Abstract
Coronary artery disease remains one of the leading causes of death. Alone in the USA, a total of 6.3 million single photon emission computed tomography (SPECT) myocardial perfusion imaging examinations are carried out annually for diagnostic, treatment planning and risk stratification. Recent advances in the development of cardiac imaging solutions like dedicated cardiac cameras based on solid-state cadmium-zinc-telluride (CZT) detectors with multi-pinhole collimators can increase the patient throughput by reduced scan times and even lower doses. Hybrid cardiac SPECT/CT imaging allows combined assessment of anatomical and functional aspects of coronary artery disease. The combination of myocardial perfusion SPECT data with CT for attenuation correction avoids inferior wall artifacts and improves diagnostic confidence. The assessment of coronary artery calcification and coronary CT angiography greatly improves sensitivity and specificity compared to myocardial perfusion SPECT imaging alone.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Coronary artery disease
- Myocardial perfusion imaging
- SPECT
- Ultra-fast cardiac camera
- CZT technology
- Pinhole imaging
- Hybrid imaging
- Attenuation correction
- Coronary artery calcification
- Coronary CT angiography
1 Introduction
Coronary artery disease is one of the major causes of morbidity and mortality in the Western civilization, although large progress was made as cardiovascular disease mortality rates declined dramatically in the last decades [1,2,3]. Besides changes of lifestyle and medical treatment, surgical revascularization of coronary arteries accounts for a big deal of the declining numbers. However, before surgical treatment, proof of myocardial ischemia is mandatory according to recent guidelines [4]. Thus, there is a need for safe, reliable, robust, fast and cost-effective procedures to detect and assess the extent of coronary artery disease.
Non-invasive cardiac imaging has witnessed huge advances in the last decades, especially for coronary artery disease where it is currently used for diagnosis, risk assessment and decision making for appropriate treatment strategies [3, 5]. As a result, the number of non-invasive cardiac imaging examinations increased over the last years which lead to an increased request of high-end devices for computed tomography (CT) and nuclear cardiology [5].
In nuclear cardiology, positron emission tomography (PET) imaging is referred as the gold standard as it is by nature a quantitative non-invasive method and allows the absolute assessment of myocardial blood flow. Additionally, PET perfusion tracers, such as Ammonia N13, have a relatively high extraction rate and a short physical half-life which allows absolute quantification. However, due to the short half-lives of the available perfusion tracer an onsite cyclotron is needed for cardiac PET imaging today. This requires large investments in equipment and infrastructure which limits the sites who can perform myocardial perfusion PET imaging.
Single photon emission computed tomography (SPECT) imaging is still the workhorse in nuclear medicine due to the commercial availability of 99mTc by Mo/Tc generators. For myocardial perfusion, two 99mTc-based tracers sestamibi (CardioLite, Du Pont) and tetrofosmin (Myoview, GE Healthcare) are available.
Myocardial perfusion SPECT imaging advanced to the most frequently used nuclear medicine test with annually 6.3 million exams in the USA [2]. Conventional SPECT imaging involves prolonged procedure times which compromise cost-effectiveness and overall diagnostic quality. Therefore, the latest development aimed to decrease required imaging time and increase patient throughput at equivalent or better levels of spatial resolution and contrast [6].
2 Ultra-Fast Cardiac Cameras Based on CZT Technology
Most recent advances in myocardial SPECT imaging were made in the development of dedicated ultra-fast cardiac cameras based on semiconductor technology of cadmium zinc tellurium (CZT) detectors. In comparison to conventional sodium iodine (NaI) detectors, CZT detectors do not contain a scintillation crystal and the radiation is converted to electrical signals directly without intermediate conversion steps. Although being integral part of conventional NaI detectors and well established for many decades, the intermediate conversion steps are responsible for significant data degradation, especially the energy resolution, and thus clinical limitations. The use of CZT detectors with direct conversion technology, converts the radiation directly, circumvents these shortcomings and recovers lost spatial and energy resolution and eliminates much of the bulk of the systems.
In conventional NaI detectors, gamma rays are absorbed and converted to ultraviolet (UV) photons. The UV photons are directed to photomultiplier tubes (PMT) by light guides and optical coupling compounds (Fig. 2.1a). The detector facing side of the PMT is equipped with a photocathode, which absorbs the UV photons and releases electrons by the photoelectric effect. These electrons are accelerated and multiplied by high voltage stages and collected on the input of a sensitive signal amplifier. This process is defined as indirect conversion. In comparison to that, in the direct conversion process as happening in CZT detectors, incident gamma rays are absorbed which directly results in an electric charge (Fig. 2.1b). This direct process happens without any steps of UV light production, transport and conversion. The 140 keV gamma photon produces 33,000 electron–hole pairs. An applied electric field causes the charges to move and induces signals on the anodes. These signals are further amplified for further readout processing. This results in a better energy resolution, as it only depends on the amplifier noise and is no longer determined by a step with low statistics. In addition to the significantly improved energy resolution, the system resolution was improved due to pixilation of the detector with 2.46 mm by 2.46 mm pixels leading to reduced uncertainty of the intrinsic resolution to the boundaries of the pixel.
Comparison of indirect and direct conversion in nuclear medicine event detection. (a) With NaI detector technology, an emitted photon is absorbed by the scintillator (i.e. NaI crystal) and a small light signal is emitted. An array of photomultiplier tubes (PMTs) collects and amplifies the light, which is then converted to 500 photo-electrons. The location of the event (location of the photon emission) is approximately calculated. (b) With the CZT detector technology a photon is directly converted into 30,000 photo-electrons. This eliminates the analog noise or signal noise. The location of the event is accurately identified, including the energy of the photon
The CZT detector has a very compact structure with a crystal dimension of 4 cm by 4 cm by 5 mm or 7.25 mm, respectively. In comparison, a conventional detector with a PMT has a vertical dimension of 7 cm, which corresponds to a reduction of size of a factor of 10. This specific design allows the packaging of multiple detectors tightly together or to develop imaging systems with improved proximity, i.e. closer to the body. This allows many detectors to be packed together in a single system which opens a completely new possibility in cardiac imaging. Figure 2.2 shows the components of a complete CZT imaging module including CZT crystal, ASIC electronics, heat sink and digital board. The fully assembled CZT detector module including cover and connectors is depicted in Fig. 2.3.
The advantages of the CZT detector in terms of energy and spatial resolution as well its extremely compact structure makes them well suited for the use in dedicated cardiac cameras. Today, two dedicated cardiac systems based on CZT technology with fundamentally different setup are commercially available. The D-SPECT camera of Spectrum Dynamics is equipped with 6 or 9 rotating detector columns with a parallel hole collimator. Each detector column consists of 4 CZT detector modules summing up to a total of 24–36 detector modules [7].
The Discovery NM 530c of GE Healthcare is equipped with 19 stationary detectors based on pinhole collimation. Each detector consists of four CZT modules summing up to a total of 76 detector modules build in the Discovery NM 530c [6, 7].
3 Pinhole Imaging
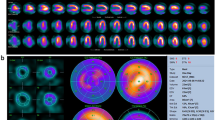
Pinhole imaging has been used historically in nuclear medicine for specialized imaging with high resolution due to the magnification of the projection of the specimen. The improvement in spatial resolution, however, comes by the cost of a reduced sensitivity [8]. In cardiac imaging, increased spatial resolution has not highest relevance due to the blurring effects of the moving heart in the cardiac cycle. However, the combination of pinhole collimation with high resolution CZT detectors provides a new means to achieve a net gain in performance without the tradeoff between spatial resolution and sensitivity. The pinhole imaging allows the high resolution of the CZT detector to be converted to high sensitivity without degrading resolution which is more valuable for cardiac imaging. The high sensitivity can then be used to optimize workflow and patient throughput, while the conserved resolution preserves the clinical character and quality of conventional images and the diagnostic protocols [6, 9, 10]. Furthermore, the multiple detectors acquire the full set of views needed for tomographic reconstruction simultaneously and without any detector motion at all (Fig. 2.4a). This capability reduces systematic error from all sources of motion blurring, including both, the detector motion and even the cardiac motion itself. Figure 2.4b shows the multi-pinhole collimator focusing the heart with each pinhole.
The principle how the combination of CZT and pinhole collimation to achieve high sensitivity and conserve resolution is depicted in Fig. 2.5. In conventional NaI imaging the pinhole resolution decreases, i.e. improves with increasing magnification factor. Thus, optimization for high resolution is achieved by moving the detectors away from the pinhole in order to increase the magnification factor. However, the small pixels with the reduced pixel size of the CZT detectors facilitate a new counterintuitive strategy to optimize for high efficiency. In this case, the detector is moved towards the pinhole. The detector surface area needed to image the object is rapidly reduced. The number of pixels in the image is preserved by use of smaller pixels. In order to preserve the resolution, the pinhole size is reduced in accordance with the small pixel size.
Principle of CZT-based pinhole imaging. Due to the high resolution of the CZT detector, the detector can be closer to the pinhole than the specimen yielding a magnification factor <1. Magnification is calculated as DH/SH or DPD/SPD. DH detector height, SH source height, DPD detector to pinhole distance, SPD source to pinhole distance
In comparison to a conventional gamma camera with low energy high resolution (LEHR) collimator pinhole imaging based on CZT detectors adds up to a five times higher system efficiency.
4 Hybrid Imaging for Dedicated Cardiac SPECT Cameras
The new design of the CZT-based dedicated cardiac cameras was reported highly efficient in multiple studies. In comparison to conventional double-head gamma cameras the new systems yield images with higher contrast and spatial resolution, acquired in shorter time and/or with reduced radiation doses to the patient [1, 7, 11,12,13,14,15]. As with conventional gamma cameras, inferior wall artifacts due to soft tissue attenuation are common. The specificity can be improved by two position imaging (supine and prone), however, attenuation correction should be applied if it is available. The CT data used for attenuation correction are embedded in the reconstruction algorithm which leads to more accurate voxel values and has the potential to provide absolute radiotracer concentrations enabling a possible flow quantification with existing tracers [3]. For the dedicated cardiac camera Discovery NM 530c there is already a straightforward approach to include external CT data for attenuation correction.
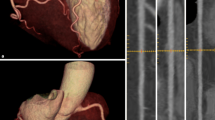
Besides attenuation correction, hybrid imaging strategies for cardiac imaging are typically synergistic and provide more information than either dataset alone, leading to improved sensitivity and specificity [16,17,18]. This is because both, the functional consequences of coronary stenosis on myocardial perfusion and metabolism and the morphological coronary artery disease are relevant. Additionally, other entities such as microvascular or endothelial dysfunction have been recognized to contribute significantly to disease pathology [5]. To achieve this, coronary artery calcification (CAC) or coronary CT angiography (CCTA) datasets are fused SPECT or PET myocardial perfusion imaging data.
CAC can be readily compared with an attenuation correction CT because a native CT scan without contrast enhancement is required. CAC adds high clinical value as it provides an estimate of coronary atherosclerotic plaque burden and correlates strongly with the overall amount of coronary plaque. The assessment of the presence of subclinical coronary atherosclerosis with CAC, which cannot be detected by MPI, provides an opportunity to identify asymptomatic patients who are at risk for developing long-term coronary artery disease. Thus, the combination of SPECT and CAC results in a significant improvement in sensitivity and specificity for the diagnosis of angiographically significant coronary artery disease compared to SPECT alone [3, 5, 19, 20].
CCTA provides an accurate morphological assessment of coronary artery anatomy and has the ability to exclude CAD through its very high sensitivity. However, CCTA is a purely anatomical imaging modality and does not offer information on the functional relevance of the coronary lesion. Therefore, the combination and fusion of SPECT and CCTA datasets significantly improve diagnostic accuracy compared to SPECT alone or SPECT and CCTA side-by-side analysis [3, 16, 21,22,23].
There are many clinical evidences that hybrid imaging adds a strong clinical benefit to myocardial perfusion imaging. This can be performed in two different ways, either by using hybrid SPECT or PET/CT systems or by consecutive SPECT and CT exams on two different systems and a dedicated post-processing. Today, commercially available hybrid SPECT systems are equipped with a CT-subsystem with a up to 2 cm detector (up to 16 rows) and PET/CT systems with a up to 4 cm detector (up to 64 rows). The in details described dedicated cardiac cameras are available as stand-alone systems only by today.
CT scans for attenuation correction and landmarking can be performed on a non-diagnostic CT scanner. CAC requires at least a diagnostic quality CT scanner and CCTA a state-of-the-art CT with at least 4 cm detector with ultra-fast tube rotation. One may ask why not every hybrid system may be equipped with a modern state-of-the-art 64 row CT, as it would enable convenient and straightforward hybrid imaging. The reason for this is of pure economic nature because every system has to be operated profitable. According to Kaufmann et al. [16], not every patient would benefit from CCTA at all and the prescription of additional CCTA has always be carefully considered, especially regarding dose exposure. If such a CT would be connected to a dedicated cardiac camera, it would be occupied with low utilization. Having a dedicated cardiac camera and a high-end CT, both systems can be operated profitable at optimal capacity utilization as patients that do not need myocardial perfusion imaging can receive their CT exam in the meantime. For those scenarios powerful post-processing was developed to easily register and fuse functional myocardial perfusion with CT data. For attenuation correction, however, an (ultra-)low dose CT combined to a cardiac camera would provide a benefit because SPECT and CT images would be inherently aligned. Although registration of SPECT and CT cardiac datasets is software assisted and a manageable region of interest, registration has to be carefully examined that no potential mismatch occurs. Here, a hybrid system could be beneficial for an improved clinical hybrid workflow.
The field of nuclear cardiac imaging underwent huge improvements by the development of CZT-based dedicated cardiac cameras. The addition of CAC or CCTA to myocardial perfusion imaging improves the diagnostic confidence of the report. Multiple post-processing tools are available to perform registration and fusion of SPECT and CT data to create meaningful SPECT/CT dataset.
References
Nkoulou R, Fuchs T, Pazhenkottil AP, Wolfrum M, Buechel RR, Gaemperli O, et al. High efficiency gamma camera enables ultra-low fixed dose stress/rest myocardial perfusion imaging. Eur Heart J. 2018;20(2):218–24.
Mensah GA, Wei GS, Sorlie PD, Fine LJ, Rosenberg Y, Kaufmann PG, et al. Decline in cardiovascular mortality: possible causes and implications. Circ Res. 2017;120(2):366–80.
Iskandrian AE, Dilsizian V, Garcia EV, Beanlands RS, Cerqueira M, Soman P, et al. Myocardial perfusion imaging: lessons learned and work to be done-update. J Nucl Cardiol. 2018;25(1):39–52.
ESCARDIO. 2019. https://www.escardio.org/Guidelines/Clinical-Practice-Guidelines/ESC-EACTS-Guidelines-in-Myocardial-Revascularisation-Guidelines-for.
Gaemperli O, Kaufmann PA, Alkadhi H. Cardiac hybrid imaging. Eur J Nucl Med Mol Imaging. 2014;41(Suppl 1):S91–103.
Bocher M, Blevis IM, Tsukerman L, Shrem Y, Kovalski G, Volokh L. A fast cardiac gamma camera with dynamic SPECT capabilities: design, system validation and future potential. Eur J Nucl Med Mol Imaging. 2010;37(10):1887–902.
Ben-Haim S, Kennedy J, Keidar Z. Novel cadmium zinc telluride devices for myocardial perfusion imaging-technological aspects and clinical applications. Semin Nucl Med. 2016;46(4):273–85.
Beekman F, van der Have F. The pinhole: gateway to ultra-high-resolution three-dimensional radionuclide imaging. Eur J Nucl Med Mol Imaging. 2007;34(2):151–61.
Sharir T, Pinskiy M, Pardes A, Rochman A, Prokhorov V, Kovalski G, et al. Comparison of the diagnostic accuracies of very low stress-dose with standard-dose myocardial perfusion imaging: automated quantification of one-day, stress-first SPECT using a CZT camera. J Nucl Cardiol. 2016;23(1):11–20.
Einstein AJ, Johnson LL, DeLuca AJ, Kontak AC, Groves DW, Stant J, et al. Radiation dose and prognosis of ultra-low-dose stress-first myocardial perfusion SPECT in patients with chest pain using a high-efficiency camera. J Nucl Med. 2015;56(4):545–51.
Imbert L, Poussier S, Franken PR, Songy B, Verger A, Morel O, et al. Compared performance of high-sensitivity cameras dedicated to myocardial perfusion SPECT: a comprehensive analysis of phantom and human images. J Nucl Med. 2012;53(12):1897–903.
Bailliez A, Lairez O, Merlin C, Piriou N, Legallois D, Blaire T, et al. Left ventricular function assessment using 2 different cadmium-zinc-telluride cameras compared with a gamma-camera with cardiofocal collimators: dynamic cardiac phantom study and clinical validation. J Nucl Med. 2016;57(9):1370–5.
Duvall WL, Sweeny JM, Croft LB, Ginsberg E, Guma KA, Henzlova MJ. Reduced stress dose with rapid acquisition CZT SPECT MPI in a non-obese clinical population: comparison to coronary angiography. J Nucl Cardiol. 2012;19(1):19–27.
Nkoulou R, Fuchs T, Pazhenkottil AP, Wolfrum M, Buechel RR, Gaemperli O, et al. High efficiency gamma camera enables ultra-low fixed dose stress/rest myocardial perfusion imaging. Eur Heart J Cardiovasc Imaging. 2019;20(2):218–24.
Nkoulou R, Pazhenkottil AP, Kuest SM, Ghadri JR, Wolfrum M, Husmann L, et al. Semiconductor detectors allow low-dose-low-dose 1-day SPECT myocardial perfusion imaging. J Nucl Med. 2011;52(8):1204–9.
Kaufmann PA, Buechel RR. Cardiac SPECT/CCTA hybrid imaging : one answer to two questions? Herz. 2016;41(5):391–7.
Grossmann M, Giannopoulos AA, Bechtiger FA, Messerli M, Schwyzer M, Benz DC, et al. Ultra-low-dose computed tomography for attenuation correction of cadmium-zinc-telluride single photon emission computed tomography myocardial perfusion imaging. J Nucl Cardiol. 2020;27(1):228–37.
Clerc OF, Fuchs TA, Possner M, Vontobel J, Mikulicic F, Stehli J, et al. Real-time respiratory triggered SPECT myocardial perfusion imaging using CZT technology: impact of respiratory phase matching between SPECT and low-dose CT for attenuation correction. Eur Heart J Cardiovasc Imaging. 2017;18(1):31–8.
Grani C, Buechel RR, Kaufmann PA, Kwong RY. Multimodality imaging in individuals with anomalous coronary arteries. JACC Cardiovasc Imaging. 2017;10(4):471–81.
Grani C, Vontobel J, Benz DC, Bacanovic S, Giannopoulos AA, Messerli M, et al. Ultra-low-dose coronary artery calcium scoring using novel scoring thresholds for low tube voltage protocols-a pilot study. Eur Heart J Cardiovasc Imaging. 2018;19(12):1362–71.
Pazhenkottil AP, Benz DC, Grani C, Madsen MA, Mikulicic F, von Felten E, et al. Hybrid SPECT perfusion imaging and coronary CT angiography: long-term prognostic value for cardiovascular outcomes. Radiology. 2018;288(3):694–702.
Pazhenkottil AP, Nkoulou RN, Ghadri JR, Herzog BA, Buechel RR, Kuest SM, et al. Prognostic value of cardiac hybrid imaging integrating single-photon emission computed tomography with coronary computed tomography angiography. Eur Heart J. 2011;32(12):1465–71.
Diaz-Zamudio M, Fuchs TA, Slomka P, Otaki Y, Arsanjani R, Gransar H, et al. Quantitative plaque features from coronary computed tomography angiography to identify regional ischemia by myocardial perfusion imaging. Eur Heart J Cardiovasc Imaging. 2017;18(5):499–507.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Krüwel, T. (2022). Industry Perspective on Hybrid Cardiac Imaging. In: Nekolla, S.G., Rischpler, C. (eds) Hybrid Cardiac Imaging. Springer, Cham. https://doi.org/10.1007/978-3-030-83167-7_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-83167-7_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-83166-0
Online ISBN: 978-3-030-83167-7
eBook Packages: MedicineMedicine (R0)