Abstract
The biota inhabiting the Suquía River Basin is described in this chapter. Comments on the species of fish, birds, invertebrates and aquatic plants registered in this system are included. Along the basin, different factors generate a noncontinuous mosaic of abiotic conditions at temporal and spatial levels, which, in turn, structure the biotic communities. The Suquía hydrological system consists of three sections: the upper basin in a mountainous area with headwaters and torrential rivers flowing into the San Roque Reservoir; the middle basin with drainage areas belonging to the eastern slope of the Sierras Chicas, together with the drainage area of the city of Córdoba; and the lower basin which is located downstream from the city of Córdoba flowing into the Mar Chiquita Lake, where the river meanders exhibit little flow. The species of the different groups change according to the characteristics of each section. In this chapter, endemic and introduced species are discussed. The bivalve species that inhabit the Suquía River are not native, and the cause of their introduction is explained. Authors also specify the anthropic factors that negatively impact water, bird and invertebrate species in the river.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Ichthyofauna
In the Suquía River Basin, 24 fish species, in permanent and casual forms, are accepted in the literature (Table 1). These species are distributed in 14 families and 8 orders [1]. The richest groups were the Characiformes (10 species) and the Siluriformes (6 species). Regarding the abundance of each order, the Cyprinodontiformes was the best represented order (50%), followed by the Siluriformes and the Characiformes (24.68% and 24.18%, respectively). However, the Cyprinodontiformes have only four species.
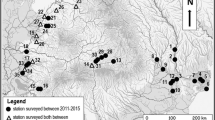
It is important to note that Phalloceros sp. was registered for the first time by Hued and Bistoni [1]. This is important because, according to Ringuelet et al. [2], the geographical distribution of this species comprises the Province of Misiones and the city of La Plata, in the Province of Buenos Aires, Argentina. It is also found in southern Brazil, Uruguay and Paraguay [2]. Hued and Bistoni [1] mentioned that only two females were found in the Villa Giardino area (upper basin) (31°02′00″S 64°29′00″O) on the San Francisco Stream (tributary of the Suquía River Basin) (Fig. 1). Further studies are necessary to determine if this species is established in the basin.
At the biogeographical level, five ichthyological provinces of the Argentinean freshwater fauna are recognised [3] (Fig. 2): Andino-Cuyana, Patagónica, Aymara, Grandes Ríos and Pampeana. According to these authors, the Pampeana province includes the Provinces of Tucumán and Córdoba, as well as an extensive area of the Provinces of Santiago del Estero, San Luis, Buenos Aires and La Pampa. It also includes the south-eastern region of the Provinces of Salta, Catamarca and La Rioja and the south-western region of the Province of Santa Fe. According to this classification, the Suquía River Basin is located fully in the Pampeana ichthyological province (Fig. 2).
Ichthyological provinces of Argentina. Adapted from López et al. [3] with permission
1.1 Distribution of the Ichthyofauna Along the Suquía River Basin
From the headwaters down, communities are structured along the abiotic factors that characterise each section, such as altitude, river order, stream gradient and distance from the source. These abiotic factors influence not only species richness but also trophic composition [4]. In the Suquía River Basin, three sections can be recognised according to their geomorphological conditions: upper, middle and lower sections (Fig. 1). The upper section extends from 1,900 m.a.s.l. to 650 m.a.s.l. and the middle section from 643 m.a.s.l. to 352 m.a.s.l. The lower section starts at 300 m.a.s.l. and runs down to the mouth of the Suquía River in the Mar Chiquita Lake (70 m.a.s.l.). Fish fauna of the middle and lower sections was comprehensively surveyed by Haro and Bistoni [5] and the upper section by Hued and Bistoni [1]. Streams located in high altitude places support cold water fish assemblages, and as streams run down the mountains, this assemblage changes to warm-water taxa. In the first case, the dominant species is trout. Trout inhabits areas near the headwaters because of their specific living requirements of oxygen, pH and temperature [6]. Thus, the headwaters can be classified as ‘trout zones’. A few kilometres downstream from the headwaters, this species rapidly declines in abundance, mainly because stream temperatures become warmer [7, 8]. Numerous studies indicate injury to native fish communities by the introduction of salmonids [9,10,11]. These studies indicate that trouts change the communities’ composition of native fish by competing for resources or predation. Further studies in the mountain river in the Province of Córdoba are necessary to confirm this aspect.
Continuous change in fish assemblages along the river seems to prevail in the middle and lower sections. Among the fish species present in downstream areas, Hoplias malabaricus and Cyprinus carpio are observed in the plains from 600 m downstream. The Siluriformes order is registered along the basin, but they are more diverse in the lower section of the basin with respect to upstream locations.
1.2 Endemism
Astyanax cordovae is a remarkable endemism in the Córdoba province, and it was described for the first time by Gunther in 1881 for Córdoba rivers (Fig. 3). Ringuelet [12] stated that this species had not been seen since its original description. In 1988, Bertolio and Gutiérrez published a detailed redescription of this fish. They note that A. cordovae has a geographical distribution restricted to the basins of the Suquía and Xanaes Rivers. Its presence is more easily observable in the Suquía River, even though it is not as common as the other two species of the same family, Astyanax eigenmanniorum and Bryconamericus iheringi. It inhabits deep wells and has also been captured in flowing water in the channelized area of the Suquía River, running through the city of Córdoba (middle basin, Fig. 1) [13].
Miquelarena et al. [14] described for the Suquía River Basin a new endemic species, Astyanax hermosus that was registered only in the San Francisco River, in the Valle Hermoso locality (37° 07’ S-64° 29’ W). This site is located at 900 m.a.s.l. and it is a typical mountain stream with a fast-flowing current and gravelly, rocky and sandy bottoms (upper basin, Fig. 1).
1.3 Introduced Fish in the Suquía River
Species that move, whether intentionally or accidentally, due to human activities, from its native area to a different region are considered alien species [15].
Out of 28 fish species, five were introduced: Cyprinus carpio, Ctenopharyngodon idella, Gambusia affinis, Odontesthes bonariensis, Oncorhynchus mykiss and Salvelinus fontinalis. The last two mentioned species were introduced in Argentina from the United States for sport fishing. They were introduced as embryonated eggs at the beginning of the twentieth century [16]. Nowadays, they are distributed in almost all mountain rivers of the Province of Córdoba. On the same hand, C. carpio, a species indigenous to China, was introduced in Argentina in 1930. It is considered a naturalised species because it sustains self-replacing populations for several life cycles. Besides, another cyprinid, C. idella, was introduced in the San Roque Dam since 1989, and it is considered a casual species because it does not form self-replacing populations in the invaded region and its persistence depends on repeated introductions. It is detected in sporadic catches by sport fishermen in the San Roque Dam.
G. affinis, a species native to Mexico and the southern region of the United States, was introduced in Argentina to fight diseases such as malaria and yellow fever, because of its recognised deficiency as a larvivorous fish. It is a small-sized fish (3–4 cm), common along the Suquía River, which inhabits pristine as well as contaminated sites, where it becomes the dominant species [17]. Silverside (O. bonariensis) is native to the Río de la Plata River (Argentina). This fish species was introduced, mainly in lakes of the Province of Córdoba, for sport fishing. Now, it is considered a naturalised species, with a permanent population in the lakes. From the San Roque Lake and through the Suquía River as a dispersal route, silverside arrived to the Mar Chiquita Lake, where it settled. This last lake showed a hypersaline condition 20 years ago [18]. At the time, the average concentration of salt in the lake was around 300 g/L (hypersaline stage), making it impossible for this species to settle there. Around 1970, the Mar Chiquita Lake received a significant amount of freshwater from their tributaries, as a result of a marked increase in precipitation throughout the basin, and its salinity decreased to 30 g/L, a concentration similar to seawater levels. This mesohaline condition allowed O. bonariensis and others species, such as Jenynsia multidentata, to colonise this lake [18]. The silverside population grew rapidly in the lake; therefore, the Government of the Province of Córdoba authorised commercial fishing in the area [19]. These authors note that in 1997 the level of the Mar Chiquita Lake decreased, and the salinity increased again. These reasons, among others like fishing pressure, population dynamics and limnology, caused a decrease in the silverside population in the lake, so commercial fishing was markedly affected. Nowadays, it is difficult for local fishermen to find silversides in the Mar Chiquita Lake (personal observation). Reati et al. [18] estimated that O. bonariensis will disappear from the Mar Chiquita Lake in a few years if the increasing trend raising the water salinity continues, probably before concentrations of 60 g/L are reached. Therefore, the presence of silverside in the Mar Chiquita Lake seems to be cyclical. During extended periods of low salinity, this species adds economic importance to the region.
1.4 Ecological Types
Ringuelet [12] made a classification integrating habitat and trophic level of freshwater fish in Argentina, and they denominated it ‘ecological types’. We modified the classification made by these authors according to our experience. Therefore, the Suquía River species can be classified into several ‘ecological types’. So ‘bottom fish’ includes species with an iliophagus diet, such as Prochilodus lineatus, and it also includes the most characteristic species of this group: the armoured catfish Rineloricaria catamarcensis, Hypostomus sp. and Corydoras paleatus. Besides, the ecological type comprising ‘fish that frequent the bottom’ encounters opportunistic carnivores, which are very common in the bottom of streams with a slow velocity. This type includes species such as catfish Rhamdia quelen, Pimelodella laticeps and Pimelodus albicans, which have long sensitive barbells that allow them to perceive their preys. Considering the diet and habitat of C. carpio, it could be included in this ecological type. The ecological type ‘open and vegetated waters’ includes the predator H. malabaricus as well as a group of small fish with an insectivorous diet. This group also includes a different genus such as Astyanax, Bryconamericus and Cheirodon. Other species included in this group are Australoheros facetum and small fish of the order Cyprinodontiformes such as J. multidentata, Cnesterodon decemmaculatus and G. affinis. In recent times, it has been observed that mosquitofish (G. affinis) is the most resistant to pollution among all the Cyprinodontiformes species, and it seems that native species decline in abundance while alien species increase. However, no studies have been conducted yet to confirm this observation. Also, a special ecological type called ‘air-breathing fish’ includes species with the ability to breathe atmospheric oxygen. Members of this ecological type found in the Suquía River Basin include Synbranchus marmoratus and C. paleatus. Finally, the ecological type ‘cold water fish’ includes Trichomycterus corduvense. This species inhabits the fastest section of the river, with rocky and sandy bottom. This little catfish shares the habitat with trouts, which have the same environmental requirements and can compete for different resources.
2 Aquatic Birds
The literature about waterbirds specifically present in the Suquía River Basin is almost non-existent; much of the concepts expressed herein correspond to observations made by the authors.
2.1 Species Richness
Birds, and particularly aquatic birds, are known for their great dispersal capability compared to other groups of vertebrates. In this way, 69% of aquatic birds cited for the Province of Córdoba can be observed in the Suquía River Basin ([20,21,22,23,24], [25, pers. obs.]), totalling 79 species belonging to 18 families in eight orders. However, it is possible that the remaining 35 species, which are rare or occasional in Córdoba, will ever be observed.
Some families are particularly rich around the world and the situation in the Suquía River Basin is not an exception. Thus, most waterbird species belong to the families Anatidae (15 species) and Scolopacidae (13 species, Table 2).
Additionally, 25 species of birds not considered as aquatic birds sensu stricto (i.e. those with physiological and anatomical adaptations to aquatic habitats) also inhabit the basin. These species, however, exhibit behavioural adaptations for living in wetlands and rivers and can hardly be observed outside such environments. Nine families, in three orders, contain species of this type in the basin, being six species of the family Tyrannidae the richest ones (Table 3).
2.2 Distribution Along the Suquía River Basin
As mentioned previously (Sect. 1), from the headwaters in the highlands of the Sierras Grandes at 1,900 m.a.s.l. to the mouth in Mar Chiquita, a saline lake at 70 m.a.s.l., the Suquía River runs through markedly different regions (Fig. 1). The upper portion of the basin is part of a highland plateau dominated by grasslands interspersed with rocky outcrops (locally named ‘Pampa de Achala’), where the headwaters flow as small streams. The flat topography in this region makes watercourses flow at a low speed and allows the formation of small wetlands with palustrine vegetation. These wetlands are inhabited by some birds that colonised them from lowland regions, as the plumbeous rail (Pardirallus sanguinolentus) and the sedge wren (Cistothorus platensis). Instead, birds like the Córdoba cinclodes (Cinclodes comechingonus), the white-winged cinclodes (C. atacamensis schocolatinus) and the Olrog’s cinclodes (C. olrogi), which travel along the rocky banks of streams, searching for small aquatic invertebrates, are endemic to the highlands of the mountains of Central Argentina [22]. Two species, the buff-necked ibis (Theristicus caudatus) and the tawny-throated dotterel (Oreopholus ruficollis), inhabit the humid grasslands near streams and constitute biogeographic singularities since they belong to local, isolated breeding populations, separated by hundreds or even thousands of kilometres from the nearest breeding populations in the lowlands of Eastern Argentina and Patagonia, respectively [22, 26]. The cast of grassland birds is completed with the widely distributed southern lapwing (Vanellus chilensis), as common here as in the lowlands.
Downstream from Pampa de Achala, watercourses become faster as the slope becomes steeper. The aquatic birds at these sites are limited to the backwaters, where fish, tadpoles and invertebrates are caught by Neotropic cormorants (Phalacrocorax olivaceous), snowy egrets (Egretta thula) and striated herons (Butorides striata). Among ducks, the speckled teal (Anas flavirostris) is by far the most common in this region. In some sites, the cliffs over rivers, both rocky and gravelly, are utilised for nesting by the Speckled Teal, the buff-necked ibis and the ringed kingfisher (Megaceryle torquata), together with several nonaquatic species (not listed in Tables 2 and 3) like the peregrine falcon (Falco peregrinus) and the introduced rock dove (Columba livia). In this section of the basin, several reservoirs of variable dimensions add a previously non-existent feature to the original landscape. These deep waters were colonised mainly by Neotropic cormorants, great grebes (Podicephorus major) and pied-billed grebes (Podilymbus podiceps). At the mouths of rivers, the marshy vegetation thrives and birds like the red-gartered coot (Fulica armillata), the white-winged coot (F. leucoptera) and some ducks are commonly associated with it.
When the river reaches the lowlands, it becomes very sinuous and forms a broad alluvial plain, with marshes and small lagoons as associated wetlands (lower basin, downstream from Córdoba City, Fig. 1). The aquatic avifauna is richer than in the previous regions. Besides Neotropic cormorants, snowy egrets and striated herons, other piscivorous birds, such as the great egret (Ardea alba) (Fig. 4) and the beautiful cocoi heron (A. cocoi), are frequently observed along the river. The lagoons exhibit a great spatial heterogeneity, and several distinct habitats (open deep waters, shallow waters, reed beds, floating plants and humid grasslands) allow the coexistence of a diverse fauna of birds. Thus, open deep waters are mainly frequented by the pied-billed grebe, the white-tufted grebe (Rollandia rolland), the white-cheeked pintail (Anas bahamensis), the lake duck (Oxyura vittata), the rosy-billed pochard (Netta peposaca), the red-gartered coot and the white-winged coot, whereas shallow waters are visited by several egrets and herons, the white-faced ibis (Plegadis chihi), the southern screamer (Chauna torquata) and the black-necked stilt (Himantopus mexicanus). The red-fronted coot (Fulica rufifrons), the common gallinule (Gallinula galeata) and the spot-flanked gallinule (G. melanops) swim through the dense reeds, whereas the plumbeous rail prefers walking, pushing through them. The limpkin (Aramus guarauna) and the snail kite (Rostrhamus sociabilis) search for amphibious snails that lay their eggs on the reeds. Reedbeds are also inhabited by a diverse fauna of small birds that eat and nest in them, like the wren-like rushbird (Phleocryptes melanops), the many-coloured rush tyrant (Tachuris rubrigastra) and the yellow-winged blackbird (Agelasticus thilius). Finally, floating plants are the substrate for the specialised wattled jacana (Jacana jacana), while humid grasslands are the home of the South American painted-snipe (Nycticryphes semicollaris), the South American snipe (Gallinago paraguaiae) and the whistling heron (Syrigma sibilatrix).
In its final section, the Suquía River is divided into two branches, both of which flow into the Mar Chiquita Lake (lower section, Fig. 1). Several small lagoons near the mouths harbour an avifauna similar to the one described above, while in the mouth itself, the silty beaches are exploited by a great number of shorebirds species, both permanent and migratory. The collared plover (Charadrius collaris) is one of the few breeding permanent species, together with the two-banded plover (C. falklandicus), which have an isolated breeding population in Mar Chiquita [27], a thousand kilometres away from the nearest breeding population in Patagonia and, thus, constituting another biogeographic singularity. The rufous-chested dotterel (C. modestus) can be observed in winter as coming from Patagonia, but the vast majority of species arrive from North America in spring, being the Wilson’s phalarope (Phalaropus tricolor), the greater yellowlegs (Tringa melanoleuca), the lesser yellowlegs (T. flavipes), the white-rumped sandpiper (Calidris fuscicollis), the Baird’s sandpiper (C. bairdii) and the pectoral sandpiper (C. melanotos) the most common ones. The brackish waters in estuaries are used by the Chilean flamingo (Phoenicopterus chilensis) for feeding, and they are visited in winter by the Andean flamingo (Phoenicoparrus andinus) and the James’s flamingo (P. jamesi), which come from their breeding grounds in the Andean puna. As beautiful as flamingos are the black-necked swans (Cygnus melancoryphus) and the coscoroba swan (Coscoroba coscoroba), which, together with the brown-hooded gull (Chroicocephalus maculipennis) and the grey-hooded gull (C. cirrocephalus), prefer deeper waters near the mouths.
2.3 Anthropic Factor Influencing the Occurrence of Waterbirds in the Basin
Contamination and deforestation are the main pressures on the biota of the Suquía River Basin. The headwaters in the upper section of the basin are the more pristine habitats; however, overgrazing magnifies water erosion, leading to an increase in the sediments transported towards the lower sections of the basin. When these nutrients reach the San Roque Reservoir, they get combined with sewage from Carlos Paz city, causing an increase in the densities of bacteria and algae, with occasional blooms, whose toxins are dangerous to all living beings, including waterbirds. Nevertheless, the main sources of contamination of the Suquía River are the industrial activities and sewage discharges of Córdoba City. Some data indicate a great reduction in richness, abundance and diversity of aquatic birds in the river in Córdoba city when compared to sections upstream and downstream [20]. This reduction, however, is also influenced by others anthropic disturbances, as the presence of homes and busy routes close to the river and the reduction in the cover of riparian vegetation. In any case, the community composition was quite different between the sections’ upstream and downstream of the city, with predominance of piscivorous species upstream from the city and of invertivorous species downstream from the city, probably reflecting a rise in invertebrate abundance and a decline of fish as a consequence of pollutants in the water [20].
Deforestation was widespread in all plains of the centre and east of the Province of Córdoba, including the Suquía River Basin. The remnant forest fragments are dispersed throughout the plains, the riparian zones of the river and the associated lagoons. These forests form a corridor that allows the penetration from the North of aquatic birds, typical of subtropical areas. In this way, in the lagoons near Río Primero city, it is possible to observe species such as the Brazilian teal (Amazonetta brasiliensis), the rufescent tiger heron (Tigrisoma lineatum) and the purple gallinule (Porphyrio martinicus), all of them common and widespread in Northern and Eastern Argentina but rare in Córdoba. Finally, in lagoons close to Córdoba City (downstream, in Chacra de la Merced, Fig. 1), species like the bare-faced ibis (Phimosus infuscatus) are observable.
3 Aquatic Macroinvertebrates
Rivers are dynamic systems where communities respond spatially and temporally to the interaction between external factors, determined by their drainage basin, and internal factors, defined by the riverbed and valley characteristics [28]. According to the size and type of lotic environment, these factors generate a noncontinuous mosaic of biotic and abiotic conditions at the temporal and spatial levels [29], which determines the distribution of the populations, especially those related to the bottom of the river [30].
The biological quality of rivers can be evaluated through the biotic communities they harbour. Macroinvertebrates, particularly insects, are an important component of both biodiversity and the functioning of freshwater ecosystems [31]. Several authors studied the communities of aquatic invertebrates in lotic environments in the Province of Córdoba, considering different aspects such as distribution and ecology [32,33,34,35,36]. Regarding the Suquía River, the most comprehensive studies were approached by Mangeaud [37].
Here, the relative composition in phyla and orders of the macroinvertebrate community is similar to other rivers in the central region of Argentina. Even though the number and abundance of the taxa differ, its comparable to rivers in other regions but under arid or semi-arid climates. Along the basin, a total of 51 taxa were recorded distributed in six phyla, nine classes and 41 families (Table 4), with diversity (H′) values ranging from 0.8 to 3.2 in different points. Diversity and number of taxa increase from the headwaters to the mouth, and when contaminants enter the basin, these variables decrease.
In the entire basin, Arthropoda is well represented (more than 88%), followed by Annelida and Mollusca with much lower relative abundances (8% and 2%, respectively). In the basin sites considered as contaminated (mainly downstream from cities), a higher proportion of Annelida and a decreased proportion of Arthropoda is observed, which might be possible since the first group is more tolerant to pollution. The non-polluted sites mark the general tendency of the basin.
Insecta is the most numerous group of macroinvertebrates in the Suquía River, with 37 recognised Families of benthic insects, belonging to eight orders, and constituting about 99% of the arthropods of the watershed. Diptera is the order with the highest abundance (more than 50% of the benthos abundance), followed by Ephemeroptera (33%), Trichoptera (10%) and Coleoptera (4%). Such composition varies in the different subbasins of the upper section (Fig. 1) with small changes in the community structure, but more important ones regarding dominant taxa. Camelobaetidius (Ephemeroptera: Baetidae) seems to be one of the dominant genus upstream in the upper section, but it is poorly represented downstream of the same section of the river. On the other hand, in this last part of the basin, the dominant genus is Leptohyphes (Ephemeroptera: Leptohyphidae), while Chironomidae is dominant in all the upper section.
3.1 Distribution Along the Suquía River Basin
Variation in diversity, number of taxa and abundance is observed when the stream order increases and the altitude decreases. Causes of this zonation are given by a complex combination of variables such as current velocity, substrate, stream flow, temperature, dissolved oxygen and nutrients, alkalinity and other chemical factors, as well as interactions with other organisms [37, 38].
The altitude range in which taxa are recorded presents great variability. Hirudinea, Annelida, Chironomidae, Acari, Elmidae, Dytiscidae, Smicridea (Trichoptera: Hidropsychidae) and Leptohyphes, among others, are well represented in the upper section of the river (from 600 up to 1,800 m.a.s.l.). Even though some taxa like Leptohyphes, Hirudinea, Hydroptila (Trichoptera: Hydroptilidae) and Dytiscidae present an inverse relationship between abundance and altitude, meaning that, although their optimal conditions come from the middle or lower sections, some individuals are able to tolerate conditions the upper section Another group develops only in the lower or middle sections area, and in the upper section up to 1,000–1,200 m.a.s.l. They are Protoptila (Trichoptera: Glossosomatidae), Zygoptera, Anisoptera (Fig. 5), Nectopsyche (Trichoptera: Leptoceridae), Marilia and Mollusca. A third group only inhabits mainly the middle section: Empididae, Maruina (Diptera: Psychodidae) and Polycentropus (Trichoptera: Polycentropodidae), while Ochrotrichia (Trichoptera: Hydroptilidae) develops in the upper section exclusively above 1,200 m.a.s.l.
Supraspecific groups such as Trichoptera, Leptohyphidae, Ephemeroptera and Mollusca show a significant decrease in abundance in relation to altitude. On the other hand, Coleoptera decreases in abundance when the order of the river increases. The abundance of Diptera and Annelida seems unrelated to these two variables.
Plecoptera is known worldwide as a species exclusive of upstream regions since their nymphs depend intimately on cold temperatures to develop [39]. In spite of the appropriate environmental conditions, this Order is poorly represented in Córdoba. Summer droughts can cause significant increases in macroinvertebrates abundance. When this occurs, algae and macrophytes that are attached to the rocky bottom of streams are not removed by summer floods, as is the case with aquatic invertebrates. An abundant layer of plants covers the bottom of streams leading to a great heterogeneity of benthic habitats [40], and the higher the plant biomass, the greater the number of macroinvertebrates [41].
To summarise, benthic macroinvertebrate communities are neither stable nor constant with regard to diversity, number of taxa and abundance in the Suquía River, presenting a great temporal and spatial variability. Community structure becomes more complex in an altitudinal gradient, from up to downstream. In the places where contaminants enter the basin, diversity and number of taxa decrease. Some taxa are more abundant in the upper section, while others increase their abundance in the middle and lower sections. Some others are present along the basin, regardless of altitude. In the upper section there is a limit (1,000–1,200 m.a.s.l.) above which a change in fauna is produced.
3.2 Invasive Bivalves
Invasive species are one of the most significant causes of biodiversity loss and changes in ecosystem services, which underline the importance of their detection and study. Freshwater systems are particularly subject to invasion by exotic invertebrate species, which use water current for dispersion throughout these systems. Among these invertebrates, bivalve molluscs are a group with high potential for invasion: they can develop massive populations in all kinds of fresh waters, consuming microalgae and substantially affecting the amount and composition of primary producers. Interactions radiating out from the primary producers can affect nearly every part of the ecosystem. Their activity also entails habitat modification, competition and extinction. Besides the characteristics of natural ecosystems, they can affect human structures, impeding domestic and industrial water supply infrastructure. Human activity, such as the construction of shipping canals for trade, building of reservoirs for water storage and power production, promotes the spreading of these species and facilitates pulses of spreading which seem to be not continuous [42].
In the last decades, two species of freshwater bivalves have been reported as invasive in inland waters of South America: the golden mussel, Limnoperna fortunei (Dunker 1857), and the Asian clam, Corbicula fluminea (Müller 1774).
In the case of Limnoperna fortunei, its sudden appearance in the Río de La Plata Estuary was first reported at the beginning of the 1990s [43], spreading into Argentina, Uruguay, Paraguay, Bolivia and Brazil. Corbicula fluminea started to colonise South America in the 1960s, when the bivalve arrived in Argentina and Brazil, through the Río de La Plata River, and subsequently spread into Venezuela, the northern part of the Pantanal in Southwest Brazil, and lower areas of the Amazon River Basin, among other areas [44]. Nowadays, they are one of the dominant freshwater benthic macrofauna in an area extending from Lake Superior in North America to Patagonia in South America. Such golden mussels and Asian clams have been reported in central Argentina. In Córdoba, their distribution is still scattered, not having been observed, for instance, in the upper reaches of the main rivers and reservoirs. It is estimated that their distribution will be more extensive in the case of species with a wide range of physiological tolerance and adaptability to different environments.
The mechanism that enabled the introduction of invasive bivalves in the Suquía River and other basins of central Argentina is uncertain, since they are geographically disconnected from the arrival area. Thus, the accidental introduction by human activities is the most probable cause. They are successful invaders that can be dispersed through natural means over large areas in a short time. Through their larvae, they could spread into reservoirs connected to the arrival area and from there, to other rivers and reservoirs like the San Roque Reservoir, most probably transported in hulls of boats for recreational or fishing purposes.
The systematics of Corbicula is controversial and especially the study of lineages in the New World, where three morphotypes have been distinguished. These are forms A and B, present throughout the continent, and form C, only present in South America and known as C. largillierti. According to Lee et al. [45], there are hybrids between the different forms. Pfenninger et al. [46] proposed that different lineages of Corbicula may be an initial state of a group of species rather than a defined species. Morton [47] considered that the observed variability could correspond to a single species. Following the analyses of different outer and inner characters suggested by previous authors [48,49,50], Reyna et al. [51] reported the presence of two corbiculid species, C. largillierti and C. fluminea in the Suquía River Basin. Among the previously reported characters, rib number was useful to differentiate the two species. Besides, conventional and geometric morphometric analyses to assess inter- and intraspecific differences in shell shape revealed a clear morphometric differentiation between C. fluminea and C. largillierti (Morán et al. in prep) (Fig. 6).
When present in high abundance, L. fortunei produces occlusions in piping systems, such as pipes or hoses, as well as in bars, turbines and water intakes with imaginable consequences. They can also affect the speed of vessels due to a loss in hydrodynamics. In all cases, a necessary step then is to spread the problem to take preventive measures. The invasions of freshwater molluscs are a real problem in different areas of the world: in the Northern Hemisphere, the zebra mussel Dreissena polymorpha produces similar effects as the golden mussel. In the United States, the use of aggregates with abundant dead Corbicula fluminea shells in the construction business caused the weakening of concrete structures. From an ecological perspective, the filtering capacity of freshwater molluscs (necessary for the feeding activity) causes a decrease in the turbidity of water bodies since they eat phytoplankton and particulate matter in general, and their droppings can be an important part of the nutrient cycling. While the golden mussels are typically epifaunal animals that normally attach themselves to hard substrates through byssal threads, the Asian clams are infaunal species. In both cases, they potentially constitute a substrate competitor for other species, since they can colonise large areas. Although native bivalves are present in other areas of Argentina (i.e. clams Cyanocyclas and Anodontites), none were reported in the Suquía River. So far, the vector of parasites that affects human health or other species is unknown. In other close areas, certain species of birds and teleost fish, such as Pterodoras granulosus and Leporinus obtusidens, predators of clams and mussels, respectively [44], can constitute natural controls for mollusc populations.
Strategies for the prevention and control of mollusc populations must be adapted to each circumstance, because once they appear, they are difficult to eliminate. Regular cleaning or the use of anti-fouling paints on the hulls of boats used for recreational or fishing purposes may limit accidental spillage. Other uses of these organisms, such as fishing bait or aquarium, should be avoided. In closed systems, biocides (chemicals), temperature or salinity alteration can be used. In natural environments, the control of these factors becomes difficult, and the predator action is limited. For this reason, the most effective method is manual removal.
Bivalve density varies with substrate type: while golden mussels typically attach themselves to natural and artificial hard substrates, clams bury themselves in sediments, preferably in sandy substrates. However, C. fluminea can colonise a wide variety of substrates: rocks, gravel, boulders, sand and clay. Darrigran [52] found that in environments with a substrate composed of silt sediment, C. largillierti dominates over C. fluminea.
Other parameters, such as fluctuation in water level and contamination, can also determine the presence and density of these animals and produce alterations in their population structure. Given the particular characteristics of the Suquía River Basin, i.e. presence of diverse substrate types, fluctuations in the flow regime and the impact of human activities, the presence, distribution and density of invasive bivalves vary throughout this extensive area.
Making a first diagnosis on the status of the invasion of corbiculids in the Suquía River Basin, Reyna et al. [51] studied their distribution and density at different sites. Variations in population structure (density, biomass, spatial distribution and size classes) during a whole year were also studied in Córdoba city. Results of that study revealed that C. fluminea is restricted to a lentic environment (the San Roque Reservoir), apparently coexisting with C. largillierti, although only empty shells of this latter species have been found in this site. In rivers and brooks, only C. largillierti was detected. The absence of living specimens and the presence of empty shells in diverse sites could be due to the water dynamics and flow, which depend on rainfall that mostly occurs during summer time in the Southern Hemisphere (from November to March). The reduction of the river flow during the dry winter season exposes the sandbanks where molluscs live buried and causes their death. The species C. largillierti showed variations in average density between the different sites and also variations in biomass and size classes throughout the year period. The average density of 302 ind./m2observed in Córdoba city was similar to the density observed in a tributary lentic environment of the Río de La Plata Basin (459 ind./m2 [52]), but it was considerably less than the mean density of Corbicula observed in the Río de La Plata Basin (2.5 ind./m2 [53]). A great number of medium-sized animals were found in Córdoba city during the year period. As stated by Boltovskoy et al. [54], the absence of small and large-sized individuals and the high density of medium-sized individuals may be caused by contamination, affecting the larvae and preventing the growth of individuals. This site is located in Córdoba city, where the river is flanked on both sides by frequently used highways and is connected to the city rainwater channels, through which garbage and sewage waters are illegally introduced.
Darrigran [52] found Corbicula in lentic environments, with C. fluminea restricted to shallow, well-oxygenated coastal waters. Besides, abundant populations of C. fluminea were detected in the headwaters of micro-basins and rivers with a stronger flow [55]. The distribution of C. fluminea, restricted to the San Roque Reservoir, within the Suquía River Basin is surprising, considering the wide, rapid spread of this species in other areas. On the other hand, C. largillierti, which was distributed in most of the sampling sites along the Suquía River, was restricted to a few sites in Argentina [52, 56]. That species seems to be better adapted to brook environments. The absence of Corbicula largillierti downstream of Córdoba city (31° 25′50″S/64°01′57″W) could be due to high contamination. The accumulation of city garbage and agricultural pesticides, sewage discharge (WWTP) of Córdoba city at Bajo Grande with low oxygen levels (5 ± 2.1 mg l−1) detected in that area [57] are probable stress conditions. They would limit the survival of juvenile and adult bivalves and, therefore, their dispersion from the upper to the lower basin. Studies on the potential distribution and the influence of contamination are necessary in order to comprehend the processes structuring changes along the Suquía River.
4 Aquatic Plants
Despite having conquered the land, some vascular plants have ventured back into fresh waters: it is estimated that they represent ca. 1% of the angiosperms and 2% of the pteridophytes, according to how strictly an aquatic plant is defined. Since plants from aquatic environments have a diversity of habitats and plasticity, it is difficult to determine a biological classification for such a heterogeneous group. However, a more generalised scheme divides them into the following categories [58,59,60]:
Hydrophytes or Strictly Aquatic Plants
They live in the water or on a substrate that is at least periodically anaerobic due to water excess, and they can be: (a) Emergent (11 genera and 19 species in the Suquía River): they are attached to submerged soils where the water depth is 50–150 cm, mainly rhizomatous perennials, usually with heterophylly, and all producing aerial reproductive organs; (b) Submerged (7 genera and 11 species): they grow on submerged soils at a water depth of ± 10 m, leaves are usually filiform and totally submerged, and reproductive organs may be submerged, floating or aerial; (c) Floating (6 genera and 9 species): they grow mainly in sheltered sites and are typically unattached, very diverse in life forms, reproductive organs floating or aerial (rarely submerged).
Riparian
(ca. 87 genera and 162 species in the Suquía River): They are completely terrestrial plants but require a high level of soil moisture, which they find on river banks. Plants have been classified by their presence in riparian areas as obligate riparian, facultative riparian and non-riparian, with some variations according to the author [61, 62].
A riparian area is the one that is adjacent to or directly influenced by a water body. Riparian means ‘belonging to the bank of a river’; therefore, it refers to biotic communities living on both sides of rivers, streams or lakes [63, 64]. Riparian areas usually maintain a high biodiversity of flora and fauna compared to other areas, working in many cases as a shelter for vulnerable species of both, plants and animals. Species richness of herbaceous plants is usually greater in the zone adjacent to the stream bank than in other zones, and species composition of herbaceous plants is statistically different from more distant zones [65]. These areas provide habitat for rich wildlife, since they function as corridors among vegetation patches in fragmented landscapes [66, 67]. Riparian areas are generally fertile and productive, with high soil quality, and are the last line of defence for the protection of water quality and aquatic ecosystems [68, 69].
Riparian vegetation is unique and different, being generally higher, denser and structurally more complex than the surrounding vegetation [68]. Microclimate in most cases is moister. The shadow produced by the riparian vegetation is critical for fluctuations in water temperature and amount of sunlight, affecting the growth of plants that live along the streams, and consequently, fish and vertebrates that feed on them [68].
Another characteristic of riparian areas is the excess of sediment and nutrients, mainly phosphorus and nitrogen from crop areas. The main functions of a riparian forest are to slow and reduce runoff by using the excess of nutrients, trapping sediments and other pollutants that flow from bare soil or crop land, protecting water bodies, and also enhancing infiltration [68].
The composition and amount of vegetation in riparian areas differ from terrestrial upland vegetation. These differences reflect the influence of water from the adjacent water body, primarily in terms of increased soil moisture in the riparian areas. At the same time, rivers or sections of a river can be characterised according to the slope as: torrential upper, swift middle and sluggish lowland. In the case of the Suquía River, two sections could be distinguished: a first one, before crossing the ravine above Bamba, where the river runs through the Pampean Hills (Sierras Grandes and Sierras Chicas), and a second one that begins when the river enters the Córdoba peneplain, where most of the city of Córdoba is located (Fig. 1). When the Suquía River leaves the city, it is already a typical lowland river. At the upper end of the Suquía River basin, riparian vegetation has elements of the Chaco Serrano biogeographical district, in which it is embedded [70, 71]. The tree stratum includes non-riparian species like Schinus areira, Manihot grahamii, Celtis ehrenbergiana, Acacia spp., Prosopis spp., Zanthoxylum coco and Lithraea molleoides, but there are also exotic species growing in the riverbanks, like Salix babylonica, Acer negundo, Gleditsia triacanthos, Melia azedarach, Ligustrum lucidum, L. sinense, Morus alba, Fraxinus pennsylvanica and Ulmus spp., which exploit the water availability and shelter of this habitat. Rubus spp. and Ligustrum lucidum are particularly troublesome and considered as dangerous invasive plants [71, 72]. A few native riparian tree species (both obligate and facultative) are dominant: Salix humboldtiana, Sapium haematospermum and Sebastiania commersoniana. When the river enters the peneplain, tree species become less frequent, being shrubby or herbaceous the dominant plants. Within the herbaceous stratum, the Poaceae, with 20 genera (e.g. Cynodon, Eragrostis, Paspalum) and ca. 45 spp. are remarkable in the Suquía Riverbank. Outstanding representatives of this family are the showy “Pampas grasses” (Cortaderia spp.) which beautify the landscape. Cyperaceae (e.g. Carex, Cyperus, Eleocharis, Bulbostylis, Fimbristylis) is also important because of its species number, while within the dicots, the Plantaginaceae (e.g. Plantago, Bacopa, Stemodia, Veronica) and many other species scattered among several families (Caryophyllaceae, Lamiaceae, Brassicaceae, Asteraceae, Apiaceae, etc.) are also worth mentioning.
Rivers have a relative constant flow; they drain a great variety of rocks and encounter geological irregularities and artificial obstacles which progressively modify their course and chemical composition, introducing a multitude of local peculiarities. As a result, it is difficult to characterise a single river as a whole. As mentioned above, slope is important in determining the type of plant communities along the river, but there are also micro-environmental variations which are very significant for the development of the Flora [59]. Among the main factors affecting the plant communities composition and the penetration of rooted vegetation are:
-
(a)
Light transmission in the water. The depth at which the limiting intensity is reached varies from site to site, according to the colour of the water (due to dissolved organic matter), the concentration of suspended particles, and the amount of phyto- and zooplankton. These factors may interact with each other and vary seasonally. Aquatic plants can be used to indicate water ecological conditions because some of them are perennial and integrate periodic changes in water clarity and nutrient status, which are reflected in the depth that plants grow down [73]. That is why in the Suquía River some plants (as Utricularia gibba or Limnobium laevigatum) can only be found in certain intact micro-habitats.
-
(b)
Water temperature: fluctuations of temperature in aquatic habitats are generally much less pronounced than in aerial environments, and are partially responsible for the extensive geographical range of many hydrophytes of all life forms. Some genera, such as Myriophyllum (submerged, Haloragaceae), Lemna (floating, Lemnaceae) and Echinocloa (emergent, Poaceae) are cosmopolitan. In fact, Lemna gibba and Echinochloa colona are worldwide spread species that can be found in the Suquía River.
-
(c)
Dissolved substances (including organic and inorganic nutrients, salts, oxygen and toxic compounds) have a notable influence on the developments of aquatic plants. Rooted emergent species obtain nutrients exclusively from substrate, whereas submerged ones may absorb ions from both, the substrate and the water. Floating species must obtain all their nutrients from water. Although some species seem to be generalists, there are some others in which the general water chemistry controls whether they can grow or not [59, 74].
4.1 Distribution Along the Suquía River Basin
The distribution of aquatics is intriguing. Although about 40% of the known aquatic plants display relatively small ranges confined within the limits of a single continent or major land mass, there are several plants that are worldwide spread, and about 25–30% are endemic [59]. South America seems to be relatively rich in endemics and poor in widespread hydrophytes. The general picture of their status and distribution with regard to the Suquía River is as follows:
-
(a)
Extensive hydrophytes. Mostly monocots (emergent, submerged and floating), some of them are among the most widely distributed of all vascular plants. Present in the Suquía River are: Lemna gibba, Phragmites spp., Zannichellia palustris, Phalaris spp., Bacopa monnieri, Cyperus digitatus, C. esculentus, C. rotundus, Echinochloa colona, E. crus-galli, Leersia hexandra, Pistia stratiotes and Utricularia gibba.
-
(b)
Hydrophytes with continental ranges. The majority of aquatic plants fall within this category and they have representatives of all life forms. Some of the most remarkable South American aquatic plants that are present in the Suquía River are Echinodorus grandiflorus, Elodea callitrichoides, Sagittaria montevidensis, Potamogeton spp., Stuckenia spp., Heteranthera spp., Marsilea ancylopoda, Bulbostylis spp., Eleocharis bonariensis, Equisetum giganteum, Ludwigia uruguayensis, Myriophyllum quitense, Schoenoplectus californicus, and Wolffia brasiliensis.
-
(c)
Endemics. By endemism is meant the possession of a remarkable confined geographical range, clearly smaller than the average for related species. The term is usually applied in a comparative sense and there is an appreciable element of arbitrary choice in its use. Since aquatic plants are generally more sporadic than terrestrials, the concept of endemic in this case is narrower. There are several species of aquatic plants endemic to Argentina or Southern South America, but just a few reach the Suquía River: Egeria densa (submerged), Hydrocotyle modesta (emergent) (Fig. 7), Cerastium rivulariastrum, Poa ligualris var. resinulosa, Sesbania punicea and Cyperus meridionalis (riparian species). Finally, there are two more riparian species, exclusive to Central Argentina, which can be found in the banks of the Suquía River: Carex bonariensis var. glabrescens and Cerastium argentinum.
-
(d)
Exotics, invasive plants or weeds. Like all other ecosystems, aquatic environments are susceptible to invasion by exotic (alien or non-indigenous) species. Exotic species are entities from one part of the world that are transported beyond their natural range and become established in a new area. Categories within exotic species are difficult to establish because of the lack of consensus and inaccuracy of terms [75, 76]. When exotic plants reproduce outside cultivation, maintaining populations over several generations without direct human intervention, but without adverse effects on the invaded habitat, they are considered as ‘adventive’ or ‘naturalised’. On the contrary, invasive plants or weeds are usually understood as exotic plants that produce new breeding individuals in large numbers, inducing significant changes in the structure, composition and functioning of ecosystems [75, 76]. Among the plants of aquatic environments, the list of species recognised as weeds is long and includes all life forms, but the most severe problems are caused by stoloniferous free-floating species, since they form large impenetrable colonies which block drainage channels and hydroelectric installations and compete with local species [59]. For the Suquía River, ca. 50 exotic species are recorded, among riparian, emergent, submerged and floating. Some of them are important for causing serious problems in other parts of the world: Alternanthera philoxeroides, Cyperus esculentus, C. rotundus and Pistia stratiotes. However, they seem not to be proliferating too much in the area. Eichhornia crassipes, an aquatic weed with critical importance over the world, has been recorded once in the river, but apparently its presence was occasional and the species would not be propagating. On the contrary, an invasive plant always associated with river banks that is present in the margins of the Suquía River is Gleditsia triacanthos, a tree which grows rapidly, replacing the natural vegetation [77].
4.2 Systematics
Within vascular plants, a few families consisting exclusively of hydrophytes (ca. 30) are observed. They are small families, each one with less than ten genera, and most of them being monotypic or with a few species. Only Podostemaceae (absent in Córdoba) and Haloragaceae have more than 100 species (two in the Suquía River). By contrast, there are many more hydrophytes, too numerous to list fully, scattered throughout other terrestrial families of ferns and angiosperms.
Considering the plants of the entire Suquía ecosystem (the river itself and the banks), there are 49 families with ca. 200 species recorded, including emergent, submerged, floating and riparian. Overall, the most important family is Poaceae, with 23 genera and 46 species, followed by Cyperaceae, with six genera and 30 species. The remaining records are scattered among several families, each one with few representatives. Strictly considering aquatic plants, the Suquía River satisfies the general rule that the majority of species are Monocots: 11 families with 30 species, out of a total of 18 families with 40 species. The remaining species are dicots, with the exception of Marsilea ancylopoda and Azolla filiculoides (ferns). Floating life form is dominated by two families of exclusively aquatic plants, Lemnaceae (5 spp.) and Pontederiaceae (2), but at the same time, A. filiculoides and Pistia stratiotes, which are native to South America but probably alien to the Suquía Basin, also live in the river. Submerged life form is also dominated by two exclusively aquatic families, Potamogetonaceae (with 6 spp.) and Hydrocharitaceae (with 3 spp.), but Utriculariagibba (Lentibulariaceae) and Zannichellia palustris (Zannichelliaceae) are also present. Finally, Emergent species reach a total of 19, scattered among 10 families, but the genus Juncus (Juncaceae) is remarkable for having the highest number of species (6).
References
Hued AC, Bistoni MA (2007) Abundancia y distribución de la fauna íctica en la cuenca del río Suquía (Córdoba, Argentina). Iheringia Ser Zool 97:286–292
Ringuelet RA, Aramburu RH, Alonso de Aramburu A (1967) Los peces Argentinos de agua dulce, Com. Inv. Cient. Prov. Buenos Aires, La Plata, 602 pp
López H, Menni R, Donato M, Miquelarena A (2008) Biogeographical revision of Argentina (Andean and Neotropical Regions): an analysis using freshwater fishes. J Biogeogr 35:1564–1579
Bistoni MA, Hued AC (2002) Patterns of fish species richness in rivers of the central region of Argentina. Braz J Biol 62:1–12
Haro JG, Bistoni MA (1986) Ictiofauna del Río Primero (Suquía) (Córdoba, Argentina). Historia Natural 6:53–63
Huet M (1983) Tratado de Piscicultura. Mundi-Prensa, Madrid, 736 pp
Hued AC, Bistoni MA (2001) Ictiofauna del río San Francisco-Cosquín en la provincia de Córdoba (Argentina) (Pisces, Osteichthyes). Iheringia Ser Zool 91:75–78
Videla M, Bistoni MA (1999) Composición y estructura de las comunidades ícticas de un río serrano a lo largo de un gradiente altitudinal. Iheringia Ser Zool 87:171–180
Fernandez HR, Fernandez LA (1995) La biodiversidad del zoobentos en ríos de montaña de Tucumán, la trucha como amenaza. In: Brown AD, Grau HR (eds) Investigación, Conservación y Desarrollo en Selvas Subtropicales de Montaña. Proyecto de desarrolloforestal, LIEY, Tucumán, pp 149–156
Krueger CC, May B (1992) Ecological and genetics effects of salmonid introductions in North America. Can J Fish Aquat Sci 48:66–77
Wegryn D, Ortubay S (1991) Nuestros salmónidos. Dirección de Pesca de la provincia de Río Negro, Viedma, 120 pp
Ringuelet RA (1975) Zoogeografía y ecología de los peces de aguas continentales de la Argentina y consideraciones sobre las áreas ictiológicas de América del Sur. Ecosur 2:1–151
Haro JG, Bistoni MA (2007) Peces de Córdoba. Universidad Nacional de Córdoba, 246 pp
Miquelarena A, Protogino L, López H (2005) Astyanax hermosus, for a new speciesfrom primero river basin, Córdoba, Argentina (Characiformes, Characidae). Revue Suisse de Zoologie 112:13–20
Richardson DM (ed) (2011) Fifty years of invasion ecology: the legacy of charles elton. Blackwell, Hoboken
Haro JG, Bistoni MA (1996) In: Di Tada I, Bucher E (eds) Biodiversidad de la Provincia de Córdoba. Fauna. Córdoba, Argentina. Universidad Nacional de Río Cuarto, Córdoba, pp 169–190
Hued AC, Bistoni MA (2005) Development and validation of a Biotic Index for evaluation of environmental quality in the central region of Argentina. Hydrobiologia 543:279–298
Reati G, Florin M, Fernandez GJ, Montes C (1997) The Laguna de Mar Chiquita (C6rdoba, Argentina): a little known, secularly fluctuating, saline lake. Int J Salt Lake Res 5:187–219
Bucher EH (ed) (2006) Bañados del Río Dulce y Laguna Mar Chiquita (Córdoba, Argentina). Academia Nacional de Ciencias, Córdoba
Andrada G (2004) Efectos de diversos disturbios sobre las comunidades de aves acuáticas del Río Suquía en la ciudad de Córdoba. Unpublished Degree Thesis, Universidad Nacional de Córdoba, 39 pp
Michelutti P, Torres R (2006) Nuevos registros y comentarios sobre aves acuáticas fuera de su rango de distribución conocido en el centro de Argentina. Nuestras Aves 51:32–34
Nores M (1996) Avifauna de la provincia de Córdoba. In: Di Tada IE, Bucher EH (eds) Biodiversidad de la provincia de Córdoba. Fauna, vol I. Universidad Nacional de Río Cuarto, Río Cuarto
Nores M, Yzurieta D, Miatello R (1983) Lista y distribución de las aves de Córdoba, Argentina. Boletín de la Academia Nacional de Ciencias, Córdoba 5:1–114
Torres R, Michelutti P (2001) Las aves de ambientes acuáticos del sistema Laguna Mar Chiquita – Bañados del río Dulce (provincias de Córdoba y Santiago del Estero, Argentina). Boletín de la Academia Nacional de Ciencias, Córdoba 66:61–73
Torres R, Michelutti P (2001) Nuevos registros de aves escasas en la región central de Argentina. Nótulas Faunísticas – Segunda Serie 1:1–5
Hancock J, Kushlan J, Kahl MP (1992) Storks, ibises and spoonbills of the world. Academic, London, 385 pp
Torres R, Michelutti P (2006) Aves acuáticas. In: Bucher EH (ed) Bañados del río Dulce y Laguna Mar Chiquita. Academia Nacional de Ciencias, Córdoba, pp 237–249
Wantzen KM, Rueda-Delgado G (2009) Técnicas de muestreo de macroinvertebradosbentónicos. In: Domínguez E, Fernández HR (eds) Macroinvertebradosbentónicos sudamericanos. Sistemática y biología. Fundación Miguel Lillo, Tucumán, pp 17–45, ISBN 978-950-668-015-2
Wantzen KM, Sá MFP, Siqueira A, Nunez da Cunha C (2006) Stream-valley systems of the Brazilian Cerrado: impactassessment and conservationscheme. AquatConserv 16:713–732
Allan JD, Castillo MM (2007) Stream ecology: structure and function of running waters. Springer, New York, 436 pp
Barba-Álvez R, De la Lanza-Espino G, Contreras-Ramos A, González-Mora I (2013) Insectos acuáticos indicadores de calidad de agua en México: casos de estudio, ríos Copalita, Zimatán y Coyula, Oaxaca. Rev MexBiodiv 84:381–383
Gualdoni CM, Duarte CA, Medeot EA (2011) Estado ecológico de dos arroyos serranos del sur de Córdoba, Argentina. Ecol Austral 21:149–162
Gualdoni CM, Oberto AM (1998) Biological quality assessment of the lotic environment of Carcarañá River tributaries (Córdoba, Argentina). VerhInternat Verein Limnol 26:1219–1222
Gualdoni CM, Oberto AM (2012) Estructura de la comunidad de macroinvertebrados del arroyo Achiras (Córdoba, Argentina): análisis previo a la construcción de una presa. Iheringia, Série Zoología, Porto Alegre 102(2):177–186
Principe RE, Gualdoni CM, Oberto AM, Raffaini GB, Corigliano MC (2010) Spatial-temporal patterns of functional feeding groups in mountain streams of Córdoba, Argentina. Ecol Austral 20:257–268
Principe RE, Raffaini GB, Gualdoni CM, Oberto AM, Corigliano MC (2007) Do hydraulic units define macroinvertebrate assemblages in mountain streams of central Argentina? Limnológica 37:323–336
Mangeaud A (1998) Macroinvertebrados bentónicos como bioindicadores de la calidad del agua en la cuenca del Suquía, Ph.D. Thesis, Facultad de Ciencias Exactas, Físicas y Naturales, Universidad Nacional de Córdoba, Córdoba, Argentina
Vannote R, Minshall G, Cummins K, Sedell J, Cushing C (1980) The river continuum concept. Can J Fish Aquat Sci 37:130–137
Roback S (1974) Insects (Arthropoda: Insecta). In: Hart CW, Fuller SH (eds) Pollution ecology of freshwater invertebrates. Academic, New York, pp 313–376
Minshall W (1984) Aquatic Insects-substratum relationships. In: Resh VH, Rosemberg DM (eds) The ecology of aquatic insects. Praeger, New York, pp 358–400
Campeau S, Murkin H, Titman R (1994) Relative importance of algae and emergent plant litter to freshwater marsh invertebrates. Can J Fish Aquat Sci 51:681–692
Karatayev AY, Padilla DK, Minchin D, Boltovskoy D, Burlakova LE (2007) Changes in global economies and trade: the potential spread of exotic freshwater bivalves. Biol Invasions 9:161–180
Pastorino G, Darrigran G, Martin SM, Lunaschi L (1993) Limnoperna fortunei (Dunker, 1857) (Mytilidae), nuevo bivalvo invasor en aguas del Río de la Plata. Neotropica 39:34
Darrigran G (2002) Potential impact of filter-feeding invaders on temperate inland freshwater environments. Biol Invasions 4:145–156
Lee T, Siripattawan S, Ituarte C, Foighle D (2005) Invasion of the clonal clams Corbicula lineages in the New World. Am Malacol Bull 20:113–122
Pfenninger M, Reinhardt F, Streit B (2002) Evidence for cryptic hybridization between different evolutionary lineages of the invasive clam genus Corbicula (Veneroida, Bivalvia). Evol Biol 15:818–829
Morton B (1986) Corbicula in Asia an updated synthesis. Am Malacol Bull 2:113–124
Ituarte C (1994) Corbicula and Neocorbicula (Bivalvia: Corbiculidae) in the Paraná, Uruguay, and Río de La Plata basins. Nautilus 107(4):129–135
Mansur M, Pereira D (2006) Bivalves límnicos da bacia do rio dos Sinos, Rio Grande do Sul, Brasil (Bivalvia, Unionoida, Veneroida e Mytiloida). Revista Brasileira de Zoologia 23(4):1123–1147
Martins DS, Veitenheimer-Mendes IL, Faccioni-Heuser C (2006) Aspectos morfológicos e de incubação em três espécies de Corbicula Mühlfeld, no lago Guaíba, Rio Grande do Sul, Brasil (Bivalvia, Corbiculidae). Biota Neotropica 6(2):1–11
Reyna P, Morán G, Tatián M (2013) Taxonomy, distribution and population structure of invasive Corbiculidae (Mollusca, Bivalvia) in the Suquía River basin, Córdoba, Argentina. Iheringia, Série Zoología 103(2):77–84
Darrigran G (1992) Nuevos datos acerca de la distribución de las especies del género Corbicula (Bivalvia, Sphaeriacea) en el área del Río de La Plata, República Argentina. Notas del Museo de La Plata 21(210):143–148
Darrigran G (1991) Competencia entre dos especies de pelecípodos invasores Corbiculafluminea, (MÜLLER, 1774) y C. largillierti (PHILlPPI, 1844) en el litoral argentino del estuario del Río de La Plata. Notas Científicas de la Segunda Reunión Argentina de Limnología 498:214–215
Boltovskoy D, Correa N, Cataldo D, Stripeikis J, Tudino M (1997) Environmental stress on Corbicula fluminea (Bivalvia) in the Paraná River Delta (Argentina): complex pollution-related disruption of population structures. Arch Hydrobiol 138:483–507
Aroceno R, Chalar G, Fabián D, De Léon L, Brugnoli E, Silva M, Rodó E, Machado I, Pacheco J, Castiglioni R, Gabito L (2008) Distribución y descripción poblacional de moluscos invasores. In: Evaluación Ecológica de Cursos de Agua y Biomonitoreo. Informe final del Convenio DINAMA – Facultad de Ciencias (Sec. Limnología), Uruguay, p 17
Torre L, Reyna P (2013) Bivalvia, Veneroidea, Corbiculidae, Corbicula largillierti (Philippi, 1844): New distribution record in the Del Valle Central basin, Catamarca Province, Argentina. Check List 9(1):165–166
Wunderlin D, Amé M, Pesce S, Hued A, Bistoni M (2001) Pattern recognition techniques for the evaluation of spatial and temporal variations in water quality. A case study: Suquía River Basin (Córdoba, Argentina). Water Res 35:2881–2894
Lovett S, Price P (1999) Riparian land management technical guidelines. In: Principles of sound management, vol 1. LWRRDC, Canberra
Sculthorpe CD (1985) The biology of aquatic vascular plants. Edward Arnold, London. Koeltz Scientific Books, Königstein
Tiner R (1991) The concept of a hydrophyte for wetland identification. BioScience 41:236–247
Johnson RR, Carothers SW, Simpson JM (1984) A riparian classification system. In: Warner RE, Hendrix KM (eds) California riparian systems: ecology, conservation, and productive management. University of California Press, Berkeley, pp 376–382
McLaughlin SP (2004) Riparian Flora. In: Baker BM, Folliott PF, Debano LF, Neary DG (eds) Riparian areas of the Southwestern United States Hydrology Ecology and Management. CRC, Boca Raton, pp 127–168
Clerici N, Weissteiner CJ, Paracchini ML, Boschetti L, Baraldi A, Strobl P (2013) Pan-European distribution modelling of stream riparian zones based on multi-source Earth Observation data. Ecol Indic 24:211–223
Naiman RJ, D’ecamps H (1997) The ecology of interfaces: riparian zones. Annu Rev Ecol Syst 28:621–658
Hagan JM, Pealer S, Whitman AA (2006) Do small headwater streams have a riparian zone defined by plant communities? Can J For Res 36:2131–2140
Naiman RJ, D’ecamps H, Pollock M (1993) The role of riparian corridors in maintaining regional biodiversity. Ecol Appl 3:209–212
Robert J, Naiman RJ, Bilby E, Peter A, Bisson P (2000) Riparian ecology and management in the pacific coastal rain forest. BioScience 50:996–1010
Lovett S, Price P (eds) (2007) Principles for riparian lands management. Land and Water Australia, Canberra
Núñez CO, Cantero JJ, Petryna L (1998) Los hidrófitos del sur de la provincia de Córdoba (Argentina). Rev UNRC 18:37–82
Cabrera AL (1971) Fitogeografía de la República Argentina. Bold Soc Argent Bot 14:1–42
Giorgis MA, Cingolani AM, Chiarini F, Chiapella J, Barboza G, Ariza Espinar L, Morero R, Gurvich DE, Tecco P, Subils R, Cabido M (2011) Composición florística del Bosque Chaqueño Serrano de la provincia de Córdoba, Argentina. Kurtziana 36:9–43
Gavier-Pizarro GI, Kuemmerle T, Hoyos LE, Stewart SI, Huebner CD, Keuler NS, Radeloff VC (2012) Monitoring the invasion of an exotic tree (Ligustrum lucidum) from 1983 to 2006 with Landsat TM/ETM+ satellite data and support vector machines in Cordoba, Argentina. Remote Sens Environ 122:134–145
Clayton J, Edwards T (2006) Aquatic plants as environmental indicators of ecological condition in New Zealand Lakes. Hydrobiologia 570:147–151
Seddon B (1965) Occurrence of Isoetes echinospora in eutrophic lakes in Wales. Ecology 46:747–748
Colautti RI, Mac Isaac HJ (2004) A neutral terminology to define ‘invasive’ species. Divers Distrib 10:135–141
Richardson DM, Pysek P, Rejmánek M, Barbour MG, Panetta FD, West CJ (2000) Naturalization and invasion of alien plants: concepts and definitions. Divers Distrib 6:93–107
Marco DE, Páez SA (2000) Invasion of Gleditsia triacanthos in Lithraea ternifolia montane forests of central Argentina. Environ Manag 26:409–419
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Bistoni, M.A., Chiarini, F., Mangeaud, A., Tatián, M., Torres, R., Visintín, A. (2016). Biota Along the Suquía River Basin. In: Wunderlin, D.A. (eds) The Suquía River Basin (Córdoba, Argentina). The Handbook of Environmental Chemistry, vol 62. Springer, Cham. https://doi.org/10.1007/698_2016_455
Download citation
DOI: https://doi.org/10.1007/698_2016_455
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-67755-2
Online ISBN: 978-3-319-67757-6
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)