Abstract
La Plata Basin, the fifth-largest basin in the world, includes areas of Argentina, Bolivia, Brazil, Paraguay, and Uruguay and is subjected to intensive human activities such as agriculture, mining, and global trade. The basin hosts 83 native bivalves (Hyriidae, Mycetopodidae, Cyrenoididae, and Sphaeriidae), including 29 endemic and at least 3 non-native species (Cyrenidae and Mytilidae). For their role as filter feeders and their dominance in biomass in benthic freshwater ecosystems, freshwater bivalves play a key role in the resilience of aquatic ecosystems. In this review, we discuss the six major global change threats to freshwater ecosystems in the Anthropocene (climate change, flow regulation, pollution, land-use change, invasive species, and overexploitation) using freshwater bivalves of La Plata Basin as a model in South America. Future directions to properly understand the effects of global change and suggestions for the conservation of the freshwater bivalves in the basin are stated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anthropocene is a term proposed to define a new epoch in the history of the Earth, a time during which humans drive the major changes on the planet (Crutzen & Stoermer, 2000; Steffen et al., 2011). These modifications refer to planetary scale modifications affecting the earth system as a whole and are known as Global Change (Muccione & Schaepman, 2023). Freshwater ecosystems are especially susceptible to global changes and can serve as bioindicators since they can evidence alterations of both, terrestrial and aquatic portions of its basins while providing essential goods and services to humankind (Dudgeon et al., 2006).
Freshwater ecosystems are particularly rich in biodiversity and endemic species due to their habitat heterogeneity (Revenga et al., 2005). In these habitats, freshwater bivalves are a guild of sessile, filter-feeding organisms that occur in speciose aggregations that dominate benthic biomass but are now among the most imperiled organisms on Earth (Aldridge et al., 2023; Lopes-Lima et al., 2023). They are considered key organisms that link benthic and pelagic compartments by performing numerous ecological functions (filter-feeding, nutrient excretion, biodeposition, bioturbation) that intervene in nutrient cycling, productivity, and ecological networks through direct and indirect pathways (Atkinson et al., 2013). Moreover, they have been proposed as indicators of anthropogenic modifications (Lydeard et al., 2004; Böhm et al., 2021). In South America, the two richest areas in freshwater bivalves are the La Plata and Amazon Basins (Pereira et al., 2014). In particular, La Plata Basin (LPB) bivalves suffer a variety of pressures in a scenario of biological, geographic, and political diversity that makes it a key case study (Mugetti et al., 2004; Coutinho et al., 2009).
Following Dudgeon (2019), the major threats to freshwater biodiversity are climate change, flow regulation, pollution, land-use change, invasive species, and overexploitation. All these modifications are promoted by man and can be pointed out as indicators of the Anthropocene. In order to organize the available information and identify information gaps as well as proposing paths for the conservation of the LPB, we discussed these six major threats to the freshwater ecosystems using the freshwater bivalves of LPB as a model in South America. First, we introduce the LPB and its bivalve diversity. Then we discuss with bibliography, our expertise, and recent fieldwork the impact of the six major threats over the bivalves of LPB. Finally, we integrate all this information, including the particulars of freshwater bivalves not only as a threatened group but as an opportunity for the resilience of freshwater ecosystems.
For this review, we used the division of the world basins proposed by Abell et al. (2008). In order to facilitate the understanding of the different nature and intensity of threats for bivalves in the basin, we have elaborated a selection of maps when suitable. To analyze the coverage of protected areas at LPB, we used the data from the World Database of Protected Areas (UNEP-WCMC & IUCN, 2023). All protected areas in the database were considered including national or provincial parks (full protection or sustainable use), private reserves, biosphere reserves, and RAMSAR sites. Several protected areas in LPB are partially or fully overlapped causing an overestimation. When overlapped, surfaces covered by protected areas in each ecoregion were discounted. There is no unified database of water quality in the LPB; for this reason, we use the number of large cities as an approximation to the pollution status of the basins since, in addition to concentrating the largest human population, they also focus industries and, horticultural areas with extensive use of agrochemicals. Therefore, the relative pollution levels were estimated by incorporating available information (city coordinates and estimated maximum population) from the Natural Earth dataset (Natural Earth, 2023). In this sense, cities with more than 300,000 inhabitants were considered large cities that could have considerable impacts on streams, rivers, and lakes. Furthermore, we mapped the reservoirs of the basin to understand how widespread this threat is in LPB. The number of LPB dams and reservoirs was extracted from GeoDAR (Wang et al., 2021) which presents a worldwide, extensive, and well-resolved database. The GeoDAR database includes information from previous works such as Paredes-Beltran et al. (2021) on South American reservoirs. Also, we included an updated distribution of the two main invasive species in the basin. For this, we used different sets of data. The distribution of Limnoperna fortunei (Dunker, 1857) incorporated in this review was extracted from the CBEIH dataset (CBEIH, 2023), while the distribution of Corbicula fluminea (Müller, 1774) was delineated from the data provided by Leal et al. (2021) for Brazil, Clavijo (2021) for Uruguay, and literature survey for Argentina. Information on freshwater bivalves in Paraguay and Bolivia is scarce compared to published information for the other three countries in the basin. Most records of invasive species in Paraguay came from international rivers at the borders of the country, and there were no published records for Bolivia, except for Darrigran (2002) who stated the probable presence of invasive bivalves there. Finally, the existence of mother-of-pearl button factories in the basin was extracted from bibliographic records or oral communications and included on a map due to their high impact on local populations (Bonetto et al., 1950; Clavijo, 2017).
Freshwater bivalves of La Plata Basin
La Plata Basin, located in the south-central portion of South America, is the fifth-largest basin in the world and the second in the continent covering 3,286,875 km2. This vast area encompasses five countries in South America: Argentina, Bolivia, Brazil, Paraguay, and Uruguay with more than 100 million inhabitants (Fig. 1). This basin includes tropical and subtropical climates (from 14° 30′ S to 36° 30′ S latitude) from sea level up to 3,600 m.a.m.s.l. and some of the largest rivers in the world such as Paraná, Paraguay, and Uruguay. The basin also includes the largest wetlands in South America (Pantanal and Iberá), one of the most productive grasslands in the world (Pampas), a semi-arid region (Chaco), rainforests (Atlantic and Paranaense) as well as mountain regions rich in minerals and the second-largest aquifer in the world (Guaraní aquifer) (CIC, 2016). Moreover, the basin includes one of the biggest waterways in the world (the Paraná–Paraguay waterway) and the second-biggest dam in the world (Itaipú Reservoir). It comprises a system of interconnected wetlands that links the Pantanal with the Delta del Paraná, at its outlet to the La Plata River, which is essential to the sustainability of an extensive area of biological diversity and productivity. According to Abell et al. (2008), the LPB include eight freshwater ecoregions: Upper Paraná (834,088 km2), Lower Paraná (636,263 km2), Iguassu (67,547 km2), Paraguay (543,295 km2), Chaco (591,424 km2), Upper Uruguay (79,546 km2), Lower Uruguay (270,059 km2), and Bonaerensean drainages (264,649 km2, Fig. 1).
Map of La Plata Basin with ecoregions according to Abell et al. (2008)
There are 83 native species of bivalves recorded in LPB (Hyriidae, Mycetopodidae, Cyrenoididae, and Sphaeriidae), representing almost half of the total bivalve species in South America, including 29 endemic, and at least 3 non-native bivalve species (Cyrenidae and Mytilidae) (Pereira et al., 2014; Cuezzo et al., 2020; Wu et al., 2023). Hyriidae and Mycetopodidae are the more diverse families in LPB and include the large freshwater mussels that in most cases need a fish host to complete their life cycle. The larvae of these species infect fishes until reaching the juvenile stage, allowing their dispersion to other suitable habitats (Wächtler et al., 2001). Thus, the conservation of freshwater bivalves is a complex process that includes not only the preservation of habitat and heedful control of invasive species but also the conservation of healthy populations of fish hosts (Modesto et al., 2018; Miyahira et al., 2022). Another fact to be considered when attending the conservation status of freshwater bivalves is that some species are long lived, sometimes with delayed sexual maturity (Haag & Rypel, 2011); therefore, population recovery after disturbances can take a long time or even be impossible to achieve due to the lack or diminution of breeding individuals. In this study, we will focus on freshwater mussels from the Hyriidae and Mycetopodidae families which, despite the scarcity of information, are the most representative taxa known to be threatened in South America, and for which there are enough data to allow discussion about threats (Lopes-Lima et al., 2018; Miyahira et al., 2022). For the minute pea clams and cyrenoidids, the information is even more scarce, and comments about the conservation of these groups must be considered as an approximation.
After the first studies made by Europeans in the nineteenth century (e.g., d’Orbigny, 1835; Ihering, 1893), the bivalves of LPB started to be studied by local researchers in the 1950s. Argentino Bonetto, Inés Ezcurra de Drago, and their colleagues studied several aspects of mussel taxonomy and biology mainly at Lower Paraná River in Santa Fe, Argentina (e.g., Bonetto, 1965; Bonetto & Ezcurra De Drago, 1965; Bonetto & Di Persia, 1975, Bonetto & Tassara, 1987). In the 1970s, some studies were conducted at the Upper Paraná River, in Brazil (e.g., Hebling & Penteado, 1974; Henry & Simão, 1985) and a few others more recently (e.g., Tomazelli et al., 2003; Avelar & Cunha, 2009). The Pantanal wetlands (Paraguay River) started to be studied in the 1990s and became one of the best studied areas of the entire basin (e.g., Serrano et al., 1998; Callil & Mansur, 2005; Callil et al., 2012; Olivera-Hyde et al., 2020; Santos et al., 2021). The Lower Paraguay River, in Paraguay, was studied in the middle twentieth century by Quintana (1982). The malacofauna of the Uruguay River was studied in 1960s (e.g., Olazarri, 1966) and more recently (e.g., Clavijo, 2009; Clavijo & Olazarri, 2009; Clavijo & Carranza, 2018). After the introduction of bivalve invasive species in LPB, they became a central theme of investigation, and studies were done all over the basin (see Darrigran et al., 2020). However, despite this solid amount of information, there are several gaps regarding taxonomy (e.g., genetic information, larval descriptions), ecology (e.g., population size) and conservation (e.g., fish host) of native bivalve species in South America (Miyahira et al., 2022).
The bivalves of the La Plata Basin in the Anthropocene
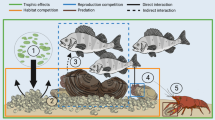
The populations of freshwater bivalves are clearly declining worldwide and numerous impacts promoted by humans are directly or indirectly responsible for these decreases (Lydeard et al., 2004; Böhm et al., 2021). However, the nature, distribution, and the effects of the threats over the conservation of native bivalves in LPB are largely unknown, even when this information is essential for the evaluation of species extinction risk, protected area selection, or impact mitigation. The impacts promoted by humans on native mussels were divided into six groups (climate change, flow regulation, pollution, land-use change, invasive species, and overexploitation) as suggested by Dudgeon (2019).
Land-use change
The extent to which humans have modified fluvial systems through alterations in land use is so extensive that it is difficult to estimate (James & Lecce, 2013). Following Winkler et al. (2021), over the past decades, one-third of the global land use has changed either once or on multiple occasions threatening the ecological integrity of freshwater ecosystems (Allan et al., 1997; Strayer et al., 2003; Winkler et al., 2021). These anthropogenic changes can significantly alter water flow, sediment concentration, and nutrient cycling, influencing the hierarchical nature of river systems, from the largest spatial scale of the basin to successively smaller scales like valley segments, individual channel units, and microhabitats.
The three main drivers that have been responsible for landscape transformation in LPB in the last few decades were international commodities related to the agricultural sector, forestry, and meat industry. Genetically modified soy and maize, sugarcane, exotic cultivated pastures (e.g., Brachiaria spp.), and trees (mostly eucalyptus and pines) have replaced vast portions of the natural biomes in the Cerrado, the Atlantic Forest, Chaco, Espinal, and the Pampas (Coutinho et al., 2009). Deforestation has been particularly intense in some regions of the LPB. For example, in the States of São Paulo and Paraná (both in Brazil; Upper Paraná and Iguassu ecoregions) and the East of Paraguay (Paraguay ecoregion), only 8.3%, 5.2%, and 15% of the original cover remain (Tucci & Clarke, 1998). In addition, the South American Pampa (Lower Uruguay, Lower Paraná, and Bonaerensean ecoregions) had a net loss of native vegetation of 16.3% in 20 years, between 2000 and 2019 (Mapbiomas Brasil, 2023). In these areas, as in several other basins, the forest was replaced by crops (see Pollution section) or pasture. Following Poole & Downing (2004), a 50% decrease in the riparian forest causes losses in the diversity of bivalve communities, while the communities of bivalves seem to remain relatively stable when the percentage of forest loss is close to 20%. Some bivalve species depend on well-preserved riparian vegetation to survive (Brainwood et al., 2006; Simeone et al., 2021; Pandolfi et al., 2022) and other species do not tolerate siltation as a result of riparian vegetation suppression (Randklev et al., 2015). Despite insufficient data in LPB, records from other locations suggest that land use negatively influences macroinvertebrate (Ourso & Frenzel, 2003; Jonsson et al., 2017; Cushway et al., 2022) and fish (Walser & Bart, 1999) assemblages by altering habitat conditions.
Additionally, these three major drivers for landscape transformation alter sedimentation processes, increase eutrophication levels and pollution, and cause other hydrologic alterations that can negatively influence bivalve populations. For example, modifications in natural sedimentation can cause the death of bivalves by gill coating. Increased turbidity decreases primary production (food availability) and reduces stream depth heterogeneity, homogenizing niches (Allan, 2004; Goldsmith et al. 2020). Goldsmith et al. (2020) related declines in mussel diversity due to an increase in sedimentation. Furthermore, changes in nutrient inputs modify community composition due to the increment of autotrophic biomass, with the proliferation of filamentous algae that are not readily consumed by bivalves and macrophytes that decrease dissolved oxygen due to the breakdown of organic material and by limitation of oxygen diffusion (Carpenter et al., 1998). Furthermore, riparian clearing and canopy opening reduce sediment trapping by vegetation, increasing bank size and channel erosion, and also reducing shading (see Lowrance et al., 1984, 1997). Livestock, widespread through all the basin's biomes, may alter stream morphology, destroy littoral vegetation, increase soil erosion, turbidity, nutrients, and fecal coliform levels, and probably cause the death of brittle-shelled bivalves by trampling (FAO, 2006).
Moreover, agriculture and land clearing are among the main anthropogenic drivers of salinization, one of the greatest threats to freshwater ecosystems and their associated biodiversity, globally (Kaushal et al., 2018). High levels of salinity cause loss of bivalve diversity and contribute to the colonization and establishment of non-native species (Cañedo-Argüelles et al., 2018; Sowa et al., 2019).
One way to offset habitat modification is the establishment of protected areas. The Aichi Target 11, included in the Convention on Biological Diversity, states that at least 17% of inland water should be protected by 2020. This goal of inland water protection was updated at COP-15 and increased to 30% (CBD, 2023). The distribution of protected areas at LPB is presented in Fig. 2. There is a relatively large number of protected areas at the basin (n = 848, Table 1). The ecoregions with a greater number of protected areas are Upper Paraná, Lower Paraná, and Paraguay. Considering only the absolute number of protected areas, a good situation at the basin can be inferred, though most of them are less than 50 km2 (Fig. 2) and several areas are partially or fully overlapped causing an overestimation. Moreover, the high number of small areas results in a severely fragmented network of protected areas. Therefore, an analysis based on the absolute number of protected areas does not seem to be the best approach. Chaco is the ecoregion with the largest surface covered by protected areas, followed by Paraguay, Upper Paraná, and Lower Paraná (Table 1). The largest protected area in the basin is Gran Chaco, which is listed twice with two different conservation nominations. The second-largest protected area, Kaa-iya del Gran Chaco, is also in the Chaco ecoregion. Despite the little sampling effort at Chaco, this ecoregion is probably an inhospitable environment for bivalves due to the intermittency of its rivers and streams, high water temperature, and high conductivity. Thus, more than 1/3 of the protected areas of LPB are of little suitability for freshwater bivalves while the two areas with the highest bivalve diversity in LPB, Lower Paraná and Lower Uruguay, are insufficiently protected. The situation is more critical at Lower Uruguay where only 2.27% of the ecoregion is protected. The Lower Uruguay was considered by Strong et al. (2007) a hotspot for freshwater gastropods; thus, this little protection coverage affects not only bivalves but also other molluscs. A similar situation was observed by Miyahira et al. (2023), who also detected a mismatch between existing protected areas and the occurrence of freshwater bivalves in Southeast Brazil.
The network of protected areas of LPB is not enough to ensure the conservation of freshwater bivalves. Considering the Aichi targets, only the Chaco ecoregion reached 17% of protected area, and Paraguay is close to achieving this goal. The other ecoregions are far from the accomplishment of the Aichi Target, and the new goal defined at COP-15 makes this task even more difficult. Finally, as evidenced by other freshwater taxa (Fagundes et al., 2016; Dagosta et al., 2020), the establishment of a protected area does not guarantee the protection of freshwater bivalves. To achieve the objective of protection of freshwater bivalves, the area must be specifically managed and designed for the conservation of these animals (Dudgeon et al., 2006; Abell et al., 2007; Miyahira et al., 2022, 2023).
Management actions that promote the reduction of nitrogen and sediment contributions, prevent trampling by livestock, and support the presence of host fishes are essential to attain the conservation aims of these areas (Strayer & Malcom, 2012; Haag & Williams, 2013; Lopes-Lima et al., 2016; Miyahira et al., 2023).
Pollution
Freshwater bivalves have been traditionally used as indicators of habitat modification (see van Hassel & Farris, 2006), but populations can disappear when modifications surpass certain thresholds (Brainwood et al., 2006; Lopes-Lima et al., 2020; Simeone et al., 2021; Miyahira et al., 2022). Unlike the Amazon, another major basin in South America where usually small towns are in between large well-preserved areas, LPB possesses some of the largest urban agglomerations in South America (Fig. 3A). There are 37 large cities in LPB, most of them in Upper Paraná (20), including the largest city in South America, São Paulo (Brazil) with more than 12 million inhabitants (Table 1). The capitals of the five countries that are part of the LPB are located on the basin: Brasilia (Brazil) is located at the headwaters of Paraná River, Sucre (Bolivia) at the headwaters of the Paraguay Basin, Asunción (Paraguay) in Lower Paraguay, Buenos Aires (Argentina), and Montevideo (Uruguay), both at the mouth of the entire basin. Except for Sucre, all other capitals have more than a half million inhabitants. Indeed, Tucci & Clarke (1998) estimated that about 50% of the population of the five countries of the basin are settled in LPB itself. This large number of cities and urban areas in the basin have a negative impact on water quality (e.g., Carvalho et al., 2015; Passig et al., 2015; Nogueira et al., 2021). The dams close to the São Paulo Metropolitan area presented poor water quality compared to other dams in the same ecoregion. As a consequence, considering the large number of big and small cities, it can be supposed that several areas of the basin are deeply polluted. This is especially true for smaller tributaries that can be easily modified by human action, but also for large rivers. The largest urban and industrial agglomeration of Argentina is located in the Lower Paraná where massive fish mortality can be frequently observed (Bonetto & Wais, 2006). If fishes are affected, mussel populations can be affected by the same stressors or by losing their hosts.
Urban and industrial pollution are pointed out as major problems in the Paraná and Paraguay Basins (CIC, 2016; Battistello Espíndola & Ribeiro, 2020). Industrial pollution can alter the local composition of freshwater bivalve populations (Hayward et al., 2022). Thus, a shift in bivalve diversity near industries can be expected in the basin. Other contaminants like metals can be persistent and influence species over a long distance. Contamination from mining activities is a problem in Paraguay Basin, especially at the headwaters of the Pilcomayo Basin in Bolivia where some tailing dams collapsed [e.g., Porco in 1996 and Santiago Apóstol in 2014 (Menéndez et al., 2014)] contaminating hundreds of kilometers of river courses, reliably causing bioaccumulation and biomagnification of metals. Although the Pilcomayo Basin is not rich in freshwater mussels, these contaminants can reach the Paraná River together with a high load of sediments (Battistello Espíndola & Ribeiro, 2020). A report from the National Water and Sanitation Agency—Brazil (Agência Nacional de Águas e Saneamento Básico—ANA, 2023) classified the Brazilian portion of LPB as high risk with a total of 475 dams: 326 at Upper Paraná, 85 at Upper Uruguay, 29 at Iguassu, 24 at Paraguay, and 11 at Lower Uruguay. This list includes large reservoirs for hydropower generation to small dams for human supply, including several other uses, such as tailing dams. Recently two tailing dams collapsed in Brazil (outside of Río de La Plata Basin) generating large environmental and social losses: Fundão and Feijão Dams (de Lima et al., 2020; Duarte et al., 2021; Koppe, 2021). Thus, the accidents reported for other areas of Brazil are also a real threat in LPB, as the data of Menéndez et al. (2014) also suggested.
Pollution was pointed as responsible for bivalve declines and even extirpation worldwide (Sheehan et al. 1989; Bogan, 1993; Pereira et al., 2014; Ferreira-Rodríguez et al., 2018; Miyahira et al., 2022). Moreover, an increase in pollution negatively affects native mussels and favors the establishment of invasive ones (Zieritz et al., 2016). Increased concentrations of heavy metals are suspected of lowering the diversity and production of aquatic communities (Mackie, 1989). High concentrations of metals like Pb, Cr, and Cu were found in several regions of LPB like Upper Paraná, Lower Uruguay, and Río de La Plata itself (Nogueira et al., 2021); several in high concentrations considered hazardous to aquatic life (Avigliano et al., 2019a, 2019b). The effects of trace metals in LPB were mostly studied in Asian clams (e.g., Bilos et al., 1998, 2009) and little information is available for native species (e.g., Vieira et al., 1995; Callil & Junk, 1999), despite it being well known that bivalves are efficient bioaccumulators of trace metals and are capable of concentrating chemicals by factors of 102 to 105 compared to surrounding water (Ribeiro Guevara et al., 2004). Anodontites trapesialis (Lamarck, 1819), the giant pearl mussel of South America, was suggested to be used as a bioindicator of some metals (Tomazelli et al., 2003; Oliveira et al., 2016). Moreover, reservoirs can act as traps for heavy metals (Palanques et al., 2014) affecting the bivalves that live in bottom substrates. This can be of special concern at Upper Paraná and its several reservoirs (see below).
In addition to habitat loss and fragmentation, agriculture also brings contamination by a wide variety of agrochemicals such as fertilizers and pesticides. Unfortunately, monitoring water quality in such a vast region is complex because pollution is diffuse due to the presence of numerous sources of contamination, so information is scattered in LPB. Pesticides were detected in water column and sediments in several points of LPB (Peruzzo et al., 2008; Dores, 2015; Etchegoyen et al., 2017; Mac Loughlin et al.. 2017). High values of pesticides were found in Paraná, Paraguay, and Bonaerensean drainages (Etchegoyen et al. 2017; Mac Loughlin et al., 2017), but in the Cuiabá River Basin, low frequency of pesticides was reported by Dores (2015), although the author also points some limitations of their analysis. The Lower Paraná River is one of the most heavily impacted ecoregions by agriculture in the basin, where glyphosate was detected in water and sediment ranging from 0.10–0.70 mg/l and 0.5–5 mg/kg, respectively, among other agrochemicals (Peruzzo et al., 2008). Surprisingly, despite the abundant information of its toxicity in non-target organisms, the effects of pesticides in native bivalve species have been poorly studied with few exceptions like Nogarol et al. (2016). The negative effect of glyphosate on invasive species of LBP (L. fortunei) was demonstrated by Miranda et al. (2021). The native species that are usually more sensitive to anthropogenic stressors than invasive ones can have worse outputs.
The level of plastic contamination is unknown in several large rivers in the world, including the Paraná River (Cera et al., 2020). The emergent plastic contamination also affects freshwater bivalves (see Berglund et al., 2019; Atici, 2022); however, little information is available for the bivalve species of LPB. Once again, the knowledge seems to be focused on invasive species (Ronda et al., 2021), contamination by microplastics has already been reported for C. fluminea and L. fortunei (Guilhermino et al., 2018; Oliveira et al., 2018; Pazos et al., 2020). The experiments done by Moreschi et al. (2020) showed that a native freshwater mussel, A. trapesialis, collected at Upper Paraguay can assimilate microplastics in its tissues. Staichak et al. (2021) suggested that the same species can be used as a sentinel of microplastic in aquatic environments of South America. Is it probable that other species in the basin were contaminated with largely unknown consequences.
Flow regulation
According to Oberdoff (2022), dams provide the most characteristic example of anthropogenic fragmentation including the creation of artificial lakes, fragmenting river networks, and distorting natural patterns of sediment transport and seasonal variation in water temperatures and flows. Dams also promote the reduction of bivalves' genetic diversity (Liu et al., 2020). In the LPB, there are a high number of established reservoirs (Tucci & Clarke, 1998; Nilsson et al., 2005), and several others planned or under construction (Zarfl et al., 2015). Nilsson et al. (2005) considered LPB as one of the most fragmented basins in South America. Although artificial lakes created by dams can be a nursery for bivalves, they often play the role of ecological traps (Paschoal et al., 2020; Sousa et al., 2021).
Studies using the BACI (Before–After-Control-Impact) methodology are still scarce and insufficient to understand the impacts of dams in LPB, including those related to the risk of introduction of invasive species. Most of the reservoirs in this area were built before the establishment of rigid environmental laws in South American countries. Biological samplings made before and after the establishment of the Salto Grande Reservoir (Argentina/Uruguay) evidenced alterations in the diversity of molluscs including local extinctions of freshwater bivalve populations (Olazarri, 1980). Also, a literature survey indicated a sharp decline of bivalves in the Tietê River Basin related to the establishment of several reservoirs in the basin (Pereira et al., 2012). Moreover, dams can also lead to a decrease in fish diversity affecting the reproductive cycle of freshwater mussels (Agostinho et al., 2008). There are some generalist species in host selection like A. trapesialis that even can use the introduced tilapia, Coptodon rendalli (Boulenger, 1897), as hosts (Silva-Souza & Eiras, 2002; Silva-Souza et al., 2011), but, according to experimental tests carried out by Canzio (1960), there are specialist mussels that are host selective. Detailed studies about fish parasitism by mussels in South America (see Pereira et al., 2014; Miyahira et al., 2017 and references therein) available so far are still insufficient to determine the effect size of variations in ichthyofauna composition on mussel diversity (Miyahira et al., 2022). However, a positive correlation between the decline of fishes and mussels is expected (Modesto et al., 2018). Moreover, fish passages do not work well in Lower Paraná, preventing a high number of migratory fish from reaching the upper part of the basin (Oldani et al., 2007). Dams can also promote ichthyofauna homogenization by local extinction, as observed after the construction of Rio Negro Dams in Uruguay, the migratory fish species disappearing in upstream dams (Serra et al., 2014), or as induced by the establishment of Itaipu Dam that connected Lower and Upper Paraná Rivers which were previously divided by Guaíra (Sete Quedas) Falls (Vitule et al., 2012). Also, the dam impacts interact with climate change exacerbating the negative effects on fishes (Peluso et al., 2022). Besides those impacts, dams also promote the modification of the margins (e.g., new villages and cities) and increase of leisure activities in the formed lake, therefore, contributing to an increase in flow of people, goods, and boats to the lake that can promote the introduction of invasive species.
The dams in LPB are concentrated in Brazil, especially at Upper Paraná. There are also a great number of dams at Chaco, Iguassu, and Upper Uruguay (Table 1; Fig. 3B). Other parts of LPB, such as the main course of Lower Paraná and Paraguay, are characterized by a smoother altitudinal gradient making them bad candidates for big hydropower dams (Tucci & Clarke, 1998; CIC, 2016). For example, Caceres (State of Mato Grosso, Brazil, at the margins of the Paraguay River) is around 3,400 km away from the sea, and about only a hundred meters above sea level. Among the three main rivers of LPB, the Paraguay River is the least affected by reservoirs and therefore less fragmented. The dams of Paraguay, Chaco, and Lower Paraná are concentrated at low order rivers, leaving extensive free-flowing areas. On the other hand, Upper Paraná, Iguassu, and Upper Uruguay ecoregions have gone through a deep fragmentation process as the dams were distributed along the entire ecoregions.
In addition, there are three large waterways in LPB: Paraguay–Paraná (3,442 km length), Tietê–Paraná (2,400 km length), and Uruguay (500 km length) (Tucci & Clarke, 1998; Oliveira et al., 2015; CIC, 2016). They include 229 ports with different sizes and purposes, i.e., commercial, recreational, and industrial (UNASUR, 2015, Fig. 3C), with a constant flow of boats and vessels that may probably promote a rapid dispersion of invasive species throughout the basin. As an example, the upstream dispersion rate for L. fortunei at Paraná River was 250 km/year (Boltovskoy et al., 2006; see invasive species below). Moreover, waterways maintenance demands frequent dredging, a task that affects water quality and current speed; and usually requires extirpation of river beds; directly affecting bivalve communities (see van Dalfsen & Essink, 2001; Hutchison et al., 2016; Castro & Arocena, 2020).
Invasive species
Invasive species are also considered as one of the major threats to freshwater bivalves since their introduction can lead to a decline of their native counterparts (Sousa et al., 2014; Ferreira-Rodríguez et al., 2018; Miyahira et al., 2022). The mechanisms by which invasive species can affect native freshwater bivalves are not completely understood, but there is evidence that they may involve competition for food resources and space (Strayer & Malcom, 2007), changes in microhabitat (Ferreira-Rodríguez et al., 2018), including increased nitrogen and oxygen stress due to tissue decomposition after mass mortality of invasive species (McDowell & Sousa, 2019), biofouling (Silva et al., 2021), dilution of host resources (Douda et al., 2013), and predation (Strayer, 1999), among others. The invasive species recognized as threats to freshwater bivalves may belong to taxonomic groups as distinct as gastropods, aquatic plants, fishes, crayfishes, and other bivalves (Strayer, 1999; Douda et al., 2013).
Some studies showed the potential of invasive species to modify the environmental conditions in La Plata and other basins bringing not only ecological but also economic problems (Boltovskoy et al., 2006; Boltovskoy & Correa, 2015; Karatayev et al., 2015; Hermes-Silva et al., 2021). The impact of invasive bivalves over native malacofauna in LPB was suggested by using historical and observational data (Clavijo & Carranza, 2018; Darrigran et al., 2020; Silva et al., 2021). For example, Clavijo & Carranza (2018), using museum information and recent field surveys, observed a sharp decline of native Cyanocyclas following the introduction of invasive species of the genus Corbicula in Uruguay.
This section will focus on C. fluminea and L. fortunei due to their already widespread distribution in the basin (Boltovskoy et al., 2006; Darrigran et al., 2020; Miyahira et al., 2020; Hermes-Silva et al., 2021; Silva et al., 2021), despite the presence of other invasive freshwater molluscs species like Corbicula largillierti (Philippi, 1844), Melanoides tuberculata (Müller, 1774), and Physella acuta (Draparnaud, 1805) (Darrigran et al., 2020; Miyahira et al., 2020). Shells morphologically attributable to other species as Corbicula sp. and Corbicula aff. fluminalis (Müller, 1774) were recorded in LPB (Santos et al., 2012; Clavijo 2014), but genetic species determination was not possible (Ludwig et al., 2023).
Records in LPB for the two invasive species included in this section were found in all freshwater ecoregions, except for Upper Uruguay and Chaco where C. fluminea is absent (Fig. 4). Most records for both species came from the ecoregions related to the main rivers: Upper Paraná, Lower Paraná, Paraguay, and Lower Uruguay, although their distribution in these ecoregions presents some variations. In the Upper Paraná, there are numerous reservoirs, cities, and an intense flow of vessels at the Tietê–Paraná waterway, thus, favoring the establishment of invasive species (Boltovskoy et al., 2006; Darrigran et al., 2020; Miyahira et al., 2020). Boltovskoy et al. (2006) related the dispersion of L. fortunei to waterways that allow a quick spreading facilitated by the constant movement of vessels and boats. In addition, waterways also provide artificial hard substrata (e.g., ports, decks, buoys, ropes) that can be colonized by the golden mussel (Boltovskoy et al., 2015). Miyahira et al. (2020) reported the occurrence of several invasive molluscs in Brazilian reservoirs mostly concentrated at Paraná Basin. The Lower Paraná and Lower Uruguay present favorable conditions for the establishment of bivalves (native or not). The Lower Uruguay is probably one of the best explored ecoregions in the LPB regarding freshwater bivalves, with a sampling effort certainly higher when compared to other ecoregions (Clavijo & Carranza, 2018; Clavijo, 2021). For the same reason (i.e., sampling effort, see Oliveira et al., 2015), Paraguay presented more records of L. fortunei than C. fluminea, so it is likely that C. fluminea presents a wider distribution in Paraguay. Also, there is a large gap in the western portion of the La Plata Basin that is related to the Chaco and northwest area of the Lower Paraná ecoregion, a naturally dry area and unsuitable for most bivalves because of the high conductivity of its waters and temporary rivers. Limnoperna fortunei presented a few records at Chaco, always related to the Paraguay River that divides the Paraguay and Chaco ecoregions. On the other hand, the Bonaerensean drainages are an ecoregion rich in coastal lagoons and temporary rivers that can be fresh or saltwater, thus, justifying the few records of invasive species in this basin, while the Upper Uruguay is an ecoregion characterized by steep terrain with several rapids, falls, and low nutrients levels (Di Persia et al., 1986). Freshwater bivalves, especially mussels, prefer flat areas with high nutrient levels (Vannote et al., 1980; Miyahira et al., 2023).
Another interesting aspect of the invasion of Corbicula related to the Anthropocene is its capacity to become an index fossil of this epoch. An index fossil is a concept used in geology to define a fossil that allows us to identify a specific epoch. For this, the index fossils must be abundant, geographically widespread, distinctive, and easily recognizable (Harries, 2015). The amplitude and intensity of invasion of Corbicula clams in LPB, indicate that they could become index fossils of the stratigraphic levels of the Anthropocene.
Overexploitation
The main use of freshwater bivalve was the extraction of mother-of-pearl from some species of freshwater mussels, especially of the genus Diplodon. In LPB, the pearl industry was mainly active between 1945 and 1960 and at the peak of this industry, nearly 72 million mussels were killed each year (Clavijo, 2017). Between 1945 and 1950 specimens suitable for industrialization were reduced to 25% and mussels were recorded as extirpated in many water bodies (Bonetto et al., 1950). By the beginning of the 1950s, the mussel banks had suffered such a decline that this industry was forced to expand northward through the Paraná River and its floodplain (Bonetto et al., 1950). At least eight cities with button factories were found in Santa Fe, Entre Ríos, and Buenos Aires Provinces in Argentina, and Montevideo in Uruguay (Fig. 5). According to historical documents and photographs, some of these factories employed approximately a hundred workers. As in the United States, the pearl button industry in South America collapsed after the introduction of plastic buttons (Resh & Rosenberg, 2015). Monitoring of mussel populations did not continue after the collapse of the industry, so it is unknown if these populations did return to pre-exploitation levels. The most recent records of the exploitation of mussels for button production in South America came from the Amazon Basin (Beasley, 2001; Miyahira et al., 2022).
Button factories in La Plata Basin during the period 1945–1965. The ecoregion delimitation follows Abell et al. (2008)
Mycetopodids and most of the hyriids of the LPB have an obligate parasitic larvae stage (Modesto et al., 2018). For that reason, fish populations are essential to fulfill their life cycle and dispersal of unionidans. In consequence, fish stockings in LPB are intrinsically related to mussel population dynamics and fish overexploitation can drive to the absence of mussel recruitment (Skawina, 2021). One of the weakest links in mussel conservation is the knowledge gap of fish host species since little is known about the specificity of this ecological relationship despite fish's fundamental role in the reproduction of many freshwater bivalve species.
Unfortunately, between 4 and 10% of all freshwater fishes in South America probably face some degree of extinction risk, and even more are currently assessed as threatened (Cappato & Yanosky, 2009; Baigún et al., 2012; Reis et al., 2016; ICMBio, 2018). Key species in LPB such as Prochilodus lineatus (Valenciennes, 1837) have experienced heavy exploitation (Baigún et al., 2013). These species represent a high portion of the fish biomass in the riverine system. They are the main prey of piscivorous species and contribute to the cycling and regulation of carbon, nutrients, and energy by feeding on organic‐rich sediment (Bowen, 1983; Bowen & Bonetto, 1984; Winemiller & Jepsen, 2004; Winemiller et al., 2006). For example, Argentina's freshwater fish exports consist principally of P. lineatus (MAGyP, 2023), which is captured in the Lower Paraná, the area including the highest bivalve diversity in LPB (Torres et al., 2018). The exports of this species reached a peak of 37,000 tons during 2004, and it has been around 15,000–20,000 per year since 2007—not including internal consumption (MAGyP, 2023). However, fish population declines are rarely associated with one particular cause but with a synergy of factors (Barletta et al., 2015; Castello et al., 2018). The relative effort necessary to harvest the same biomass of fish has greatly increased after the construction of dams in the Upper Paraná River (Hoeinghaus et al., 2009). In addition, fish population declines have been repeatedly observed in large-bodied species ascribed to the genera Pseudoplatystoma, Zungaro, Colossoma, Piaractus, and Prochilodus (Scarabotti et al., 2021).
Climate change
Climate change consists of a complex amalgam of stressors, including elevated atmospheric CO2, alterations in temperature, and increased frequency and intensity of droughts and extreme flow events (Woodward et al., 2010). These alterations are anticipated to amplify many of the five major freshwater threats identified herein (Reid et al., 2019). From a global perspective, these effects are not evenly distributed and systems at higher latitudes and altitudes experience higher rates of extreme events (Millennium Ecosystem Assessment, 2005). In this sense, systems like LPB could be considered as a “sentinel system,” since they could provide early warnings compared to more complex and thus more stable systems in warmer regions (Woodward et al., 2009; Layer et al., 2010; Perkins et al., 2010).
In freshwater ecosystems, climate change alters biodiversity by increasing acidity and modifying flow regimes and water availability through variations in precipitation and/or temperature (Döll & Zhang, 2010; Blöschl et al., 2017; Knouft & Ficklin, 2017). Compared to other more mobile organisms, extreme climate events may have longer and more acute effects on sedentary organisms, such as freshwater bivalves, although these animals can disperse mainly during their brief larval stage (Strayer, 2009).
Ectotherms like bivalves are known to be more vulnerable to environmental warming, in part because of their inability to acclimate to rapidly changing temperature regimes (Stillman, 2003; Deutsch et al., 2008). In addition, it is important to highlight that their larval stage is particularly sensitive to warm temperatures; therefore, any future increases in water temperature, as predicted by climatology, could be extremely challenging for the larvae of some Unionida species (Khan et al., 2019). With the ongoing increase in global average temperature and its impacts on Earth's climate system, if water bodies become too warm too fast for freshwater species to adapt, dispersal to cooler habitats will be mandatory, although not without the subjection to the limitations imposed by species biology, topography, and habitat availability among others (Dudgeon, 2019). Also, increased water temperatures often lead to progressive shifts in the structure and composition of assemblages explained by modifications in species metabolic rates, body size, fish migration timing, recruitment, species size range, and interactions (Oberdorff, 2022). Projections of climate change impacts in the region, including the Pantanal and its watershed, remain highly variable (Marengo et al., 2015). These uncertainties limit model reliability, although any significant change in rainfall patterns will profoundly impact local ecology and socioeconomic dynamics. In a study in a nearby area of LPB, the filtration rates of freshwater mussels were affected by temperature (Marroni et al., 2021), influencing food and oxygen acquisition. Modification of temperature regimes can also affect bivalves' gamete maturation (Galbraith & Vaughn, 2009) and survival (Falfushynska et al., 2014).
Paschoal et al. (2020) observed that extreme droughts in the Upper Paraná Basin led to a significant decline of bivalve abundances, richness, and occupied sites; floods, on the other hand, alter community composition and spatial distribution with dominance of bivalve species that are more tolerant to disturbances (Tarter et al., 2022). Sotola et al. (2021) reported a significant reduction in the abundance of freshwater bivalve populations after severe floods. The bivalves of the Pantanal wetland (Paraguay ecoregion) already deal with a natural flood pulse (Callil et al., 2018) and are adapted to water level variations. However, in a scenario with a decrease in frequency and intensity of flood events, the time lapse between flood events may be insufficient for recolonization and recovery of bivalves’ populations. Still, flooding's negative effects may be buffered by connectivity with backwater areas forming large wetlands, which usually favors the reproduction and recruitment of fish species (Agostinho et al., 2004; Rabuffetti et al., 2020) that could serve as hosts for mussel larvae, improving bivalve reproduction. In addition, floods transport nutrients from terrestrial to aquatic ecosystems supporting aquatic food webs by boosting productivity, which may benefit some bivalve species (Atkinson et al., 2013).
Furthermore, the acute effects of climate change can be exacerbated by other climatic phenomena like El Niño-Southern Oscillation (ENSO) and countless additional anthropogenic stressors to which many fresh waters are subjected (Malmqvist et al., 2008). In most parts of the LPB, La Niña (included in ENSO) is often accompanied by drought, and the Niña event during the current period (2019–2022) has ranked among the driest events in southeastern South America since the 1950s (Naumann et al., 2021). Massive die-offs of freshwater bivalves due to droughts were commonly reported in the last decades related to the effects of climate change (Sousa et al., 2012, 2018; Paschoal et al., 2020). This event can be related to prolonged droughts at the Paraguay River, which can be a side effect of global change. Fires are also associated with droughts in LPB. Recently, Pantanal (Paraguay ecoregion), and Iberá (Lower Paraná) had one-third and one-half of their area, respectively, consumed by fires associated with severe droughts (Berlinck et al., 2021; SNMF, 2023). In this context, the association of global change (e.g., severe droughts) with human-induced habitat modification like fires may lead to profound alterations of terrestrial and aquatic ecosystems in the LPB domain. Recently, Lawrence et al. (2022) prompted wildfires and subsequent ash and sediment runoff as the cause of the local extinction of mussel populations. Moreover, extracts of ashes to represent post-fire runoff, caused mortality in C. fluminea, a rather tolerant species (Silva et al., 2016). Nonetheless, the effect of fires in freshwaters remains largely unstudied (Cochrane & Laurance, 2008; Peltzer et al., 2023), especially in native, typically more sensitive, benthic bivalve communities.
Conclusions
LPB is diverse in habitats, biodiversity, and global change pressures. Impacts in LPB can be evaluated with certain limitations due to the wide gaps in information, the lack of databases that gather information from the entire basin, and the non-unification of criteria and timing in monitoring (CIC, 2016). According to Dudgeon (2019), freshwaters are especially susceptible to changes arising from the “tragedy of the commons.” This is particularly relevant in LPB, where management is distributed between five countries, some with federal political systems like Argentina and Brazil, or with local autonomies, like Bolivia, where political authority is divided between two autonomous sets of governments with different levels of sovereignty over its natural resources and biodiversity which could delay decision making and agreements.
Due to LPB extension and geographical conditions, stressors are not uniformly distributed. In Paraná River, the stressors are widespread. The Upper Paraná concentrates the worst values for all stressors evaluated. The highest number of large cities, ports, dams, and records of invasive species are found at Upper Paraná. Despite the intermediate value of freshwater bivalve diversity, there are endemic species in this basin, e.g., Diplodon caipira (Ihering, 1893) that were never studied in detail. The species inhabiting this basin can be considered of higher priority for conservation efforts. The Lower Paraná is in a slightly better situation, as all stressors presented high to intermediate values. The diversity is greater compared to Upper Paraná, but some species are shared with Paraguay and Lower Uruguay. The percentage of coverage of protected areas in the two ecoregions of the Paraná River presents intermediate values in the ranking of the LPB ecoregions. The ecoregions of Upper Uruguay and the Bonaerensean drainages are the most pristine of LPB. All stressors have their best values in Bonaerensean drainages, except for the records of C. fluminea. On the other hand, this ecoregion presented the lowest protected area coverage in the LPB and a low number of native bivalves. The Upper Uruguay presented a good value for almost all stressors, except for the number of dams. In contrast, the main problems in Lower Uruguay are ports and records of invasive species. The Paraguay River presents intermediate to high values for almost all stressors, only the number of dams is not an issue in this ecoregion.
Although stressors in the Anthropocene are usually studied separately, they frequently feedback and act in synergism. As an example, land-use change is caused by the production of commodities and urbanization. The production of these commodities is related to processes that include the use of agrochemicals, activities, and industries that generate pollution. At the same time, production and economic development require energy, for which a large part of the LPB basin uses hydroelectric energy that generates modifications in the flow regime. Moreover, the importation of supplies and exportation of produced goods will generate the movement of boats that promote the dispersal of invasive species. Simultaneously, the increase in human population generates more demand and overexploitation of biodiversity. And finally, all these drivers and effects interact with climate change and other climatic phenomena, intensifying extreme events.
Future directions to properly understand the effects of global change in the basin include (1) filling knowledge gaps on freshwater bivalves in LPB including identity, distribution, population status, life history, and ecology of species (for detailed information see Table 2 in Miyahira et al., 2022), (2) investigation of the unknown or underexplored sub-basins (e.g., Chaco and Paraguay), (3) the evaluation of the conservation status of native bivalve species, (4) the establishment of continuous, unified, and basin-wide monitoring, and (5) studying the effects of global change on bivalves as for its key role in freshwater ecosystems.
In addition, some suggestions for the conservation of the freshwater bivalves include (1) a clear policy for ballast water and flow of goods along La Plata waterways to avoid new introductions or the dispersion of invasive species to new areas, (2) a more biodiversity based regulation of the flow management of reservoirs, (3) the establishment of new protected areas that include bivalves and fishes as focal objects of conservation, (4) a joint plan of LPB countries for the basin and biodiversity management, and (5) resources to support research on bivalve biology for fulfill the knowledge gaps.
Finally, for their key roles and their dominance in biomass in freshwater ecosystems, the protection of the diverse native communities of bivalves is a central part of the resilience of aquatic ecosystems of La Plata Basin.
Data availability
Enquiries about data availability should be directed to the authors.
References
Abell, R., J. D. Allan & L. Bernhard, 2007. Unlocking the potential of protected areas for freshwater. Biological Conservation 134: 48–63. https://doi.org/10.1016/j.biocon.2006.08.017.
Abell, R., M. L. Thieme, C. Revenga, M. Bryer, M. Kottelat, N. Bogutskaya, B. Coad, N. Mandrak, S. C. Balderas, W. Bussing, et al., 2008. Freshwater ecoregions of the world: a new map of biogeographic units for freshwater biodiversity conservation. Bioscience 58: 403–414. https://doi.org/10.1641/b580507.
Agência Nacional de Águas e Saneamento Básico (ANA), 2023. ANA [available on internet at https://www.gov.br/ana/pt-br].
Agostinho, A. A., L. C. Gomes, S. Veríssimo & E. K. Okada, 2004. Flood regime, dam regulation and fish in the Upper Paraná River: effects on assemblage attributes, reproduction and recruitment. Reviews in Fish Biology and Fisheries 14: 11–19. https://doi.org/10.1007/s11160-004-3551-y.
Agostinho, A. A., F. M. Pelicice & L. C. Gomes, 2008. Dams and the fish fauna of the Neotropical region: impacts and management related to diversity and fisheries. Brazilian Journal of Biology 68: 1119–1132. https://doi.org/10.1590/s1519-69842008000500019.
Aldridge, D. C., I. S. Ollard, Y. V. Bespalaya, I. N. Bolotov, K. Douda, J. Geist, W. R. Haag, M. W. Klunzinger, M. Lopes-Lima, M. C. Mlambo, N. Riccardi, R. Sousa, D. L. Strayer, S. H. Torres, C. C. Vaughn, T. Zając & A. Zieritz, 2023. Freshwater mussel conservation: a global horizon scan of emerging threats and opportunities. Global Change Biology 29: 575–589. https://doi.org/10.1111/gcb.16510.
Allan, J. D., 2004. Landscapes and riverscapes: the influence of land use on stream ecosystems. Annual Review of Ecology, Evolution, and Systematics 35: 257–284. https://doi.org/10.1146/annurev.ecolsys.35.120202.110122.
Allan, D., D. Erickson & J. Fay, 1997. The influence of catchment land use on stream integrity across multiple spatial scales. Freshwater Biology 37: 149–161. https://doi.org/10.1046/j.1365-2427.1997.d01-546.x.
Atici, A. A., 2022. The first evidence of microplastic uptake in natural freshwater mussel, Unio stevenianus from Karasu River, Turkey. Biomarkers 27: 118–126. https://doi.org/10.1080/1354750X.2021.2020335.
Atkinson, C. L., C. C. Vaughn, K. J. Forshay & J. T. Cooper, 2013. Aggregated filter-feeding consumers alter nutrient limitation: consequences for ecosystem and community dynamics. Ecology 94: 1359–1369. https://doi.org/10.1890/12-1531.1.
Avelar, W. E. P. & A. D. Cunha, 2009. The anatomy and functional morphology of Diplodon rhombeus fontainianus (Orbigny, 1835) (Mollusca Bivalvia, Hyriidae). Brazilian Journal of Biology 69: 1153–1163. https://doi.org/10.1590/s1519-69842009000500021.
Avigliano, E., C. Clavijo, P. Scarabotti, S. Sánchez, S. Llamazares Vegh, F. R. del Rosso, J. D. Caffetti, J. F. Facetti, A. Domanico & A. V. Volpedo, 2019a. Exposure to 19 elements via water ingestion and dermal contact in several South American environments (La Plata Basin): from Andes and Atlantic Forest to sea front. Microchemical Journal 149: 103986. https://doi.org/10.1016/j.microc.2019.103986.
Avigliano, E., M. V. Monferrán, S. Sánchez, D. A. Wunderlin, J. Gastaminza & A. V. Volpedo, 2019b. Distribution and bioaccumulation of 12 trace elements in water, sediment and tissues of the main fishery from different environments of the La Plata Basin (South America): risk assessment for human consumption. Chemosphere 236: 124394. https://doi.org/10.1016/j.chemosphere.2019.124394.
Baigún, C. R. M., D. Colautti, H. L. López, P. A. Van Damme & R. E. Reis, 2012. Application of extinction risk and conservation criteria for assessing fish species in the lower La Plata River Basin, South America: extinction risk in La Plata Basin. Aquatic Conservation: Marine and Freshwater Ecosystems 22: 181–197. https://doi.org/10.1002/aqc.2223.
Baigún, C., P. Minotti & N. Oldani, 2013. Assessment of sábalo (Prochilodus lineatus) fisheries in the Lower Paraná River Basin (Argentina) based on hydrological, biological, and fishery indicators. Neotropical Ichthyology 11: 199–210. https://doi.org/10.1590/s1679-62252013000100023.
Barletta, M., V. E. Cussac, A. A. Agostinho, C. Baigún, E. K. Okada, A. Carlos Catella, N. F. Fontoura, P. S. Pompeu, L. F. Jiménez-Segura, V. S. Batista, et al., 2015. Fisheries ecology in South American river basins. In Craig, J. F. (ed), Freshwater Fisheries Ecology Wiley, Chichester: 311–348.
Battistello Espíndola, I. & W. C. Ribeiro, 2020. Transboundary waters, conflicts and international cooperation – examples of the La Plata Basin. Water International 45: 329–346. https://doi.org/10.1080/02508060.2020.1734756.
Beasley, C. R., 2001. The impact of exploitation on freshwater mussels (Bivalvia: Hyriidae) in the Tocantins River. Studies on Neotropical Fauna and Environment 36: 159–165. https://doi.org/10.1076/snfe.36.2.159.2137.
Berglund, E., V. Fogelberg, P. A. Nilsson & J. Hollander, 2019. Microplastics in a freshwater mussel (Anodonta anatina) in Northern Europe. Science of the Total Environment 697: 134192. https://doi.org/10.1016/j.scitotenv.2019.134192.
Berlinck, C. N., L. H. A. Lima, A. M. M. Pereira, E. A. R. Carvalho Jr., R. C. Paula, W. M. Thomas & R. G. Morato, 2021. The Pantanal is on fire and only a sustainable agenda can save the largest wetland in the world. Brazilian Journal of Biology 82: e244200. https://doi.org/10.1590/1519-6984.244200.
Bilos, C., J. C. Colombo & M. J. Presa, 1998. Trace metals in suspended particles, sediments and Asiatic clams (Corbicula fluminea) of the Río de la Plata Estuary, Argentina. Environmental Pollution 99: 1–11. https://doi.org/10.1016/s0269-7491(97)00177-2.
Bilos, C., J. C. Colombo, C. N. Skorupka, S. O. Demichelis & L. M. Tatone, 2009. Size-related trace metal bioaccumulation in Asiatic clams (Corbicula fluminea) from the Rio de la Plata Estuary, Argentina. International Journal of Environmental Research 3: 390–409. https://doi.org/10.1504/ijenvh.2009.030110.
Blöschl, G., J. Hall, J. Parajka, R. A. P. Perdigão, B. Merz, B. Arheimer, G. T. Aronica, A. Bilibashi, O. Bonacci, M. Borga, et al., 2017. Changing climate shifts timing of European floods. Science 357: 588–590. https://doi.org/10.1126/science.aan2506.
Bogan, A. E., 1993. Freshwater bivalve extinctions (Mollusca: Unionoida): a search for causes. American Zoologist 33: 599–609. https://doi.org/10.1093/icb/33.6.599.
Böhm, M., N. I. Dewhurst-Richman, M. Seddon, S. E. H. Ledger, C. Albrecht, D. Allen, A. E. Bogan, J. Cordeiro, K. S. Cummings, A. Cuttelod, et al., 2021. The conservation status of the world’s freshwater molluscs. Hydrobiologia 848: 3231–3254. https://doi.org/10.1007/s10750-020-04385-w.
Boltovskoy, D. & N. Correa, 2015. Ecosystem impacts of the invasive bivalve Limnoperna fortunei (golden mussel) in South America. Hydrobiologia 746: 81–95. https://doi.org/10.1007/s10750-014-1882-9.
Boltovskoy, D., N. Correa, D. Cataldo & F. Sylvester, 2006. Dispersion and ecological impact of the invasive freshwater bivalve Limnoperna fortunei in the Río de la Plata Watershed and beyond. Biological Invasions 8: 947–963. https://doi.org/10.1007/s10530-005-5107-z.
Boltovskoy, D., M. Xu & D. Nakano, 2015. Impacts of Limnoperna fortunei on man-made structures and control strategies: General overview. In Boltovskoy, D. (ed), Limnoperna fortunei, Invading Nature: Springer Series in Invasion Ecology, Vol. 10. Springer, Berlin: 375–393. https://doi.org/10.1007/978-3-319-13494-9_21.
Bonetto, A. A., 1965. Las especies del género Diplodon en el sistema hidrográfico del Río de La Plata. In Proceedings of II Congreso Latinoamericano de Zoologia, São Paulo, Brasil, 1965.
Bonetto, A. A. & D. H. Di Persia, 1975. Las poblaciones de pelecípodos del arroyo Ayuí Grande (prov. Entre Ríos) y los factores que regulan su distribución y estructura. Ecosur 2: 123–151.
Bonetto, A. A. & I. Ezcurra De Drago, 1965. Estudio comparado de las formas larvales de Mutelidae Ortmann y su significación sistemática y zoogeográfica. In Proceedings of II Congreso Latinoamericano de Zoologia, São Paulo, Brasil, 1965.
Bonetto, A. A. & M. P. Tassara, 1987. Contribución al conocimiento de dos náyades sudamericanas. Revista Del Museo Argentino De Ciencias Naturales 24: 163–170.
Bonetto, A. A. & I. R. Wais, 2006. Southern South American streams and rivers. In Cushing, C. E., K. W. Cummins & G. Wayne Minshall (eds), River and Stream Ecosystems of the World: With a New Introduction University of California Press, Berkeley: 257–293.
Bonetto, A. A., M. L. Miranda, G. A. Iglesias & A. Pravisani, 1950. Las Almejas Productoras de Nácar. Ministerio de Hacienda y Economía, Departamento General de Industria Comercio y Abastecimiento, División de Caza, Pesca y Piscicultura, Santa Fe.
Bowen, S. H., 1983. Detritivory in Neotropical fish communities. Environmental Biology of Fishes 9: 137–144. https://doi.org/10.1007/bf00690858.
Bowen, S. H. & A. A. O. Bonetto, 1984. Microorganisms and detritus in the diet of a typical Neotropical riverine detritivore Prochilodus platensis (Pisces: Prochilodontidae). Limnology and Oceanography 29: 1120–1122.
Brainwood, M., S. Burgin & M. Byrne, 2006. Is the decline of freshwater mussel populations in a regulated coastal river in south-eastern Australia linked with human modification of habitat? Aquatic Conservation: Marine and Freshwater Ecosystems 16: 501–516. https://doi.org/10.1002/aqc.758.
Callil, C. T. & W. J. Junk, 1999. Concentração e incorporação de mercúrio por moluscos bivalves (Anodontites trapesialis Lamark, 1819 e Castalia ambigua Lamark, 1819) do Pantanal de Poconé - MT, BRASIL. Biociências (porto Alegre) 7: 3–28.
Callil, C. T. & M. C. D. Mansur, 2005. Ultrastructural analysis of the shells of Anodontites trapesialis (Lamarck) and Anodontites elongatus (Swaison) (Mollusca, Bivalvia, Etherioidea) from the Mato Grosso Pantanal Region, Brazil. Revista Brasileira De Biologia 22: 724–734. https://doi.org/10.1590/s0101-81752005000300033.
Callil, C. T., D. Krinski & F. A. Silva, 2012. Variations on the larval incubation of Anodontites trapesialis (Unionoida, Mycetopodidae): synergetic effect of the environmental factors and host availability. Brazilian Journal of Biology 72: 545–552. https://doi.org/10.1590/s1519-69842012000300017.
Callil, C. T., M. C. S. Leite, L. A. F. Mateus, et al., 2018. Influence of the flood pulse on reproduction and growth of Anodontites trapesialis (Lamarck, 1819) (Bivalvia: Mycetopodidae) in the Pantanal Wetland, Brazil. Hydrobiologia 810: 433–448. https://doi.org/10.1007/s10750-017-3097-3.
Cañedo-Argüelles, M., B. Kefford & R. Schäfer, 2018. Salt in freshwaters: causes, effects and prospects – introduction to the theme issue. Philosophical Transactions of the Royal Society B: Biological Sciences 374: 20180002. https://doi.org/10.1098/rstb.2018.0002.
Canzio, O. A., 1960. Contribución al Estudio Bioeconómico de las Especies de Almejas Nacaríferas del Río Paraná, Secretaría de Estado de Agricultura y Ganadería de la Nación, Buenos Aires:
Cappato, J. & A. Yanosky, 2009. Uso Sostenible de Peces en la Cuenca del Plata. Fundación Proteger, Argentina.
Carpenter, S. R., N. F. Caraco, D. L. Correll, R. W. Howarth, A. N. Sharpley & V. H. Smith, 1998. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecological Applications 8: 559. https://doi.org/10.2307/2641247.
Carvalho, K. Q., S. B. Lima, F. H. Passig, L. K. Gusmão, D. C. Souza, C. Kreutz, A. D. Belini & E. J. Arantes, 2015. Influence of urban area on the water quality of the Campo River Basin, Paraná State, Brazil. Brazilian Journal of Biology 75: S96–S106. https://doi.org/10.1590/1519-6984.00413suppl.
Castello, L., L. L. Hess, R. Thapa, D. G. McGrath, C. C. Arantes, V. F. Renó & V. J. Isaac, 2018. Fishery yields vary with land cover on the Amazon River Floodplain. Fish and Fisheries 19: 431–440. https://doi.org/10.1111/faf.12261.
Castro, M. & R. Arocena, 2020. Río de la Plata native Erodona mactroides (Bivalvia, Erodonidae) and exotic bivalves respond differently to sediment supply. Pan-American Journal of Aquatic Sciences 15: 163–172.
CBD, 2023. Kunming–Montreal Global Biodiversity Framework. CBD [available on internet athttps://prod.drupal.www.infra.cbd.int/sites/default/files/2022-12/221222-CBD-PressRelease-COP15-Final.pdf?_gl=1*1jmi9ew*_ga*MjkxNTIxOTQ3LjE3MDIxNjE0MzU.*_ga_7S1TPRE7F5*MTcwMjE2MTQzNS4xLjAuMTcwMjE2MTQ0My41Mi4wLjA].
CBEIH Collaborative Database, 2023. CBEIH [available on internet at https://base.cbeih.org/]. Accessed 4 Jan 2023.
Cera, A., G. Cesarini & M. Scalici, 2020. Microplastics in freshwater: what is the news from the world? Diversity 12: 276. https://doi.org/10.3390/d12070276.
CIC, 2016. CIC Transboundary Diagnostic Analysis (TDA) and Strategic Action Program (SAP) for the La Plata Basin – Executive Summary, CIC, Buenos Aires:
Clavijo, C., 2009. Distribución del género Anodontites (Mollusca: Bivalvia: Mycetopodidae) en Uruguay. Comunicaciones De La Sociedad Malacológica Del Uruguay 9: 201–210.
Clavijo, C., 2014. Biodiversidad y conservación de Cyanocyclas (Bivalvia, Cyrenidae) en Uruguay. Doctoral Thesis, Universidad de la República, Uruguay.
Clavijo, C., 2017. The pearl industry and pioneering research in biology and conservation of pearl mussels (Unionoida) in the Río de la Plata Basin. Tentacle 25: 14–15.
Clavijo, C., 2021. 40 años después bivalvos del género Corbicula en Uruguay. In Bresciano, D., E. Brugnoli & M. Iturburu (eds), Especies exóticas invasoras en Uruguay: distribución, impactos socioambientales y estrategias de gestión Retema-UdelaR/CEEI, Ministerio de Ambiente, Montevideo: 163–170.
Clavijo, C. & A. Carranza, 2018. Critical reduction of the geographic distribution of Cyanocyclas (Cyrenidae: Bivalvia) in Uruguay. Aquatic Conservation: Marine and Freshwater Ecosystems 28: 1249–1252. https://doi.org/10.1002/aqc.2941.
Clavijo, C. & J. Olazarri, 2009. Mollusca, Bivalvia, Mycetopodidae, Anodontites trigonus: southern dispersion in the Uruguay River. Checklist 5: 530–532. https://doi.org/10.15560/5.3.530.
Cochrane, M. A. & W. F. Laurance, 2008. Synergisms among fire, land use, and climate change in the Amazon. Ambio 37: 522–527. https://doi.org/10.1579/0044-7447-37.7.522.
Coutinho, H. L. C., E. Noellemeyer, E. Jobbagy, M. Jonathan & J. Paruelo, 2009. Impacts of land use change on ecosystems and society in the Rio de la Plata Basin. In Tiessen, H. & J. W. B. Stewart (eds), Applying Ecological Knowledge to Land Use Decisions Inter-American Institute for Global Change Research and SCOPE, Paris: 56–64.
Crutzen, P. J. & E. F. Stoermer, 2000. The Anthropocene. Global Change Newsletter 41: 17–18. https://doi.org/10.1007/s10888-011-9190-3.
Cuezzo, M. G., D. E. G. Gregoric, J. P. Pointier, A. A. Vázquez, C. Ituarte, M. C. D. Mansur, et al., 2020. Phylum Mollusca. In Rogers, D. C., C. Damborenea & J. Thorp (eds), Thorp and Covich’s Freshwater Invertebrates: Keys to Neotropical and Antarctic Fauna, Vol. 5. Academic, Cambridge: 261–430. https://doi.org/10.1016/B978-0-12-804225-0.00011-3.
Cushway, K. C., N. S. Ring, D. K. Patton & D. A. Woolnough, 2022. Landscape associations with native and invasive freshwater mussels. Hydrobiologia 849: 2449–2462. https://doi.org/10.1007/s10750-022-04850-8.
Dagosta, F. C., M. de Pinna, C. A. Peres & V. A. Tagliacollo, 2020. Existing protected areas provide a poor safety-net for threatened Amazonian fish species. Aquatic Conservation: Marine and Freshwater Ecosystems 31: 1167–1189. https://doi.org/10.1002/aqc.3461.
Darrigran, G., 2002. Potential impact of filter-feeding invaders on temperate inland freshwater environments. Biological Invasions 4: 145–156. https://doi.org/10.1023/a:1020521811416.
Darrigran, G., I. Agudo-Padrón, P. Baez, C. Belz, F. Cardoso, A. Carranza, G. Collado, M. Correoso, M. G. Cuezzo, A. Fabres, et al., 2020. Non-native mollusks throughout South America: emergent patterns in an understudied continent. Biological Invasions 22: 853–871. https://doi.org/10.1007/s10530-019-02178-4.
de Lima, R. E., J. de Lima Picanço, A. F. da Silva & F. A. Acordes, 2020. An anthropogenic flow type gravitational mass movement: the Córrego do Feijão Tailings Dam Disaster, Brumadinho, Brazil. Landslides 17: 2895–2906. https://doi.org/10.1007/s10346-020-01450-2.
de Oliveira, L. F., M. T. Cabral, C. E. D. Vieira, M. H. Antoniazzi, W. E. Risso & C. B. dos Reis Martinez, 2016. Metals bioaccumulation and biomarkers responses in the Neotropical freshwater clam Anodontites trapesialis: implications for monitoring coal mining areas. Science of the Total Environment 571: 983–991. https://doi.org/10.1016/j.scitotenv.2016.07.086.
Deutsch, C. A., J. J. Tewksbury, R. B. Huey, K. S. Sheldon, C. K. Ghalambor, D. C. Haak & P. R. Martin, 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proceedings of the National Academy of Sciences of USA 105: 6668–6672. https://doi.org/10.1073/pnas.0709472105.
Di Persia, D. H., J. J. Neiff & J. Olazarri, 1986. The Uruguay River system. In Junk, W. (ed), The Ecology of River Systems Springer, Dordrecht: 599–629.
Döll, P. & J. Zhang, 2010. Impact of climate change on freshwater ecosystems: a global-scale analysis of ecologically relevant river flow alterations. Hydrology and Earth System Sciences 14: 783–799. https://doi.org/10.5194/hess-14-783-2010.
d’Orbigny, A., 1835. Synopsis terrestrium et fluviatilium molluscorum, In suo per Americam meridionalem itinere. Magasin De Zoologie 5: 1–44.
Dores, E. F. G., 2015. Pesticides in the Pantanal. In Bergier, I. & M. Assine (eds), Dynamics of the Pantanal Wetland in South America. The Handbook of Environmental Chemistry, Vol. 37. Springer, Cham. https://doi.org/10.1007/698_2015_356.
Douda, K., M. Lopes-Lima, M. Hinzmann, J. Machado, S. Varandas, A. Teixeira & R. Sousa, 2013. Biotic homogenization as a threat to native affiliate species: fish introductions dilute freshwater mussel’s host resources. Diversity and Distributions 19: 933–942. https://doi.org/10.1111/ddi.12044.
Duarte, E. B., M. A. Neves, F. B. de Oliveira, M. E. Martins, C. H. R. de Oliveira, D. L. Burak, M. T. D. Orlando & C. V. G. T. Rangel, 2021. Trace metals in Rio Doce sediments before and after the collapse of the Fundão Iron Ore Tailing Dam Southeastern Brazil. Chemosphere 262: 127879. https://doi.org/10.1016/j.chemosphere.2020.127879.
Dudgeon, D., 2019. Multiple threats imperil freshwater biodiversity in the Anthropocene. Current Biology 29: R960–R967. https://doi.org/10.1016/j.cub.2019.08.002.
Dudgeon, D., A. H. Arthington, M. O. Gessner, Z.-I. Kawabata, D. J. Knowler, C. Lévêque, R. J. Naiman, A.-H. Prieur-Richard, D. Soto, M. L. J. Stiassny, et al., 2006. Freshwater biodiversity: importance, threats, status and conservation challenges. Biological Reviews of the Cambridge Philosophical Society 81: 163–182. https://doi.org/10.1017/S1464793105006950.
Etchegoyen, M. A., A. E. Ronco, P. Almada, M. Abelando & D. J. Marino, 2017. Occurrence and fate of pesticides in the Argentine stretch of the Paraguay-Paraná Basin. Environmental Monitoring and Assessment. https://doi.org/10.1007/s10661-017-5773-1.
Fagundes, C. K., R. C. Vogt & P. De Marco Júnior, 2016. Testing the efficiency of protected areas in the Amazon for conserving freshwater turtles. Diversity and Distributions 22: 123–135. https://doi.org/10.1111/ddi.12396.
Falfushynska, H., L. Gnatyshyna, I. Yurchak, A. Ivanina, O. Stoliar & I. Sokolova, 2014. Habitat pollution and thermal regime modify molecular stress responses to elevated temperature in freshwater mussels (Anodonta anatina: Unionidae). Science of the Total Environment 500–501: 339–350. https://doi.org/10.1016/j.scitotenv.2014.08.112.
FAO, 2006. Livestock’s Long Shadow: Environmental Issues and Options. Food and Agriculture Organization of the United Nations (FAO) [available on internet at https://www.fao.org/docrep/010/a0701e/a0701e.pdf].
Ferreira-Rodríguez, N., R. Sousa & I. Pardo, 2018. Negative effects of Corbicula fluminea over native freshwater mussels. Hydrobiologia 810: 85–95. https://doi.org/10.1007/s10750-016-3059-1.
Galbraith, H. S. & C. C. Vaughn, 2009. Temperature and food interact to influence gamete development in freshwater mussels. Hydrobiologia 636: 35–47. https://doi.org/10.1007/s10750-009-9933-3.
Goldsmith, A. M., F. H. Jaber, H. Ahmari & C. R. Randklev, 2020. Clearing up cloudy waters: a review of sediment impacts to unionid freshwater mussels. Environmental Reviews 29: 100–108. https://doi.org/10.1139/er-2020-0080.
Guilhermino, L., L. R. Vieira, D. Ribeiro, A. S. Tavares, V. Cardoso, A. Alves & J. M. Almeida, 2018. Uptake and effects of the antimicrobial florfenicol, microplastics and their mixtures on freshwater exotic invasive bivalve Corbicula fluminea. Science of the Total Environment 622–623: 1131–1142. https://doi.org/10.1016/j.scitotenv.2017.12.020.
Haag, W. R. & A. L. Rypel, 2011. Growth and longevity in freshwater mussels: evolutionary and conservation implications. Biological Reviews of the Cambridge Philosophical Society 86: 225–247. https://doi.org/10.1111/j.1469-185X.2010.00146.x.
Haag, W. & J. Williams, 2013. Biodiversity on the brink: an assessment of conservation strategies for North American freshwater mussels. Hydrobiologia 735: 1–16. https://doi.org/10.1007/s10750-013-1524-7.
Harries, P., 2015. Index fossil. In Jack Rink, W. & J. W. Thompson (eds), Encyclopedia of Scientific Dating Methods, Encyclopedia of Earth Sciences Series. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-6304-3_77.
Hayward, E. E., P. L. Gillis, C. J. Bennett, R. S. Prosser, J. Salerno, T. Liang, S. Robertson & C. D. Metcalfe, 2022. Freshwater mussels in an impacted watershed: influences of pollution from point and non-point sources. Chemosphere 307: 135966. https://doi.org/10.1016/j.chemosphere.2022.135966.
Hebling, N. J. & A. M. G. Penteado, 1974. Anatomía funcional de Diplodon rotundus gratus Wagner, 1827 (Mollusca, Bivalvia). Revista Brasileira De Zoologia 34: 67–80.
Henry, R. & C. A. Simão, 1985. Spatial distribution of a bivalve population (Diplodon delodontus expansus) (Küster, 1856) in a small tropical reservoir. Revista Brasileira De Biologia 45: 407–415.
Hermes-Silva, S., J. Ribolli, S. de Ávila-Simas, E. Zaniboni-Filho, G. F. M. Cardoso & A. P. O. de Nuñer, 2021. Limnoperna fortunei – updating the geographic distribution in the Brazilian watersheds and mapping the regional occurrence in the Upper Uruguay River Basin. Biota Neotropica 21: e20201175. https://doi.org/10.1590/1676-0611-bn-2020-1175.
Hoeinghaus, D. J., A. A. Agostinho, L. C. Gomes, F. M. Pelicice, E. K. Okada, J. D. Latini, E. A. L. Kashiwaqui & K. O. Winemiller, 2009. Effects of river impoundment on ecosystem services of large tropical rivers: embodied energy and market value of artisanal fisheries. Conservation Biology 23: 1222–1231. https://doi.org/10.1111/j.1523-1739.2009.01248.x.
Hutchison, Z. L., V. J. Hendrick, M. T. Burrows, B. Wilson & K. S. Last, 2016. Buried alive: the behavioural response of the mussels, Modiolus modiolus and Mytilus edulis to sudden burial by sediment. PLoS ONE 11: e0151471. https://doi.org/10.1371/journal.pone.0151471.
ICMBio, 2018. Livro Vermelho da Fauna Brasileira Ameaçada de Extinção, VII - Invertebrados. ICMBio, Brasilia.
Ihering, H., 1893. Najaden von S. Paulo und die geographische Verbreitung der süsswasser Faunen von Südamerika. Archiv Für Naturgeschichte 1: 45–140.
James, L. A. & S. A. Lecce, 2013. Impacts of land-use and land-cover change on river systems. In Shroder, J. F. (ed), Treatise on Geomorphology Elsevier, Amsterdam: 768–793. https://doi.org/10.1016/B978-0-12-374739-6.00264-5.
Jonsson, M., R. M. Burrows, J. Lidman, E. Fältström, H. Laudon & R. A. Sponseller, 2017. Land use influences macroinvertebrate community composition in boreal headwaters through altered stream conditions. Ambio 46: 311–323. https://doi.org/10.1007/s13280-016-0837-y.
Karatayev, A. Y., D. Boltovskoy, L. E. Burlakova & D. K. Padilla, 2015. Parallels and contrasts between Limnoperna fortunei and species of Dreissena. In Boltovskoy, D. (ed), Limnoperna fortunei, Invading Nature – Springer Series in Invasion Ecology, Vol. 10. Springer, Berlin: 261–297. https://doi.org/10.1007/978-3-319-13494-9_15.
Kaushal, S. S., G. E. Likens, M. L. Pace, R. M. Utz, S. Haq, J. Gorman & M. Grese, 2018. Freshwater salinization syndrome on a continental scale. Proceedings of the National Academy of Sciences of USA 115: E574–E583. https://doi.org/10.1073/pnas.1711234115.
Khan, J. M., M. Hart, J. Dudding, C. R. Robertson, R. Lopez & C. R. Randklev, 2019. Evaluating the upper thermal limits of glochidia for selected freshwater mussel species (Bivalvia: Unionidae) in central and east Texas, and the implications for their conservation. Aquatic Conservation: Marine and Freshwater Ecosystems 29: 1202–1215. https://doi.org/10.1002/aqc.3136.
Knouft, J. H. & D. L. Ficklin, 2017. The potential impacts of climate change on biodiversity in flowing freshwater systems. Annual Review of Ecology, Evolution, and Systematics 48: 111–133. https://doi.org/10.1146/annurev-ecolsys-110316-022803.
Koppe, J. C., 2021. Lessons learned from the two major tailings dam accidents in Brazil. Mine Water and the Environment 40: 166–173. https://doi.org/10.1007/s10230-020-00722-6.
Lawrence, A. J., C. Matuch, J. J. Hancock, A. L. Rypel & L. A. Eliassen, 2022. Potential local extirpation of an imperiled freshwater mussel population from wildfire runoff. Western North American Naturalist 82: 695–703. https://doi.org/10.3398/064.082.0405.
Layer, K., A. Hildrew, D. Monteith & G. Woodward, 2010. Long-term variation in the littoral food web of an acidified mountain lake: long-term variation in an acidified lake food web. Global Change Biology 16: 3133–3143. https://doi.org/10.1111/j.1365-2486.2010.02195.x.
Leal, M. F., L. R. L. de Simone, A. C. F. Lacerda, E. L. da Silva & T. Gimenez Pinheiro, 2021. Current distribution of the invasive mollusk Corbicula fluminea (O.F. Müller, 1774) (Bivalvia, Cyrenidae) in Brazil, including a new record from the State of Piauí. Checklist 17: 151–157. https://doi.org/10.15560/17.1.151.
Liu, X., R. Wu, X. Chen, Y. Zhou, L. Yang, S. Ouyang & X. P. Wu, 2020. Effects of dams and their environmental impacts on the genetic diversity and connectivity of freshwater mussel population in Poyang Lake Basin, China. Freshwater Biology 65: 264–277. https://doi.org/10.1111/fwb.13419.
Lopes-Lima, M., D. R. Sousa, J. Geist, D. C. Aldridge, R. Araujo, J. Bergengren, Y. Bespalaya, E. Bódis, L. Burlakova, D. Van Damme, et al., 2016. Conservation status of freshwater mussels in Europe: state of the art, perspectives and future challenges. Biological Reviews 92: 572–607. https://doi.org/10.1111/brv.12244.
Lopes-Lima, M., L. E. Burlakova, A. Y. Karatayev, K. Mehler, M. Seddon & R. Sousa, 2018. Conservation of freshwater bivalves at the global scale: diversity, threats and research needs. Hydrobiologia 810: 1–14. https://doi.org/10.1007/s10750-017-3486-7.
Lopes-Lima, M., M. Hinzmann, S. Varandas, E. Froufe, J. Reis, C. Moreira, S. Araújo, F. Miranda, D. V. Gonçalves, P. Beja, et al., 2020. Setting the stage for new ecological indicator species: a holistic case study on the Iberian dolphin freshwater mussel Unio delphinus Spengler, 1793. Ecological Indicators 111: 105987. https://doi.org/10.1016/j.ecolind.2019.105987.
Lopes-Lima, M., J. Reis, M. G. Alvarez, P. M. Anastácio, F. Banha, P. Beja, P. Castro, M. Gama, M. G. Gil, A. Gomes-dos-Santos, F. Miranda, J. Garrido Nogueira, R. Sousa, A. Teixeira, S. Varandas & E. Froufe, 2023. The silent extinction of freshwater mussels in Portugal. Biological Conservation. https://doi.org/10.1016/j.biocon.2023.110244.
Lowrance, R., R. Todd, J. Fail Jr., O. Hendrickson Jr., R. Leonard & L. Asmussen, 1984. Riparian forests as nutrient filters in agricultural watersheds. Bioscience 34: 374–377. https://doi.org/10.2307/1309729.
Lowrance, R., L. S. Altier, J. D. Newbold, R. R. Schnabel, P. M. Groffman, J. M. Denver, D. L. Correll, J. W. Gilliam, J. L. Robinson, R. B. Brinsfield, et al., 1997. Water quality functions of riparian forest buffers in Chesapeake Bay Watersheds. Environmental Management 21: 687–712. https://doi.org/10.1007/s002679900060.
Ludwig, S., G. Darrigran & W. Boeger, 2023. Opening the black box of the invasion of Corbicula clams (Bivalvia, Corbiculidae) in South America: a genetic and morphological evaluation. Hydrobiologia. https://doi.org/10.1007/s10750-023-05378-1.
Lydeard, C., R. H. Cowie, W. F. Ponder, A. E. Bogan, P. Bouchet, S. A. Clark, K. S. Cummings, T. J. Frest, O. Gargominy, D. G. Herbert, et al., 2004. The global decline of nonmarine mollusks. Bioscience 54: 321–330.
Mackie, G. L., 1989. Tolerances of five benthic invertebrates to hydrogen ions and metals (Cd, Pb, Al). Archives of Environmental Contamination and Toxicology 18: 215–223. https://doi.org/10.1007/BF01056206.
Mac Loughlin, T. M., L. Peluso & D. J. G. Marino, 2017. Pesticide impact study in the peri-urban horticultural area of Gran La Plata, Argentina. Science of the Total Environment 598: 572–580. https://doi.org/10.1016/j.scitotenv.2017.04.116.
MAGyP, 2023. Estadísticas de productos de río por origen y destino. MAGyP [available on internet at https://www.magyp.gob.ar/sitio/areas/pesca_continental/estadisticas/].
Malmqvist, B., S. D. Rundle, A. P. Covich, A. G. Hildrew, C. T. Robinson & C. R. Townsend, 2008. Prospects for streams and rivers: an ecological perspective. In Polunin, N. V. C. (ed), Aquatic Ecosystems: Trends and Global Prospects Cambridge University Press, Cambridge: 19–29. https://doi.org/10.1017/CBO9780511751790.004.
Marengo, J. A., G. S. Oliveira & L. M. Alves, 2015. Climate change scenarios in the Pantanal. In Bergier, I. & M. Assine (eds), Dynamics of the Pantanal Wetland in South America. The Handbook of Environmental Chemistry, Vol. 37. Springer, Cham. https://doi.org/10.1007/698_2015_357.
Mapbiomas Brasil. Mapbiomas.org. Mapbiomas [available on internet at https://mapbiomas.org/en/south-american-pampas-has-lost-163-of-native-vegetation-in-20-years-mostra-mapeamento-inedito-abrangendo-brasil-argentina-e-uruguai-1].
Marroni, S., N. Mazzeo & C. Iglesias, 2021. Effects of temperature and food availability on the filtration and excretion rates of Diplodon parallelopipedon (Hyriidae). International Review of Hydrobiology 106: 249–258. https://doi.org/10.1002/iroh.202002066.
McDowell, W. G. & R. Sousa, 2019. Mass mortality events of invasive freshwater bivalves: current understanding and potential directions for future research. Frontiers in Ecology and Evolution 7: 331. https://doi.org/10.3389/fevo.2019.00331.
Menéndez, A. N., S. Gerbec, M. Kazimierski & L. D. Badano, 2014. Onda de contaminación por rotura de diques de colas en la alta cuenca del río Pilcomayo. Instituto Nacional del Agua, Comisión Trinacional para el desarrollo de la cuenca del río Pilcomayo.
Millennium Ecosystem Assessment, 2005. Ecosystems and Human Well-Being: Current State and Trends, Island Press, Washington, DC:
Miranda, C. E., C. D. Clauser, V. L. Lozano, D. H. Cataldo & H. N. Pizarro, 2021. An invasive mussel is in trouble: how do glyphosate, 2, 4-D and its mixture affect Limnoperna fortuneiʹs survival? Aquatic Toxicology 239: 105957. https://doi.org/10.1016/j.aquatox.2021.105957.
Miyahira, I. C., S. B. dos Santos & M. C. D. Mansur, 2017. Freshwater mussels from South America: state of the art of Unionida, specially Rhipidodontini. Biota Neotropica 17(4): e20170341. https://doi.org/10.1590/1676-0611-BN-2017-0341.
Miyahira, I. C., L. Pereira & L. dos Santos, 2020. Non-native freshwater molluscs in the Neotropics: what can be learned from Brazilian reservoirs? Aquatic Invasions 15: 455–472. https://doi.org/10.3391/ai.2020.15.3.06.
Miyahira, I. C., C. Clavijo, C. T. Callil, M. G. Cuezzo, G. Darrigran, S. R. Gomes, C. A. Lasso, M. C. D. Mansur, M. S. Pena, R. Ramírez, et al., 2022. The conservation of non-marine molluscs in South America: where we are and how to move forward. Biodiversity and Conservation 31: 2543–2574. https://doi.org/10.1007/s10531-022-02446-1.
Miyahira, I. C., M. C. D. Mansur, L. E. M. de Lacerda, I. C. B. Gonçalves, G. G. Sant’Anna & S. B. dos Santos, 2023. Protected areas and native freshwater bivalves are not in the same place in south-east Brazil. Aquatic Conservation: Marine and Freshwater Ecosystems 33: 102–114. https://doi.org/10.1002/aqc.3904.
Modesto, V., M. Ilarri, A. T. Souza, M. Lopes-Lima, K. Douda, M. Clavero & R. Sousa, 2018. Fish and mussels: importance of fish for freshwater mussel conservation. Fish and Fisheries 19: 244–259. https://doi.org/10.1111/faf.12252.
Moreschi, A. C., C. T. Callil, S. W. Christo, A. L. Ferreira Junior, C. Nardes, É. de Faria & P. Girard, 2020. Filtration, assimilation and elimination of microplastics by freshwater bivalves. Case Studies in Chemical and Environmental Engineering 2: 100053. https://doi.org/10.1016/j.cscee.2020.100053.
Muccione, V. & M. Schaepman, 2023. Terminology Brief Series. Uzh.ch [available on internet at https://www.gcb.uzh.ch/dam/jcr:ffffffff-8f53-4fa0-0000-00002189557f/Terminology_Brief_Global_Change.pdf]. Accessed 4 Jan 2023.
Mugetti, A. C., A. T. Calcagno, C. A. Brieva, M. S. Giangiobbe, A. Pagani & S. Gonzalez, 2004. Aquatic habitat modifications in La Plata River Basin, Patagonia and associated marine areas. AMBIO: A Journal of the Human Environment 33: 78–87.
Natural Earth – Free vector and raster map data at 1:10 m, 1:50 m, and 1:110 m scales. Naturalearthdata.com [available on internet at https://www.naturalearthdata.com/].
Naumann, G., G. Podestá, J. Marengo, J. Luterbacher, D. Bavera, C. Arias-Muñoz, P. Marinho Ferreira Barbosa, C. Cammalleri, L. Chamorro, L. A. Cuartas, et al., 2021. The 2019–2021 Extreme Drought Episode in La Plata Basin: A Joint Report from EC-JRC, CEMADEN, SISSA and WMO. Joint Report. https://doi.org/10.2760/773.
Nilsson, C., C. A. Reidy, M. Dynesius & C. Revenga, 2005. Fragmentation and flow regulation of the world’s large river systems. Science 308: 405–408. https://doi.org/10.1126/science.1107887.
Nogarol, L. R., A. L. Brossi-Garcia, R. Bastão de Souza & C. S. Fontanetti, 2016. Histopathological effects of the herbicide atrazine on gills of the Brazilian endemic bivalve Diplodon expansus. International Journal of Environmental Analytical Chemistry 96: 387–403. https://doi.org/10.1080/03067319.2016.1150461.
Nogueira, M. G., G. Perbiche-Neves, D. de Oliveira Naliato, S. M. Caglierani Casanova, J. R. Debastiani-Júnior & E. G. Espíndola, 2021. Limnology and water quality in La Plata Basin (South America) – Spatial patterns and major stressors. Ecological Indicators 120: 106968. https://doi.org/10.1016/j.ecolind.2020.106968.
Oberdorff, T., 2022. Time for decisive actions to protect freshwater ecosystems from global changes. Knowledge and Management of Aquatic Ecosystems 423: 19. https://doi.org/10.1051/kmae/2022017.
Olazarri, J., 1966. Los moluscos de Agua Dulce Del Depto. de Colonia, Uruguay. Part I: Pelecypoda. Uruguay. Part I: Pelecypoda. Comunicaciones De La Sociedad Malacológica Del Uruguay 2: 15–37.
Olazarri, J., 1980. La formación del Embalse de Salto Grande y sus efectos sobre la malacofauna fluvial. Jornadas De Ciencias Naturales (montevideo) 1: 21–22.
Oldani, N. O., C. R. M. Baigún, J. M. Nestler & R. A. Goodwin, 2007. Is fish passage technology saving fish resources in the Lower La Plata River Basin? Neotropical Ichthyology 5: 89–102. https://doi.org/10.1590/s1679-62252007000200002.
Oliveira, M. D., M. C. S. Campos, E. M. Paolucci, M. C. D. Mansur & S. K. Hamilton, 2015. Colonization and spread of Limnoperna fortunei in South America. In Boltovskoy, D. (ed), Limnoperna fortunei, Invading Nature: Springer Series in Invasion Ecology, Vol. 10. Springer, Berlin: 333–355. https://doi.org/10.1007/978-3-319-13494-9_19.
Oliveira, P., L. G. Antão Barboza, V. Branco, N. Figueiredo, C. Carvalho & L. Guilhermino, 2018. Effects of microplastics and mercury in the freshwater bivalve Corbicula fluminea (Müller, 1774): filtration rate, biochemical biomarkers and mercury bioconcentration. Ecotoxicology and Environmental Safety 164: 155–163. https://doi.org/10.1016/j.ecoenv.2018.07.062.
Olivera-Hyde, M., E. Hallerman, R. Santos, J. Jones, B. Varnerin, G. da Cruz Santos Neto, M. C. D. Mansur, P. Moraleco & C. T. Callil, 2020. Phylogenetic assessment of freshwater mussels Castalia ambigua and C. inflata at an ecotone in the Paraguay River Basin, Brazil shows that inflated and compressed shell morphotypes are the same species. Diversity 12: 481. https://doi.org/10.3390/d12120481.
Ourso, R. T. & S. A. Frenzel, 2003. Identification of linear and threshold responses in streams along a gradient of urbanization in Anchorage, Alaska. Hydrobiologia 501: 117–131. https://doi.org/10.1023/A:1026211808745.
Palanques, A., J. Grimalt, M. Belzunces, F. Estrada, P. Puig & G. Guillén, 2014. Massive accumulation of highly polluted sedimentary deposits by river damming. Science of the Total Environment 497–498: 369–381. https://doi.org/10.1016/j.scitotenv.2014.07.091.
Pandolfi, G. S., J. W. Mays & M. M. Gangloff, 2022. Riparian land-use and in-stream habitat predict the distribution of a critically endangered freshwater mussel. Hydrobiologia 849: 1763–1776. https://doi.org/10.1007/s10750-022-04826-8.
Paredes-Beltran, B., A. Sordo-Ward & L. Garrote, 2021. Dataset of georeferenced dams in South America (DDSA). Earth System Science Data 13: 213–229. https://doi.org/10.5194/essd-13-213-2021.
Paschoal, L. R. P., D. P. Andrade, D. M. Pimpao, S. Torres & G. Darrigran, 2020. Massive mortality of the giant freshwater mussel Anodontites trapesialis (Lamarck, 1819) (Bivalvia: Mycetopodidae) during a severe drought in a Neotropical reservoir. Anais Da Academia Brasileira De Ciências 92: e20180811. https://doi.org/10.1590/0001-3765202020180811.
Passig, F. H., S. B. Lima, K. Q. Carvalho, M. C. R. Halmeman, P. C. Souza & L. K. Gusmão, 2015. Monitoring of urban and rural basins: water quality of Mourão Basin. Brazilian Journal of Biology 75: S158–S164. https://doi.org/10.1590/1519-6984.01213suppl.
Pazos, R. S., F. Spaccesi & N. Gómez, 2020. First record of microplastics in the mussel Limnoperna fortunei. Regional Studies in Marine Science 38: 101360. https://doi.org/10.1016/j.rsma.2020.101360.
Peltzer, P. M., A. P. Cuzziol Boccioni, R. E. Lorenzón, A. Bortoluzzi, N. Peña, A. M. Attademo, A. Bassó, E. J. León, A. H. Beltzer & R. C. Lajmanovich, 2023. Effects of man-made fires on wetlands of the Paraná River in Argentina: perspectives of ecological restoration. Oecologia Australis 27: 344–357. https://doi.org/10.4257/oeco.2023.2704.01.
Peluso, L. M., L. Mateus, J. Penha, D. Bailly, F. Cassemiro, Y. Suárez, I. Fantin-Cruz, E. Kashiwaqui & P. Lemes, 2022. Climate change negative effects on the Neotropical fishery resources may be exacerbated by hydroelectric dams. Science of the Total Environment 828: 154485. https://doi.org/10.1016/j.scitotenv.2022.154485.
Pereira, D., M. C. D. Mansur & D. M. Pimpao, 2012. Identificação e diferenciação dos bivalves límnicos invasores dos demais bivalves nativos do Brasil. In Mansur, M. C. D., C. P. Santos, D. Pereira, I. C. P. Paz, M. L. L. Zurita, M. T. R. Rodriguez & M. V. E. A. Nehrke (eds), Moluscos Límnicos Invasores no Brasil: Biologia, Prevenção e Controle Redes Editora, Porto Alegre: 75–94.
Pereira, D., M. C. D. Mansur, L. D. S. Duarte, A. S. de Oliveira, D. M. Pimpão, C. T. Callil, C. Ituarte, E. Parada, S. Peredo, G. Darrigran, et al., 2014. Bivalve distribution in hydrographic regions in South America: historical overview and conservation. Hydrobiologia 735: 15–44. https://doi.org/10.1007/s10750-013-1639-x.
Perkins, D. M., J. Reiss, G. Yvon-Durocher & G. Woodward, 2010. Global change and food webs in running waters. Hydrobiologia 657: 181–198. https://doi.org/10.1007/s10750-009-0080-7.
Peruzzo, P. J., A. A. Porta & A. E. Ronco, 2008. Levels of glyphosate in surface waters, sediments and soils associated with direct sowing soybean cultivation in north pampasic region of Argentina. Environmental Pollution 156: 61–66. https://doi.org/10.1016/j.envpol.2008.01.015.
Poole, K. E. & J. A. Downing, 2004. Relationship of declining mussel biodiversity to stream-reach and watershed characteristics in an agricultural landscape. Journal of the North American Benthological Society 23: 114–125.
Quintana, M. G., 1982. Catálogo preliminar de la malacofauna del Paraguay. Revista Del Museo Argentino De Ciencias Naturales 11: 61–158.
Rabuffetti, A. P., L. A. Espínola, E. Abrial, M. L. Amsler, M. C. M. Blettler, M. F. Eurich & E. G. Eberle, 2020. Commercial fisheries in a mega unregulated floodplain river: assessment of the most favourable hydrological conditions for its preservation. Journal of Fish Biology 96: 59–73. https://doi.org/10.1111/jfb.14184.
Randklev, C. R., H. H. Wang, J. E. Groce, W. E. Grant, S. Robertson & N. Wilkins, 2015. Land use relationships for a rare freshwater mussel species endemic to Central Texas. Journal of Fish and Wildlife Management 6: 327–337. https://doi.org/10.3996/012015-JFWM-003.
Reid, A. J., A. K. Carlson, I. F. Creed, E. J. Eliason, P. A. Gell, P. T. J. Johnson, K. A. Kidd, T. J. MacCormack, J. D. Olden, S. J. Ormerod, J. P. Smol, W. W. Taylor, K. Tockner, J. C. Vermaire, D. Dudgeon & S. J. Cooke, 2019. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biological Reviews 94: 849–873. https://doi.org/10.1111/brv.12480.
Reis, R. E., J. S. Albert, F. Di Dario, M. M. Mincarone, P. Petry & L. A. Rocha, 2016. Fish biodiversity and conservation in South America: fish biodiversity and conservation. Journal of Fish Biology 89: 12–47. https://doi.org/10.1111/jfb.13016.
Resh, V. & D. Rosenberg, 2015. Economic aspects of freshwater invertebrates. In Thorp, J. H. & D. C. Rogers (eds), Thorp and Covich's Freshwater Invertebrates. 93–109. https://doi.org/10.1016/B978-0-12-385026-3.00006-1.
Revenga, C., I. Campbell, R. Abell, P. de Villiers & M. Bryer, 2005. Prospects for monitoring freshwater ecosystems towards the 2010 targets. Philosophical Transactions of the Royal Society b: Biological Sciences 360: 397–413. https://doi.org/10.1098/rstb.2004.1595.
Ribeiro Guevara, S. R., D. Bubach, P. Vigliano, G. Lippolt & M. Arribére, 2004. Heavy metal and other trace elements in native mussel Diplodon chilensis from Northern Patagonia Lakes, Argentina. Biological Trace Element Research 102: 245–263. https://doi.org/10.1385/BTER:102:1-3:245.
Ronda, A. C., A. H. Arias, G. N. Rimondino, A. F. Pérez, A. Harte & J. E. Marcovecchio, 2021. Plastic impacts in Argentina: a critical research review contributing to the global knowledge. Current Environmental Health Reports 8: 212–222. https://doi.org/10.1007/s40572-021-00323-7.
Santos, S., S. Thiengo, M. Fernandez, I. Miyahira, I. Gonçalves, R. Ximenes, M. C. D. Mansur & D. Pereira, 2012. Espécies de moluscos límnicos invasores no Brasil. In Mansur, M. C. D., C. P. Santos, D. Pereira, I. C. P. Paz, M. L. L. Zurita, M. T. R. Rodriguez & M. V. E. A. Nehrke (eds), Moluscos Límnicos Invasores no Brasil: Biologia, Prevenção e Controle Redes Editora, Porto Alegre: 25–49.
Santos, R. C. L., A. W. Michiura & C. T. Callil, 2021. Host attraction behaviour: the red exhalant aperture extension of the Neotropical freshwater mussel Castalia ambigua. Journal of Molluscan Studies 87: eyab025. https://doi.org/10.1093/mollus/eyab025.
Scarabotti, P. A., L. O. Lucifora, L. A. Espínola, A. P. Rabuffetti, J. Liotta, J. E. Mantinian, J. P. Roux, N. Silva, L. Balboni, F. Vargas, et al., 2021. Long-term trends of fishery landings and target fish populations in the Lower La Plata Basin. Neotropical Ichthyology 19: e210013. https://doi.org/10.1590/1982-0224-2021-0013.
Serra, S., J. Bessonart, F. Texeira de Mello, A. Duarte, L. Malabarba & M. Loureiro, 2014. Peces del Río Negro. MGAP-DINARA, Montevideo. ISBN 978-9974-594-19-7.
Serrano, M. A. S., R. S. Tietböhl & M. C. D. Mansur, 1998. Sobre a ocorrência de moluscos Bivalvia no pantanal de Mato Grosso, Brasil. Biociências 6: 131–144.
Sheehan, R. J., R. J. Neves & H. E. Kitchel, 1989. Fate of freshwater mussels transplanted to formerly polluted reaches of the Clinch and North Fork Holston Rivers, Virginia. Journal of Freshwater Ecology 5: 139–149. https://doi.org/10.1080/02705060.1989.9665224.
Silva, V., N. Abrantes, R. Costa, J. J. Keizer, F. Gonçalves & J. L. Pereira, 2016. Effects of ash-loaded post-fire runoff on the freshwater clam Corbicula fluminea. Ecological Engineering 90: 180–189. https://doi.org/10.1016/j.ecoleng.2016.01.043.
Silva, I., E. Brugnoli, C. Clavijo, A. D. Anatro, D. A. Naya, F. Teixeira de Mello, G. Tesitore & I. González-Bergonzoni, 2021. Interacciones entre el mejillón dorado y macroinvertebrados bentónicos nativos del Río Uruguay. Innotec 22: e573. https://doi.org/10.26461/22.04.
Silva-Souza, A. T. & J. C. Eiras, 2002. The histopathology of the infection of Tilapia rendalli and Hypostomus regani (Osteichthyes) by lasidium larvae of Anodontites trapesialis (Mollusca, Bivalvia). Memórias Do Instituto Oswaldo Cruz 97: 431–433. https://doi.org/10.1590/s0074-02762002000300029.
Silva-Souza, A. T., P. Guardia-Felipi & N. R. Arrebola, 2011. Embryonic development of Anodontites trapesialis (Lamarck, 1819) (Bivalvia: Mycetopodidae). Brazilian Journal of Biology 71: 139–144. https://doi.org/10.1590/s1519-69842011000100020.
Simeone, D., C. H. Tagliaro & C. R. Beasley, 2021. Novel insights into habitat suitability for Amazonian freshwater mussels linked with hydraulic and landscape drivers. Ecology and Evolution 11: 11786–11798. https://doi.org/10.1002/ece3.7947.
Skawina, A., 2021. Evolutionary history of bivalves as parasites. In De Baets, K. & J. W. Huntley (eds), The Evolution and Fossil Record of Parasitism Springer, Berlin: 153–207. https://doi.org/10.1007/978-3-030-42484-8_5.
SNMF, 2023. Manejo del fuego. Reporte de fuegos. Ministerio de Medio ambiente y desarrollo sostenible, Argentina [available on internet at https://www.argentina.gob.ar/ambiente].
Sotola, V. A., K. T. Sullivan, B. M. Littrell, N. H. Martin, D. S. Stich & T. H. Bonner, 2021. Short-term responses of freshwater mussels to floods in a southwestern U.S.A. river estimated using mark–recapture sampling. Freshwater Biology 66: 349–361. https://doi.org/10.1111/fwb.13642.
Sousa, R., S. Varandas, R. Cortes, A. Teixeira, M. Lopes-Lima, J. Machado & L. Guilhermino, 2012. Massive die-offs of freshwater bivalves as resource pulses. Annales De Limnologie 48: 105–112. https://doi.org/10.1051/limn/2012003.
Sousa, R., A. Novais, R. Costa & D. L. Strayer, 2014. Invasive bivalves in fresh waters: impacts from individuals to ecosystems and possible control strategies. Hydrobiologia 735: 233–251. https://doi.org/10.1007/s10750-012-1409-1.
Sousa, R., A. Ferreira, F. Carvalho, M. Lopes-Lima, S. Varandas & A. Teixeira, 2018. Die-offs of the endangered pearl mussel Margaritifera margaritifera during an extreme drought. Aquatic Conservation: Marine and Freshwater Ecosystems 28: 1244–1248. https://doi.org/10.1002/aqc.2945.
Sousa, R., D. Halabowski, A. M. Labecka, K. Douda, O. Aksenova, Y. Bespalaya, I. Bolotov, J. Geist, H. A. Jones, E. Konopleva, et al., 2021. The role of anthropogenic habitats in freshwater mussel conservation. Global Change Biology 27: 2298–2314. https://doi.org/10.1111/gcb.15549.
Sowa, A., M. Krodkiewska, D. Halabowski & I. Lewin, 2019. Response of the mollusc communities to environmental factors along an anthropogenic salinity gradient. Natural Sciences 106: 60. https://doi.org/10.1007/s00114-019-1655-4.
Staichak, G., A. L. Ferreira Jr., A. C. Moreschi Silva, P. Girard, C. T. Callil & S. W. Christo, 2021. Bivalves with potential for monitoring microplastics in South America. Case Studies in Chemical and Environmental Engineering 4: 100119. https://doi.org/10.1016/j.cscee.2021.100119.
Steffen, W., A. Persson, L. Deutsch, J. Zalasiewicz, M. Williams, K. Richardson, C. Crumley, P. Crutzen, C. Folke, L. Gordon, M. Molina, V. Ramanathan, J. Rockström, M. Scheffer, H. J. Schellnhuber & U. Svedin, 2011. The Anthropocene: from global change to planetary stewardship. Ambio 40: 739–761. https://doi.org/10.1007/s13280-011-0185-x.
Stillman, J. H., 2003. Acclimation capacity underlies susceptibility to climate change. Science 301: 65. https://doi.org/10.1126/science.1083073.
Strayer, D. L., 1999. Effects of alien species on freshwater mollusks in North America. Journal of the North American Benthological Society 18: 74–98.
Strayer, D. L., 2009. Freshwater Mussel Ecology: A Multifactor Approach to Distribution and Abundance. Springer, Berlin; University of California, Press, Oakland. https://doi.org/10.1525/california/9780520255265.001.0001.
Strayer, D. L. & H. M. Malcom, 2007. Effects of zebra mussels (Dreissena polymorpha) on native bivalves: the beginning of the end or the end of the beginning? Journal of North American Benthological Society 26: 111–122. https://doi.org/10.1899/0887-3593(2007)26[111:EOZMDP]2.0.CO;2.
Strayer, D. & H. Malcom, 2012. Causes of recruitment failure in freshwater mussel populations in southeastern New York. Ecological Applications: A Publication of the Ecological Society of America 22: 1780–1790. https://doi.org/10.2307/41722893.
Strayer, D. L., R. E. Beighley, L. C. Thompson, S. Brooks, C. Nilsson, G. Pinay & R. J. Naiman, 2003. Effects of land cover on stream ecosystems: roles of empirical models and scaling issues. Ecosystems 6: 407–423. https://doi.org/10.1007/pl00021506.
Strong, E., O. Gargominy, W. Ponder & P. Bouchet, 2007. Global diversity of gastropods (Gastropoda; Mollusca) in freshwater. Hydrobiologia 595: 149–166. https://doi.org/10.1007/s10750-007-9012-6.
Tarter, A. A., D. F. Ford, D. E. Symonds, N. B. Ford & A. N. Schwalb, 2022. Impact of extreme climatic events on unionid mussels in a subtropical river basin. Hydrobiologia 850: 1427–1442. https://doi.org/10.1007/s10750-022-04819-7.
Tomazelli, A. C., L. A. Martinelli, W. E. P. Avelar, P. B. de Camargo, A.-H. Fostier, E. S. B. Ferraz, F. J. Krug & D. Santos Júnior, 2003. Biomonitoring of Pb and Cd in two impacted watersheds in Southeast Brazil, using the freshwater mussel Anodontites trapesialis (Lamarck, 1819) (Bivalvia: Mycetopodidae) as a biological monitor. Brazilian Archives of Biology and Technology 46: 673–684. https://doi.org/10.1590/s1516-89132003000400022.
Torres, S., L. Cao, D. E. Gutiérrez Gregoric, M. de Lucía, F. Brea & G. Darrigran, 2018. Distribution of the Unionida (Bivalvia, Paleoheterodonta) from Argentina and its conservation in the Southern Neotropical Region. PLoS ONE 13: e0203616. https://doi.org/10.1371/journal.pone.0203616.
Tucci, C. E. M. & R. T. Clarke, 1998. Environmental issues in the La Plata Basin. International Journal of Water Resources Development 14: 157–173. https://doi.org/10.1080/07900629849376.
UNASUR, 2015. Sistema de Información Geográfica del COSIPLAN, UNASUR, Buenos Aires:
UNEP-WCMC & IUCN, 2023. Protected Planet: The World Database on Protected Areas (WDPA) and World Database on Other Effective Area-Based Conservation Measures (WD-OECM), December 2023. UNEP-WCMC and IUCN, Cambridge [available on internet at www.protectedplanet.net].
van Dalfsen, J. A. & K. Essink, 2001. Benthic community response to sand dredging and shoreface nourishment in Dutch coastal waters. Senckenbergiana Maritima 31: 329–332. https://doi.org/10.1007/bf03043041.
Van Hassel, J. H. & J. Farris, 2006. A review of the use of unionid mussels as biological indicators of ecosystem health. In Farris, J. L. & J. H. van Hassel (eds), Freshwater Bivalve Ecotoxicology CRC Press, Boca Raton: 19–49. https://doi.org/10.1201/9781420042856.ch2.
Vannote, R. L., G. W. Minshall, K. W. Cummins, J. R. Sedell & C. E. Cushing, 1980. The river continuum concept. Canadian Journal of Fisheries and Aquatic Sciences 37: 130–137. https://doi.org/10.1139/f80-017.
Vieira, L. M., J. R. C. Alho & G. A. L. Ferreira, 1995. Contaminação por mercúrio em sedimento e em moluscos do Pantanal, Mato Grosso, Brasil. Revista Brasileira De Zoologia 12: 663–670. https://doi.org/10.1590/S0101-81751995000300022.
Vitule, J. R. S., F. Skóra & V. Abilhoa, 2012. Homogenization of freshwater fish faunas after the elimination of a natural barrier by a dam in Neotropics: biotic homogenization in Neotropics. Diversity and Distributions 18: 111–120. https://doi.org/10.1111/j.1472-4642.2011.00821.x.
Wächtler, K., M. C. Dreher-Mansur & T. Richter, 2001. Larval types and early postlarval biology in naiads (unionoida). In Bauer, G. & K. Wachtler (eds), Ecology and Evolution of the Freshwater Mussels Unionoida Springer, Berlin: 93–125. https://doi.org/10.1007/978-3-642-56869-5_6.
Walser, C. A. & H. L. Bart, 1999. Influence of agriculture on in-stream habitat and fish community structure in Piedmont Watersheds of the Chattahoochee River System. Ecology of Freshwater Fish 8: 237–246.
Wang, J., B. A. Walter, F. Yao, C. Song, M. Ding, A. S. Maroof, J. Zu, C. Fan, J. M. McAlister, S. Sikder, Y. Sheng, G. H. Allen, J. F. Crétaux & Y. Wada, 2021. GeoDAR: georeferenced global dam and reservoir dataset for bridging attributes and geolocations. Earth System Science Data 14: 1–52. https://doi.org/10.5194/essd-14-1869-2022.
Winemiller, K. O. & D. B. Jepsen, 2004. Migratory Neotropical fish subsidize food webs of oligotrophic blackwater rivers. In Polis, G. A., M. E. Power & G. R. Huxel (eds), Food Webs at the Landscape Level University of Chicago Press, Chicago: 115–132.
Winemiller, K. O., J. V. Montoya, D. L. Roelke, C. A. Layman & J. B. Cotner, 2006. Seasonally varying impact of detritivorous fishes on the benthic ecology of a tropical floodplain river. Journal of the North American Benthological Society 25: 250–262.
Winkler, K., R. Fuchs, M. Rounsevell & M. Herold, 2021. Global land use changes are four times greater than previously estimated. Nature Communications 12: 2501. https://doi.org/10.1038/s41467-021-22702-2.
Woodward, G., N. Friberg & A. G. Hildrew, 2009. The need for scientific rigor in biomonitoring and conservation of fresh waters. In de Carlo, F. & A. Bassano (eds), Freshwater Ecosystems: Biodiversity, Management and Conservation Nova Science Publishers, New York: 277–288.
Woodward, G., D. M. Perkins & L. E. Brown, 2010. Climate change and freshwater ecosystems: impacts across multiple levels of organization. Philosophical Transactions of the Royal Society b: Biological Sciences 365: 2093–2106. https://doi.org/10.1098/rstb.2010.0055.
Wu, R., L. Liu, X. Liu, Y. Ye, X. Wu, Z. Xie, Z. Liu & Z. Li, 2023. Towards a systematic revision of the superfamily Cyrenoidea (Bivalvia: Imparidentia): species delimitation, multi-locus phylogeny and mitochondrial phylogenomics. Invertebrate Systematics 37: 607–622. https://doi.org/10.1071/is23015.
Zarfl, C., A. E. Lumsdon, J. Berlekamp, L. Tydecks & K. A. Tockner, 2015. Global boom in hydropower dam construction. Aquatic Sciences 77: 161–170. https://doi.org/10.1007/s00027-014-0377-0.
Zieritz, A., M. Lopes-Lima, A. E. Bogan, R. Sousa, S. Walton, K. A. Rahim, J. J. Wilson, P. Y. Ng, E. Froufe & S. McGowan, 2016. Factors driving changes in freshwater mussel (Bivalvia, Unionida) diversity and distribution in Peninsular Malaysia. Science of the Total Environment 15: 1069–1078. https://doi.org/10.1016/j.scitotenv.2016.07.098.
Acknowledgements
Our gratitude goes to anonymous reviewers for improving an initial version of the manuscript. The authors also like to extend our thanks to the artist COF for his contributions to the enhancement of the graphical abstract (http://cof.ar).
Funding
ICM has the support of Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ E-26/201.347/2021); CC has the support of Agencia Nacional de Investigación e Innovación (ANII), Programa de Desarrollo de las Ciencias Básicas (PEDECIBA) and Mohamed bin Zayed Species Conservation Fund (MBZ 202524562).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: André M. Amado, Roberto J. P. Dias, Sthefane D'ávila, & Simone J. Cardoso / Emergent Issues of Neotropical Aquatic Ecosystems in the Anthropocene
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Clavijo, C., Miyahira, I.C. & Bassó, A. The freshwaters bivalves of La Plata Basin in the Anthropocene. Hydrobiologia (2024). https://doi.org/10.1007/s10750-024-05679-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10750-024-05679-z