Abstract
The hospital antibiotic policy should be implemented to rationalize the antibiotic use and to decrease the risk of spread of resistant bacteria. The aim of this study was to describe the antibiotic consumption patterns in a single oncosurgery ward before and after the implementation of hospital antibiotic policy. We conducted a retrospective analysis of the antibiotic use at the oncosurgery ward in Warsaw, Poland, in the years 2011–2016. Calculations were based on daily defined doses (DDD), DDD/100 hospitalizations, and DDD/100 person-days. Drug utilization rates (DU 90% and DU 100%) were also analyzed. After the implementation of hospital antibiotic policy, a total antibiotic consumption increased (365.35 DDD in 2011 vs. 1359.22 DDD in 2016). A significant change was observed in the antibiotic consumption patterns: the use of amoxicillin clavulanate and carbapenems or glycopeptides decreased significantly (p < 0.05), while the use of ciprofloxacin and aminoglycosides increased (p < 0.05). The DU100% rate varied from 6 in 2011 to 12 in 2016; while DU 90% rate varied from 2 in 2011 to 3–5 in 2013–2016. Although the implementation of hospital antibiotic policy did not result in a decrease in the antibiotic consumption, it seems to provide a favorable change into the antibiotic consumption pattern.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Antibiotic resistance
- Antibiotic stewardship
- Antibiotic use pattern
- Hospital antibiotic policy
- Oncosurgery ward
1 Introduction
Bacterial resistance to antibiotics is best illustrated by the occurrence of the methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus, extended-spectrum beta-lactamases (ESBL), and carbapenemase-producing bacteria Klebsiella pneumoniae (Davies and Davies 2010; Schwaber and Carmelli 2008). In light of the growing drug resistance, the development of new antibacterial substances is not a promising occurrence. Moreover, breakthroughs in the area of research in the next few years are rather improbable. Therefore, a rational antibiotic therapy aiming at decreasing risk of drug resistance should be implemented on a large scale, in both hospitals and outpatient settings. An example of this activity might be the implementation of the hospital antibiotic policy. The aim of this policy is to introduce a rational antibiotic therapy and do it with the utmost consideration for patient care. Antibiotics should be prescribed in proper doses, for optimum duration of therapy, with a minimum risk of adverse effects or the development of antibiotic resistance, and with a consideration for the lowest possible therapy cost (Dik et al. 2015; Davey et al. 2005). The aim of this study was to retrospectively evaluate the implementation of hospital antibiotic policy in the Oncosurgery Ward during a 6-year observation time, a subject that was hitherto only sparingly dealt with in the relevant medical literature.
2 Methods
The study was approved by a local Bioethics Committee of Warsaw Medical University in Warsaw, Poland. A retrospective quantitative and qualitative analysis of the antibiotic use, before and in the consecutive 5 years after the implementation of the hospital antibiotic policy, was conducted in the Oncosurgery Ward of the St. Family Hospital in Warsaw. The hospital is a second level referral hospital, and the Oncosurgery Ward has 20 beds. The number of hospitalizations and patient-days in the analyzed years are presented in Table 1. The ward specializes in breast cancer surgery and thyroid surgeries. Surgical procedures performed in the period above outlined are summarized in Table 2. There was a shift towards breast cancer surgery, whereas thyroid surgery declined.
Responsibility for the implementation of the hospital antibiotic policy was placed on the Infection Control Team that consisted of an epidemiologist, pharmacist, microbiologist, and epidemiological nurse. One of the key goals of the policy was to create a Hospital Antibiotics List divided into three groups: ‘first-line’ antibiotics (prescribed by all physicians at all times, e.g. cefazolin, amoxicillin, and doxycycline); ‘controlled antibiotics’ (prescribed by all physicians, but a continuation of therapy required a permission from the head physician, e.g., amoxicillin clavulanate, cefuroxime, ceftriaxone, clindamycin, ciprofloxacin, piperacillin with tazobactam, gentamycin, amikacin); and ‘restricted antibiotics’, prescribed with a written permission of the Infection Control Team head, e.g., carbapenems (imipenem and meropenem) and glycopeptides (teicoplanin and vancomycin).
We evaluated the antibiotic use according to the methodology described previously (Nitsch-Osuch et al. 2015a, b). The evaluation included the Hospital Pharmacy data consisting of the ordered and prescribed antibiotics, the type of antibiotic, the route of administration, and the dose as well as the Medical Statistics Department data consisting of the number of hospitalizations and patient-days. Moreover, antibiotic consumption pattern was defined, specifying the proportion of antibiotics used in terms of daily defined dose (DDD), which is the average daily dose of a drug, specified with a code of the Anatomical Therapeutic Chemical Classification, used in therapy as the main indication. The following formula was employed: DDD = (number of doses × g/dose) / DDD value (g). Other parameters used in the assessment of antibiotic use were as follows:
-
DDD/100 hospitalizations;
-
DDD/100 person-days;
-
Drug Utilization 100% (DU 100%);
-
Drug Utilization 90% (DU 90%).
The DDD/100 hospitalizations and DDD/100 person-days enable the inter-departmental comparisons and also describe the trends in antibiotic consumption in a ward at different time intervals. The DU100% and DU90% are considered to reflect a correctness of antibiotic treatment application. The DU100% shows how many groups of antibiotics are used, while DU90% shows which groups of antibiotics comprise 90% of the antibiotics used.
Statistical evaluation was performed using a chi-squared test. A p-value of <0.05 defined statistically significant differences. We also calculated the odds ratios (OR) and 95% confidence intervals (95%CI) using an online statistical calculator available at www.medcalc.3000.
3 Results
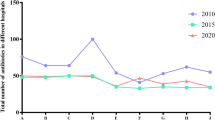
Consumption of antibiotics was steadily increasing; from 365.4 DDD in 2011 before the implementation of the hospital antibiotic policy to 1359.2 DDD in the fifth year of observation on the hospital antibiotic policy in 2016 (Table 3). A similar upward trend concerned also the use of antibiotics expressed in DDD/100 person-days (Table 4) and DDD/100 hospital admissions (Table 5). There was a significant change in the antibiotic consumption pattern after the implementation of the hospital antibiotic policy in 2012–2016 (Fig. 1). There was a decrease in the use of penicillin with inhibitors, mainly amoxicillin clavulanate, 64% DDD in 2011 vs. 26.5% in 2016, and cephalosporins (mainly cefuroxime and ceftriaxone), 22.5% DDD in 2011 vs. 6.6% in 2016. Furthermore, the use of restricted antibiotics (carbapenems and glycopeptides) decreased from 7.0% in 2011 vs. 1.0% in 2016. The most spectacular change was the increased consumption of ciprofloxacin from 5.0% in 2011 to 57.3% in 2016 and aminoglycosides from 0% in 2011 to 8.5% in 2016. The detailed antibiotic consumption patterns period are presented in Table 6. The DU 100% rate rose initially after the implementation of the hospital antibiotic policy HAP; in 2011 it amounted to 6 antibiotics, whereas in 2014 it was 11 antibiotics, only to drastically decrease in 2015 (DU100% 5). The DU ratio of 90% were 2 in 2011, and then 3 or 5 antibiotics in 2012–2016 (Table 7).
4 Discussion
4.1 Effects of Implementation of the Hospital Antibiotic Policy on Antibiotic Consumption Patterns
The implementation of the hospital antibiotic policy did not reduce a total consumption of antibiotics in the ward. On the contrary, antibiotic consumption steadily increased. This seemingly surprising observation may be caused by a different profile of patients being admitted to the ward. While before the HAP implementation, mainly thyroidectomies and mastectomies were carried out, breast cancer surgeries predominated after the implementation. Patients after mastectomy often required antibiotic prophylaxis against infections, notably with ciprofloxacin, or antibiotic treatment for nosocomial, mainly surgical site, and infections. According to HAP, ciprofloxacin was prescribed for 5–7 days after surgery, especially for patients who had expanders. Increased antibiotic use could also be linked to a longer hospital stay in the time after HAP implementation in subsequent years (Table 1). The results observed were not influenced by the more frequent use of cephazoline as a preoperative antibiotic, since this antibiotic was given in the surgical theater room, not in the ward.
Antibiotic consumption in our ward was lower than that in similar oncological hospitals in France (39 DDD/100 person-days) (Dumarin et al. 2010), which can be explained by the predominance of planned admissions in our ward. Antibiotic consumption is greater in wards having more than 25% of ‘acute admission’ beds (Miliani et al. 2008). Likewise, in an Iranian study of Ayazi et al. (2015) antibiotic consumption has been greater than that in the present study; it amounted to 5–7 DDD/100 person-days and showed an upward trend in the follow-up period. In line with the present findings, HAP implementation did not result in a lower antibiotic consumption pattern in a Chinese study of Bao et al. (2015). Previous Polish studies related to other than surgical profiles have also provided data indicating a growing trend in the total DDD use of antibiotics after HAP implementation (Nitsch-Osuch et al. 2015a, b).
In the present study, HAP implementation had a significant effect on the scope of antibiotics used: consumption of penicillin with inhibitors, mainly amoxicillin clavulanate, of cephalosporins, mainly cefuroxime, and of restricted antibiotics, mainly carbapenems and glycopeptides, decreased, whereas the use of ciprofloxacin and aminoglycosides increased. All these changes were statistically significant and in our opinion they were in a positive direction in terms of antibiotic consumption patterns. Firstly, a reduction in the use of carbapenems may be viewed as a favorable result of HAP. Carbapenems are wide spectrum antibiotics often called ‘last chance treatment’. Zilberberg et al. (2017) have shown that the infection with carbapenemase resistant Enterobacteriaceae is associated with a fourfold increased risk of receiving previous inappropriate empiric treatment, which, in turn, increases mortality, length of hospitalization, and costs. Increased consumption of carbapenems is associated with increased rate of carbapenem-resistant bacteria, including Acinetobacter baumannii, as observed by Mascarello et al. (2017). Secondly, a lower use of betalactam antibiotics, including cephalosporins of the second and third generations and penicillin with inhibitors could be viewed as a favorable result as well. The excessive use of third generation cephalosporins is a risk factor for the occurrence and spread of extended-spectrum beta-lactamases (ESBL)-producing bacterial strains. Effective control of their use is associated with a successful restriction in the spread of resistant bacteria (Kim et al. 2008; Urbánek et al. 2007) and of Clostridium difficile infections (Sullivan 2014).
Consumption of ciprofloxacin in the ward was higher as compared to hospitals of a similar profile in Great Britain. Schelenz et al. (2015) have shown that ciprofloxacin consumption is 14.9/100 hospitalizations, whereas in our ward it was 65.6 DDD/100 hospitalizations. Ciprofloxacin is a widely used antibiotic in hematology and oncology wards, but its overuse is conducive to antibiotic resistance among Gram-negative bacteria. Hence, it is advisable to rotate its use (Schelenz et al. 2015). In the light of these results, it appears appropriate to conclude that in our ward, ciprofloxacin may be an overused antibiotic, and this hypothesis is backed by the literature data. Hammuda et al. (2013) have shown that fluoroquinolones constitute 9% of the antibiotics consumed in a ward of similar profile, which is significantly less than the 65.5% noted in 2015 in the present study. A wide use of ciprofloxacin in our ward may be explained by the procedure of using this antibiotic in all patients who underwent breast prosthesis or were being prepared for breast prosthesis with skin expanders. The loss of prosthesis due to hospital acquired infection is one of the most dramatic and serious complications of the breast reconstruction surgery. The efficacy of ciprofloxacin for infection treatment after breast augmentation is well known. It has been shown that 40% of bacteria isolated in patients with a surgical site infection is resistant to commonly used antibiotics, but 86% of strains are sensitive to gentamicin, and 63% strains were sensitive to ciprofloxacin (Weichman et al. 2013).
4.2 Effects of Implementation of the Hospital Antibiotic Policy on the DU90% and DU100% Indicators
A positive result of HAP implementation in our ward also was the increased DU90% index, from 2 antibiotics in 2011 to 3–5 antibiotics in the years 2013–2016. Changes in the profile of patients admitted to the ward afore-mentioned and the creation and implementation of recommendations regarding infection treatment could lead to a growth of this index. The use of only a few antibiotics in the ward may suggest a suboptimal compliance to the antibiotic therapy recommendations, which may result in antibiotic resistance build-up (Birger et al. 2015; Roca et al. 2015). In this context, the reduction in the DU90% index to three in 2015 should be considered alarming, as it implies the use of too narrow a range of antibiotics in the ward.
4.3 Study Strength and Weakness
The strength of our study is that we obtained results that can be used to compare the antibiotic consumption patterns in wards of similar treatment profile in Poland as well as in other countries. We show that HAP implementation provided favorable changes in the pattern of antibiotic consumption, even when a total number of antibiotic doses did not decrease.
One of the main limitations of the study is the methodology used to determine the antibiotic consumption, i.e., the calculation based on defined daily doses (DDD). This method is recommended by the WHO, but it is not devoid of drawbacks as illustrated in Table 8.
A DDD scheme can be used not only to compare drug consumption in wards, hospitals, regions, or countries, but also across different time periods, as we did in the present study. A calculation of DDD/100 or 1000 patient-days enables the exclusion of differences stemming from the different ward bed count and occupancy. Thus, DDD-assessed antibiotic consumption is a reliable tool to compare drug consumption between similar wards (Sabaté et al. 2015; Muller et al. 2006). However, the value of DDD/100 hospitalizations or DDD/100 patient-days is influenced by many factors, e.g., the referral level of the hospital, the incidence of infectious diseases in the studied period, the epidemic foci in the hospital, the prescription habits in the ward, the promotion of certain drugs, and even the season of year. Thus, assessment should be treated with caution, taking note of sudden and large increases in DDD/100 patients-days and of therapy costs of a single person-day, which we considered in the present study. Moreover, our assessment concerned the consumed DDD and not the hospital pharmacy data that do not take into account the number of expired and damaged drugs or the surplus of drugs in the ward. We conclude that there is an obvious need for the continuous evaluation and monitoring of the hospital antibiotic policy.
References
Ayazi K, Khabaz A, Ayazi L, Ghorbani B, Eslami M, Ebrahimi M (2015) Changes in antibiotic use in a general surgery unit over a 5-year period. East Mediterr Health J 21:134–139
Bao L, Peng R, Wang Y, Ma R, Ren X, Meng W (2015) Significant reduction of antibiotic consumption and patients’ costs after an action plan in China, 2010–2014. PLoS One 13(10):e0118868
Birger RB, Kouyos RD, Cohen T, Griffiths EC, Huijben S, Mina MJ, Volkova V, Grenfell B, Metcalf CJE (2015) The potential impact of coinfection on antimicrobial chemotherapy and drug resistance. Trends Microbiol 23(9):537–544
Davey P, Brown E, Fenelon L, Finch R, Gould I, Hartman G, Holmes A, Ramsay C, Taylor E, Wilcox M, Wiffen P (2005) Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Datebase Syst Rev 4:CD003543
Davies J, Davies D (2010) Origins and evaluation of antibiotic resistance. Microbiol Mol Biol Rev 74:417–433
Dik JW, Vemer P, Friedrich A, Hendrix R, Lo-Ten Foe JR, Sinha B, Postma MJ (2015) Financial evaluations of antibiotic stewardship programs – a systematic review. Front Microbiol 6:546–555
Dumarin C, L’Heriteau F, Pefau M (2010) Antibiotic use in 530 French hospitals: results from a surveillance network at hospital and ward levels in 2007. J Antimicrob Chemother 65:2028–2036
Hammuda A, Hayder S, Elazzazy S, Black E (2013) Point prevalence survey of antimicrobial utilization in oncology patients. J Infect Dev Ctries 7:990–993
Kim JY, Sohn JW, Park DW, Yoon YK, Kim YM, Kim MJ (2008) Control of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae using a computer-assisted management program to restrict third-generation cephalosporin use. J Antimicrob Chemother 62:416–421
Mascarello M, Simonetti O, Knezevich A, Carniel LI, Monticelli J, Busetti M, Schincariol P, Torelli L, Luzzati R (2017) Correlation between antibiotic consumption and resistance of bloodstream bacteria in a University Hospital in North Eastern Italy, 2008–2014. Infection 45(4):459–467
Miliani K, L’Hériteau F, Alfandari S, Arnaud I, Costa Y, Delière E (2008) Antimicrobial surveillance network. Specific control measures for antibiotic prescription are related to lower consumption in hospitals: results from a French multicentre pilot study. J Antimicrob Chemother 62:823–829
Muller A, Monnet DL, Talon D, Hénon T, Bertrand X (2006) Discrepancies between prescribed daily doses and WHO defined daily doses of antibacterials at a university hospital. Br J Clin Pharmacol 61:585–591
Nitsch-Osuch A, Kurpas D, Kuchar E, Zycińska K, Zielonka T, Wardyn K (2015a) Antibiotic consumption pattern in the neonatal special care unit before and after implementation of the hospital’s antibiotic policy. Adv Exp Med Biol 835:45–51
Nitsch-Osuch A, Kuchar E, Życińska K, Gyrczuk E, Miśkiewicz K, Korzeniewski K (2015b) Implementation of hospital’s antibiotic policy decreases antimicrobial use in the general pediatric ward. Adv Exp Med Biol 857:67–74
Roca I, Akova M, Baquero F, Carlet J, Cavaleri M, Coenen S (2015) The global threat of antimicrobial resistance: science for intervention. New Microbes New Infect 6:22–29
Sabaté M, Ferrer P, Ballarín E, Rottenkolber M, Amelio J, Schmiedl S (2015) Drug utilization in Europe: nationwide data sources and a review of publications on a selected group of medicines (PROTECT project). Basic Clin Pharmacol Toxicol 116:201–211
Schelenz A, Nwaka D, Hunter P (2015) Longitudinal surveillance of bacteraemia in haematology and oncology patients at a UK cancer centre and the impact of ciprofloksacin use on antimicrobial resistance. J Antimicrob Chemother 12:1431–1438
Schwaber M, Carmelli Y (2008) Carbapenem-resistant Enterobacteriaceae: a potential threat. JAMA 300:2911–2913
Sullivan T (2014) Antibiotic overuse and Clostridium difficile: a teachable moment. JAMA Intern Med 174:1219–1220
Urbánek K, Kolár M, Lovecková Y, Strojil J, Santavá L (2007) Influence of third-generation cephalosporin utilization on the occurrence of ESBL-positive Klebsiella pneumoniae strains. J Clin Pharm Ther 32:403–408
Weichman KE, Levine SM, Wilson SC, Choi M, Karp NS (2013) Antibiotic selection for the treatment of infectious complications of implant-based breast reconstruction. Ann Plast Surg 71:140–143
Zilberberg MD, Nathanson BH, Sulham K, Fan W, Shorr AF (2017) Carbapenem resistance, inappropriate empiric treatment and outcomes among patients hospitalized with Enterobacteriaceae urinary tract infection, pneumonia and sepsis. BMC Infect Dis 17:279
Conflicts of Interest
The authors declare no conflict of interests in relation to this article.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Nitsch-Osuch, A., Okruciński, D., Dawgiałło, M., Gołębiak, I., Kuchar, E. (2017). Evaluation of the Implementation of Hospital Antibiotic Policy in Oncosurgery Ward: A Six-Year Experience. In: Pokorski, M. (eds) Clinical Investigation. Advances in Experimental Medicine and Biology(), vol 1047. Springer, Cham. https://doi.org/10.1007/5584_2017_122
Download citation
DOI: https://doi.org/10.1007/5584_2017_122
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-74079-9
Online ISBN: 978-3-319-74080-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)