Abstract
Cardiovascular computed tomography (CT) has undergone significant technical developments over the past decade. The introduction of multi-detector row computed tomography (MDCT) with wider detector coverage, faster gantry rotation speed, multiple X-ray sources, electrocardiographic (ECG)-based tube current modulation, and integration of new iterative reconstruction algorithms has allowed for tangible improvements in diagnostic accuracy. Utilizing these technical advancements, recent attempts have been made to develop CT myocardial perfusion (CTP) imaging strategies. Moreover, the evaluation of myocardial perfusion defects on routine coronary CT angiography (cCTA) has been shown to be of additional value above that of assessing coronary anatomy alone, particularly in the acute chest pain setting. Unfortunately, there are many limitations that currently hinder CT perfusion with single-energy CT imaging, including artifacts. This chapter provides an overview of the role of single-source dual-energy CT in the evaluation of the myocardial perfusion and the current state of Rapid-kVp switching dual-energy CT.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Myocardial Perfusion
- Coronary Compute Tomography Angiography
- Fractional Flow Reserve
- Invasive Coronary Angiography
- Obstructive Coronary Artery Disease
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Coronary CT angiography (cCTA) is an established noninvasive modality that has shown a high diagnostic accuracy for the detection or exclusion of coronary artery disease (CAD) (Meijboom et al. 2008b). Although highly accurate in estimating the degree of luminal stenosis, cCTA has a limited role in determining the hemodynamic significance of detected CAD. This limitation is not surprising, as invasive coronary angiography (ICA) shares similar limitations, largely to the same degree. This unreliable relationship between anatomical stenosis and both global and lesion specific ischemia has raised concerns that the use of cCTA may encourage unnecessary ICA and coronary revascularization. Prior multicenter randomized trials have delineated the clinical and economic effectiveness of an integrated anatomic-physiologic approach to CAD by invasive methods, as compared to an anatomic approach alone. Incremental to stenosis assessment by ICA, the addition of fractional flow reserve (FFR) for physiologic determination of lesion-specific ischemia improves identification of patients who benefit from coronary revascularization by restricting revascularization to individuals with severe stenosis that specifically cause ischemia. In the Fractional Flow Reserve versus Angiography for Multicenter Evaluation (FAME) trial of patients with anatomically obstructive CAD, a combined diagnostic approach of stenosis- and ischemia-guided revascularization by ICA and FFR, respectively, was superior to stenosis-guided revascularization alone for enhancing event-free survival (Harvey and Hecht 2009; Meijboom et al. 2008a; Schuijf et al. 2011).
In addition, the FFR-guided group had shorter length of hospitalization, thereby leading to lower healthcare costs and similar or improved post-procedural quality of life (Pijls et al. 2010). On the basis of this date the ability to combine both anatomical and functional evaluation of CAD utilizing a single noninvasive modality is highly desired in clinical practice. Such noninvasive testing offers the potential to improve the selection of patients with CAD who may benefit from invasive testing and revascularization (Shaw et al. 2008; Brian et al. 2011; Pijls et al. 2010; Tonino et al. 2010).
2 Clinical Background
Cardiovascular computed tomography (CT) has undergone tremendous technical developments over the past decade. The introduction of multi-detector row computed tomography (MDCT), wider detector coverage, faster gantry rotation speed, multiple X-ray sources, electrocardiographic (ECG)-based tube current modulation, and integration of new iterative reconstruction algorithms have allowed improvements in diagnostic accuracy and have enabled progressive radiation dose reduction (Hsiao et al. 2010; Entrikin et al. 2011; Beckmann 2006; Goldman 2008). Utilizing these technical advancements, recent attempts have been made to develop CT myocardial perfusion (CTP) imaging strategies. Several single center studies have shown that CTP imaging has good diagnostic accuracy as compared to single-photon emission computed tomography (SPECT), cardiac magnetic resonance (CMR), and FFR. (Gaemperli et al. 2008; Melikian et al. 2010).
Moreover, the evaluation of myocardial perfusion defects on routine coronary CTA has been shown to be of additional value above that of assessing coronary anatomy alone, particularly in the acute chest pain setting. (Tops et al. 2008; Weininger et al. 2010). While these early results are promising, significant improvements still need to be made to overcome associated technological limitations.
3 Limitations of CTP with Single-Energy CT
Myocardial hypoperfusion manifests as an area of hypoattenuation (i.e., myocardial perfusion defect) on intravenous (IV) contrast-enhanced CT studies. Different qualitative and quantitative strategies have been used to detect areas of myocardial perfusion defects during the first-pass diffusion of contrast material into the left ventricular myocardial segments (Brian et al. 2011). Unfortunately, there are many limitations that currently hinder CT perfusion with single-energy CT imaging, including artifacts, radiation exposure, and contrast load. The most commonly encountered artifact is that of beam-hardening (Kitagawa et al. 2010). Beam hardening is inherent in single-energy CT acquisitions that produce an X-ray beam composed of photons with a wide range of energy levels (i.e., polychromatic beam). As the X-ray beam is transmitted through a high-density object, preferential absorption of lower-energy photons occurs, creating a filtered beam containing high-energy photons. This can distort myocardial Hounsfield units (HU, i.e., attenuation units), with both artificial reduction and elevation of these values being possible, depending on the path of the X-ray beam. This artifactual distortion of myocardial attenuation can act to confound CT assessment of myocardial perfusion (Kitagawa et al. 2010; Zatz and Alvarez 1977).
The issue of beam-hardening is particularly problematic in CTP imaging, as the high iodine concentration in the aorta and cardiac chambers contributes a high burden of artifact. These artifacts are most commonly identified in the apical and inferobasal left ventricular myocardial segments, where HU measurements are typically reduced Fig. 1. In addition, paradoxically increased HU measurements in the ventricular septum are commonly encountered as a result of the same phenomenon having an opposite result. These artifacts not only reduce reader confidence, but can also result in erroneous iodine quantification in dynamic CTP imaging using dual-source single-energy technology (Rodríguez-Granillo et al. 2010a; So et al. 2009).
A 58-year-old female patient with no prior cardiac history. Single-energy CCTA reconstructed in a basal two-chambers short-axis, and b basal four-chambers transverse axial plane. The images demonstrate transmural hypo-attenuation in the inferobasal left ventricular myocardial segment (arrow) consistent with beam-hardening artifact in this region in the absence of significant coronary artery disease. Normal attenuation is identified in the remaining of the myocardium
In a recent study evaluating asymptomatic patients without history of or CT findings for obstructive CAD, beam-hardening related differences between the inferobasal myocardial segment and the reminder of the myocardium were identified in 72 % of patients, mimicking a perfusion defect (Rodríguez-Granillo et al. 2010a, b).
4 Technical Background of Dual-Energy technique in Myocardial perfusion
Dual-energy CT has been recently introduced into clinical practice offering the potential to help reduce these artifacts and their impact on image quality and diagnostic accuracy of CTP imaging. Currently, dual-energy CT has been commercially introduced with two separate mechanisms. One method implements a dual-source system, in which two X-ray tube-detector pairs are mounted onto the same rotating gantry at an angular offset of 90° (or 94° for the 2nd generation scanner), with one tube operating at a peak kilovoltage (kVp) of 80 or 100 kVp, and the other operating at 140 kVp (So et al. 2011; Karcaaltincaba and Aykut 2010).
A second approach has been more recently introduced, which generates single-source dual-energy X-rays based on ultrafast, submillisecond kVp switching (Ko et al. 2012; Silva et al. 2011). The fundamental basis of rapid kVp switching is the acquisition of two different tube voltages alternating on a view-by-view basis with a minimal delay between low (e.g., 80 kVp) and high (e.g., 140 kVp) projections during a single x-ray gantry rotation. This offers the potential for precise temporal registration of views, thereby minimizing the misalignment error from cardiac motion. This technique also relies on new detector and scintillator materials that have ultrafast decay times, enabling the reduction of image noise between projection views. The reduction in noise allows both high- and low-energy data sets to be acquired simultaneously for axial and helical acquisitions at the full 50 cm field of view (Chandra 2011; Wu et al. 2009).
5 Material Decomposition
In diagnostic imaging, X-ray photons interact with different tissues by two distinct mechanisms: the photoelectric effect and Compton scatter. The total attenuation of photons through a specific material is dependent on the contributions of these two effects. Depending on the tissue attenuation coefficient and the energy of the photons delivered, the relative contribution of these interactions to image generation will vary. Compton scatter predominates as the photon energy increases. On the other hand, the photoelectric interaction predominates when lower-energy photons interact with large atomic number materials such as iodine based IV contrast material. Thus, CT numbers (i.e., attenuation values, HU) will vary dramatically with high atomic number materials, whereas with soft tissues, blood and collagen, the CT numbers tend to be less variable and therefore display similar behavior at different photon energies. Recognizing different tissue atomic numbers, attenuation coefficients, and their behavior with variable photon energies, material differentiation can be achieved through spectral monochromatic photon energy (Johnson et al. 2007; Ko et al. 2012).
Owing to the tightly synchronized acquisition, dual-energy single-source/detector CT offers the potential of projection-based monochromatic imaging. To do this, the alternating 80 and 140 kVp raw data (i.e., projection views) are transformed into density (or amount) of the two selected basis materials: water and iodine (So et al. 2011), which can then be reconstructed into corresponding water and iodine images. These two materials are selected owing to their abundance in the typical imaged field of view and due to the significant differences in the way they interact with variable X-ray energy levels. From these material density images, projection-based monochromatic energy images can be generated from their linear combination (Matsumoto et al. 2011).
This process of converting the measured attenuations into density projections, reconstructing the density images, and further processing to obtain a monochromatic attenuation coefficient at any desired photon energy is known as “material decomposition”, which offers the potential for material quantification and characterization and may allow for dynamic quantified myocardial perfusion Fig. 2a, b (So et al. 2012).
Transverse axial images of a myocardial phantom consisting of two inner chambers filled with contrast representing the ventricular chambers and a contrast-filled cylinder for the aorta. a, b Beam-hardening artifact became evident within the myocardial area when the images of the phantom were obtained with 80 kVp single-energy CT and 140 kVp single-energy CT. c, e The same artifact was constant with image-based reconstructing mono energies at 60 and 70 keV. d, f However, the hypo-attenuation area became less evident at projection-based reconstructing mono energies of 60 and 70 keV, which show homogenous enhancement of the myocardial phantom
Importantly, these monochromatic images are reconstructed from raw projection data, which is unique to the single-source dual-energy platform. In addition, the projection-based technique applied in single-source dual-energy CT requires the 80 kVp and 140 kVp projection sets to be obtained from the same angle, which is only possible for single-source dual-energy systems (So et al. 2012).
Essentially, each monochromatic dataset is a representation of how the imaged object would appear if the X-ray source produced only monochromatic X-ray photons.
6 Iodine Mapping and Quantitative Measurement of Myocardial perfusion with Rapid kVp Switching Dual-Energy CT
Initial experiments in both phantom and animal models have shown great promise for the reduction of beam-hardening artifacts utilizing single-source rapid-kVp-switching dual-energy scanning. Additionally, there is the capacity for material density imaging from dual-energy data to quantify iodine as a surrogate for myocardial blood, facilitating quantification of myocardial perfusion. Ting Lee and colleagues recently used a myocardial phantom consisting of two inner chambers filled with contrast, representing the ventricular chambers, and a contrast-filled cylinder for the aorta to evaluate the efficacy of single-source dual-energy scanning to reduce beam-hardening artifacts Fig. 3 (So et al. 2011). Using the phantom, a region of simulated ischemic defect was placed in the basal lateral wall of the left ventricle. Both chambers and the aortic cylinder were placed in water-filled or diluted contrast-filled rings. The images were acquired with two single-energy techniques at 80 and 140 kVp using the dual-energy scan protocol with rapid kVp switching, allowing projection-based reconstruction monochromatic energy imaging.
Projection-based dual-energy CT showed significantly greater uniformity of the mean attenuation throughout the myocardium suggesting a significant reduction of beam-hardening. Subsequently, this same group performed similar experiments using a Gammex tissue characterization phantom then followed this experiment to determine optimal imaging parameters for iodine sensitivity detection or contrast-to-noise ratio. Rather than using a single iodine concentration, this phantom model consisted of multiple iodine-filled chambers of variable concentrations of iodine solutions. Using the same imaging techniques that were applied to the myocardial phantom, projection-based monochromatic energy imaging (i.e., 70 kilo electron volt ‘keV’) showed the highest sensitivity for iodine detection and also achieved the highest contrast-to-noise ratio compared to imaged-based single-energy or dual-energy techniques (So et al. 2011).
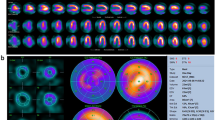
Building on this, additional studies have been performed with a porcine model, in which normal (non-ischemic) pigs were scanned and evaluated with rapid kVp switching dual-energy CT with the use of a Discovery CT750 HD scanner (GE Health care, Waukasha, WI). Two scans were performed, using 140 and 80 kVp alternating at 0.2-ms intervals, 630 mA, and 0.5-s gantry rotation period initiated at 3–4 s after IV contrast injection Fig. 4. The projection-based dual-energy 70 keV images and the corresponding 140 kVp single-energy imaging sets were then analyzed using CTP software. Myocardial average attenuation maps and corresponding aortic and myocardial time-density curve perfusion maps and values were derived and calculated across the maximal cross section of the left ventricular lateral, apical, and septal walls, as well as the aorta. Beam-hardening artifacts were greatly reduced, with the projection-based dual-energy 70 keV images resulting in uniform enhancement throughout the myocardium; this allowed more robust and accurate time-attenuation curve derivation and ultimately accurate quantification of myocardial perfusion defects.
A 62-year-old patient known to have hypertension, dyslipidemia, and diabetes mellitus came with atypical chest pain. Reconstructed short-axis views of the basal left ventricle, top row, and the corresponding images in transverse axial plane, bottom row a average map, b material decomposition of water, and c material decomposition of iodine equivalent density images. Reconstructed images obtained with d 60 keV, e 70 keV, and f 80 keV. The beam-hardening effect was significantly reduced in the 70 keV map
These initial experiences suggest that projection-based monochromatic energy imaging is superior to polychromatic imaging and image-based monochromatic imaging in reducing beam-hardening and in iodine quantification.
7 Early Human Experiences
Early human experiences with rapid kVp switching dual-energy CT have shown promising results. While the degree of beam-hardening reduction is not as profound as noted in the in vitro studies, there was a significant reduction observed, particularly in the basal inferior wall and the mid septum. In a matched cohort of 30 patients without obstructive CAD who underwent both a clinical single-energy coronary CTA and an experimental rapid kVp switching CT, there was 50 % reduction in beam-hardening artifact in the basal inferior wall and mid septum at 80 keV Fig. 2c, d, and e. In a larger cohort of 49 patients undergoing dual-energy CT alone, contrast-to-noise and signal-to-noise ratios were found to be optimized at 70–80 keV for the myocardium and 65 keV for coronary analysis (Leipsic et al. unpublished data). At the time of the submission of this chapter, experiences with the use of rapid kVp switching CT in the assessment of pathological perfusion cases were extremely limited, precluding any comment on the potential impact on the diagnostic accuracy of CTP imaging using such a technique.
8 Future Outlook: the Potential for Clinical Application
Single-source dual-energy CTP imaging has only recently moved beyond the phantom and animal experiment sphere and is now being utilized in patients. As stated above, initial human experiences have supported the prior data from both phantom and animal experiments, showing a reduction in beam-hardening and consistent ability to routinely quantify iodine in the myocardium. Continued study is needed to evaluate the impact, if any, of this beam-hardening reduction on the diagnostic accuracy of CTP. Additionally, future trials are needed to investigate the potential of material decomposition to characterize and more robustly segment coronary artery plaques Fig. 5. Initial experiences suggest that projection-based monochromatic imaging might reduce partial volume averaging of calcified plaque, allowing more accurate luminal assessment in the setting of calcified plaques. However, this experience remains limited and further studies involving larger multicenter cohorts with invasive angiographic, and likely, intravascular ultrasound correlation is needed to confirm these findings.
References
Beckmann EC (2006) CT scanning the early days. Br J Radiol 79(937):5–8
Brian SK et al (2011) CT stress myocardial perfusion imaging using multidetector CT—a review. J Cardiovasc Comput Tomogr 5(6):345–356
Chandra N, Langan DA (2011) Gemstone Detector: dual energy imaging via fast kVp switching. In: Johnson T, Fink C, Schönberg SO, Reiser MF (eds) Dual energy CT in clinical practice, Medical Radiology, Springer, Heidelberg, pp 35–41, ISBN 978-3-642-01739-1. doi:10.1007/174_2010_35, url:http://dx.doi.org/10.1007/174_2010_35
Entrikin DW, Leipsic JA, Carr JJ (2011) Optimization of radiation dose reduction in cardiac computed tomographic angiography. Cardiol Rev 19(4):163–176
Gaemperli O et al (2008) Functionally relevant coronary artery disease: comparison of 64-section CT angiography with myocardial perfusion SPECT. Radiology 248(2):414–423
Goldman LW (2008) Principles of CT: multislice CT. J Nucl Med Technol 36(2):57–68 quiz 75–76
Harvey SH (2009) Is coronary computed tomographic angiography the “gold standard” for coronary artery disease? J Cardiovasc Comput Tomogr 3(5):334–339
Hsiao EM, Rybicki FJ, Steigner M (2010) CT coronary angiography: 256-slice and 320-detector row scanners. Curr Cardiol Rep 12(1):68–75
Johnson TRC et al (2007) Material differentiation by dual energy CT: initial experience. Eur Radiol 17(6):1510–1517
Karcaaltincaba M, Aykut A (2010) Dual-energy CT revisited by multidetector CT: review of principles and clinical applications. Diagn Interv Radiol 17(3):181–194. doi:10.4261/1305-3825.DIR.3860-10.0
Kitagawa K et al (2010) Characterization and correction of beam-hardening artifacts during dynamic volume CT assessment of myocardial perfusion. Radiology 256(1):111–118
Ko JP et al (2012) Dual-energy computed tomography: concepts, performance, and thoracic applications. J Thorac Imaging 27(1):7–22
Matsumoto K et al (2011) Virtual monochromatic spectral imaging with fast kilovoltage switching: improved image quality as compared with that obtained with conventional 120-kVp CT. Radiology 259(1):257–262
Meijboom WB, Van Mieghem CAG et al (2008a) Comprehensive assessment of coronary artery stenoses. J Am Coll Cardiol 52(8):636–643
Meijboom WB, Meijs MFL et al (2008b) Diagnostic accuracy of 64-slice computed tomography coronary angiography. J Antimicrob Chemother 52(25):2135–2144
Melikian N et al (2010) Fractional flow reserve and myocardial perfusion imaging in patients with angiographic multivessel coronary artery disease. JCIN 3(3):307–314
Pijls NH, Fearon WF, Tonino PA, Siebert U, Ikeno F, Bornschein B, van't Veer M, Klauss V, Manoharan G, Engstrøm T, Oldroyd KG, Ver Lee PN, MacCarthy PA, De Bruyne B (2010) Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease: 2-year follow-up of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) study investigators. J Am Coll Cardiol 56(3):177–84. doi:10.1016/j.jacc.2010.04.012
Rodríguez-Granillo GA, Rosales MA, Degrossi E, Rodriguez AE (2010a) Signal density of left ventricular myocardial segments and impact of beam hardening artifact: implications for myocardial perfusion assessment by multidetector CT coronary angiography. Int J Cardiovasc Imaging 26(3):345–354
Rodríguez-Granillo GA, Ingino CA, Lylyk P (2010b) Myocardial perfusion imaging and infarct characterization using multidetector cardiac computed tomography. World J Cardiol 2(7):198–204
Schuijf JD et al (2011) Current applications and limitations of coronary computed tomography angiography in stable coronary artery disease. Heart 97(4):330–337
Shaw LJ et al (2008) Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the clinical outcomes utilizing revascularization and aggressive drug evaluation (COURAGE) trial nuclear substudy. Circulation 117(10):1283–1291
Silva AC et al (2011) Dual-energy (spectral) CT: applications in abdominal imaging. Radiographics 31(4):1031–1046 discussion 1047–1050
So A, Lee T-Y (2011) Quantitative myocardial CT perfusion: a pictorial review and the current state of technology development. J Cardiovasc Comput Tomogr 5(6):467–481
So A et al (2009) Beam hardening correction in CT myocardial perfusion measurement. Phys Med Biol 54(10):3031–3050
So A et al (2011) Quantitative myocardial perfusion imaging using rapid kVp switching dual energy CT: preliminary experience. J Cardiovasc Comput Tomogr 5(6):430–442
So A et al (2012) Prospectively ECG triggered rapid kV-switching dual energy CT for quantitative imaging of myocardial perfusion. JACC Cardiovasc Imaging 5(8):829–836. doi:10.1016/j.jcmg.2011.12.026
Tonino PAL et al (2010) Angiographic versus functional severity of coronary artery stenoses in the FAME study. JAC 55(25):2816–2821
Tops LF et al (2008) Noncoronary applications of cardiac multidetector row computed tomography. JACC Cardiovasc Imaging 1(1):94–106
Weininger M et al (2010) Adenosine-stress dynamic real-time myocardial perfusion CT and adenosine-stress first-pass dual-energy myocardial perfusion CT for the assessment of acute chest pain: initial results. Eur J Radiol 81(12):3703-3710. doi:10.1016/j.ejrad.2010.11.022
Wu X, Langan DA, Xu D et al (2009) Monochromatic CT image representation via fast switching dual kVp. Proc SPIE 7258:725845
Zatz LM, Alvarez RE (1977) An inaccuracy incomputed tomography: the energy dependence dependence of CT values. Radiology 124(1):91–97
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Al-Hassan, D.A., Ajlan, A.M., Leipsic, J. (2013). CT Evaluation of the Myocardial Supply-Fast kV-Switching Dual-Energy CT. In: Schoepf, U., Bamberg, F., Ruzsics, B., Vliegenthart, R., Bastarrika, G. (eds) CT Imaging of Myocardial Perfusion and Viability. Medical Radiology(). Springer, Berlin, Heidelberg. https://doi.org/10.1007/174_2012_791
Download citation
DOI: https://doi.org/10.1007/174_2012_791
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-33878-6
Online ISBN: 978-3-642-33879-3
eBook Packages: MedicineMedicine (R0)