Abstract
Purpose
The spread of multidrug-resistant bacteria is a worrisome problem worldwide. This study investigated the correlation between antibiotic consumption and antimicrobial resistance trends of the most important bacteria causing bacteremia at the University hospital of Trieste, Italy, from 2008 to 2014.
Methods
Antibiotic consumption (Defined Daily Dose—DDD—per 100 patient/days) and antibiotic resistance (percentage of antibiotic intermediate o resistant isolates) were analyzed independently with linear correlation by year. Potential correlations between antibiotic consumption and bacteria resistance rates were investigated through the Pearson’s correlation.
Results
The overall consumption of antibiotic grew from 80 to 97 DDD 100 patient/days (p = 0.005) during the study period. The increased consumption of amoxicillin/clavulanate and piperacillin/tazobactam was associated with the reduction of MRSA rate from 48.5 to 25.9% (p = 0.007 and p = 0.04, respectively). The increased consumption of piperacillin/tazobactam was associated with the reduction of ESBL-positive Enterobacteriaceae rate from 28.9 to 20.9% (p = 0.01). The increased consumption of carbapenems was associated with the increased rate of carbapenem-resistant Acinetobacter baumannii from 0 to 96.4% (p = 0.03). No carbapenem-resistant Enterobacteriaceae isolates were reported. The consumption of vancomycin grew significantly (p = 0.005). A dramatic spread of vancomycin-resistant Enterococcus faecium occurred in 2014. The consumption of fluoroquinolones and extended-spectrum cephalosporins remained stable.
Conclusions
An antibiotic stewardship program targeted to limit the consumption of extended-spectrum cephalosporins and fluoroquinolones in favor of amoxicillin/clavulanate and piperacillin/tazobactam correlates with a decreasing rate of MRSA and ESBL-positive Enterobacteriaceae. The analysis of correlations between antibiotic consumption and bacterial resistance rates is a useful tool to orient antimicrobial stewardship policies at local level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The misuse of antibiotics is considered the main trigger for the selection of antibiotic-resistant bacteria in the hospital setting. Several studies have demonstrated that previous antibiotic therapy is a strong risk factor for colonization and infection due to drug-resistant pathogens enhancing selective pressure on bacteria and selecting resistant strains [1].
Infections caused by resistant bacteria in hospital setting are a worrisome problem worldwide, leading to increased length of hospital stay, morbidity, mortality and healthcare costs [2, 3]. The spread of bacterial resistance in the hospital setting is determined by many different factors such as infection control practice, clonal spread of multidrug-resistant bacterial strains, inter-hospital transfer of resistance and community contribution [4]. This study aims to investigate the correlation between antibiotic consumption and antimicrobial resistance trends of most important bacteria causing bacteremia at the University hospital of Trieste, Italy, from 2008 to 2014.
Materials and methods
We conducted a retrospective analysis of correlation between antibiotic consumption and antimicrobial resistance of the bacteria most commonly causing bacteremia. The University hospital of Trieste is an acute, tertiary care, 840-bed hospital, in north eastern Italy, admitting almost 24,000 adult patients each year. This hospital includes medicine, surgical, hematology, oncology and geriatric departments, two dialysis units, four intensive care units and no pediatric, gynecology, obstetrics or transplant units. Since 2006 the hospital was under accreditation by the Joint Commission International (JCI) and implemented the practices of infection control according with JCI. In 2008, 2011 and 2014, the hospital received the JCI accreditations. Therefore, we chose 2008 as a starting point of our study to minimize the potential bias of infection control practices in the evaluation of the correlations between antibiotic consumption and resistance. Data on antibiotic consumption prospectively collected were obtained from the databases of the hospital pharmacy. Antibiotic consumption was defined as the number of Defined Daily Dose (DDD) and was normalized per 100 patient-days. DDD is the assumed average maintenance dose per day for a drug used for its main indication in adults, according to the WHO ATC/DDD classification [5]. Data on consumption of parenteral penicillins J01C (oxacillin, ampicillin, amoxicillin, amoxicillin/clavulanate) and anti pseudomonal penicillin/β-lactamase inhibitor J01CR05 (piperacillin/tazobactam), extended-spectrum cephalosporins J01D (ceftriaxone, cefotaxime, ceftazidime and cefepime), carbapenems J01DH (imipenem, meropenem and ertapenem), aminoglycosides J01G (gentamicin and amikacin), fluoroquinolones J01 M (ciprofloxacin and levofloxacin) and glycopeptides J01XA (vancomycin and teicoplanin) were analyzed. We defined bacteremia as the presence of at least one positive blood culture for a bacterial organism. We considered all consecutive bloodstream isolates of Staphylococcus aureus, Enterococcus faecalis, Enterococcus faecium, Escherichia coli, Klebsiella spp., Proteus spp., Pseudomonas aeruginosa and Acinetobacter baumannii. We decided to exclude coagulase-negative staphylococci isolates considering their potential role as blood culture contaminants. Data on bloodstream isolates were prospectively collected and provided by the Clinical Microbiology Laboratory of the hospital. Duplicate isolates of the same patient were excluded. Only bloodstream isolates of patients admitted to the hospital were included, outpatients isolates were excluded. Blood cultures were performed by BactAlert system (bioMérieux, France). Antibiotic susceptibility tests were performed by an automated system (VITEK® 2, bioMérieux, France); MIC of multidrug-resistant bacteria was confirmed by a microdiluition method (Sensitre panels tests TREK Part of Thermo Fisher Scientific, Oakwood Village, USA). Data on antimicrobial susceptibility were extracted by a dedicated software (VIGI@ct™, bioMérieux, France); cumulative antibiogram reports were performed on an annual base. Susceptibility data were interpreted using Clinical and Laboratory Standards Institute (CLSI) breakpoints, data after July 2012 using the European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoints [6]. Isolates with intermediate susceptibility were considered resistant. A. baumannii was considered multidrug resistant (MDR) when resistant to at least 1 agent in 3 or more of the following antimicrobial categories: aminoglycosides, carbapenems, fluoroquinolones, extended-spectrum cephalosporins, antipseudomonal penicillin/β-lactamase inhibitor, folate pathway inhibitors, tigecycline, penicillins/β-lactamase inhibitors, polymyxins and tetracycline [7]. The incidence density of isolates was calculated as the number of isolates per 10,000 patient-days. Antibiotic-resistant rates were calculated as the number of non-susceptible isolates (resistant or intermediate isolates) divided by the total number of isolates of the same species tested against the corresponding antibiotic, multiplied by 100. Trends of bacterial resistance rates and antibiotic consumption were analyzed independently with linear correlation by year. Pearson’s correlation coefficient was used to describe the relationship between antibiotic consumption and bacterial resistance rates. We used R Software (Free Software Foundation’s GNU General Public License), specifically R commander package (R, version 3.2.2). A p value ≤0.05 was considered statistically significant. This study was exempt for approval by our ethic committee because of the anonymous storage of data.
Results
Trends of antibiotic consumption
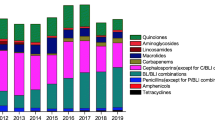
During the study period, the total consumption of antibiotics grew significantly from 80 DDD/100 patient-days in 2008 to 97 DDD/100 patient-days in 2014 (p = 0.005) (Table 1). In 2014, penicillins (oxacillin, ampicillin, amoxicillin and amoxicillin/clavulanate) were the main antibiotics consumed followed by fluoroquinolones, extended-spectrum cephalosporins, piperacillin/tazobactam and others. Considering the single antibiotic categories, a significant increase in consumption was found for amoxicillin/clavulanate (p = 0.0005), piperacillin/tazobactam (p = 0.02), carbapenems (p = 0.007) especially meropenem (p = 0.00004) and vancomycin (p = 0.005). Conversely, a significant reduction of consumption was observed for gentamicin (p = 0.02). The consumption of total extended-spectrum cephalosporins and anti-P.aeruginosa cephalosporins remained stable. The consumption of fluoroquinolones decreased substantially although not significantly.
Trends of blood culture isolates
We analyzed a total of 3389 consecutive bloodstream isolates (Table 2). The most frequently isolated microorganisms were: E. coli (43.4%), S. aureus (19.6%) and Enterococcus spp. (14.6%). The incidence density (n. isolates/10,000 patients days) during the study period increased for E. faecalis (from 1.61 in 2008 to 2.39 in 2014, p = 0.05), E. coli (from 6.81 in 2008 to 9.4 in 2014, p = 0.03) and A. baumannii (from 0.11 in 2008 to 1.07 in 2014, p = 0.02), while S. aureus showed a decreasing trend, although not significant (from 3.88 in 2008 to 3.63 in 2014, p = 0.06).
Trends of antibiotic resistance
Trends of antibiotic resistance are summarized in Table 3. The rate of methicillin-resistant S. aureus (MRSA) significantly decreased during the study period, from 48.5% in 2008 to 25.9% in 2014 (p = 0.02). A reduction rate of S. aureus resistant to levofloxacin (p = 0.03) and gentamicin (p = 0.03) was also observed. Similarly, rates of extended-spectrum beta-lactamase (ESBL)-positive Klebsiella spp. significantly decreased from 21.7% in 2008 to 1.7% in 2014 (p = 0.04). The rate of ESBL-positive E. coli decreased since 2010 but the trend during the overall study period was not significant (p = 0.1). Rates of ESBL-positive Proteus spp remained stable. When considering all the ESBL-positive Enterobacteriaceae isolated, we found a significant reduction rate (p = 0.04). No significant variations were observed in the resistance rates to ciprofloxacin, piperacillin/tazobactam and carbapenems in E. coli, Klebsiella spp., Proteus spp. and P. aeruginosa. During the first three years of the study period, only few cases of bacteremia caused by A. baumannii occurred in our hospital. Since 2011, the absolute number of bacteremia caused by A. baumannii increased owing to an outbreak of MDR A.baumannii. Moreover, we observed a significant increase of piperacillin/tazobactam and carbapenem resistance rates in A. baumannii (p = 0.03 and p = 0.01, respectively).
More than two-thirds of A.baumannii strains isolated in 2008, 2009, 2012 and 2014 were MDR strains; all the A. baumannii isolated in 2010, 2011 and 2013 were MDR. Resistance rates of E. faecalis and E. faecium remained stable during the study period; nevertheless, we observed an important increase in the percentage of vancomycin-resistant E. faecium (VREm) in 2014 (from 0% in 2008 to 10.4% in 2014; p value not applicable).
Correlations between antibiotic consumption and resistance rates
The main correlations between antibiotic consumption ad resistance rates are summarized in Table 4. We observed a significative correlation between the increasing consumption of total penicillins, amoxicillin/clavulanate and piperacillin/tazobactam and the reduction rate of oxacillin resistance in S. aureus (p = 0.002, p = 0.007 and p = 0.04, respectively). A significative correlation was also found between the increased consumption of piperacillin/tazobactam and the reduction of ESBL-positive Enterobacteriaceae rates (p = 0.01) and ESBL-positive E. coli (p = 0.02). On the other hand, we did not find a significant increase in the resistance rate to piperacilin/tazobactam of E. coli, Proteus spp., Klebsiella spp. and P.aeruginosa. Moreover, we did not find significant association between the increased use of piperacillin/tazobactam and the spread of MDR A. baumannii. The decreasing use of gentamicin was associated with reduced resistance of E. coli and Klebsiella spp. to gentamicin (p = 0.03 and p = 0.02, respectively).
The increasing consumption of carbapenems, observed during the study period, was significantly associated with an increasing rate of carbapenem-resistant A. baumannii (CRAB) (p = 0.03) and MDR A.baumannii (p = 0.05), while we did not observe an increase of carbapenem-resistant P. aeruginosa. No cases of carbapenem-resistant E. coli, Proteus spp. or Klebsiella spp. were isolated during the study period.
Discussion
The present study shows a substantial increase of antibiotics consumption during a 7-year period in a referral Italian hospital. Unfortunately, this finding is known to be commonly reported in a number of hospitals of many countries [8]. In detail, we observed a significant increase in the consumption of penicillins, amoxicillin/clavulanate and piperacillin/tazobactam. The increasing consumption of these antibiotics results from a widespread adherence to the local guidelines of antibiotic therapy of major infectious syndromes, implemented by our hospital infection control committee since 2006. Unexpectedly we observed a worrying increasing consumption of carbapenems despite the decreasing rate of ESBL-positive Enterobacteriaceae. Similarly, an increased consumption of vancomycin was observed despite the decreasing resistance to oxacillin of S. aureus. It is important to notice that the consumption of other antibiotics classes with high potential selection pressure for resistance as extended-spectrum cephalosporins remained stable and fluoroquinolones decreased, although not significantly. During the study period, we observed a significant reduction of bacteremia caused by MRSA. This finding is consistent with the decreasing trend observed in Italy and reported by ECDC [9]. This phenomenon has been mainly attributed to improvement of infection control practices rather than antibiotic consumption. Contrary to what was expected, we found a negative correlation between the MRSA rate and the increased use of amoxicillin/clavulanate and piperacillin/tazobactam. In previous studies, the consumption of these antibiotics has been positively correlated with increasing MRSA prevalence and it has been identified as an independent risk factor for acquisition of methicillin resistance [10–12]. In vitro tests showed that amoxicillin/clavulanate, at concentrations achievable in human serum, has significant activity against some strains of MRSA [13]. It could be argued that the combination of aminopenicillin-β-lactamase inhibitor may play a protective role against MRSA selection; nevertheless, there is no evidence for significant clinical activity of amoxicillin/clavulanate against MRSA. It is well known that amoxicillin/clavulanate is not recommended in the treatment of MRSA infections. We believe that further research is needed to clarify this potentially favorable association. Fluoroquinolones and, in particular, ciprofloxacin are known to have an important impact in selecting nosocomial MRSA [11, 14]. In our setting, the decreasing consumption of fluoroquinolones could have played a role in the decreasing rate of MRSA. The decreasing use of gentamicin during the study period significantly correlated with reduced resistance of E. coli and Klebsiella spp. to gentamicin. This observation confirms the important role of aminoglycosides in selecting aminoglycosides-resistant strains [15, 16].
In this study, we observed a steady decline of ESBL-positive Enterobacteriaceae in particular among Klebsiella spp. isolates. We found that the reduction rate of ESBL-positive Enterobacteriaceae was significantly correlated with the increased consumption of piperacillin/tazobactam. Moreover, unlike what reported by a previous study [16], we found that the increasing use of piperacillin/tazobactam did not correlate with increased resistance to piperacillin/tazobactam among Enterobacteriaceae. As previously suggested by other authors, our results confirm that the use of piperacillin/tazobactam, instead of cephalosporins, has a potential ecological benefit and is associated with a reduction of ESBL-producing Enterobacteriaceae rates without clear increase in piperacillin/tazobactam resistance [17]. The replacement of extended-spectrum cephalosporins by piperacillin/tazobactam has been suggested as a strategy for reducing prevalence of ESBL-producing Gram-negative bacteria in healthcare settings [18]. Piperacillin/tazobactam has been recently recommended as an alternative to carbapenems for the treatment of infections caused by ESBL-producing Enterobacteriaceae, if in vitro susceptibility is documented [19]. Although A. baumannii caused only a relatively small number of bacteremia, in this study the increasing use of carbapenems was significantly associated with increasing rates of CRAB and MDR A.baumannii. Previous studies demonstrated that the prior use of carbapenems is an independent risk factor for CRAB infection and/or colonization at individual level and that the increased consumption of carbapenems correlated with the increase of CRAB isolates [20, 21]. Selective pressure from antibiotic exposure has a key role in the onset of CRAB; on the other hand, the lack of adherence to the infection control practices is the leading factor in the spread of this organism among patients. In hospital setting, A. baumannii is frequently responsible for outbreaks commonly caused by a limited number of successful clones. Indeed, during the study period, an epidemic dissemination of a CRAB clone carrying armA occurred in our hospital [23]. We did not find significant association between the increased use of piperacillin/tazobactam and the spread of MDR A. baumannii, as previously reported by others [23].
Despite the significant increase in the carbapenems consumption observed during the study period, we did not observe an increase of carbapenem-resistant P. aeruginosa. No cases of carbapenem-resistant E. coli, Proteus spp. or Klebsiella spp. were isolated during the study period. Interestingly, these findings are not consistent with other studies that found a correlation between increasing carbapenem use and increasing resistance in Enterobacteriacea and P. aeruginosa [24, 25]. A steady increase in consumption of vancomycin was observed in our hospital. In the same period, we observed a steady decline in the MRSA incidence rate. This observation differs from previous studies that showed a positive correlation between prevalence of MRSA and use of glycopeptides [11]. An increasing consumption of MRSA-active drugs, without increasing MRSA, has been reported in studies conducted in acute care hospitals in Catalonia and in 55 ICUs in Germany [26, 27]. In the study conducted in ICUs in Germany, the authors observed an increasing consumption of “new MRSA-active antibiotics” such as linezolid and daptomycin and an increasing consumption of vancomycin an “old MRSA-active antibiotic” similarly to what observed in our settings [27]. The discrepancy between the burden of MRSA and the consumption of vancomycin may be indicative of an overestimation of MRSA as the causative agent of infections treated empirically in our hospital. We can argue that a considerable share of vancomycin may have been inappropriately prescribed in our hospital.
In previous studies, the consumption of glycopeptides was found to be associated with an increasing incidence of vancomycin-resistant enterococci [28, 29]. Despite the constant increase in the vancomycin consumption in our hospital, Enterococcus spp. isolates remained susceptible to vancomycin until 2014 when we observed a rapid spread of a VREm clone in different hospital wards. Further research is needed to clarify this potential association in our setting.
This study has some limitations. The design of the study is retrospective and potential confounders such as changes in the length of hospital stay, staffing level and hand hygiene compliance could not be evaluated although the JCI accreditation of our hospital, during the study period, might have reduced some of these discrepancies. Secondly, we have considered all consecutive blood cultures including those isolated within 48 h after hospital admission; as a consequence, community-acquired isolates have not been excluded. Thirdly, we measured antibiotic use by DDD/100 patient-days; this is a useful measure unit for inter-hospital comparison but it may not reflect the real antibiotic use not taking into account factors such as proper dosage in specific groups of patients (i.e., patients with renal insufficiency). However, the impact should be limited in this study because those specific patients groups are only a small fraction of the study patients and the effect would be diluted equally in each year. A further limitation is that we used yearly aggregation of time points to find a relationship between antibiotic consumption and bacteria resistance rate. This timing may not be sensitive enough to reflect subtle changes in the complex interaction between bacterial resistance and antibiotic consumption. Notwithstanding these limitations, we believe that the methodology of this study is sound since data on bacterial resistance and antibiotic consumption were prospectively collected by the microbiological laboratory and the pharmacy, respectively.
The spread of drug-resistant bacteria in an hospital setting is a multifaceted, complex phenomenon, not driven by antibiotic consumption alone but also influenced by clonal spread of strains, resistance mechanism that might differ by species and adherence to infection control practices. Nevertheless, the analysis of correlation between antibiotic use and bacterial resistance has shown that an antibiotic stewardship program targeted to limit the consumption of extended-spectrum cephalosporins and fluoroquinolones in favor of amoxicillin/clavulanate and piperacillin/tazobactam correlates with decreasing rates of MRSA and ESBL-positive Enterobacteriaceae bacteremia. It is well known that the adherence to infection control practices is crucial to limit the spread of MRSA, A. baumannii and VRE in the hospital setting. Nevertheless, we believe that the unjustified increase of carbapenem and vancomycin consumptions, occurred in our hospital, may also have played a role in the increasing rates of MDR A. baumannii and VREm, respectively. Surveillance of the burden and trends of bacterial resistance and monitoring hospital consumption of antibacterial agents constitute essential parts of antimicrobial stewardship programs [30]. We believe that the knowledge of correlations between antibiotic consumption and bacterial resistance rates is a useful tool to orient antimicrobial stewardship policies and antibiotic prescription guidelines at local level.
Part of this study has been presented at the 2016 ECCMID (posters section).
References
Tacconelli E, De Angelis G, Cataldo MA, et al. Antibiotic usage and risk of colonization and infection with antibiotic-resistant bacteria: a hospital population-based study. Antimicrob Agents Chemother. 2009;53:4264–9.
Macedo-Viñas M, De Angelis G, Rohner P, et al. Burden of meticillin-resistant Staphylococcus aureus infections at a Swiss University hospital: excess length of stay and costs. J Hosp Infect. 2013;84:132–7.
Cosgrove SE, Carmeli Y. The impact of antimicrobial resistance on health care and economic outcomes. Clin Infect Dis. 2003;36:1433–7.
Sedláková MH, Urbánek K, Vojtová V, et al. Antibiotic consumption and its influence on the resistance in Enterobacteriaceae. BMC Res Notes. 2014;7:454.
WHO Collaborating Centre for Drug Statistics Methodology. Guidelines for ATC classification and DDD assignment 2014. Oslo; 2014. http://www.sifac.it/sites/default/files/dice1838.pdf. Accessed 07 July 2015.
The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 5.0; 2015. http://www.eucast.org/clinical_breakpoints/. Accessed 08 July 2015.
Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbial Infect. 2012;18:268–81.
Bitterman R, Hussein K, Leibovici L, et al. Systematic review of antibiotic consumption in acute care hospitals. Clin Microbiol Infect. 2016;22:561.e7–562.e19.
European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe 2014. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). Stockholm: ECDC; 2015. http://ecdc.europa.eu/en/publications/publications/antimicrobial-resistance-europe-2014.pdf. Accessed 07 July 2015.
Muller AA, Mauny F, Bertin M, et al. Relationship between spread of methicillin-resistant Staphylococcus aureus and antimicrobial use in French University hospital. Clin Infect Dis. 2003;36:971–8.
Tacconelli E, De Angelis G, Cataldo MA, et al. Does antibiotic exposure increase the risk of methicillin-resistant Staphylococcus aureus (MRSA) isolation? A systematic review and meta-analysis. J Antimicrob Chemother. 2008;61:26–38.
Aldeyab MA, Monnet DL, López-Lozano JM, et al. Modelling the impact of antibiotic use and infection control practices on the incidence of hospital-acquired methicillin-resistant Staphylococcus aureus: a time-series analysis. J Antimicrob Chemother. 2008;62:593–600.
Prieto J, Aguilar L, Giménez MJ, et al. In vitro activities of co-amoxiclav at concentrations achieved in human serum against the resistant subpopulation of heteroresistant Staphylococcus aureus: a controlled study with vancomycin. Antimicrob Agents Chemother. 1998;42:1574–7.
Weber SG, Gold HS, Hooper DC, et al. Fluoroquinolones and the risk for methicillin-resistant Staphylococcus aureus in hospitalized patients. Emerg Infect Dis. 2003;9:1415–22.
Joseph NM, Bhanupriya B, Shewade DG, Harish BN. Relationship between antimicrobial consumption and the incidence of antimicrobial resistance in Escherichia coli and Klebsiella pneumoniae isolates. J Clin Diagn Res. 2015;9:DC08–12.
Lai CC, Wang CY, Chu CC, et al. Correlation between antibiotic consumption and resistance of Gram-negative bacteria causing healthcare-associated infections at a university hospital in Taiwan from 2000 to 2009. J Antimicrob Chemother. 2011;66:1374–82.
Livermore DM, Hope R, Reynolds R, et al. Declining cephalosporin and fluoroquinolone non susceptibility among bloodstream Enterobacteriaceae from the UK: links to prescribing change? J Antimicrob Chemother. 2013;68:2667–74.
Bantar C, Vesco E, Heft C, et al. Replacement of broad-spectrum cephalosporins by piperacillin/tazobactam: impact on sustained high rates of bacterial resistance. Antimicrob Agents Chemother. 2004;48:392–5.
Gutiérrez-Gutiérrez B, Pérez-Galera S, Salamanca E, et al. A multinational, preregistered cohort study of β-Lactam/β-Lactamase inhibitor combinations for treatment of bloodstream infections due to extended-spectrum-β-Lactamase-producing enterobacteriace. Antimicrob Agents Chemother. 2016;60:4159–69.
Lee SO, Kim NJ, Choi SH, et al. Risk factors for acquisition of imipenem-resistant Acinetobacter baumannii a case control study. Antimicrob Agents Chemother. 2004;48:224–8.
Su CH, Wang JT, Hsiung CA, et al. Increase of carbapenem- resistant Acinetobacter baumannii infection in acute care hospitals in Taiwan: association with hospital antimicrobial usage. PLoS One. 2012;7:e37788.
Milan A, Furlanis L, Cian F, et al. Epidemic dissemination of a carbapenem-resistant Acinetobacter baumannii clone carrying armA two years after its first isolation in an Italian hospital. Microb Drug Resist. 2016;22:668–74.
Chen IL, Lee CH, Su LH, et al. Antibiotic consumption and healthcare—associated infections caused by multidrug-resistant gram negative bacilli at a large medical center in Taiwan from 2002 to 2009: implicating the importance of antibiotic stewardship. PLoS One. 2013;8:e65621.
McLaughlin M, Advincula MR, Malczynski M, et al. Correlations of antibiotic use and carbapenem resistance in enterobacteriaceae. Antimicrob Agents Chemother. 2013;57:5131–3.
Lepper PM, Grusa E, Reichl H, et al. Consumption of imipenem correlates with β-lactam resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2002;46:2920–5.
Grau S, Fondevilla E, Freixas E, et al. Relationship between consumption of MRSA-active antibiotics and burden of MRSA in acute care hospitals in Catalonia. Spain J Antimicrob Chemother. 2015;70:1193–7.
Meyer E, Schwab F, Schroeren-Boersch B, et al. Increasing consumption of MRSA-active drugs without increasing MRSA in German ICUs. Intensive Care Med. 2011;37:1628–32.
Forstner C, Diab-Elschahawi M, Kivaranovic D, et al. Non-linear significant relationship between use of glycopeptides and isolation of vancomycin-resistant Enterococcus species in a university hospital setting. Antimicrob Resist Infect Control. 2015;4:25.
Kolar M, Urbanek K, Vagnerova I, et al. The influence of antibiotic use on the occurrence of vancomycin-resistant enterococci. J Clin Pharm Ther. 2006;31:67–72.
Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62:e51–77.
Acknowledgements
The authors thank the members of the hospital Infection Control Committee of the University Hospital of Trieste, Italy, for their cooperation in the implementation of local guidelines of antibiotic therapy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
This study was exempt for approval by our ethic committee because of the anonymous storage of data.
Funding
This study was not sponsored by external sources, no funding was received.
Rights and permissions
About this article
Cite this article
Mascarello, M., Simonetti, O., Knezevich, A. et al. Correlation between antibiotic consumption and resistance of bloodstream bacteria in a University Hospital in North Eastern Italy, 2008–2014. Infection 45, 459–467 (2017). https://doi.org/10.1007/s15010-017-0998-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-017-0998-z