group, pH, etc.) and its adsorption capacity compared to pristine char (Mohamed et al. 2016; Rajapaksha et al. 2016; Yao et al. 2013).
Figure 4 depicts the impact of different modification methods on physicochemical properties of engineered char materials. Char engineering allows for the modification of properties that are optimal for specific applications and/or conditions. This would result in harnessing the beneficial properties of char and increasing its efficiency while minimising the existing trade-offs. While activated carbon derived from biomass is one of the types, the definition of engineered char is much broader. Majority of char engineering methods are either more convenient or less expensive than traditional carbon activation processes. The properties of chars for adsorbing inorganic and organic ions (i.e. particle size and specific surface area) could be engineered through physical ball milling (Cai et al. 2016a, b; Lyu et al. 2018). Through an optimised planetary ball mill process, for example, the surface area of a corn-stover-based biochar could be increased by more than 3.2 times, reaching 194 m2/g (Peterson et al. 2012). In addition to micropores and surface area, the functional groups within the char could be modified during ball milling in the presence of an appropriate chemical, a process known as chemical ball milling and is represented in Fig. 5. Through ball milling, nano-sized char with organic and inorganic contaminants removal performance comparable to carbon nanotubes and activated carbon could be produced (Shan et al. 2016).
A model of porous engineered biochar containing different functional groups (Lee et al. 2017)
The positive properties of char could be nearly doubled by activating it with gas/steam, such as air, CO2, water vapour, and others. In terms of agriculture, this method produces chars with higher nutrient retention, which plants may absorb (Borchard et al. 2012). This activation method also removes incomplete combustion products and other impurities from char. Overall, gas/steam activation could be used as a preliminary treatment to increase the surface area of char before moving on to a second technique aimed at positively modifying char functional groups. The use of microwave pyrolysis is a relatively new method of speeding up the process. Furthermore, microwave irradiation pyrolysis eliminates the need for biomass shredding and drying. When compared to conventional biochar, microwave pyrolysis-derived biochar would have a larger surface area (Brunauer–Emmett–Teller, BET, of up to 450–800 m2/g) and more functional groups (Wang et al. 2018a, b, c). If this engineered char material is used as an agent in sustainable environmental management, it has the potential to improve soil water retention and cation exchange capacity. The functional groups in char are enriched through this strategy, which improves its adsorption selectivity and capacity to desired chemical species (i.e. heavy metals). Some functional groups containing coordinate atoms (e.g. N, O, or S) that can chelate with metal ions are amino and amide, carboxyl, hydroxyl, sulfhydryl, and sulfonyl (Zhou et al. 2018). Because of ion exchange, electrostatic interaction (between the positively charged metal cation and negatively charged carboxyl anion), and complexation, the carboxyl group has a strong affinity to coordinate with metal ions (Yu et al. 2017; Zhou et al. 2018). Chemical-based methods involve activating biomass/char in the presence of chemicals and inert gas. More specifically, acids or bases could be used to oxidise char, improving micropores, surface area, cation exchange capacity, and the availability of functional groups in chars. Masoumi and Dalai (2020) found that the chemical activation of algal hydrochar using K2CO3 or KOH resulted in the increase of specific surface area from 4 to 2100 m2/g which was used as catalysts/catalyst support. Chemical modification in the presence of an appropriate oxidant effectively increases char's sorption capacity and heavy metals uptake. More specifically, acid treatment results in more carboxyl groups on char, which is suitable for better Cu, Pb, and Zn adsorption (Uchimiya et al. 2012a), sulfamethazine (Vithanage and Rajapaksha 2015), Cd and oxytetracycline (Aghababaei et al. 2017). Alkaline treatment, on the other hand, increases surface graphite C and/or aromatic functional groups (e.g. hydroxyl groups) while decreasing surface electrostatic attraction, p-p interaction, surface precipitation, and/or surface complexation, which is better for As, Cd, tannic acid, and chloramphenicol. The use of oxygen plasma to activate char is a more recent technique for achieving rapid and cost-effective activation at temperatures lower than 150 °C. Gupta et al. (2015) found that 5-min oxygen plasma activation improved the supercapacitor characteristics (171.4 F/g) of yellow pine biochar. Capacitance improvements of 185 per cent and 72.3 per cent were observed when pristine and conventional base activated biochar’s were compared.
Table 5 summarises the impact of physical modification techniques on the properties of engineered char materials. Acidic functionalisation of hydrochar with the introduction of SO3OH or –COOH functional groups into the hydrochar matrix is one of the most common techniques of modification making it suitable as a solid acid catalyst for cellulose hydrolysis or biodiesel production (Masoumi et al. 2021). For instance, Huang et al. (2016) reported the highest biodiesel yield of 95.4% from oleic acid transesterification using catalysts obtained from carbonising lignin in supercritical ethanol sulfonated with H2SO4. The surface area of char could also be increased by incorporating different nanoparticles or metal oxides onto its surface using various chemical impregnation/coating techniques at various thermochemical processing steps (i.e. in-situ or post-modifications). Takaya et al. (2016b) used the versatility of the clay-coating technique to recover phosphate from wastewaters using Mg impregnated biochar. As a result, the phosphate adsorption capacity of biochar made from oak wood increased by 31.6–33.5 times, reaching 70.3%. Synthesis of magnetic carbon composites generally involves impregnation of chars with iron salts followed by chemical co-precipitation of iron oxide nano particles (IONPs) (Reynel-Ávila et al. 2021). Their affinity for specific substances helps in the separation/purification of targeted contaminants and their magnetic properties can be advantageous for easy recovery from fluids using magnetic decantation (Franzreb 2020). For instance, Patiño et al. (2021) have synthesised a novel magnetic hydrochar with super paramagnetic properties (saturation magnetisation of 55.21 emu/g) which has a potential application in environmental remediation.
Microbial digestion of char materials, on the other hand, can alter the redox potential and pH values of the feedstock biomass, which creates a digested char with relatively higher pH, surface area, CEC, anion exchange capacity (AEC), and hydrophobicity, along with a negative surface charge as compared to pristine char (Inyang et al. 2010). The improvement of CEC and AEC suggests that biologically activated char could be used as ion exchangers, sequestering both positively and negatively charged ions from water.
Energy and environmental applications of pristine and engineered carbon materials
Application in composting
Compost, just like biochar/hydrochar, uses biodegradable organic wastes as feedstock for recycling carbon and nutrients in the soil. While high moisture content (60–70%) and low lignin content feedstocks such as food waste were considered ideal for composting, low moisture content (15–25%) and high lignin content feedstocks such as agricultural residues, and lignocellulosic biomasses are usually preferred for biochar production (Gajalakshmi and Abbasi 2008; Pang and Mujumdar 2010). In contrast, hydrothermal carbonisation prefers feedstocks with high moisture content and lignin content.
The addition of char-based materials as co-substrates in composting has recently gained prominence as their mutual interactions depicted the potential to maximise the benefits of both the materials (Wu et al. 2017). The possible chemical processes of adding char during composting are represented in Fig. 6. Addition of char during composting was reported to improve aeration, accelerate compost development, reduce odour, greenhouse gas emissions (CH4 and N2O), promote nutrient retention, immobilise heavy metals, and improve the overall quality of compost itself (Abujabhah et al. 2016; Fischer and Glaser 2012). Moreover, its positive priming effect in stimulating microbial activity and its negative priming effect of stabilising labile organic matter were also reported (Fischer and Glaser 2012). These synergistic effects could be majorly attributed to the char’s porous nature, large surface area, and high water holding capacity. Moreover, Dias et al. (2010) reported addition of biochar as a bulking agent resulted in 70% degradation of the organic matter and intense humification of the material, thereby producing mature composts with a high fertiliser value. This could be attributed to the recalcitrance, negative surface charge, and cation exchange capacity of chars itself help in charging its surface with the supply of nutrients from the compost and thereby increase its nutrient retentivity and loading of the final compost (Fischer and Glaser 2012). This further leads to increase in the overall reactivity of biochar surfaces with composting (Fischer and Glaser 2012; Lehmann 2012). This in turn increases its chemical adsorption potential, which was reported to reduce bioavailable HMs and remediate contaminants in the soil matrix (Borchard et al. 2012). Although hydrochar can also be a source of labile carbon and support nutrient retention, its relatively low stability can be a limiting factor for long-term C sequestration (Busch and Glaser 2015). Moreover, hydrochar was found to enhance N2O and CO2 emissions possibly due to the enhanced microbial activity and lower stability in the soil (Kammann et al. 2012). So, the potential environmental risks of hydrochar co-composting must be carefully evaluated before application.
The possible chemical processes of adding chars during composting (Adapted with modifications from (Godlewska et al. 2017))
So, engineered chars with enhanced surface microstructure, greater ion exchange capacity promoted by more surface functional groups can further promote composting efficiency. For instance, Ye et al. (2019) demonstrated that co-composting of contaminated soil with activated biochar addition showed efficient performance for decontamination and detoxification of soil polluted with metals and PAHs in tidal wetlands. Similarly, Chen et al. (2022a, b, c) reported decrease in the concentration of diethylenetriamine pentaacetic acid extractable heavy metals during composting with H3PO4 modified biochar by 15.15% (Cu), and 36.50% (Zn) as compared to 12.04% (Cu), and 26.91% (Zn) in the pristine biochar. Further, the effects of 10% H2O2 modified cornstalk biochar to reduce ammonia emissions from compost (by 61.69%) by increasing the number of ammonia-oxidising bacteria and decreasing urease activity were also depicted by Zhou et al. (2021). However, all these effects are also highly dependent on the proportion of chars in the mixture. At very low rates of application, chars probably cannot induce any significant changes in the properties; an excessive dosage, on the other hand, could interfere with the biodegradation of the composting material. This necessitates future research on dose optimisation for improved synergism between both the materials. Moreover, the surface functionality and morphological changes of conventional biochar during composting have been depicted by some previous studies (Wu et al. 2016, 2017), understanding the mechanisms of interactions affecting the physicochemical characteristics and abiotic/biotic oxidation of different engineered chars during composting need to be further research.
Application in soil amendment
Earliest scientific evidence on improved properties of soil with the application of carbon-derived organic materials can be associated with fertile terra preta soils of the central amazon (Lehmann 2012). Although direct application of organic feedstocks was reported to improve the overall quality of soil (Li et al. 2019a, b; Soon and Lupwayi 2012; Surekha et al. 2003), their rapid decomposition in soil limits their utility for long-term amendment.
Engineered carbon substances such as biochar/hydrochar due to their porous surface morphology and superior physicochemical characteristics are reported to promote carbon sequestration, nutrient retention, reduced bioavailability of contaminants, improved water holding capacity, and create suitable habitat creation for microbial population when applied as a soil amendment (Islam et al. 2021; Lehmann 2012) (Fig. 7). Notably, these substances are reported to have a significant influence on the biochemical and biophysical mechanisms governing interactions among soil microorganisms, mesofauna, and macrofauna, which in turn has profound effects on both aboveground and belowground soil ecosystem (Ameloot et al. 2013; Lehmann et al. 2011). However, the extent of these effects is contingent upon variable factors including soil type, feedstock, hydrology, rate of application, and climatic conditions (Islam et al. 2021; Mukherjee and Lal 2013).
Porosity, surface morphology, oxygen-containing functional groups, cation exchange capacity, and nutrient content of chars are important factors governing their soil amending properties (Awasthi 2022). The porous surface structure of chars aids in reduced hydraulic conductivity of soil, thereby relatively increasing its water holding capacity (Devereux et al. 2012). Especially, the capillary action of micropores promote water retentivity with longer retention times in chars than macropores (larger than 10–20 µm) (Lehmann 2012). Moreover, the effects of secondary parameters such as zeta potential and cation exchange capacity are associated with adsorption of hydrated ions on the surface (Batista et al. 2018). The microporous structure of the chars serves as a conducive environment for the microbial community, serving as a stable source of nutrients and facilitating their prosperity (Joseph et al. 2013; Quilliam et al. 2013). However, the presence of volatile organic carbons, environmentally persistent free radicals and phytotoxic compounds could turn out lethal to the survival of microbes (Lehmann et al. 2011). For instance, George et al. (2012) attributed the presence of phytotoxic compounds including organic acids and phenols for the negative effects of hydrochar on the abundance of arbuscular mycorrhiza fungal root colonisation. Moreover, char’s positive influence over the physicochemical properties of soil like pH, nutrient content, aeration, carbon recycling, etc., also seemed to improve the soil quality (Islam et al. 2021; Quilliam et al. 2013). The strong adsorptive capacity of chars for ionic solutes significantly contributes to nutrient retention in soil. Field and column studies reported char’s ability in soil to reduce the leaching of soluble nutrients such as ammonium (Takaya et al. 2016a; Yao et al. 2012), nitrate (Yao et al. 2012), sulphate (Zhao et al. 2019), phosphate (Trazzi et al. 2016), etc. Further, the same phenomena aids in bioremediation that helps to reduce the bioavailability and phyto-availability of several heavy metals and organic pollutants in a contaminated soil (Ogbonnaya and Semple 2013; Qin et al. 2013; Sun et al. 2018).
So, engineering chars to enhance these surface and physicochemical characteristics is necessary to further enhance their soil amending properties. These engineered char amendments should consequently assume a multifaceted role, contributing to carbon sequestration, water conservation, nutrient retention, microbial growth, and the immobilisation of heavy metals in soil (Khan et al. 2023). The carbon sequestration potential of char depends on its yield and recalcitrance when added to soil. Studies suggest that with increase in pyrolysis temperature, the easily mineralisable aliphatic carbons transform into aromatic compounds which oxidise relatively slowly (Mimmo et al. 2014; Zimmerman 2010). Zimmerman (2010) estimated half-lives of biochar ranging from 102 to 107 years with carbon losses of 3–26% in 100 years. Moreover, this is true even in the case of hydrochar, which has a significantly higher proportion of labile carbon, thereby having relatively low stability in the soil (Dicke et al. 2014). De Jager et al. (2022) depicted greater interaction and association between hydrochar carbon and soil organic matter in hydrochar produced at higher temperatures. However, the study emphasised that this kind of C stabilisation may not be enough to counteract or balance the losses observed in hydrochar amended soils due to initial positive priming effect, leading to loss of labile carbon fraction. While pristine biochar, as a carbon-negative technology, has garnered considerable attention, there remains a gap in the comprehensive study of the long-term stability of various engineered chars in soil. This gap is particularly significant due to conflicting findings in some studies, where an increase in thermal stability is observed alongside lower chemical stability in soil, as seen in the case of biochar-based fertilisers produced from co-pyrolysis of H3PO4, MgO, coffee husk, and poultry litter (Carneiro et al. 2018). So, there is a crucial need for long-term research focussing on understanding the intricate interactive effects of different engineered chars with soil organic matter across various soil types.
The improvement in soil quality and plant growth with char amendments can be a factor of improved water holding capacity, nutrient supply, and nutrient retentivity. Cation exchange capacity, surface negative charge, and covalent interaction are the most influential reasons for the nutrient absorbability of the chars (Clough et al. 2013; Gao and DeLuca 2016; Takaya et al. 2016a; Van Zwieten et al. 2010). Nutrient retentivity can moreover be a factor of porosity, bulk density, water holding capacity of the char (Sun and Lu 2014). Although some of the studies have indicated no signficant effects of hydrochars on water holding capacities in soil (Kalderis et al. 2019), hydrochar produced using feedstocks of small-sized particles and low reaction temperatures (≈180 °C) favour higher water retention capacities (Eibisch et al. 2015). Since chars are not sustainable sole source of nutrient supply, production of nutrient-enriched biochars—which are engineered biochar-inorganic/organic fertiliser complexes—have become prominent in the recent years (Sim et al. 2021). For instance, a meta-analysis by Melo et al. (2022) on the effects of biochar-based fertiliser (BBF) on crop productivity depicted an average increase by 10% with low application rates (mean of 0.9 t ha−1) compared with fertilised controls and 186% compared with non-fertilised controls. Further, Zhang et al. (2022) also successfully depicted the potential of poly(acrylic acid)-grafted chitosan and biochar composite amendment for improved nitrogen cycling. The study depicted the potential of this engineered biochar composite in significantly promoting soil ammonium retention, and reduction in nitrate accumulation, nitrous oxide emission, and ammonia volatilisation during the rice cultivation. The effect of adding biochar along with mineral fertilisers also proved to reduce their leaching and hence lower the associated problems of eutrophication and hypoxia of both inland and coastal waters (Yao et al. 2012).
The bioremediation potential of metal–biochar (nano)composites in contaminated soils remediation was well documented through pot culture and field experiments. Metal sorption in char amendments occurs through precipitation, electrostatic interactions, complexation, chemical reduction, and cation exchange (Li et al. 2017). The successful application of metal-modified biochar composites in immobilisation of various heavy metals, such as Fe–Mn modified biochar composite (As), Fe-biochar (As, Cd), sulphur, and sulphur-iron modified biochar (Cd), MgO-coated biochar (Pb), and MnO-modified biochar (As), depicts the efficacy in contaminated soils remediation. The mechanisms followed in organic pollutants adsorption are hydrogen bonding, hydrophobicity, electrostatic interactions, and π–π interaction (Inyang et al. 2014; Qiu et al. 2009). Application of engineered chars functionalised by steam/CO2 activation, ball milling, oxidising, iron materials, LDH, organic surfactants, and bacteria loading for the remediation of various organic pollutants (e.g. pesticides, antibiotics, plasticiser, PAHs, and phenols) in soil has been successfully depicted (Chen et al. 2022a, b, c).
The effects of biochar composites were also highly dependent on the type and intrinsic properties of the soil. The differences in the water retentivity of sandy and clayey soils were reported to affect the effectiveness of char water holding capacity (WHC). While Yu et al. (2013) reported doubling of WHC of sandy loam soil with 9% (w/w) biochar addition, research on the effects of chars on clayey soil depicted mixed results (Castellini et al. 2015; Devereux et al. 2012; Tryon 1948). Further, Vijay et al. (2021) depicted better performance of char amendments in tropical over temperate regions to improve overall soil quality and crop yield. Meta-analyses conducted by Jeffery et al. (2017) and Thomas and Gale (2015) also revealed significantly higher effects of char amendments on crop yield and tree growth in tropical regions relative to temperate zones. The liming effect, enhanced nutrient availability, and WHC following char addition were attributed as the reasons for the yield increase in tropical soils with inherently poor soil quality characteristics (Vijay et al. 2021). Similarly, field study-based char amendments depicted soil CEC increase in Indonesian tropical soils (Islami et al. 2011), while in Australian subtropical ferralsols (highly weathered acidic soil), no significant effects were observed (Slavich et al. 2013). Moreover, in temperate zones, research indicated a positive CEC response to char amendments in non-calcareous soils (Yamato et al. 2006; Laird et al. 2010; Peng et al. 2011). Conversely, in calcareous soils, no discernible effect of biochar on CEC was observed (Van Zwieten et al. 2010; Kumari et al. 2014).
However, these effects can exhibit variability contingent upon factors such as the char's feedstock, modifications, inherent characteristics, and the application rate which might provide diverse effects in different soil types. Along with engineering char for different types of soil conditions, research should also be focussed on optimisation of the process parameters in char application for variable conditions (Vanapalli et al. 2021a, b). Consequently, this data can enable engineering char amendment strategies specific to climatic conditions, soil types, pH levels, nutrient content, and char properties to specific crops and their yields, accounting for variations in these parameters.
Application as an electrode material
Use of carbon materials such as carbon nanotubes, graphene, activated carbon, and graphite granule as electrodes is well established (Li et al. 2011). Char is a low-cost carbon material with appreciable nitrogen as ammonia, rich carbon percentage, large specific surface area, excellent cycling stability and high power density has the potential to replace the conventional electrode materials successfully (Rizwan et al. 2016). However, intrinsic properties of chars determine their potential for electrode application. For example, chars with high electrical conductivity, porosity, and stability at lower temperatures are preferred as electrode material in microbial fuel cells (Huggins et al. 2014). Chars containing relatively high bound oxygen groups are preferred in direct carbon fuel cells (Kacprzak et al. 2014). Char with high porosity and structural bound nitrogen groups is preferred in the development of supercapacitors (Titirici et al. 2012).
The ideal electrode materials require rich porous structure and high surface area in order to provide enough active sites for electrochemical oxidation (Wang and Wang 2019b). Various porous carbons derived from biomass have been developed through physical, chemical, acid/alkali modifications, microwave-assisted pre-treatment (Panahi et al. 2020a; b; Rajapaksha et al. 2016; Wang et al. 2018a, b, c). Figure 8 depicts the modifications executed for developing the electrochemical properties of char materials. Recently, Cheng (2021) produced porous carbon materials from corn glue meal waste followed by KOH activation at 700 °C. The material exhibited a high specific surface area of 3353 m2/g along with a good energy-storage capacity of 488 F/g at 0.5 A/g and excellent cycling stability. Hydrochar-based porous carbons for supercapacitors were created by (Ding et al. 2013), utilising H3PO4, NaOH, and KOH activation. This resulted in augmented BET surface areas ranging from 1355 to 3322 m2/g and increased pore volumes ranging from 1.45 to 2.53 cm3/g. The highest specific capacitance of 179.4 F/g with a current density of 6.25 A/g was observed with KOH activated hydrochar, which depicted promising electrode material prospects for supercapacitors.
Sun et al. (2021) synthesised a porous sheet-like graphitic carbon via a simultaneous activation–graphitisation route from coconut shell. In the synthesis process, the activating agent (ZnCl2) and graphitic catalyst precursor (FeCl3) were firstly introduced into the skeleton of the coconut shell through coordination of the metal precursor with the functional groups in the coconut shell. Then, the Zn2+/Fe2+ loaded coconut shell was heat treated under an inert atmosphere. During the pyrolytic process, the ZnCl2 can act as an activating agent to produce porous structure, while the iron can catalyse the formation of the graphene-like structure. The results showed that the porous sheet-like graphitic carbon possesses high specific surface area (SSA) (1874 m2g−1), large pore volume (1.21 cm3g−1), and good electrical conductivity due to the high graphitic degree. When used as a supercapacitor electrode, the as-synthesised carbon material exhibits a high specific capacitance of 268 Fg−1at 1 Ag−1, which is much higher than that of activated carbon (210 Fg−1) fabricated by only activation with ZnCl2 and graphitic carbon (117Fg−1) by only graphitisation with FeCl3.
He and Chen (2015) tested the prospects of flexible carbon cloth coated with MnO2 nanosheets (MnO2/CFC) as supercapacitor electrode, and observed that it exhibited a high specific capacitance of 683.7 Fg−1 at 2 Ag−1and still retained 269 Fg−1at 300 Ag−1, which is much high than that of bare CFC (0.56 Fg−1at 5 Ag−1). Moreover, the MnO2/CFC electrode showed relatively high stability with 94% capacitance retention after 1000 cycles. Also, Thines et al. (2016) demonstrated that the synthesised magnetic biochar composite developed a highest specific capacitance of 615 F/g at 10 mV/s and energy density of 76.88 Wh/kg than original biochar composite. Similarly, Arenas Esteban et al. (2020) demonstrated the utility of carbon/gold nano grapes (C/Au NGs) prepared from hydrothermal polymerisation of glucose-stabilised gold nanoparticles as supercapacitors with high volumetric capacitance.
It is well known that the low carbonisation temperature of biomass to produce biochar/hydrochar brings in high yield, high density, and abundant functional groups (Liu et al. 2015a, b). This helps in the char materials to become promising supercapacitor electrode materials with high volumetric specific capacitance, whereas the inherent poor conductivity hinders its further development. These porous carbons possess large specific surface area but generally low specific capacitance of less than 300 F/g, due to the dominant electrical double layer capacitance (EDLC) contribution. Thus, exploring conductive char materials is required to develop high electrochemical performance supercapacitor electrode materials. Hetero atoms (N, S, P, B, etc.) doping is an important approach to enhance the specific capacitance of porous carbon. N doping can not only provide large pseudo-capacitance but also improve the conductivity and wettability of porous carbon materials, which makes it the most promising choice to optimise the electrochemical performance for carbon materials (Dong et al. 2021). N-doped porous carbons derived from biomass are generally prepared through two approaches. One is to treat N-containing biomass precursors (such as corn gluten meal waste (Cheng et al. 2021), poplar catkins (Su et al. 2017), puffed rice (Hou et al. 2017), chitosan (Huang et al. 2021), water hyacinth (Zheng et al. 2017), silkworm excrement (Lei et al. 2018), coconut shell and sewage sludge (Peng et al. 2018), platanus fruit (Tan et al. 2021), tofu (Liu et al. 2015a, b), dried distillers grains (Jin et al. 2015) with carbonisation and chemical activation (KOH, ZnCl2, Ni (NO3)2, etc.) process.
The other is to introduce N-containing molecule (such as polyacrylonitrile, urea, acetyl trimethyl ammonium bromide, CN2H4S, trithiocyanuric acid, etc.) into N-free biomass materials such as apricot shell (Shu et al. 2017), dandelion fluff (Zhao et al. 2021), rice straw (Liu et al. 2018), corncob sponge (Materials et al. 2018), pomelo peels (Wang et al. 2018a, b, c), bagasse (Zou et al. 2018), (Tang et al. 2018; Wang et al. 2016a, b) etc. followed by chemical activation. The reported N-doped porous carbons show greatly enhanced gravimetric specific capacitance. However, the preparation process usually requires high activation temperature, leading to limited N-doped content, large energy consumption, and environmental pollution (Peng et al. 2018). In addition, these N-doped porous carbons usually present low volumetric specific capacitance, due to their low density (generally less than 0.6 g/cm3) (Long et al. 2015; Wang et al. 2021; Wang 2016). The study also showed that nitrogen enriched graphite polymers employed as high-performance supercapacitor electrodes. The prepared single-electrode exhibits remarkable gravimetric specific capacitance, ultrahigh volumetric capacitance (950 F/cm3 at 1 A/g), and excellent cycling stability in 1 M H2SO4 electrolyte. Furthermore, a superior volumetric energy density of 42.8 Wh/L is achieved for the assembled symmetric supercapacitor with 1 M Na2SO4 electrolyte. The excellent electrochemical performance of synthesised material demonstrates that the designed strategy provides an effective approach to prepare high-performance N-enriched conductive char-based materials from biomass for sustainable energy-storage devices (Zhang et al. 2019a, b, c, d, e).
Application as a catalyst
Catalysts are highly utilised to convert not only carbonaceous precursors like petroleum, coal, and natural gas, but also renewable materials (biomass) into value-added products such as chemicals and fuels. Over decades carbon materials are used in reactions of heterogeneous catalysis due to their capable qualities for catalyst support and the same act as direct catalysts in many industrial applications (Rodriguez-reinoso 1998). Apart from the usage of synthesised char materials, scientists have recently discovered several modification methods to prolong their activation (Qian et al. 2015; Sik et al. 2015). Char-based catalysts have been successful in different reaction processes as the removal of tar in bio-oil and syngas, production of biodiesel, syngas production, deNOx, and biomass hydrolysis. The catalytic properties of the char materials depend on its properties including SSA, porous nature, surface functional groups and acidic nature which are factors of feedstock properties, and pre-/post-modifications.
To enhance and optimise catalytic characteristics of char for specific processes, more efforts should be kept to control feedstock type, operating conditions, and post-treatment conditions. For instance, the potential of sulfonated biochar in achieving highest yield of biodiesel products (88%) from cooking oil has been depicted (Li et al. 2014). Also, when the catalyst of 3wt% was used then the yield of methyl esters was achieved as 99% at 65 °C. However, after several reuses, both catalysts tended to deactivate. During transesterification, the base catalyst was poisoned by undesirable by-products produced by reactions between CaO and the feed (Kouzu et al. 2008; Liu et al. 2008). Also, to use char materials as a biodiesel catalyst, the stability of the catalyst material must be improved to avoid the post-separation steps for removing S or Ca. Similarly, study by (Quevedo-Amador et al. 2022) used KOH-functionalised hydrochar-based catalysts for biodiesel production through oil transesterification. The study reported the highest fatty acid methyl esters yields of 98.7% where transesterification was endothermic and chemically controlled with an activation energy of 47.9 kJ/mol.
Biochar-supported base metal (e.g. Ni and Fe) catalysts, on the other hand, performed better tar removal compared to conventional mineral catalysts (e.g. olivine and dolomite) in the biomass gasification process (Kastner et al. 2015; Shen et al. 2014; Wang et al. 2011). A catalyst made of a physical mixture of NiO and wood-biochar, for example, removed 97% of the real tars produced during sawdust gasification, resulting in an increase in syngas production due to catalytic reforming of the tars (Wang et al. 2011). Study by Gai et al. (2017) also observed excellent catalytic performance of hydrochar-supported Ni nanoparticles composite during the catalytic gasification of sewage sludge. The study reported 72.5% selectivity for hydrogen and 78.7 g H2yield per kg of hydrochar, with minimal tar formation, even at moderately low temperatures (700–800 °C). Similarly, Ni–Fe bimetallic catalysts supported on rice husk biochar produced seven times less tars than raw biochar and monometallic catalysts during biomass pyrolysis (Shen et al. 2014). The NiO/biochar catalyst mixture was stable for 8 h on-stream. The addition of Fe to biochar reduced the activation energy (Ea) of toluene decomposition from 90.6 to 48.4 kJ mol/L (Kastner et al. 2015; Mani et al. 2013; Shen et al. 2014; Wang et al. 2011). According to the experimental results, biochar is a promising alternative for removing tar in gasification processes. The reaction temperature is one of the drawbacks of biochar and metal/biochar catalysts for tar removal, as its removal occurs only at temperatures above 700 °C. Tar removal, on the other hand, can be initiated at lower temperatures (e.g. 560 °C) using the conventional Ni catalyst (Libs et al. 2007; Mani et al. 2013) while biochar (as a catalyst) is not yet effective at the low temperatures (Mani et al. 2013). As a result, future efforts must focus on overcoming these limitations and broadening the application of biochar as a catalyst.
Ren et al. (2014) also claimed that using a biochar catalyst increased syngas yield from biomass pyrolysis. Because biochar has properties similar to activated carbon, a few studies have reported its use as a catalyst to support deNOx reactions (Cha et al. 2010; Shen et al. 2015; Singh et al. 2013). The MnOx/rice straw biochar demonstrated high NOx removal efficiency at 250 °C (85%). Furthermore, even at 50 °C, the MnOx/rice straw biochar had an 84% NOx removal efficiency. Zhuang et al. (2022) synthesised hydrochar-supported catalysts from glucose and analysed its catalytic activity towards the production of functional amines. The study reported 93.7% conversion efficiency of benzaldehyde to benzylamine under the optimal reaction conditions with catalysts prepared from impregnation method.
Aside from these, further research into the catalytic properties of chars will be required to design active, selective, and stable char-based catalysts. Furthermore, for char materials to be viable substitutes for industrial heterogeneous catalysts, an industrial-scale biochar/hydrochar production systems are highly desirable. Furthermore, securing stable sources of supply for raw char materials is difficult to maintain consistent properties for large-scale production. This could help to replace expensive and non-environmentally benign catalysts that have been used in the past for a variety of purposes.
Promoting direct interspecies electron transfer
Biogas produced from AD of organic waste is a renewable energy source. In last few decades, the research on theory and fundamental aspects of AD has been investigated deeply. The major disadvantage of AD process is the longer digestion period (45–75 days). The longer digestion period is due to the slow growing microorganisms; further, the energy gain during the metabolism must be divided to hydrolytic bacteria, acidogenic bacteria, acetogenic bacteria, and methanogenic bacteria. Syntrophic interaction between bacteria and methanogens is the foundation to make AD process efficient. The transfer of electron for energy between bacteria and methanogens is usually carried by interspecies electron transfer (by acetate, H2, format, humic substances, quinones, or phenazines) or direct interspecies electron transfer (by cellular structure or conductive material) represented in Fig. 9.
The interspecies electron transfer is one of the constrictions in AD process as it depends on thermodynamics and microbial community of AD process. In recent decades, a novel pathway for electron transfer called direct interspecies electron transfer (DIET) was discovered as a potential substitute to interspecies electron transfer through conductive materials (nanowires, biochar, hydrochar, graphite rods/plates/pillars, carbon cloth, carbon nanotube, and carbon fabric coated with nanoparticles). The direct transfer of electron would enhance production of methane by the reduction of CO2 without any electron shuttle. In recent studies, it was found that DIET has the potential to resist acidic shock load. The transfer of electron from electron-donating and electron-accepting microbes by DIET reduces the load on anaerobic microbes which ultimately results in improvement of AD process. The conductive material such as chars promotes DIET in AD process through the conduction-based mechanism, wherein electrons transferred through char from electron-donating and electron-accepting microbes. Interestingly, it was found that char materials can indemnify the pili of microbes and soluble chemical compounds involved in electron transfer.
The electron transfer through char was 106 times faster than the conventional interspecies electron transfer which results in degradation of organic substances at a faster rate (Cruz Viggi et al. 2015). In the study of Chen et al. (2014), it was observed that biochar can degrade the ethanol to methane equivalent to the theoretical value calculated by using stoichiometry. Biochar derived from saw dust at a dosage of 15 g/L mitigated volatile fatty acids accumulation and improved microbial activities of Tepidimicrobium spp. and Methanothermobacter spp. These two types of microbes may be able to transmit electrons outside of cells. The presence of biochar enabled DIET by substituting Thermincola spp. and Methanothermobacter spp. on anode and cathode, respectively. The study ascertained the capability of biochar to uphold DIET in a way like that previously reported for granular activated carbon. However, biochar investigated by the authors was found thousand times less conductive than granular activated carbon. The authors stimulated direct interspecies electron transfer by using ethanol as an electron donor with consortium of G. metallireducens with G. sulfurreducens or M. barkeri. The study reported that biochar can stimulate direct interspecies electron transfer as an imperative factor while modifying soils with biochar.
The study conducted by Wang et al. (2022) confirmed the exact function of biochar and its primary role in the digestion process. Results indicated that the total pore volume and adsorption capacity of biochar played significant role. Comparably, direct interspecies electron transfer was not found dominant due to the insufficient electrical conductivity and electron-donating and accepting capacities of chars. Additionally, the microbial analysis further ascertained that mediated interspecies electron transfer remained the primary mechanism rather than direct interspecies electron transfer. Ren et al. (2020) also found evidence for DIET in hydrochar facilitated anaerobic digestion mediated through surface oxygen-containing functional groups. The study reported an enhanced production of methane by 37% from hydrogenotrophic methanogenesis possibly due to DIET mechanism by converting H+, e−, and CO2 to methane.
Despite evidence for improved biogas production, the research on occurrence of DIET in char-mediated AD system requires further microbial analysis. Future research should focus on specific strains involved in DIET. There is a significant research gap on direction of electron transfer Geobacter sp. and Methanogenic archaea, and micromechanism of electrons absorption by methanogens archaea. The production of biochar/hydrochar requires energy and material; hence, it is necessary to investigate the environmental benefits of char-mediated AD process by life-cycle assessment.
Application in the removal of contaminants from wastewater
Use of carbon materials such as graphene oxide, carbon nanotubes, and activated carbon as adsorbents for wastewater treatment has been extensively popular in the recent past. Biochar and hydrochar have gained popularity lately for being low-cost carbonaceous materials with large surface area, high porosity, catalytic activity, and cation exchange capacity which helps in the removal of a range of contaminants (Gupta et al. 2020). The affinity of chars to adsorb inorganic (heavy metals) and organic pollutants (phenols, pharmaceutical compounds, pesticides, dyes, and nitrogen/phosphorus-based organics) in the wastewater has been demonstrated by previous studies (Hu et al. 2020; Kapoor et al. 2021; Karić et al. 2022). The adsorption mechanism of char-based materials for different contaminants and their removal efficiencies might vary depending on the properties and interrelationships between the contaminant and the char material.
Heavy metal removal
The major heavy metal absorption mechanisms include physical adsorption, ion exchange, electrostatic adsorption, precipitation, complexation, and reduction reactions. These mechanisms can act independently or in conjunction with each other which can help in decontamination of wastewater.
Physical adsorption is the phenomenon where the heavy metal ions may either bind to the surface of the char or diffuse into pores using Vander Waals forces. The high surface area and porous skeletal structure of the char particles greatly expand the physical adsorption ability of heavy metals in water and their fixation and passivation in solution. For instance, heavy metal ions, including As, Cd, and Zn, were physically immobilised on the surface of biochar via adsorption, according to research conducted by (Beesley et al. 2014) which decreased the mobility and availability of these metal ions. Successful removal of Cu and Ur from water through physical adsorption through biochar made from pine (700 °C) and switch grass (300 °C) was also reported (Liu et al. 2010).
Ion exchange and surface complexation are a result of the columbic forces between the negatively charged surface groups on chars and the positively charged heavy metal ions. This mechanism has a limited adsorption capacity and is a non-specific adsorption process. The presence of oxygen-containing functional groups like hydroxyl, carbonyl, and carboxyl groups on the char’s surface results in the formation of stable complexes that immobilise the heavy metal ions. In a study on Cd2+ and K+ biochar-sorption analysis in water, Harvey et al. (2011) reported that K+ was largely adsorbed on deprotonated functional groups via ion exchange with the adsorption molar temperatures, while cation-bonding processes led to Cd2+ adsorption. Similarly, Tong et al. (2011) reported the predominance of surface complexation mechanism in the removal of Cu2+using biochar made from different types of agricultural straw charcoal. Similar effects of ion exchange and surface complexation mechanism were depicted on Cr (VI) removal by Eucalyptus globulus bark biochar (Choudhary and Paul 2018), Pb2+ removal by magnesium oxide coated watermelon rind biochar (Zhang et al. 2020a, b, c), As (III) and As (V) using Tectona and Lagerstroemia speciosa leaves litter biochar (Verma and Singh 2019) where the respective highest adsorption capacities of 21.3 mg/g, 558 mg/g, 666.7 μg/g, and 1250 μg/g were reported in aqueous media.
Electrostatic interactions between the negative surface charges on chars and positively charged heavy metals could lead to decontamination of water (Uchimiya et al. 2012b). The pH of the solution (Dong et al. 2011), valence state of the heavy metals, ionic radius, and zero potential of chars (Mukherjee et al. 2011) are all strongly correlated with the strength of electrostatic interactions. The predominance of electrostatic interaction during the adsorption of Cu2+ (Park et al. 2016), Pb2+ (Qiu et al. 2008), and Cr6+ (Hsu et al. 2009) was reported. Especially, Hsu et al. (2009) reported that Cr6+ was initially adsorbed on the surface of the biochar under the influence of electrostatic forces, then reduced to Cr3+ by elemental carbon on the surface of the biochar or H+ in solution, and finally complexed with functional groups on the surface of the biochar. This depicts the complexity of multiple mechanisms formulated by char media acting simultaneously in the removal of heavy metals from water.
The presence of soluble mineral ions in char materials can form precipitable compounds with the heavy metals in the water which helps in their removal. For instance, PO43− and CO32− can co-precipitate with lead and cadmium and other heavy metal ions to create relatively stable minerals (such as lead and cadmium carbonate etc.) in water (Han et al. 2017). For instance, in a study by Cairns et al. (2021) biochar co-amended with wood ash was found to be immobilise metal ions (lead, copper, zinc, and cadmium) and precipitate on the surface of biochar. Similarly, Kong et al. (2011) who studied Hg sorption using biochar made from soybean stalks, suggested that Hg2+ was reduced to Hg2Cl2 in the presence of Cl, which was then precipitated on the surface of the biochar, in addition to cation exchange, complexation, and Hg(OH)2 precipitation. The heavy metal adsorption mechanisms of chars along with the removal efficiencies of different heavy metals as reported by previous studies are listed in Table 6.
Organic pollutant removal
Positive effects of char-based carbon materials in the removal of various organic contaminants, such as phenols, textile dyes, antibiotics, pesticides, and herbicides, have been reported by previous literature as depicted in Table 7. The mechanisms of adsorption could be chemical or physical but specifically they depend on variety of intrinsic factors including the aromatic content, the presence of functional groups and polarity of both the organic pollutant and char material. The chemical adsorption mainly depends on the formation of bonds such as hydrogen bonds, π bonds, and coordination bonds, while physical adsorption depends on the strength of electrostatic force and the nature of intermolecular gravitation between the contaminant and the char (Tan et al. 2021). The most frequent organic pollutant adsorption methods onto biochar may involve hydrophobic interaction, pore-filling, partitioning, electron donor and acceptor (EDA) interaction, and electrostatic attraction (Hu et al. 2020). The variable adsorption mechanisms of organic and inorganic contaminants in aqueous media using char materials are depicted in Fig. 10.
The pore-filling mechanism, which depends on the microporous and microporous nature of the char, makes it possible to adsorb a lot of polar and nonpolar organic pollutants. For instance, the sorption of catechol by gamma grass, oak, and loblolly pine biochar was caused by a dominant micropore-filling process (Kasozi et al. 2010). Similarly, the sorption of 1,2,4-trichlorobenzene and 1,4-dichlorobenzene was attributed to the low molecular diameter of pitch pine pores through a pore-filling mechanism (Nguyen et al. 2007). Especially in chars with little volatile matter content, the pore-filling process promotes the sorption of organic molecules even at a low concentration of solute (Kasozi et al. 2010).
In the partitioning mechanism, the properties of the carbonised (graphene and crystalline-like fractions) and noncarbonised (organic carbon, non-crystalline, amorphous) fractions of char determine how well organic pollutants are absorbed. The first step in partitioning is the diffusion of sorbates into the pores or into the organic matter of the char's non-carbonised fraction. Afterwards, to improve the sorption, these organic components solubilised within the char's organic matter matrix during partition. Organic compounds in char partition onto the carbon amorphous phase, which contains aliphatic and polyaromatic chemicals like ketones, sugars, phenols, etc. (Keiluweit et al. 2010). For instance, dairy and swine char generated at 200 °C and 350 °C, respectively, are found to be suitable for the sorption of atrazine by sorbate partitioning on organic carbon fractions of biochar (Fruehwirth et al. 2020). The sorption of norflurazon and fluoridone was also enhanced by the organic carbon fractions of wood and grass biochar through partitioning (Sun et al. 2011).
The primary mechanism for the adsorption of several organic pollutants on the graphene structure of char particles was hydrophobic interactions (Ersan et al. 2016). By employing both hydrophobic interaction and partitioning mechanisms, hydrophobic biochar was found to be effective in the sorption of both neutral and hydrophobic organic molecules. For instance, (Li et al. 2018) depicted the predominance of hydrophobic interactions in the adsorption of ionisable organic pollutants such as p-chlorobenzoic acid, o-chlorobenzoic acid, and benzoic acid. Similar observations of hydrophobic interaction predominance was made in the sorption of perfluorooctane sulfonate with biochar made from willow and maize straw (Militao et al. 2021). The absorbability due to hydrophobic interactions is directly proportional to hydrophobicity of the organic pollutants.
The sorption of ionisable and ionic organic molecules occurs mostly by electrostatic interactions (Kah et al. 2017). The cationic sorbates often combine with the negatively charged surface of char particles, whereas anionic sorbates typically bond with the positively charged surface. The fate of attracting and repellent electrostatic forces in the sorption of the organic pollutants is determined by ionic strength and pH (Patra et al. 2020). The net charge on the surface of the char is regulated by pH. At low pH, the char surface maintains a positive charge, but at high pH, the surface acquires a net negative charge (Uchimiya et al. 2017). Increased ionic strength of the sorbate solution eventually improves the sorption during the repulsive electrostatic contact between sorbates and sorbent; however, when there is an attractive electrostatic relationship, it is likely to reduce the sorption of organic sorbate. The electrostatic interaction process was also implicated in the sorption of methyl violet and methylene blue dyes through charcoal (Patra et al. 2020).
The aromatic nature of the char system functions as an electron acceptor or a donor which also helps in the sorption of aromatic chemicals (Han et al. 2017). For instance, studies on the removal of antibiotics such as tetracycline, ciprofloxacin, ofloxacin, and delafloxacin using biochar in their aqueous phase mainly depicted the dominance of hydrogen bonding, π–π electron transition, and cationic interactions in the adsorption mechanisms (Akhtar et al. 2021). The study conducted by Vyavahare et al. (2019) revealed electrostatic attraction as a predominant adsorption mechanism in the study of methyl violet adsorption capacity of biochar, especially on its surface hydrophilic and –COO-sites. Similarly, electrostatic interaction mechanism was reported to be predominant in the adsorption of tetracycline (Wei et al. 2019) and three fluoroquinolones (Dang et al. 2022) as reported by other studies. The maximum biochar adsorption capacities of 593.84 mg/g (tetracycline), 399.6 μg/g (ciprofloxacin), 306 μg/g (ofloxacin), and 93.9 μg/g (delafloxacin) were reported by these studies.
Techno-economic assessment of char production systems
Techno-economic assessment is a technical and economic evaluation of a system which typically includes design engineering, process modelling, energy balance, and economic evaluation (Kumar et al. 2020). With the perspective of char production technologies, energy balance is an important parameter to evaluate the economic viability of the process, which also provides a detail reference for economic investments and commercial possibilities. The net energy of the system can either be positive or negative depending on the operating parameters and feedstock. For instance, Boateng et al. (2010) reported 90% energy recovery in a soybean straw-based biochar system with simultaneous energy output from all the by-products in the system including steam, bio-oil, biochar, and non-condensable gases. Roberts et al. (2010) also reported positive net energy for biochar production which ranged from 3044 to 4899 MJ/t depending on the feedstock. Another study by Zhai et al. (2017) who evaluated the energy balance of hydrothermal carbonisation (HTC), reported an energy recovery rate of 47–71.6% from different biomasses, and suggested a carbonisation temperature greater than 260 °C for maximum energy efficiency of the process. Similarly, Reza et al. (2016) also reported a net positive energy recovery with HTC of pulp waste at 220 °C for 30 min which was sufficient to supply energy requirements for this process at relatively low running costs.
Techno-economic analysis is crucial to recognise the cost competitiveness of the char production process at large scale. This includes detailed evaluation of all the cost parameters of the process including overall capital cost, operational cost including feedstock collection, labour cost, equipment, manufacturing costs, etc., (Kumar et al. 2020). Various researchers have reported the economic assessment of char-based treatment systems for their performance, suitability, and economical sustainability (Khan et al. 2021; Maroušek and Trakal 2022; Zhang et al. 2021). For instance, in a techno-economic study by Kung et al. (2013), the value of biochar varied by 10.98 $/t and 2.85 $/t using slow and fast pyrolysis techniques. Further, the study depicted a net loss of 21$/t and 27$/t of feedstock for slow and fast pyrolysis process, respectively. It might be attributed to higher electricity production costs and lesser economic and environmental profits for fast pyrolysis process. In another study by Campbell et al. (2018), the financial viability of biochar and biofuel production from forest residues as a substrate was evaluated. The results revealed that the coproduction cost (biochar and biofuel) scenario showed revenue of − 24.2 million $ at the average historic market biofuel price. In a waste management scenario, the total revenue was also − 5.5 million $. On the other end, the net revenue from hearth-based biochar was 45.1 million $ at the waste management scenario and decreased to 27.3 million $ at 80 $/t feedstock cost. A techno-economic assessment study by Sahoo et al. (2021), on the other hand, also revealed that economically feasibility of portable systems which can be technologically improved there by reducing the production cost of biochar by 470 $/oven-dry metric ton.
In the case of hydrochar, Saqib et al. (2019) depicted that the current production technology of hydrochar cannot compete with fossil fuel derived carbon materials unless integrated with the anaerobic digestion of process water for biogas production and additional costs of mitigated greenhouse gas have not been considered. Cao et al. (2019) also suggested the process of microwave-assisted hydrothermal treatment to be economically viable (net revenue of 1015 $) only when co-recovery of levulinic acid and hydrochar is done. Shabangu et al. (2014) also depicted the economic viability of the system with the co-recovery of methanol and biochar from the process. The sensitivity analysis revealed a breakeven price of 220–280 $/t of biochar. Zeymer et al. (2017) also estimated the minimum cost of hydrochar derived from sewage sludge to be 169.5 $/t without pelletisation. The study suggested cost reductions through heat recovery from waste fractions and recycling back into the system.
Optimisation of plant capacity, feedstock choice, logistic supply, and other process parameters (such as temperature, residence time, and pressure), marketability of products, play a significant role in improving the economic efficiency of the char production technologies. Similarly, the economic efficacy and recyclability of char-based systems in water treatment varies according to the type of biomass or feedstocks, process conditions, type of contaminant and its concentration, degree of water treatment required or carbon credits reflecting the social value for mitigating greenhouse gas mitigation (Sahoo et al. 2021). However, since most of these studies were based on laboratory and small-scale pilot plants, industrialisation and large-scale application of char production technologies may incur many other practical challenges, and further research is necessitated in this area.
Biochar/hydrochar industry and sustainable development goals (SDGs)
Production of char from biomass results in the sequestration of roughly 50% of the initial carbon, in contrast to the little amounts of carbon retained after burning (3%) and biological breakdown (less than 10–20% after 5–10 years). Thus, the introduction of chars to agricultural soil can mitigate the climate change by stabilising carbon storage and lowering GHG emissions up to 4 Gt of carbon/year which is equivalent to the current carbon flux emitted from burning of all fossil fuels (Kong et al. 2014) which contribute into the achievement of the 13th sustainable development goal (SDG) stated by the United Nations in 2015. The high specific surface area and high porosity of numerous chars can enhance crops growth via enhancing chemical and physical properties of soil, such as nutrient retention, water retention, cation exchange, and pH. However, the impact of char as soil amendment is strongly dependent on soil fertility and fertiliser control. Some chars reported promising results in ammonium adsorption and lowering leaching of the major nutrients such as nitrate and phosphates from soil, subsequently increase the crops yields and quality as well as keep the soil from deterioration (Kong et al. 2014) which has a significant contribution to SDG 2, i.e. no hunger. The use of biochar/hydrochar developed from biomass in the adsorption and removal of several organic and inorganic contaminants in water and soil has a positive impact on SDG6 (clean water and sanitation), SDG14 (life below water) and SDG15 (life on land), where the toxic contaminants entering the food cycle through water pollution or landfilling will be restricted. Moreover, the phosphate attached to the surface of the biochar is slowly release to the plants and hence minimise the intensive use of fertilisers which are considered one of the major sources of contamination in agricultural sector (Shyam et al. 2022).
Challenges, opportunities, and research directions
Production and application of engineered carbon materials encounter numerous challenges including type and characteristics of the feedstock, logistical and economic factors, and public and market acceptance. The following sections will highlight all these challenges.
Technical challenges
Although laboratory and pilot scale-based studies depict positive results, production of chars incurs huge challenges when applied at large commercial scale. The initial characteristics of biomass such as high moisture content, along with seasonal variations and ecosystem functions also act as technical constraints for char production (Kong et al. 2014). Homogeneity of the feedstock is another challenge where the different biomass used to develop biochar or hydrochar are varied in composition depending on agricultural conditions such as soil, climate, and region (Seo et al. 2022). Prolonged storage time has negative impact on biomass quality and quantity owning to the moisture content which may results in natural decay via bacterial or fungi actions. Hence, storage time should be shortened (Kong et al. 2014). Efficient storage of the biomass is a technical challenge that needs to be faced for ensuring the continuous supply of the biomass to the processing plants. In this concern, some innovative solutions like mobile reactors recorded promising results as cost-effective solutions for the char production (Rajpoot et al. 2022).
Assessments of materials, energy balance, optimising energy conservation, and recovery should be evaluated to boost the large-scale applications of biochar/hydrochar. Optimisation of the process conditions has significant effect on the properties and yield of the final product. The yields of the biochar for gasification, fast pyrolysis, moderate pyrolysis, slow pyrolysis (Gargiulo et al. 2018), hydrothermal carbonisation, and torrefaction and pyrolysis are estimated as 10, 12, 25, 35, 50–80, and 80%. Hence, it is preferable to improve the processes with low yield. On the other hand, integration of the above-mentioned thermal processes results in economic and environmental merits (Hoang et al. 2022).
Different reactor configurations have been used for biochar production such as fixed, ablative, auger, and fluidised bed reactors. However, circulating fixed bed and dual fluidised bed are superior to others since the sustainability of the thermal treatment is guaranteed (Seo et al. 2022). Thermochemical conversion techniques are endothermic processes; hence, they require high energy, and future studies on heat transfer, energy enhancement are required. Introduction of renewable energy systems such as solar system would be an economic and sustainable option (Rajpoot et al. 2022). Additionally, the recycling of pyrolysis gas and process water from HTC enhances the overall process efficiency and promotes resource recovery. Hence, further studies are required for developing of the current reactors and developing new one to increase the energy enhancement, and to control dust, wastewater and other pollutants (Seo et al. 2022). Production of chars from industrial wastes such as de-oiled cakes of soybean and cotton seeds need to be explored (Rajpoot et al. 2022).
On the other hand, scaling-up the energy production from biochar requires improvement of catalyst efficiency, understanding the mechanism of the catalyst, and catalyst deactivation. Therefore, future research on material design and process optimisation is still needed (Seo et al. 2022). Such variations affect the yields and physical and chemical properties of the products even in similar reaction conditions and treatments. However, further studies should investigate the kinetics and mechanism involved in the development of biochar/hydrochar from numerous biomasses (Shyam et al. 2022). Much more studies are still required for reusing the sorbent/catalyst for multiple cycles in order to estimate their lifetimes. The reusability studies are important to minimise the need for fresh sorbents/catalysts which is subsequently lowering the overall cost. The management of the exhausted sorbents/catalysts after their applications should be discussed in term of toxicity. One of the major driving forces to overcome the above-mentioned challenges is the cost-effectiveness of biochar and hydrochar production from biomass which is estimated to varied from range $0.3–3.1 kg−1 and $0.1–0.2 kg−1, for biochar and hydrochar, respectively, compared to porous metal oxides ($3–6 kg−1 and hybrid ion exchange resins ($15–20 kg−1) (Shyam et al. 2022).
Economic challenges
Although char production mostly adopts residues and waste materials which are of low-cost and very little value, the high production costs involved in thermal treatment, transportation, labour, and other pre- and post-processing steps increase the end cost of the product (Issaka et al. 2022). Especially, some of these biomasses need pre-treatment such as drying, size reduction (chopping, shredding, and grinding), and steam sterilisation which increase the overall cost of treatment (Kong et al. 2014). Although it is possible to make agreements for short-term deliveries, it is highly challenging to make long-term plans to guarantee supply at a fair price (Kong et al. 2014).
Additional costs incurred in collection and transportation of these wastes from cultivated areas to char production plants need extra machinery operations and labour which will increase the overall cost. Therefore, more economically viable strategies for biomass processing should be enhanced in the future (Seo et al. 2022). For instance, promoting char regeneration in contaminant removal via adsorption applications can be a cost-effective strategy for reducing the overall cost of material requirement. For instance, biochar used in the adsorption of trichloro ethylene was reused for up to eight cycles which made the treatment process more economic (Issaka et al. 2022).
Environmental challenges
Excessive utilisation of agricultural residue in char production can negatively impact the nutrient cycles, soil organic carbon, and organic matter balance in the soil which decrease the crop production. Hence, proper management of these residues is necessary (Seo et al. 2022). Life-cycle assessment of chars is important to detect and mitigate the environmental impacts. For example, developing biochar from forestry wastes could lower the CO2-eq emissions to 2.74 kg/kg (Hoang et al. 2022). Recently, biochar is reused in modern technologies as bio-fertiliser. However, risk and toxicological studies of char used as soil amendment should be carried out to assess the impact on human health on the long run with special focus after loading with different contaminants from water and soil (Issaka et al. 2022). Improper storage could lead to self-decomposition of the biomass resulting in CH4, N2O, H2S, and CO2 which cause environmental problems. Further studies need to be focused on life-cycle assessment of thermochemical processes, environmental effects, and carbon cycle, which is currently very limited in the literature.
Social challenges
Enhancing the demand and acceptability of biochar/hydrochar in the local market is crucial, as highlighted by (Rajpoot et al. 2022). Despite its potential benefits in soil fertility, water holding capacity, water and wastewater treatment, and energy production, several challenges hinder its widespread adoption. Addressing these challenges is not only essential for market acceptance but also requires farmer acceptance.
Key challenges include the lack of quantitative knowledge regarding the effects of biochar on various aspects, such as soil fertility and water holding capacity (Kong et al. 2014). Additionally, social challenges play a significant role in shaping the acceptability of biochar. These challenges encompass awareness and education, cultural beliefs and practices, perceived risks and unknowns, limited access to technology, economic considerations, community engagement, policy and regulation, demonstration projects, social perceptions of novel technologies, and local engagement and participation. Leach et al. (2014) claimed that awareness of the economic benefits of biochar application for soil amendment can make the farmers winners in the market. Promoting the application of biochar involves considering social criteria like public perception, social acceptance, and job creation potential, as outlined in studies (Khalaj et al. 2020). Highlighting positive impact biochar on specific applications, such as odour control in landfills and wastewater treatment plants, can bolster its social acceptability (Kamalai et al. 2022). Limited studies, like the successful use of peanut shell-derived biochar for odour control (Wong et al. 2017), indicate the potential of biochar to address such social challenges.
Lack of awareness poses a significant obstacle, emphasising the need for educating communities and farmers about the positive environmental and agricultural impacts of biochar. Addressing concerns about safety and unknowns associated with biochar through transparent communication is vital for overcoming scepticism. Limited access to technology for production and application, coupled with economic feasibility concerns, can be barriers that need to be addressed to ensure widespread adoption.
Future research directions
Future scientific research and technical development should focus on developing innovating technologies at low capital cost which depend on utilisation and management co-products, by-products and wastes generated from agricultural and industrial sectors (Kong et al. 2014). According to the principle of the circular economy, the co-products (syngas and bio-oil) from the pyrolysis process (process water and syngas) from HTC should be included in the life-cycle-assessment of chars. Sustainable co-recovery of chars along with other by-products can maximise the economic values of biomass while mitigating the environmental burdens of biochar production (Zhu et al. 2022). The economic viability, renewability, and regeneration are strongly required to be studied for the commercialisation of char products. The development of new polices and legislative law is required for encouragement of the production and industrial utilisation of biochar and hydrochar. Extensive research should focus on integrating different techniques for production and utilisation of biochar and hydrochar to maximise the overall efficiency of the production process and minimise the energy consumption during production and application. Much more techno-economic analyses should be carried out to evaluate the cost of the final product; hence, pilot scale studies are of high priority (Shyam et al. 2022).
Conclusion
In this review, we thoroughly examined recent progress in carbonaceous materials derived from organic waste, aiming to establish a connection between organic waste and applications in the environmental, energy, and agricultural sectors. The study extensively compared the thermochemical transformations of two common chars, biochar and hydrochar both produced from organic waste. The paper explored the diverse potential applications of biomass-derived carbon materials, particularly highlighting the recognition of biomass-based activated carbons. These activated carbons, known for their excellent physical properties and cost-effectiveness, are increasingly acknowledged for their role in air and wastewater pollutant removal, as well as carbon sequestration. Given the inherent limitations of organic waste-derived materials, such as low pore volume and limited functional groups on their surfaces, activation treatment and/or surface modification become essential. These processes are crucial for producing porous carbons with abundant surface functional groups, enabling their utilisation in energy storage, conversion, and environmental conservation. The resulting porous carbons, post-activation, and surface functionalisation, offer versatile applications in wastewater treatment, soil improvement, gas capture, and serve as promising materials for fuel cell electrodes, batteries, supercapacitors, catalysts, and catalyst supports. The transformation of organic waste into value-added products with potential applications plays a pivotal role in establishing a sustainable society and circular economy. The review emphasises the significance of carbon materials, particularly engineered char, as a soil conditioner, electrode material, catalyst, and wastewater treatment agent for various pollutant sources (organic/inorganic). While current research has extensively covered the theory and development of engineered carbon materials at laboratory and bench scales, future investigations should focus on understanding the mechanisms and effects of engineered chars on the environment.
Availability of data and materials
All the relevant data and material are available in the main manuscript.
References
Abujabhah IS, Bound SA, Doyle R, Bowman JP (2016) Effects of biochar and compost amendments on soil physico-chemical properties and the total community within a temperate agricultural soil. Appl Soil Ecol 98:243–253
Aghababaei A, Ncibi MC, Sillanpää M (2017) Optimized removal of oxytetracycline and cadmium from contaminated waters using chemically-activated and pyrolyzed biochars from forest and wood-processing residues. Biores Technol 239:28–36
Akhtar L, Ahmad M, Iqbal S, Abdelhafez AA, Mehran MT (2021) Biochars’ adsorption performance towards moxifloxacin and ofloxacin in aqueous solution: Role of pyrolysis temperature and biomass type. Environ Technol Innov 24:101912
Alfredo Quevedo-Amador R, Elizabeth Reynel-Avila H, Ileana Mendoza-Castillo D, Badawi M, Bonilla-Petriciolet A (2022) Functionalized hydrochar-based catalysts for biodiesel production via oil transesterification: Optimum preparation conditions and performance assessment. Fuel 312:122731. https://doi.org/10.1016/j.fuel.2021.122731
Ameloot N, Graber ER, Verheijen FGA, Neve SD (2013) Interactions between biochar stability and soil organisms: review and research needs. Eur J Soil Sci 64:379–390. https://doi.org/10.1111/ejss.12064
Arenas Esteban D, Guerrero Martínez A, Carretero González J, Birss VI, Otero-Díaz LC, Ávila Brande D (2020) Tunable supercapacitor materials derived from hydrochar/gold nanograpes. ACS Appl Energy Mater 3:9348–9359. https://doi.org/10.1021/acsaem.0c01711
Awasthi MK (2022) Engineered biochar: a multifunctional material for energy and environment. Environ Pollut 298:118831
Bargmann I, Rillig MC, Buss W, Kruse A, Kuecke M (2013) Hydrochar and biochar effects on germination of spring barley. J Agron Crop Sci 199:360–373. https://doi.org/10.1111/jac.12024
Batista EMCC, Shultz J, Matos TTS, Fornari MR, Ferreira TM, Szpoganicz B, de Freitas RA, Mangrich AS (2018) Effect of surface and porosity of biochar on water holding capacity aiming indirectly at preservation of the Amazon biome. Sci Rep 8:1–9
Beesley L, Inneh OS, Norton GJ, Moreno-Jimenez E, Pardo T, Clemente R, Dawson JJC (2014) Assessing the influence of compost and biochar amendments on the mobility and toxicity of metals and arsenic in a naturally contaminated mine soil. Environ Pollut 186:195–202
Boateng AA, Mullen CA, Goldberg NM, Hicks KB, Devine TE, Lima IM, James E (2010) Sustainable production of bioenergy and biochar from the straw of high-biomass soybean lines via fast pyrolysis. Environ Prog Sustain Energy 29:175–183. https://doi.org/10.1002/ep.10446
Borchard N, Wolf A, Laabs V, Aeckersberg R, Scherer HW, Moeller A, Amelung W (2012) Physical activation of biochar and its meaning for soil fertility and nutrient leaching: a greenhouse experiment. Soil Use and Manag 177:184. https://doi.org/10.1111/j.1475-2743.2012.00407.x
Busch D, Glaser B (2015) Stability of co-composted hydrochar and biochar under field conditions in a temperate soil. Soil Use Manag 31:251–258. https://doi.org/10.1111/sum.12180
Cai H, Xu L, Chen G, Peng C, Ke F, Liu Z, Li D, Zhang Z, Wan X (2016a) Removal of fluoride from drinking water using modified ultrafine tea powder processed using a ball-mill. Appl Surf Sci 375:74–84
Cai J, Li B, Chen C, Wang J, Zhao M, Zhang K (2016b) Hydrothermal carbonization of tobacco stalk for fuel application. Bioresour Technol 220:305–311. https://doi.org/10.1016/j.biortech.2016.08.098
Cai T, Liu X, Zhang J, Tie B, Lei M, Wei X, Peng O, Du H (2021) Silicate-modified oiltea camellia shell-derived biochar: a novel and cost-effective sorbent for cadmium removal. J Clean Prod 281:125390
Cairns S, Chaudhuri S, Sigmund G, Robertson I, Hawkins N, Dunlop T, Hofmann T (2021) Wood ash amended biochar for the removal of lead, copper, zinc and cadmium from aqueous solution. Environ Technol Innov 24:101961
Cairns S, Robertson I, Sigmund G, Street-Perrott A (2020) The removal of lead, copper, zinc and cadmium from aqueous solution by biochar and amended biochars. Environ Sci Pollut Res 27:21702–21715
Campbell RM, Anderson NM, Daugaard DE, Naughton HT (2018) Financial viability of biofuel and biochar production from forest biomass in the face of market price volatility and uncertainty. Appl Energy 230:330–343
Cao L, Yu IKM, Cho D-W, Wang D, Tsang DCW, Zhang S, Ding S, Wang L, Ok YS (2019) Microwave-assisted low-temperature hydrothermal treatment of red seaweed (Gracilaria lemaneiformis) for production of levulinic acid and algae hydrochar. Biores Technol 273:251–258. https://doi.org/10.1016/j.biortech.2018.11.013
Carneiro JSDS, Lustosa Filho JF, Nardis BO, Ribeiro-Soares J, Zinn YL, Melo LCA (2018) Carbon stability of engineered biochar-based phosphate fertilizers. ACS Sustain Chem Eng 6(11):14203–14212
Castellini M, Giglio L, Niedda M, Palumbo AD, Ventrella D (2015) Impact of biochar addition on the physical and hydraulic properties of a clay soil. Soil Tillage Res 154:1–13
Cha JS, Choi J-C, Ko JH, Park Y-K, Park SH, Jeong K-E, Kim S-S, Jeon J-K (2010) The low-temperature SCR of NO over rice straw and sewage sludge derived char. Chem Eng J 156:321–327
Chang C-F, Chang C-Y, Tsai W-T (2000) Effects of burn-off and activation temperature on preparation of activated carbon from corn cob agrowaste by CO2 and steam. J Colloid Interface Sci 232:45–49
Chen D, Cen K, Zhuang X, Gan Z, Zhou J, Zhang Y, Zhang H (2022a) Insight into biomass pyrolysis mechanism based on cellulose, hemicellulose, and lignin: evolution of volatiles and kinetics, elucidation of reaction pathways, and characterization of gas, biochar and bio-oil. Combust Flame 242:112142. https://doi.org/10.1016/j.combustflame.2022.112142
Chen H, Gao Y, Li J, Fang Z, Bolan N, Bhatnagar A, Wang H (2022b) Engineered biochar for environmental decontamination in aquatic and soil systems: a review. Carbon Res 1(1):4
Chen S, Rotaru A-E, Shrestha PM, Malvankar NS, Liu F, Fan W, Nevin KP, Lovley DR (2014) Promoting interspecies electron transfer with biochar. Sci Rep 4:1–7
Chen Z, Bao H, Wen Q, Wu Y, Fu Q (2022c) Effects of H3PO4 modified biochar on heavy metal mobility and resistance genes removal during swine manure composting. Biores Technol 346:126632
Cheng B (2021) Preparation of high performance supercapacitor materials by fast pyrolysis of corn gluten meal waste. Sustain Energy Fuels 2017(1):891–898. https://doi.org/10.1039/C7SE00029D
Cheng B, Tian K, Zeng RJ, Jiang H (2021) Sustainable Energy & Fuels Supplementary files 250, 5–8
Choi Y-K, Kan E (2019) Effects of pyrolysis temperature on the physicochemical properties of alfalfa-derived biochar for the adsorption of bisphenol A and sulfamethoxazole in water. Chemosphere 218:741–748
Choudhary B, Paul D (2018) Isotherms, kinetics and thermodynamics of hexavalent chromium removal using biochar. J Environ Chem Eng 6:2335–2343
Clough TJ, Condron LM, Kammann C, Müller C (2013) A review of biochar and soil nitrogen dynamics. Agronomy 3:275–293. https://doi.org/10.3390/agronomy3020275
Cruz Viggi C, Presta E, Bellagamba M, Kaciulis S, Balijepalli SK, Zanaroli G, Petrangeli Papini M, Rossetti S, Aulenta F (2015) The “Oil-Spill Snorkel”: an innovative bioelectrochemical approach to accelerate hydrocarbons biodegradation in marine sediments. Front Microbiol 6:881
Cuong DV, Matsagar BM, Lee M, Hossain MdSA, Yamauchi Y, Vithanage M, Sarkar B, Ok YS, Wu KC-W, Hou C-H (2021) A critical review on biochar-based engineered hierarchical porous carbon for capacitive charge storage. Renew Sustain Energy Rev 145:111029. https://doi.org/10.1016/j.rser.2021.111029
Dang B-T, Gotore O, Ramaraj R, Unpaprom Y, Whangchai N, Bui X-T, Maseda H, Itayama T (2022) Sustainability and application of corncob-derived biochar for removal of fluoroquinolones. Biomass Convers Biorefinery 12:913–923
de Jager M, Schröter F, Wark M, Giani L (2022) The stability of carbon from a maize-derived hydrochar as a function of fractionation and hydrothermal carbonization temperature in a Podzol. Biochar 4(1):52
Devereux RC, Sturrock CJ, Mooney SJ (2012) The effects of biochar on soil physical properties and winter wheat growth. Earth Environ Sci Trans R Soc Edinburgh 103:13–18. https://doi.org/10.1017/S1755691012000011
Dias BO, Silva CA, Higashikawa FS, Roig A, Sánchez-Monedero MA (2010) Use of biochar as bulking agent for the composting of poultry manure: effect on organic matter degradation and humification. Biores Technol 101:1239–1246
Dicke C, Lanza G, Mumme J, Ellerbrock R, Kern J (2014) Effect of hydrothermally carbonized char application on trace gas emissions from two sandy soil horizons. J Environ Qual 43(5):1790–1798
Dieguez-Alonso A, Funke A, Anca-Couce A, Rombolà AG, Ojeda G, Bachmann J, Behrendt F (2018) Towards biochar and hydrochar engineering—Influence of process conditions on surface physical and chemical properties, thermal stability, nutrient availability, toxicity and wettability. Energies 11:496
Ding L, Zou B, Li Y, Liu H, Wang Z, Zhao C, Su Y, Guo Y (2013) The production of hydrochar-based hierarchical porous carbons for use as electrochemical supercapacitor electrode materials. Colloids Surf, A 423:104–111. https://doi.org/10.1016/j.colsurfa.2013.02.003
Dong X, Ma LQ, Li Y (2011) Characteristics and mechanisms of hexavalent chromium removal by biochar from sugar beet tailing. J Hazard Mater 190:909–915
Dong X, Wang R, Zhang J, Feng X, Yan C, Chen S (2021) High volumetric capacitance. Ultralong Life Supercapacitors Enabled Waxberry Derived Hierarchical Porous Carbon Mater 8:1–18
Eibisch N, Durner W, Bechtold M, Fuß R, Mikutta R, Woche SK, Helfrich M (2015) Does water repellency of pyrochars and hydrochars counter their positive effects on soil hydraulic properties? Geoderma 245–246:31–39. https://doi.org/10.1016/j.geoderma.2015.01.009
Ersan G, Kaya Y, Apul OG, Karanfil T (2016) Adsorption of organic contaminants by graphene nanosheets, carbon nanotubes and granular activated carbons under natural organic matter preloading conditions. Sci Total Environ 565:811–817
Fang J, Zhan L, Ok YS, Gao B (2018) Minireview of potential applications of hydrochar derived from hydrothermal carbonization of biomass. J Ind Eng Chem 57:15–21. https://doi.org/10.1016/j.jiec.2017.08.026
Fernandes JO, Bernardino CAR, Mahler CF, Santelli RE, Braz BF, Borges RC, da Cunha Veloso MC, Romeiro GA, Cincotto FH (2021) Biochar generated from agro-industry sugarcane residue by low temperature pyrolysis utilized as an adsorption agent for the removal of thiamethoxam pesticide in wastewater. Water Air Soil Pollut 232:1–13
Fischer D, Glaser B (2012) Synergisms between compost and biochar for sustainable soil amelioration. Manag Organic Waste 1:167–198
Foong SY, Liew RK, Yang Y, Cheng YW, Yek PNY, Mahari WAW, Lee XY, Han CS, Vo D-VN, Van Le Q (2020) Valorization of biomass waste to engineered activated biochar by microwave pyrolysis: Progress, challenges, and future directions. Chem Eng J 389:124401
Franzreb M (2020) New classes of selective separations exploiting magnetic adsorbents. Curr Opin Colloid Interface Sci Spec Top Colloidal Interfacial Challenges Relat Sep Anal Recycling 46:65–76. https://doi.org/10.1016/j.cocis.2020.03.012
Fruehwirth M, Sbizzaro M, Rosa DM, Sampaio SC, dos Reis RR (2020) Adsorption of atrazine by biochars produced from byproducts of the wood industry. Engenharia Agrícola 40:769–776
Fu M-M, Mo C-H, Li H, Zhang Y-N, Huang W-X, Wong MH (2019) Comparison of physicochemical properties of biochars and hydrochars produced from food wastes. J Clean Prod 236:117637
Funke A, Ziegler F (2010) Hydrothermal carbonization of biomass: a summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod Biorefin 4(2):160–177
Gai C, Zhang F, Guo Y, Peng N, Liu T, Lang Q, Xia Y, Liu Z (2017) Hydrochar-supported, in situ-generated nickel nanoparticles for sorption-enhanced catalytic gasification of sewage sludge. ACS Sustain Chem Eng 5:7613–7622. https://doi.org/10.1021/acssuschemeng.7b00924
Gajalakshmi S, Abbasi SA (2008) Solid waste management by composting: state of the art. Crit Rev Environ Sci Technol 38:311–400
Gao S, DeLuca TH (2016) Influence of biochar on soil nutrient transformations, nutrient leaching, and crop yield. Adv Plants Agric Res 4:1–16
Gao W (2022) Porous biomass carbon derived from clivia miniata leaves via NaOH activation for removal of dye. Materials 15:1285
Gargiulo V, Gomis-Berenguer A, Giudicianni P, Ania CO, Ragucci R, Alfè M (2018) Assessing the potential of biochars prepared by steam-assisted slow pyrolysis for CO2 adsorption and separation. Energy Fuels 32:10218–10227
George C, Wagner M, Kücke M, Rillig MC (2012) Divergent consequences of hydrochar in the plant–soil system: Arbuscular mycorrhiza, nodulation, plant growth and soil aggregation effects. Appl Soil Ecol 59:68–72. https://doi.org/10.1016/j.apsoil.2012.02.021
Godlewska P, Schmidt HP, Ok YS, Oleszczuk P (2017) Biochar for composting improvement and contaminants reduction. A Review. Bioresource Technol 246:193–202
Gupta RK, Dubey M, Kharel P, Gu Z, Fan QH (2015) Biochar activated by oxygen plasma for supercapacitors. J Power Sources 274:1300–1305
Gupta S, Sireesha S, Sreedhar I, Patel CM, Anitha KL (2020) Latest trends in heavy metal removal from wastewater by biochar based sorbents. J Water Process Eng 38:101561
Hairuddin MN, Mubarak NM, Khalid M, Abdullah EC, Walvekar R, Karri RR (2019) Magnetic palm kernel biochar potential route for phenol removal from wastewater. Environ Sci Pollut Res 26:35183–35197
Han L, Qian L, Yan J, Chen M (2017) Effects of the biochar aromaticity and molecular structures of the chlorinated organic compounds on the adsorption characteristics. Environ Sci Pollut Res 24:5554–5565
Harvey OR, Herbert BE, Rhue RD, Kuo L-J (2011) Metal interactions at the biochar-water interface: energetics and structure-sorption relationships elucidated by flow adsorption microcalorimetry. Environ Sci Technol 45:5550–5556
Hasan MM, Rasul MG, Khan MMK, Ashwath N, Jahirul MI (2021) Energy recovery from municipal solid waste using pyrolysis technology: a review on current status and developments. Renew Sustain Energy Rev 145:111073
He M, Xu Z, Hou D, Gao B, Cao X, Ok YS, Rinklebe J, Bolan NS, Tsang DCW (2022) Waste-derived biochar for water pollution control and sustainable development. Nat Rev Earth Environ 3:444–460
He S, Chen W (2015) Application of biomass-derived flexible carbon cloth coated with MnO 2 nanosheets in supercapacitors. J Power Sources 294:150–158. https://doi.org/10.1016/j.jpowsour.2015.06.051
Hoang AT, Goldfarb JL, Foley AM, Lichtfouse E, Kumar M, Xiao L, Ahmed SF, Said Z, Luque R, Bui VG (2022) Production of biochar from crop residues and its application for anaerobic digestion. Bioresource Technol 363:127970
Hoekman SK, Broch A, Robbins C (2011) Hydrothermal carbonization (HTC) of lignocellulosic biomass. Energy Fuels 25:1802–1810
Hoslett J, Ghazal H, Ahmad D, Jouhara H (2019) Removal of copper ions from aqueous solution using low temperature biochar derived from the pyrolysis of municipal solid waste. Sci Total Environ 673:777–789
Hou J, Jiang K, Tahir M, Wu X, Idrees F, Shen M, Cao C (2017) Tunable porous structure of carbon nanosheets derived from puffed rice for high energy density supercapacitors. J Power Sources 371:148–155
Hsu N-H, Wang S-L, Liao Y-H, Huang S-T, Tzou Y-M, Huang Y-M (2009) Removal of hexavalent chromium from acidic aqueous solutions using rice straw-derived carbon. J Hazard Mater 171:1066–1070
Hu B, Ai Y, Jin J, Hayat T, Alsaedi A, Zhuang L, Wang X (2020) Efficient elimination of organic and inorganic pollutants by biochar and biochar-based materials. Biochar 2:47–64
Huang J, Liang Y, Liu Y, Hu H, Liu S, Cai Y, Dong H, Zheng M, Xiao Y (2021) Journal of Materials Chemistry A Supplementary files 1–5
Huang M, Luo J, Fang Z, Li H (2016) Biodiesel production catalyzed by highly acidic carbonaceous catalysts synthesized via carbonizing lignin in sub- and super-critical ethanol. Appl Catal B 190:103–114. https://doi.org/10.1016/j.apcatb.2016.02.069
Huggins T, Wang H, Kearns J, Jenkins P, Jason Z (2014) Bioresource Technology Biochar as a sustainable electrode material for electricity production in microbial fuel cells. Biores Technol 157:114–119. https://doi.org/10.1016/j.biortech.2014.01.058
Ighalo JO, Iwuchukwu FU, Eyankware OE, Iwuozor KO, Olotu K, Bright OC, Igwegbe CA (2022) Flash pyrolysis of biomass: a review of recent advances. Clean Technol Environ Policy 24(8):2349–2363
Inyang M, Gao B, Pullammanappallil P, Ding W, Zimmerman AR (2010) Biochar from anaerobically digested sugarcane bagasse. Biores Technol 101:8868–8872
Inyang M, Gao B, Zimmerman A, Zhang M, Chen H (2014) Synthesis, characterization, and dye sorption ability of carbon nanotube–biochar nanocomposites. Chem Eng J 236:39–46
Islam MA, Limon MSH, Romić M, Islam MA (2021) Hydrochar-based soil amendments for agriculture: a review of recent progress. Arab J Geosci 14:102. https://doi.org/10.1007/s12517-020-06358-8
Islami T, Guritno B, Basuki N, Suryanto A (2011) Biochar for sustaining productivity of cassava based cropping systems in the degraded lands of East Java, Indonesia. J Trop Agric 49:40–46
Issaka E, Fapohunda FO, Amu-Darko JNO, Yeboah L, Yakubu S, Varjani S, Ali N, Bilal M (2022) Biochar-based composites for remediation of polluted wastewater and soil environments: challenges and prospects. Chemosphere 297:134163
Jadhav SK, Thorat SR (2022) Adsorption of azo dyes using biochar prepared from regional crop waste material. Biosci, Biotechnol Res Asia 19:141–151
Jahirul MI, Rasul MG, Chowdhury AA, Ashwath N (2012) Biofuels production through biomass pyrolysis—a technological review. Energies 5(12):4952–5001
Jeffery S, Abalos D, Prodana M, Bastos AC, Van Groenigen JW, Hungate BA et al (2017) Biochar boosts tropical but not temperate crop yields. Environ Res Lett 12:053001. https://doi.org/10.1088/1748-9326/aa67bd
Jin H, Wang X, Gu Z, Fan Q, Luo B (2015) A facile method for preparing nitrogen-doped graphene and its application in supercapacitors. J Power Sources 273:1156–1162
Joseph S, Graber ER, Chia C, Munroe P, Donne S, Thomas T, Nielsen S, Marjo C, Rutlidge H, Pan G, Li L, Taylor P, Rawal A, Hook J (2013) Shifting paradigms: development of high-efficiency biochar fertilizers based on nano-structures and soluble components. Carbon Manag 4:323–343. https://doi.org/10.4155/cmt.13.23
Jung K-W, Lee SY, Choi J-W, Lee YJ (2019) A facile one-pot hydrothermal synthesis of hydroxyapatite/biochar nanocomposites: adsorption behavior and mechanisms for the removal of copper (II) from aqueous media. Chem Eng J 369:529–541
Kacprzak A, Koby RWR, Bis Z (2014) The effect of fuel type on the performance of a direct carbon fuel cell with molten alkaline electrolyte 255. https://doi.org/10.1016/j.jpowsour.2014.01.012
Kah M, Sigmund G, Xiao F, Hofmann T (2017) Sorption of ionizable and ionic organic compounds to biochar, activated carbon and other carbonaceous materials. Water Res 124:673–692
Kalderis D, Papameletiou G, Kayan B (2019) Assessment of orange peel hydrochar as a soil amendment: impact on clay soil physical properties and potential phytotoxicity. Waste Biomass Valor 10:3471–3484. https://doi.org/10.1007/s12649-018-0364-0
Kamali M, Sweygers N, Al-Salem S, Appels L, Aminabhavi TM, Dewil R (2022) Biochar for soil applications-sustainability aspects, challenges and future prospects. Chem Eng J 428:131189
Kammann C, Ratering S, Eckhard C, Müller C (2012) Biochar and hydrochar effects on greenhouse gas (carbon dioxide, nitrous oxide, and methane) fluxes from soils. J Environ Qual 41:1052–1066. https://doi.org/10.2134/jeq2011.0132
Kang S, Li X, Fan J, Chang J (2012) Characterization of hydrochars produced by hydrothermal carbonization of lignin, cellulose, D-xylose, and wood meal. Ind Eng Chem Res 51:9023–9031
Kantakanit P, Tippayawong N, Koonaphapdeelert S, Pattiya A (2018) Hydrochar generation from hydrothermal carbonization of organic wastes. IOP Conf Ser Earth Environ Sci 159:012001. https://doi.org/10.1088/1755-1315/159/1/012001
Kapoor RT, Danish M, Singh RS, Rafatullah M (2021) Exploiting microbial biomass in treating azo dyes contaminated wastewater: Mechanism of degradation and factors affecting microbial efficiency. J Water Process Eng 43:102255
Karić N, Maia AS, Teodorović A, Atanasova N, Langergraber G, Crini G, Ribeiro ARL, Đolić M (2022) Bio-waste valorisation: Agricultural wastes as biosorbents for removal of (in) organic pollutants in wastewater treatment. Chem Eng J Adv 9:100239
Kasozi GN, Zimmerman AR, Nkedi-Kizza P, Gao B (2010) Catechol and humic acid sorption onto a range of laboratory-produced black carbons (biochars). Environ Sci Technol 44:6189–6195
Kastner JR, Mani S, Juneja A (2015) Catalytic decomposition of tar using iron supported biochar. Fuel Process Technol 130:31–37
Katiyar R, Patel AK, Nguyen T-B, Singhania RR, Chen C-W, Dong C-D (2021) Adsorption of copper (II) in aqueous solution using biochars derived from Ascophyllum nodosum seaweed. Biores Technol 328:124829
KazemiShariatPanahi H, Dehhaghi M, Ok YS, Nizami A-S, Khoshnevisan B, Mussatto SI, Aghbashlo M, Tabatabaei M, Lam SS (2020) A comprehensive review of engineered biochar: production, characteristics, and environmental applications. J Clean Prod 270:122462. https://doi.org/10.1016/j.jclepro.2020.122462
Keiluweit M, Nico PS, Johnson MG, Kleber M (2010) Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ Sci Technol 44:1247–1253
Khalaj M, Kamali M, Costa MEV, Capela I (2020) Green synthesis of nanomaterials-A scientometric assessment. J Clean Prod 267:122036
Khan N, Chowdhary P, Gnansounou E, Chaturvedi P (2021) Biochar and environmental sustainability: emerging trends and techno-economic perspectives. Biores Technol 332:125102
Khan Z, Yang XJ, Fu Y, Joseph S, Khan MN, Khan MA, Shen H (2023) Engineered biochar improves nitrogen use efficiency via stabilizing soil water-stable macroaggregates and enhancing nitrogen transformation. Biochar 5(1):52
Kong H, He J, Gao Y, Wu H, Zhu X (2011) Cosorption of phenanthrene and mercury (II) from aqueous solution by soybean stalk-based biochar. J Agric Food Chem 59:12116–12123
Kong S-H, Loh S-K, Bachmann RT, Rahim SA, Salimon J (2014) Biochar from oil palm biomass: a review of its potential and challenges. Renew Sustain Energy Rev 39:729–739
Kouzu M, Kasuno T, Tajika M, Yamanaka S, Hidaka J (2008) Active phase of calcium oxide used as solid base catalyst for transesterification of soybean oil with refluxing methanol. Appl Catal A 334:357–365
Kumar A, Saini K, Bhaskar T (2020) Hydochar and biochar: Production, physicochemical properties and techno-economic analysis. Biores Technol 310:123442. https://doi.org/10.1016/j.biortech.2020.123442
Kumari KGID, Moldrup P, Paradelo M, Elsgaard L, Hauggaard-Nielsen H, de Jonge LW (2014) Effects of biochar on air and water permeability and colloid and phosphorus leaching in soils from a natural calcium carbonate gradient. J Environ Qual 43:647–657. https://doi.org/10.2134/jeq2013.08.0334
Kung C-C, McCarl BA, Cao X (2013) Economics of pyrolysis-based energy production and biochar utilization: a case study in Taiwan. Energy Policy 60:317–323
Kyi PP, Quansah JO, Lee C-G, Moon J-K, Park S-J (2020) The removal of crystal violet from textile wastewater using palm kernel shell-derived biochar. Appl Sci 10:2251
Laird DA, Fleming P, Davis DD, Horton R, Wang B, Karlen DL (2010) Impact of biochar amendments on the quality of a typical midwestern agricultural soil. Geoderma 158:443–449. https://doi.org/10.1016/j.geoderma.2010.05.013
Leach M, Fairhead J, Fraser J (2014) Green grabs and biochar: revaluing African soils and farming in the new carbon economy. In: Green grabbing: a new appropriation of nature. Routledge, pp 49–72
Lee J, Kim K, Kwon EE (2017) Biochar as a Catalyst. Renew Sustain Energy Rev 77:70–79. https://doi.org/10.1016/j.rser.2017.04.002
Lehmann DJ (2012) Biochar for environmental management: science and technology. Earthscan
Lehmann J, Cowie A, Masiello CA, Kammann C, Woolf D, Amonette JE, Cayuela ML, Camps-Arbestain M, Whitman T (2021) Biochar in climate change mitigation. Nat Geosci 14:883–892
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effects on soil biota–a review. Soil Biol Biochem 43:1812–1836
Lei S, Chen L, Zhou W, Deng P, Liu Y, Fei L, Lu W (2018) Tetra-heteroatom self-doped carbon nanosheets derived from silkworm excrement for high-performance supercapacitors. J Power Sources 379:74–83. https://doi.org/10.1016/j.jpowsour.2018.01.032
Li H, Dong X, da Silva EB, de Oliveira LM, Chen Y, Ma LQ (2017) Mechanisms of metal sorption by biochars: biochar characteristics and modifications. Chemosphere 178:466–478
Li H, Pan L, Lu T, Zhan Y, Nie C, Sun Z (2011) A comparative study on electrosorptive behavior of carbon nanotubes and graphene for capacitive deionization. J Electroanal Chem 653:40–44
Li J, Li L, Suvarna M, Pan L, Tabatabaei M, Ok YS, Wang X (2022) Wet wastes to bioenergy and biochar: a critical review with future perspectives. Sci Total Environ 152921
Li M, Zheng Y, Chen Y, Zhu X (2014) Biodiesel production from waste cooking oil using a heterogeneous catalyst from pyrolyzed rice husk. Biores Technol 154:345–348
Li S, Chan CY, Sharbatmaleki M, Trejo H, Delagah S (2020a) Engineered biochar production and its potential benefits in a closed-loop water-reuse agriculture system. Water 12:2847
Li S, Lyons-Hart J, Banyasz J, Shafer K (2001) Real-time evolved gas analysis by FTIR method: an experimental study of cellulose pyrolysis. Fuel 80:1809–1817
Li X, Zhang M, Luo J, Zhang S, Yang X, Deshani A, Sik Y, Tsang DCW, Sze C, Lin K (2019a) Efficient succinic acid production using a biochar-treated textile waste hydrolysate in an in situ fibrous bed bioreactor. Biochem Eng J 149:107249. https://doi.org/10.1016/j.bej.2019.107249
Li Y, Xing B, Ding Y, Han X, Wang S (2020b) A critical review of the production and advanced utilization of biochar via selective pyrolysis of lignocellulosic biomass. Biores Technol 312:123614. https://doi.org/10.1016/j.biortech.2020.123614
Li Y, Zhang Y, Wang G, Li S, Han R, Wei W (2018) Reed biochar supported hydroxyapatite nanocomposite: characterization and reactivity for methylene blue removal from aqueous media. J Mol Liq 263:53–63
Li Z, Li D, Ma L, Yu Y, Zhao B, Zhang J (2019b) Effects of straw management and nitrogen application rate on soil organic matter fractions and microbial properties in North China Plain. J Soils Sediments 19:618–628
Liang J, Xu X, Qamar W, Hu X, Zhao L, Qiu H (2019) Different mechanisms between biochar and activated carbon for the persulfate catalytic degradation of sulfamethoxazole : roles of radicals in solution or solid phase. Chem Eng J 375:121908. https://doi.org/10.1016/j.cej.2019.121908
Libs S, Courson C, Kiennemann A (2007) Steam Reforming of tar from a biomass gasification process over ni/olivine catalyst using toluene as a model compound. Appl Catal Environ 74:211–222. https://doi.org/10.1016/j.apcatb.2007.01.017
Lin Q, Tan X, Almatrafi E, Yang Y, Wang W, Luo H, Qin F, Zhou C, Zeng G, Zhang C (2022) Effects of biochar-based materials on the bioavailability of soil organic pollutants and their biological impacts. Sci Total Environ 826:153956. https://doi.org/10.1016/j.scitotenv.2022.153956
Lingamdinne LP, Choi J-S, Angaru GKR, Karri RR, Yang J-K, Chang Y-Y, Koduru JR (2022) Magnetic-watermelon rinds biochar for uranium-contaminated water treatment using an electromagnetic semi-batch column with removal mechanistic investigations. Chemosphere 286:131776
Liu S, Zhao Y, Zhang B, Xia H, Zhou J, Xie W, Li H (2018) Nano-micro carbon spheres anchored on porous carbon derived from dual-biomass as high rate performance supercapacitor electrodes. J Power Sources 381:116–126
Liu W, Jiang H, Yu H (2015) materials : a review and future directions 4888–4907. https://doi.org/10.1039/c5gc01054c
Liu W-J, Jiang H, Yu H-Q (2015b) Development of biochar-based functional materials: toward a sustainable platform carbon material. Chem Rev 115:12251–12285
Liu X, Piao X, Wang Y, Zhu S (2008) Calcium ethoxide as a solid base catalyst for the transesterification of soybean oil to biodiesel. Energy Fuels 22:1313–1317
Liu X, Zhai Y, Li S, Wang B, Wang T, Liu Y, Qiu Z, Li C (2020) Hydrothermal carbonization of sewage sludge: effect of feed-water pH on hydrochar’s physicochemical properties, organic component and thermal behavior. J Hazard Mater 388:122084
Liu Y, Liu X, Ren N, Feng Y, Xue L, Yang L (2019) Effect of pyrochar and hydrochar on water evaporation in clayey soil under greenhouse cultivation. Int J Environ Res Public Health 16:2580
Liu Z, Zhang F-S, Wu J (2010) Characterization and application of chars produced from pinewood pyrolysis and hydrothermal treatment. Fuel 89:510–514
Long C, Chen X, Jiang L, Zhi L, Fan Z (2015) Porous layer-stacking carbon derived from in-built template in biomass for high volumetric performance supercapacitors. Nano Energy 12:141–151
Luo M, Lin H, He Y, Li B, Dong Y, Wang L (2019) Bioresource Technology Efficient simultaneous removal of cadmium and arsenic in aqueous solution by titanium-modified ultrasonic biochar. Bioresource Technol 284:333–339. https://doi.org/10.1016/j.biortech.2019.03.108
Lyu H, Gao B, He F, Zimmerman AR, Ding C, Huang H, Tang J (2018) Effects of ball milling on the physicochemical and sorptive properties of biochar: experimental observations and governing mechanisms. Environ Pollut 233:54–63
Malav LC, Yadav KK, Gupta N, Kumar S, Sharma GK, Krishnan S, Rezania S, Kamyab H, Pham QB, Yadav S (2020) A review on municipal solid waste as a renewable source for waste-to-energy project in India: Current practices, challenges, and future opportunities. J Clean Prod 277:123227
Mani S, Kastner JR, Juneja A (2013) Catalytic decomposition of toluene using a biomass derived catalyst. Fuel Process Technol 114:118–125
Mao Q, Zhou Y, Yang Y, Zhang J, Liang L, Wang H (2019) Experimental and theoretical aspects of biochar-supported nanoscale zero- valent iron activating H 2 O 2 for cipro fl oxacin removal from aqueous solution. J Hazard Mater 380:120848. https://doi.org/10.1016/j.jhazmat.2019.120848
Maroušek J, Trakal L (2022) Techno-economic analysis reveals the untapped potential of wood biochar. Chemosphere 291:133000
Masoumi S, Borugadda VB, Nanda S, Dalai AK (2021) Hydrochar: a review on its production technologies and applications. Catalysts 11:939. https://doi.org/10.3390/catal11080939
Masoumi S, Dalai AK (2020) Optimized production and characterization of highly porous activated carbon from algal-derived hydrochar. J Clean Prod 263:121427. https://doi.org/10.1016/j.jclepro.2020.121427
Materials H, Liu Y, Wang Z, Sun Q, Yin B, Cheng J, Zhu S, Yang J (2018) International Journal of Refractory Metals Tribological behavior and wear mechanism of pure WC at wide range temperature from 25 to 800 ° C in vacuum and air environment. Int J Refract Metal Hard Mater 71:160–166. https://doi.org/10.1016/j.ijrmhm.2017.11.024
Melo LCA, Lehmann J, Carneiro JSDS, Camps-Arbestain M (2022) Biochar-based fertilizer effects on crop productivity: a meta-analysis. Plant Soil 472(1–2):45–58
Miandad R, Barakat MA, Aburiazaiza AS, Rehan M, Nizami AS (2016) Catalytic pyrolysis of plastic waste: a review. Process Saf Environ Prot 102:822–838. https://doi.org/10.1016/j.psep.2016.06.022
Militao IM, Roddick FA, Bergamasco R, Fan L (2021) Removing PFAS from aquatic systems using natural and renewable material-based adsorbents: a review. J Environ Chem Eng 9:105271
Mimmo T, Panzacchi P, Baratieri M, Davies CA, Tonon G (2014) Effect of pyrolysis temperature on miscanthus (Miscanthus × giganteus) biochar physical, chemical and functional properties. Biomass Bioenerg 62:149–157. https://doi.org/10.1016/j.biombioe.2014.01.004
Mohamed BA, Ellis N, Soo C, Bi X, Emam AE (2016) Science of the Total Environment Engineered biochar from microwave-assisted catalytic pyrolysis of switchgrass for increasing water-holding capacity and fertility of sandy soil. Sci Total Environ 566–567:387–397. https://doi.org/10.1016/j.scitotenv.2016.04.169
Mukherjee A, Lal R (2013) Biochar impacts on soil physical properties and greenhouse gas emissions. Agronomy 3:313–339. https://doi.org/10.3390/agronomy3020313
Mukherjee A, Zimmerman AR, Harris W (2011) Surface chemistry variations among a series of laboratory-produced biochars. Geoderma 163:247–255
Munir MT, Mansouri SS, Udugama IA, Baroutian S, Gernaey KV, Young BR (2018) Resource recovery from organic solid waste using hydrothermal processing: opportunities and challenges. Renew Sustain Energy Rev 96:64–75
Nguyen TH, Cho H-H, Poster DL, Ball WP (2007) Evidence for a pore-filling mechanism in the adsorption of aromatic hydrocarbons to a natural wood char. Environ Sci Technol 41:1212–1217
Ogbonnaya U, Semple KT (2013) Impact of biochar on organic contaminants in soil: A tool for mitigating risk? Agronomy 3:349–375
Panahi HKS, Dehhaghi M, Ok YS, Nizami A-S, Khoshnevisan B, Mussatto SI, Aghbashlo M, Tabatabaei M, Lam SS (2020) A comprehensive review of engineered biochar: production, characteristics, and environmental applications. J Clean Prod 270:122462
Panchasara H, Ashwath N (2021) Effects of pyrolysis bio-oils on fuel atomisation—a review. Energies 14(4):794
Pang S, Mujumdar AS (2010) Drying of woody biomass for bioenergy: drying technologies and optimization for an integrated bioenergy plant. Drying Technol 28:690–701
Park J-H, Ok YS, Kim S-H, Cho J-S, Heo J-S, Delaune RD, Seo D-C (2016) Competitive adsorption of heavy metals onto sesame straw biochar in aqueous solutions. Chemosphere 142:77–83
Parshetti GK, Balasubramanian R (2014) Evaluation of hydrothermally carbonized hydrochar in improving energy security and mitigating greenhouse gas emissions. In: Green catalysts for energy transformation and emission control. ACS Publications, pp 23–48
Patel B, Guo M, Izadpanah A, Shah N, Hellgardt K (2016) A review on hydrothermal pre-treatment technologies and environmental profiles of algal biomass processing. Biores Technol 199:288–299
Patiño AAB, Lassalle VL, Horst MF (2021) Magnetic hydrochar nanocomposite obtained from sunflower husk: a potential material for environmental remediation. J Mol Struct 1239:130509. https://doi.org/10.1016/j.molstruc.2021.130509
Patra DK, Pradhan C, Patra HK (2020) Toxic metal decontamination by phytoremediation approach: concept, challenges, opportunities and future perspectives. Environ Technol Innov 18:100672
Peng L, Liang Y, Dong H, Hu H, Zhao X, Cai Y, Xiao Y, Liu Y, Zheng M (2018) Super-hierarchical porous carbons derived from mixed biomass wastes by a stepwise removal strategy for high-performance supercapacitors. J Power Sources 377:151–160
Peng X, Ye LL, Wang CH, Zhou H, Sun B (2011) Temperature- and duration-dependent rice straw-derived biochar: characteristics and its effects on soil properties of an Ultisol in Southern China. Soil Tillage Res 112:159–166. https://doi.org/10.1016/j.still.2011.01.002
Peterson SC, Jackson MA, Kim S, Palmquist DE (2012) Increasing biochar surface area: optimization of ball milling parameters. Powder Technol 228:115–120
Premarathna KSD, Rajapaksha AU, Sarkar B, Kwon EE, Bhatnagar A, Ok YS, Vithanage M (2019) Biochar-based engineered composites for sorptive decontamination of water: a review. Chem Eng J 372:536–550
Promdej C, Matsumura Y (2011) Temperature effect on hydrothermal decomposition of glucose in sub-and supercritical water. Ind Eng Chem Res 50:8492–8497
Qasim HM, Abudi ZN, Alzubaidi LA (2022) Cobalt ion removal using magnetic biochar obtained from conocarpus erectus leaves. Biomass Conversion and Biorefinery 1–11
Qian K, Kumar A, Zhang H, Bellmer D, Huhnke R (2015) Recent advances in utilization of biochar. Renew Sustain Energy Rev 42:1055–1064
Qin G, Gong D, Fan M-Y (2013) Bioremediation of petroleum-contaminated soil by biostimulation amended with biochar. Int Biodeterior Biodegrad 85:150–155
Qiu Y, Cheng H, Xu C, Sheng GD (2008) Surface characteristics of crop-residue-derived black carbon and lead (II) adsorption. Water Res 42:567–574
Qiu Y, Zheng Z, Zhou Z, Sheng GD (2009) Effectiveness and mechanisms of dye adsorption on a straw-based biochar. Biores Technol 100:5348–5351
Quilliam RS, Glanville HC, Wade SC, Jones DL (2013) Life in the “charosphere” - Does biochar in agricultural soil provide a significant habitat for microorganisms? Soil Biol Biochem 65:287–293. https://doi.org/10.1016/j.soilbio.2013.06.004
Rajapaksha AU, Chen SS, Tsang DCW, Zhang M, Vithanage M, Mandal S, Gao B, Bolan NS, Ok YS (2016) Engineered/designer biochar for contaminant removal/immobilization from soil and water: potential and implication of biochar modification. Chemosphere 148:276–291
Rajpoot L, Tagade A, Deshpande G, Verma K, Geed SR, Patle DS, Sawarkar AN (2022) An overview of pyrolysis of de-oiled cakes for the production of biochar, bio-oil, and pyro-gas: current status, challenges, and future perspective. Bioresource Technol Rep 101205
Rashidi NA, Chai YH, Ismail IS, Othman MFH, Yusup S (2022) Biomass as activated carbon precursor and potential in supercapacitor applications. Biomass Conv Bioref. https://doi.org/10.1007/s13399-022-02351-1
Ren S, Lei H, Wang L, Bu Q, Chen S, Wu J (2014) bio-oil upgrading over biochar catalysts 10731–10737. https://doi.org/10.1039/c4ra00122b
Ren S, Usman M, Tsang DCW, Thong S, Angelidaki I, Zhu X, Zhang S, Luo G (2020) Hydrochar-facilitated anaerobic digestion: evidence for direct interspecies electron transfer mediated through surface oxygen-containing functional groups. Environ Sci Technol 54:5755–5766. https://doi.org/10.1021/acs.est.0c00112
Reynel-Ávila HE, Camacho-Aguilar KI, Bonilla-Petriciolet A, Mendoza-Castillo DI, González-Ponce HA, Trejo-Valencia R (2021) Engineered magnetic carbon-based adsorbents for the removal of water priority pollutants: an overview. Adsorpt Sci Technol 2021:e9917444. https://doi.org/10.1155/2021/9917444
Reza MT, Coronella C, Holtman KM, Franqui-Villanueva D, Poulson SR (2016) Hydrothermal carbonization of autoclaved municipal solid waste pulp and anaerobically treated pulp digestate. ACS Sustain Chem Eng 4:3649–3658. https://doi.org/10.1021/acssuschemeng.6b00160
Rizwan M, Ali S, Qayyum MF, Ibrahim M, Zia-ur-Rehman M, Abbas T, Ok YS (2016) Mechanisms of biochar-mediated alleviation of toxicity of trace elements in plants: a critical review. Environ Sci Pollut Res 23:2230–2248
Roberts KG, Gloy BA, Joseph S, Scott NR, Lehmann J (2010) Life cycle assessment of biochar systems: estimating the energetic, economic, and climate change potential. Environ Sci Technol 44:827–833. https://doi.org/10.1021/es902266r
Rodriguez-reinoso F (1998) The role of carbon materials catalysis * in heterogeneous 36, 159–175
Sahoo K, Upadhyay A, Runge T, Bergman R, Puettmann M, Bilek E (2021) Life-cycle assessment and techno-economic analysis of biochar produced from forest residues using portable systems. Int J Life Cycle Assess 26:189–213
Saqib NU, Sharma HB, Baroutian S, Dubey B, Sarmah AK (2019) Valorisation of food waste via hydrothermal carbonisation and techno-economic feasibility assessment. Sci Total Environ 690:261–276. https://doi.org/10.1016/j.scitotenv.2019.06.484
Seo JY, Tokmurzin D, Lee D, Lee SH, Seo MW, Park Y-K (2022) Production of biochar from crop residues and its application for biofuel production processes-An overview. Bioresource Technol 127740
Shabangu S, Woolf D, Fisher EM, Angenent LT, Lehmann J (2014) Techno-economic assessment of biomass slow pyrolysis into different biochar and methanol concepts. Fuel 117:742–748. https://doi.org/10.1016/j.fuel.2013.08.053
Shaikh WA, Alam MA, Alam MO, Chakraborty S, Owens G, Bhattacharya T, Mondal NK (2020) Enhanced aqueous phase arsenic removal by a biochar based iron nanocomposite. Environ Technol Innov 19:100936
Shan D, Deng S, Zhao T, Wang B, Wang Y, Huang J, Yu G, Winglee J, Wiesner MR (2016) Preparation of ultrafine magnetic biochar and activated carbon for pharmaceutical adsorption and subsequent degradation by ball milling. J Hazard Mater 305:156–163
Sharma HB, Sarmah AK, Dubey B (2020) Hydrothermal carbonization of renewable waste biomass for solid biofuel production: a discussion on process mechanism, the influence of process parameters, environmental performance and fuel properties of hydrochar. Renew Sustain Energy Rev 123:109761
Shen B, Chen J, Yue S, Li G (2015) A comparative study of modified cotton biochar and activated carbon based catalysts in low temperature SCR. Fuel 156:47–53
Shen DK, Gu S, Bridgwater AV (2010) Study on the pyrolytic behaviour of xylan-based hemicellulose using TG–FTIR and Py–GC–FTIR. J Anal Appl Pyrolysis 87:199–206
Shen Y, Zhao P, Shao Q, Ma D, Takahashi F (2014) In-situ catalytic conversion of tar using rice husk char-supported nickel-iron catalysts for biomass pyrolysis/gasification. Appl Catal Environ 152:140–151. https://doi.org/10.1016/j.apcatb.2014.01.032
Shi J, Fan X, Tsang DCW, Wang F, Shen Z, Hou D, Alessi DS (2019) Removal of lead by rice husk biochars produced at different temperatures and implications for their environmental utilizations. Chemosphere 235:825–831
Shu Y, Maruyama J, Iwasaki S, Maruyama S, Shen Y, Uyama H (2017) Nitrogen-doped biomass/polymer composite porous carbons for high performance supercapacitor. J Power Sources 364:374–382
Shukla P, Giri BS, Mishra RK, Pandey A, Chaturvedi P (2021) Lignocellulosic biomass-based engineered biochar composites: a facile strategy for abatement of emerging pollutants and utilization in industrial applications. Renew Sustain Energy Rev 152:111643. https://doi.org/10.1016/j.rser.2021.111643
Shyam S, Arun J, Gopinath KP, Ribhu G, Ashish M, Ajay S (2022) Biomass as source for hydrochar and biochar production to recover phosphates from wastewater: a review on challenges, commercialization, and future perspectives. Chemosphere 286:131490
Sik Y, Chang SX, Gao B, Chung H (2015) Environmental Technology & Innovation SMART biochar technology: a shifting paradigm towards advanced materials and healthcare research. Environ Technol Innov 4:206–209. https://doi.org/10.1016/j.eti.2015.08.003
Sim DHH, Tan IAW, Lim LLP, Hameed BH (2021) Encapsulated biochar-based sustained release fertilizer for precision agriculture: a review. J Clean Prod 303:127018
Singh R, Dutta RK, Naik DV, Ray A, Kanaujia PK (2021) High surface area Eucalyptus wood biochar for the removal of phenol from petroleum refinery wastewater. Environ Challenges 5:100353
Singh S, Nahil MA, Sun X, Wu C, Chen J, Shen B, Williams PT (2013) Novel application of cotton stalk as a waste derived catalyst in the low temperature SCR-deNOx process. Fuel 105:585–594
Slavich PG, Sinclair K, Morris SG, Kimber SWL, Downie A, Van Zwieten L (2013) Contrasting effects of manure and green waste biochars on the properties of an acidic ferralsol and productivity of a subtropical pasture. Plant Soil 366:213–227. https://doi.org/10.1007/s11104-012-1412-3
Soon YK, Lupwayi NZ (2012) Straw management in a cold semi-arid region: impact on soil quality and crop productivity. Field Crop Res 139:39–46
Su X, Cheng M, Fu L, Yang J, Zheng X (2017) Superior supercapacitive performance of hollow activated carbon nanomesh with hierarchical structure derived from poplar catkins. J Power Sources 362:27–38. https://doi.org/10.1016/j.jpowsour.2017.07.021
Sun F, Lu S (2014) Biochars improve aggregate stability, water retention, and pore-space properties of clayey soil. J Plant Nutr Soil Sci 177:26–33
Sun K, Keiluweit M, Kleber M, Pan Z, Xing B (2011) Sorption of fluorinated herbicides to plant biomass-derived biochars as a function of molecular structure. Biores Technol 102:9897–9903
Sun L, Tian C, Li M, Meng X, Wang L, Wang R, Yin J, Fu H (2021) Journal of Materials Chemistry A Supplementary files 1–5
Sun W, Zhang S, Su C (2018) Impact of biochar on the bioremediation and phytoremediation of heavy metal(loid)s in soil. Adv Bioremed Phytoremed. https://doi.org/10.5772/intechopen.70349
Suo F, You X, Ma Y, Li Y (2019) Rapid removal of triazine pesticides by P doped biochar and the adsorption mechanism. Chemosphere 235:918–925
Surekha K, Padma Kumari AP, Narayana Reddy M, Satyanarayana K, Sta Cruz PC (2003) Crop residue management to sustain soil fertility and irrigated rice yields. Nutr Cycl Agroecosyst 67:145–154
Tag AT, Duman G, Ucar S, Yanik J (2016) Effects of feedstock type and pyrolysis temperature on potential applications of biochar. J Anal Appl Pyrol 120:200–206
Takaya CA, Fletcher LA, Singh S, Anyikude KU, Ross AB (2016a) Phosphate and ammonium sorption capacity of biochar and hydrochar from different wastes. Chemosphere 145:518–527
Takaya CA, Fletcher LA, Singh S, Okwuosa UC, Ross AB (2016b) Recovery of phosphate with chemically modified biochars. Biochem Pharmacol 4:1156–1165. https://doi.org/10.1016/j.jece.2016.01.011
Tan H, Wang X, Jia D, Hao P, Sang Y, Liu H (2021) Journal of Materials Chemistry A 1–5
Tan X, Liu Y, Gu Y, Xu Y, Zeng G, Hu X (2016) Bioresource Technology Biochar-based nano-composites for the decontamination of wastewater : a review. Biores Technol 212:318–333. https://doi.org/10.1016/j.biortech.2016.04.093
Tang Z, Pei Z, Wang Z, Li H, Zeng J, Ruan Z, Huang Y, Zhu M, Xue Q, Yu J (2018) Highly anisotropic, multichannel wood carbon with optimized heteroatom doping for supercapacitor and oxygen reduction reaction. Carbon 130:532–543
Thines KR, Abdullah EC, Ruthiraan M, Mubarak NM, Tripathi M (2016) A new route of magnetic biochar based polyaniline composites for supercapacitor electrode materials. J Anal Appl Pyrol 121:240–257
Thomas SC, Gale N (2015) Biochar and forest restoration: a review and meta-analysis of tree growth responses. New for 46:931–946. https://doi.org/10.1007/s11056-015-9491-7
Titirici M-M, White RJ, Falco C, Sevilla M (2012) 2012 “Black perspectives for a green future: hydrothermal carbons for environment protection and energy storage.” Energy Environ Sci 5(5):6796–6822. https://doi.org/10.1039/c2ee21166a
Tomczyk A, Sokołowska Z, Boguta P (2020) Biochar physicochemical properties: pyrolysis temperature and feedstock kind effects. Rev Environ Sci Biotechnol 19:191–215. https://doi.org/10.1007/s11157-020-09523-3
Tong X, Li J, Yuan J, Xu R (2011) Adsorption of Cu (II) by biochars generated from three crop straws. Chem Eng J 172:828–834
Trazzi PA, Leahy JJ, Hayes MHB, Kwapinski W (2016) Adsorption and desorption of phosphate on biochars. J Environ Chem Eng 4:37–46
Tryon EH (1948) Effect of charcoal on certain physical, chemical, and biological properties of forest soils. Ecol Monogr 18:81–115. https://doi.org/10.2307/1948629
Uchimiya M, Bannon DI, Wartelle LH (2012a) Retention of heavy metals by carboxyl functional groups of
Uchimiya M, Bannon DI, Wartelle LH (2012b) Retention of heavy metals by carboxyl functional groups of biochars in small arms range soil. J Agric Food Chem 60:1798–1809
Uchimiya M, Pignatello JJ, White JC, Hu S-L, Ferreira PJ (2017) Surface interactions between gold nanoparticles and biochar. Sci Rep 7:1–9
Van Zwieten L, Kimber S, Morris S, Downie A, Berger E, Rust J, Scheer C (2010) Influence of biochars on flux of N2O and CO2 from Ferrosol. Aust J Soil Res 48:555–568. https://doi.org/10.1071/SR10004
Vanapalli KR, Bhattacharya J, Samal B, Chandra S, Medha I, Dubey BK (2021a) Single-use LDPE-Eucalyptus biomass char composite produced from co-pyrolysis has the properties to improve the soil quality. Process Saf Environ Prot 149:185–198
Vanapalli KR, Bhattacharya J, Samal B, Chandra S, Medha I, Dubey BK (2021b) Inhibitory and synergistic effects on thermal behaviour and char characteristics during the co-pyrolysis of biomass and single-use plastics. Energy 235:121369. https://doi.org/10.1016/j.energy.2021.121369
Varsha M, Kumar PS, Rathi BS (2022) A review on recent trends in the removal of emerging contaminants from aquatic environment using low-cost adsorbents. Chemosphere 287:132270
Verma L, Singh J (2019) Synthesis of novel biochar from waste plant litter biomass for the removal of Arsenic (III and V) from aqueous solution: a mechanism characterization, kinetics and thermodynamics. J Environ Manage 248:109235
Vigneshwaran S, Sirajudheen P, Karthikeyan P, Meenakshi S (2021) Fabrication of sulfur-doped biochar derived from tapioca peel waste with superior adsorption performance for the removal of Malachite green and Rhodamine B dyes. Surf Interfaces 23:100920
Vijay V, Shreedhar S, Adlak K, Payyanad S, Sreedharan V, Gopi G, Aravind PV (2021) Review of large-scale biochar field-trials for soil amendment and the observed influences on crop yield variations. Front Energy Res 9:710766
Vijayaraghavan K (2019) Recent advancements in biochar preparation, feedstocks, modification, characterization and future applications. Environ Technol Rev 8:47–64
Vithanage M, Rajapaksha AU (2015) Acid-activated biochar increased sulfamethazine retention in soils 2175–2186. https://doi.org/10.1007/s11356-014-3434-2
Vítková M, Sylva C, Trakal L, Veselská V, Šafar I (2016) Lead and cadmium sorption mechanisms on magnetically modified biochars. Bioresour Technol 203:318–324. https://doi.org/10.1016/j.biortech.2015.12.056
Vyavahare G, Jadhav P, Jadhav J, Patil R, Aware C, Patil D, Gophane A, Yang Y-H, Gurav R (2019) Strategies for crystal violet dye sorption on biochar derived from mango leaves and evaluation of residual dye toxicity. J Clean Prod 207:296–305
Vyavahare GD, Gurav RG, Jadhav PP, Patil RR, Aware CB, Jadhav JP (2018) Response surface methodology optimization for sorption of malachite green dye on sugarcane bagasse biochar and evaluating the residual dye for phyto and cytogenotoxicity. Chemosphere 194:306–315
Wan Y, Chen P, Zhang B, Yang C, Liu Y, Lin X, Ruan R (2009) Microwave-assisted pyrolysis of biomass: catalysts to improve product selectivity. J Anal Appl Pyrol 86:161–167
Wang B, Gao B, Fang J (2017) Recent advances in engineered biochar productions and applications. Crit Rev Environ Sci Technol 47:2158–2207
Wang B, Gao B, Fang J, Wang B (2018) Technology Recent advances in engineered biochar productions and applications 3389. https://doi.org/10.1080/10643389.2017.1418580
Wang D, Yuan W, Ji W (2011) Char and char-supported nickel catalysts for secondary syngas cleanup and conditioning. Appl Energy 88:1656–1663
Wang J, Nie P, Ding B, Dong S, Hao X, Dou H, Zhang X (2021) Journal of Materials Chemistry A 4–7
Wang J, Wang S (2019a) Preparation, modification and environmental application of biochar: a review. J Clean Prod 227:1002–1022
Wang J, Wang S (2019b) Preparation, modification and environmental application of biochar : a review. J Clean Prod 227:1002–1022. https://doi.org/10.1016/j.jclepro.2019.04.282
Wang K, Cao Y, Wang X, Castro MA, Luo B, Gu Z, Liu J, Hoefelmeyer JD, Fan Q (2016a) Rod-shape porous carbon derived from aniline modified lignin for symmetric supercapacitors. J Power Sources 307:462–467
Wang K, Xu M, Gu Y, Gu Z, Hua Q (2016b) Symmetric supercapacitors using urea-modified lignin derived N- doped porous carbon as electrode materials in liquid and solid electrolytes. J Power Sources 332:180–186. https://doi.org/10.1016/j.jpowsour.2016.09.115
Wang Q (2016) Environmental Science and opportunities 729–762. https://doi.org/10.1039/c5ee03109e
Wang T, Zhai Y, Zhu Y, Li C, Zeng G (2018b) A review of the hydrothermal carbonization of biomass waste for hydrochar formation: process conditions, fundamentals, and physicochemical properties. Renew Sustain Energy Rev 90:223–247
Wang Z, Tan Y, Yang Y, Zhao X, Liu Y, Niu L, Tichnell B, Kong L, Kang L, Liu Z (2018c) Pomelo peels-derived porous activated carbon microsheets dual-doped with nitrogen and phosphorus for high performance electrochemical capacitors. J Power Sources 378:499–510
Wang Z, Zhang C, Watson J, Sharma BK, Si B, Zhang Y (2022) Adsorption or direct interspecies electron transfer? A comprehensive investigation of the role of biochar in anaerobic digestion of hydrothermal liquefaction aqueous phase. Chem Eng J 435:135078
Wei X, Zhang R, Zhang W, Yuan Y, Lai B (2019) High-efficiency adsorption of tetracycline by the prepared waste collagen fiber-derived porous biochar. RSC Adv 9:39355–39366
Wong JTF, Chen Z, Chen X, Ng CWW, Wong MH (2017) Soil-water retention behavior of compacted biochar-amended clay: a novel landfill final cover material. J Soils Sediments 17:590–598
Wu H, Lai C, Zeng G, Liang J, Chen J, Xu J, Wan J (2017) The interactions of composting and biochar and their implications for soil amendment and pollution remediation: a review. Crit Rev Biotechnol 37(6):754–764
Wu H, Zeng G, Liang J, Chen J, Xu J, Dai J, Wan J (2016) Responses of bacterial community and functional marker genes of nitrogen cycling to biochar, compost and combined amendments in soil. Appl Microbiol Biotechnol 100(19):8583–8591
Xia C, Cai L, Zhang H, Zuo L, Shi SQ, Lam SS (2021) A review on the modeling and validation of biomass pyrolysis with a focus on product yield and composition
Yamato M, Okimori Y, Wibowo IF, Anshori S, Ogawa M (2006) Effects of the application of charred bark Ofacacia Mangiumon the yield of maize, cowpea and peanut, and soil chemical properties in South Sumatra. Indonesia Soil Sci Plant Nutr 52:489–495. https://doi.org/10.1111/j.1747-0765.2006.00065.x
Yang F, Du Q, Sui L, Cheng K (2021) One-step fabrication of artificial humic acid-functionalized colloid-like magnetic biochar for rapid heavy metal removal. Biores Technol 328:124825
Yang X, Liu J, McGrouther K, Huang H, Lu K, Guo X, He L, Lin X, Che L, Ye Z (2016) Effect of biochar on the extractability of heavy metals (Cd, Cu, Pb, and Zn) and enzyme activity in soil. Environ Sci Pollut Res 23:974–984
Yankovych H, Novoseltseva V, Kovalenko O, Behunova DM, Kanuchova M, Vaclavikova M, Melnyk I (2021) New perception of Zn (II) and Mn (II) removal mechanism on sustainable sunflower biochar from alkaline batteries contaminated water. J Environ Manage 292:112757
Yao Y, Gao B, Chen J, Zhang M, Inyang M, Li Y, Alva A, Yang L (2013) Engineered carbon (biochar) prepared by direct pyrolysis of Mg-accumulated tomato tissues: characterization and phosphate removal potential. Biores Technol 138:8–13
Yao Y, Gao B, Zhang M, Inyang M, Zimmerman AR (2012) Effect of biochar amendment on sorption and leaching of nitrate, ammonium, and phosphate in a sandy soil. Chemosphere 89:1467–1471
Ye S, Zeng G, Wu H, Liang J, Zhang C, Dai J, Yu J (2019) The effects of activated biochar addition on remediation efficiency of co-composting with contaminated wetland soil. Resources Conserv Recycling 140:278–285
Yu O-Y, Raichle B, Sink S (2013) Impact of biochar on the water holding capacity of loamy sand soil. Int J Energy Environ Eng 4:44. https://doi.org/10.1186/2251-6832-4-44
Yu Z, Dang Q, Liu C, Cha D, Zhang H, Zhu W, Zhang Q, Fan B (2017) Preparation and characterization of poly (maleic acid ) -grafted cross-linked chitosan microspheres for Cd (II) adsorption. Carbohyd Polym 172:28–39. https://doi.org/10.1016/j.carbpol.2017.05.039
Zeymer M, Meisel K, Clemens A, Klemm M (2017) Technical, economic, and environmental assessment of the hydrothermal carbonization of green waste. Chem Eng Technol 40:260–269. https://doi.org/10.1002/ceat.201600233
Zhai Y, Peng C, Xu B, Wang T, Li C, Zeng G, Zhu Y (2017) Hydrothermal carbonisation of sewage sludge for char production with different waste biomass: Effects of reaction temperature and energy recycling. Energy 127:167–174. https://doi.org/10.1016/j.energy.2017.03.116
Zhang A, Li X, Xing J, Xu G (2020a) Adsorption of potentially toxic elements in water by modified biochar: a review. J Environ Chem Eng 8:104196
Zhang H, Yue X, Li F, Xiao R, Zhang Y, Gu D (2018) Preparation of rice straw-derived biochar for efficient cadmium removal by modification of oxygen-containing functional groups. Sci Total Environ 631:795–802
Zhang J, Hou D, Shen Z, Jin F, O’Connor D, Pan S, Ok YS, Tsang DCW, Bolan NS, Alessi DS (2020b) Effects of excessive impregnation, magnesium content, and pyrolysis temperature on MgO-coated watermelon rind biochar and its lead removal capacity. Environ Res 183:109152
Zhang L, Tang S, He F, Liu Y, Mao W, Guan Y (2019a) Highly efficient and selective capture of heavy metals by poly (acrylic acid) grafted chitosan and biochar composite for wastewater treatment. Chem Eng J 378:122215
Zhang S, Zhu X, Zhou S, Shang H, Luo J, Tsang DC (2019) Hydrothermal carbonization for hydrochar production and its application. In: Biochar from biomass and waste. Elsevier, pp 275–294
Zhang W, Zou Y, Yu C, Zhong W (2019c) Nitrogen-enriched compact biochar-based electrode materials for supercapacitors with ultrahigh volumetric performance. J Power Sources 439:227067
Zhang X, Wang Y, Cai J, Wilson K, Lee AF (2020c) Bio/hydrochar sorbents for environmental remediation. Energy Environ Mater 3:453–468. https://doi.org/10.1002/eem2.12074
Zhang Y, Cui Y, Chen P, Liu S, Zhou N, Ding K, Ruan R (2019) Gasification technologies and their energy potentials. In Sustainable resource recovery and zero waste approaches. Elsevier, pp 193–206
Zhang Z, Delcroix B, Rezazgui O, Mangin P (2021) Simulation and techno-economic assessment of bio-methanol production from pine biomass, biochar and pyrolysis oil. Sustain Energy Technol Assess 44:101002
Zhang Z, Zhu Z, Shen B, Liu L (2019e) Insights into biochar and hydrochar production and applications: a review. Energy 171:581–598. https://doi.org/10.1016/j.energy.2019.01.035
Zhao B, Xu H, Ma F, Zhang T, Nan X (2019) Effects of dairy manure biochar on adsorption of sulfate onto light sierozem and its mechanisms. RSC Adv 9:5218–5223
Zhao J, Li Y, Wang G, Fan Z, Wei T, Liu Z, Cheng K, Ye K, Zhu K, Cao D (2021) Journal of Materials Chemistry A Supplementary files 2017, 1–5
Zhao X, Song Z, Liu H, Li Z, Li L, Ma C (2010) Microwave pyrolysis of corn stalk bale: a promising method for direct utilization of large-sized biomass and syngas production. J Anal Appl Pyrol 89:87–94
Zheng K, Li Y, Zhu M, Yu X, Zhang M, Shi L (2017) The porous carbon derived from water hyacinth with well-designed hierarchical structure for supercapacitors. J Power Sour 366:270–277. https://doi.org/10.1016/j.jpowsour.2017.09.034
Zhou S, Wen X, Cao Z, Cheng R, Qian Y, Mi J, Wu Y (2021) Modified cornstalk biochar can reduce ammonia emissions from compost by increasing the number of ammonia-oxidizing bacteria and decreasing urease activity. Bioresource Technol 319:124120
Zhou X, Zhou J, Liu Y, Guo J, Ren J, Zhou F (2018) Preparation of iminodiacetic acid-modified magnetic biochar by carbonization, magnetization and functional modification for Cd (II) removal in water. Fuel 233:469–479. https://doi.org/10.1016/j.fuel.2018.06.075
Zhu X, Labianca C, He M, Luo Z, Wu C, You S, Tsang DCW (2022) Life-cycle assessment of pyrolysis processes for sustainable production of biochar from agro-residues. Bioresource Technol 127601
Zhu Z, Xu Z (2020) The rational design of biomass-derived carbon materials towards next-generation energy storage: a review. Renew Sustain Energy Rev 134:110308. https://doi.org/10.1016/j.rser.2020.110308
Zhuang X, Liu J, Ma L (2022) Facile synthesis of hydrochar-supported catalysts from glucose and its catalytic activity towards the production of functional amines. Green Energy Environ. https://doi.org/10.1016/j.gee.2022.01.012
Zimmerman AR (2010) Abiotic and microbial oxidation of laboratory-produced black carbon (biochar). Environ Sci Technol 44:1295–1301
Zou K, Deng Y, Chen J, Qian Y, Yang Y, Li Y, Chen G (2018) Hierarchically porous nitrogen-doped carbon derived from the activation of agriculture waste by potassium hydroxide and urea for high-performance supercapacitors. J Power Sources 378:579–588
Acknowledgements
The authors would like to thank Venna Saikrishna, National Institute of Technology, Warangal, for providing initial materials during first draft preparation.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Hari Bhakta Sharma was involved in writing—original draft preparation, editing. Kumar Raja Vanapalli helped in writing—original draft preparation, editing. Deepika Bhatia helped in writing—original draft preparation. Simranjeet Singh helped in writing—original draft preparation. Gaurav Arora was involved in writing—original draft preparation. Sagarika Panigrahi assisted in writing—original draft preparation. Brajesh Dubey helped in reviewing, editing, supervision. Praveen C Ramamurthy contributed to reviewing, editing, supervision. Bijayananda Mohanty helped in reviewing, supervision.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The authors have no relevant ethics approval to take because the manuscript does not involve research involving humans and/or animals.
Consent for publication
This research does not involve any human subjects for taking informed consent. All the authors approve the contents of this manuscript for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, H.B., Vanapalli, K.R., Bhatia, D. et al. Engineered biochar/hydrochar derived from organic wastes for energy, environmental, and agricultural applications. Clean Techn Environ Policy (2024). https://doi.org/10.1007/s10098-024-02863-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10098-024-02863-6