Abstract
Plant-parasitic nematodes are costly burdens to crop production because of their intricate relationship with the host plants, wide host range, and the level of postinfection damage. Limitations on the use of chemical pesticides have brought increasing attention in studies on alternative methods for nematode control. Among the strategies of nonchemical nematode management is the identification and implementation of host resistance. Plant resistance involves the production of morphological barriers to prevent pathogens from entry into host cells or may include the synthesis of certain biochemicals that interfere with the subsequent development of pathogens. Among plant biochemical responses to infection is the synthesis of important and diverse compounds from the shikimate and the phenylpropanoid pathways. Many of these compounds are bioactive, playing important roles in defense against biotic and abiotic tresses. This review gathers information from across a large body of studies focusing on the role of the shikimate and the phenylpropanoid pathways in plant-nematode interactions.
Communicated by Francisco M. Cánovas.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
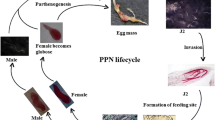
Plant-parasitic nematodes (PPNs) are agricultural pests that cause significant crop loss, estimated at up to $US 125 billion globally per annum (McCarter 2009; Nicol et al. 2011). There are over 4,100 species of PPN described to date (Decraemer and Hunt 2006), and they are classified into three groups, Triplonchida, Dorylaimida, and Tylenchida, with the majority of agriculturally damaging nematodes belonging to the last order (Tytgat et al. 2000).
PPNs deploy different parasitic strategies; they are either migratory or sedentary and can be either endoparasitic (enter the host and migrate through host tissues causing extensive damage) or ectoparasitic (nematodes never enter the host but simply migrate through the soil, using roots as an ephemeral food source as they encounter them) (Wyss and Grundler 1992; Sijmons et al. 1994; Tytgat et al. 2000). The feeding process damages the root system and reduces the plant’s ability to absorb water and nutrients. Typical nematode damage symptoms are a reduction in root biomass, a distortion of root structure, and/or enlargement of the root. Nematode damage of the plant’s root system also provides an opportunity for other plant pathogens to invade the root and thus further damage the plant. The most economically important nematodes, which belong to the sedentary endoparasitic group, are the root-knot nematodes (RKN, Meloidogyne species) and cyst nematodes (Globodera and Heterodera species), followed by the migratory endoparasites, the root lesion nematodes (Pratylenchus species), and the burrowing nematodes (Radopholus species) (Bird and Kaloshian 2003; Jones et al. 2013).
PPNs have evolved a highly specialized feeding structure, termed stylet (a protrusible hollow mouth spear) (Fig. 1), used to penetrate cells to allow feeding on plant tissues (Kikuchi et al. 2017). Parasitic nematodes secrete an array of effectors into host cells through their stylet to initiate the formation of specialized feeding structures known as syncytia for cyst nematodes and giant cells for root-knot nematodes (Fig. 1) (Wyss and Grundler 1992; Golinowski et al. 1996; Mitchum et al. 2013). These plant feeding cells, which are characterized by dense cytoplasm, enlarged multiple nuclei, a fragmented central vacuole, and proliferation of organelles (Jones 1981; Golinowski et al. 1996), act as the permanent source of nutrients for further nematode development. These secretions are accompanied by an extensive alteration of gene expression of parasitized plant cells and roots including genes related to defense responses, cell wall modifications, and metabolic pathways (Puthoff et al. 2003; Jammes et al. 2005; Alkharouf et al. 2006; Ithal et al. 2007; Gheysen and Mitchum 2009; Szakasits et al. 2009; Barcala et al. 2010). Two of the plant metabolic pathways that are involved in plant interactions with parasitic nema todes are the shikimate and the phenylpropanoid pathways.
A model of potential interactions of secreted nematode effectors into a host cell. Secretions are released into the host cells through the nematode’s feeding spear (stylet). Cell wall (CW)-modifying proteins are secreted during migration of infective juveniles through host plant tissues. Other nematode secretions are involved in the formation of specialized feeding cells by the nematode, including effects on host cell metabolism by secreted CM. Modified from Davis et al. (2004)
2 The Shikimate Pathway
The shikimate pathway (Fig. 2) connects primary metabolism to aromatic acids (phenylalanine, tyrosine, and tryptophan) biosynthesis. In a sequence of seven metabolic reactions, phosphoenolpyruvate and erythrose 4-phosphate are converted to chorismate. The enzyme chorismate mutase (CM) then coverts chorismate to prephenate, which is the first step of phenylalanine and tyrosine biosynthesis.
Although the shikimate pathway does not exist in animals, CM gene has been found in the root-knot nematode (Lambert et al. 1999; Doyle and Lambert 2003; Gao et al. 2003; Huang et al. 2005), potato cyst nematode (Jones et al. 2003), sugar beet cyst nematode (Heterodera schachtii) (Vanholme et al. 2009), and soybean cyst nematode (Bekal et al. 2003). Although these species of nematodes possess either a single or two CM genes, they still lack the other genes of the shikimate and aromatic amino acid biosynthesis pathways (Lambert et al. 1999; Popeijus et al. 2000; Bekal et al. 2003). Because plants have the shikimate pathway and nematodes have a secreted form of CM, this enzyme is thought to interfere with the plant’s endogenous shikimate pathway and alter the regulation of this pathway for the benefit of the parasite. The secreted nematode CM may increase the flow through the cytosolic branch of the shikimate pathway, thereby decreasing the biosynthesis of plastid-derived phenolics. Increased flux through the cytosolic branch of the shikimate pathway may be one strategy the nematode uses to decrease accumulation of plastid-derived phenolic compounds known to mediate plant defense responses, thereby suppressing plant defense.
Transgenic expression of the Meloidogyne javanica nematode MjCM1 gene in plant suppresses lateral rot formation and the development of the vascular system, which can be rescued by exogenous application of indoleacetic acid, suggesting that the expression of MjCM1 reduces auxin levels (Doyle and Lambert 2003). Since chorismate is also a precursor for the synthesis of the plant hormones auxin and salicylate, the expression of the MjCM1 in plant cells is thought to competitively reduce the fluxes toward (1) the synthesis of Trp and its downstream hormone auxin and (2) the synthesis of salicylate directly from chorismate.
3 The Phenylpropanoid Metabolism
The phenylalanine ammonia lyase (PAL) is a key enzyme in the phenylpropanoid pathway; it catalyzes the non-oxidative deamination of phenylalanine (one of the end products of the shikimate pathway) to trans-cinnamate and directs the carbon flow from the shikimate pathway to the various branches of the phenylpropanoid metabolism, including lignols (precursor to lignin and lignocellulose), flavonoids, isoflavonoids, anthocyanins, and stilbenes, coumarins, hydroxycinnamic acid conjugates, and lignans (Fig. 3) (D’Auria and Gershenzon 2005).
Expression of PAL gene upon nematode infection has been documented in different studies. Transcription of PAL gene increased in resistant, but not in susceptible, soybean cultivars after Heterodera glycines and M. incognita infections (Edens et al. 1995). In addition, induced resistance in tomato plants activates the expression of PAL gene when the plant is infected with M. incognita (Vasyukova et al. 2007) and with the potato cyst nematode (Globodera rostochiensis) (Uehara et al. 2010). Similarly, in maize-resistant lines, expression of PAL4 gene was highly induced upon infection with M. incognita compared to susceptible lines (Starr et al. 2014).
Moreover, activity of PAL enzyme after nematode infection has also been reported. PAL enzyme activity increased in resistant tomato roots infected with RKN (Breuske 1980), in resistant soybean cultivars after H. glycines and M. incognita infection (Edens et al. 1995), and in resistant potato plants infected with H. rostochiensis (Giebel 1973).
Recent studies using comparative microarray analysis of an incompatible and compatible response of soybean plants to H. glycines indicated no major change in expression of genes encoding PAL in the compatible interaction. However, at 3, 6, and 9 days after infection in the incompatible interaction, genes encoding PAL increased in expression 20 to more than 40 times, indicating an increased metabolic flow into the pathway (Klink et al. 2007a).
The observed increased PAL gene expression and PAL enzyme activity upon nematode infection may induce the phenylpropanoid pathway for the timely synthesis of flavonoids, lignin, and phenolics to protect the resistant plants from nematode infection, or it may induce synthesis of salicylic acid as a signal transducer to induce defense mechanisms (Silverman et al. 1993; Vernooij et al. 1994). Though these results reflect whole plant response to nematode infection in terms of PAL gene expression or PAL enzyme activity, they do not necessarily reflect the expression level of the gene inside the nematode feeding structures. Transgenic plants transformed with promoter: GUS reporter gene offer the advantage of directly observing the gene expression level inside the nematode feeding structures. Transgenic tobacco and Arabidopsis plants expressing the PAL promoter: GUS indicated that PAL gene was strongly downregulated in both H. schachtii and M. incognita feeding structures (Goddijn et al. 1993). Absence of PAL activity could be a prerequisite for nematode feeding site development since phenylpropanoids play key roles in the protection against pathogens. The effect of PAL overexpression, inside the nematode feeding site, on nematode development remains to be examined.
4 Lignin
From phenylalanine, lignin biosynthesis proceeds via a series of side-chain modifications and ring hydroxylations and O-methylations leading to the production of hydroxycinnamyl alcohols, also known as monolignols. Monolignols are the building blocks of lignin, which confers structural support, vascular integrity, and resistance to plants against pathogens.
Lignin synthesis plays a role in plant-nematode interactions. It has been reported that resistance to migratory nematodes correlates with increased lignin deposition in the cell walls of resistant banana plants (Wuyts et al. 2006; Dhakshinamoorthy et al. 2014). In addition, susceptible tomato plants treated with benzothiadiazole (BTH), which acts as a priming agent in plant defense leading to a reduction in penetration and development of the root-knot nematode M. incognita, revealed accumulation of higher lignin levels at later infection stages compared to non-treated plants (Veronico et al. 2018).
The timing of its synthesis influences strongly plants’ susceptibility to nematode infection. Expression of lignin synthesis-related genes was faster and greater in resistant tomato cv. Rossol roots after infection with RKN compared to susceptible tomato cv. Roma (Veronico et al. 2018; Wuyts et al. 2006).
In soybean plants, upregulation of genes involving lignin biosynthesis has also been shown in the nematode feeding structures. Global analysis of gene expression changes in soybean plants infected with nematode revealed that genes involved in lignin biosynthesis are upregulated in both giant cell and syncytium such as the gene encoding quercetin 3-O-methyltransferase (OMT) (Ithal et al. 2007) and caffeoyl-coA-O-methyltransferase genes (CCAO-OMT12 and 10) (Ibrahim et al. 2011). In addition, soybean genes encoding a family of 21 extensin peroxidases that participate in lignin biosynthesis were also upregulated in M. incognita-formed giant cells (Ibrahim et al. 2011).
In Arabidopsis, it has been shown that root-knot nematodes activate expression of Arabidopsis caffeic acid O-methyltransferse 1 (COMT1) gene early in cells of the swelling gall, in giant cells, and in surrounding dividing cells (Quentin et al. 2009). COMT1 expression was maintained in the mature root gall until 21 days after inoculation, in both the giant cells and their neighboring cells. However, knockout of COMT1 did not have any observable effect on the mean number of galls established 3 weeks after inoculation (Quentin et al. 2009).
Induced expression of gene involved in lignin biosynthesis inside the nematode feeding sites is not surprising, as both giant cells and outer walls of syncytia induce thickening of the cell wall through callose or lignin deposition (Grundler et al. 1998) and peroxidase activity (Ithal et al. 2007) probably to withstand the rising pressure of feeding sites.
5 Flavonoids
Flavonoids constitute a large class of secondary carbon-based metabolites present in all land plants. More than 10,000 different types of flavonoids have been described from a variety of plant species. Flavonoids are induced in response to pathogen attack including fungi (Christensen et al. 1998), bacteria (Shirley 1996), and insects (Misra et al. 2010; Diaz Napal et al. 2010; Thoison et al. 2004).
Flavonoids are also induced in response to nematode infections. Specifically, flavonoids have been shown to be induced in response to infection with the root-knot nematodes (Hutangura et al. 1999) and also in or around developing syncytia of H. schachtii early during infection and around galls formed by Xiphinema diversicaudatum (Jones et al. 2007). However, mutant lines defective in various parts of the flavonoid biosynthetic pathway did not show a reduction in nematode development; on the contrary, these plants were more susceptible to nematode infection than the control plants suggesting that flavonoids are produced in plants as part of the defense response to nematode infection.
There are several flavonoid subgroups based on their structural properties, including the chalcones, flavones, flavonols, flavandiols, anthocyanins, condensed tannins, aurones, isoflavonoids, and pterocarpans (Winkel-Shirley 2001; Hassan and Mathesius 2012).
6 Flavonols
Flavonols, a subclass of flavonoids, are the most abundant flavonoids. They possess potent-free radical scavenging activity (Braca et al. 2003) and have insecticidal properties (Simmonds 2003). In fact, soybean (Glycine max) genotype PI 227687, which accumulates the flavonol rutin, has been used widely in breeding programs as a source of insect resistance (Hoffmann-Campo et al. 2006). Flavonols are also involved in modulating auxin transport and signaling (Bohm 1998; Harborne and Williams 2000; Kobayashi et al. 2004). Several studies support a role of auxin in nematode feeding site formation of both cyst and root-knot nematode (Gheysen and Mitchum 2011; Goverse et al. 2000; Hutangura et al. 1999; Karczmarek et al. 2004). Moreover, flavonols have been shown to have direct effect on chemotaxis, motility, and egg hatching of many nematode species (Wuyts et al. 2006). Kaempferol (flavonol) has been shown to inhibit egg hatching in Radopholus similis (Wuyts et al. 2006). The flavonols (kaempferol, quercetin, and myricetin) repelled and slowed M. incognita juveniles (Wuyts et al. 2006). Patuletin, patulitrin, quercetin, and rutin were shown to kill the juveniles of H. zeae at various concentrations and duration of exposure (Faizi et al. 2011).
Flavonol effect on PPRs is species-specific. Using similar concentrations of flavonols, kaempferol, quercetin, and myricetin repelled M. incognita and R. similis juveniles but not Pratylenchus penetrans, whereas the flavonols inhibited the motility of M. incognita juveniles, but not R. similis and P. penetrans juveniles (Wuyts et al. 2006).
The differences in flavonol effects in different nematode species are likely due to the differences in chemosensory receptors, flavonoid receptor binding affinities, cell signaling cascade, and solute permeability across the cuticle in different species.
In Arabidopsis, a gene encoding flavonol synthase1 (FLS1) enzyme is strongly downregulated in both syncytia and giant cells as indicated by histochemical analysis of GUS gene expression (Fig. 4), suggesting that downregulation of this gene is important in the formation and/or maintenance of the nematodes’ feeding sites. Downregulation of flavonol synthase gene in the nematode feeding sites is surprising, as nematodes have been shown to induce reactive oxygen species formation in their feedings sites (Siddique et al. 2014; Melillo et al. 2006) and flavonols have very strong scavenging activity. It would be interesting to see if overexpression of FLS1 and fls1 mutant has any effect on nematode’s development.
7 Isoflavonoids
Isoflavonoids also play a role in plant-nematode interaction. It has been reported that isoflavonoids are produced in infected roots of both H. glycines-resistant Hartwig and susceptible Essex soybean (Kennedy et al. 1999). A recent study by Chin et al. (2018) showed that isoflavonoids are elicited in high amounts in Medicago truncatula in response to M. javanica infection. Examination of transgenic M. truncatula plants which over- and under-produced isoflavonoids indicated that the early production or very high accumulation of isoflavonoids resulted in less severe infection. Specifically, the isoflavonoid, afromosin, and an isoflavonoid derivative, medicarpin, were effective in inhibiting nematode motility and in repelling nematodes in vitro, and the accumulation of the isoflavonoid formononetin and medicarpin in the roots of resistant white clover is believed to exhibit a defensive role on the stem nematode Ditylenchus dipsaci (Cook et al. 1995); the isoflavonoid glyceolin was found to accumulate close to the nematode’s head in a resistant cultivar but not in susceptible plants (Huang and Barker 1991).
Expression of genes involved in isoflavonoid production has been shown to increase during nematode infection. Klink et al. (2007b) examined gene expression in roots of soybean cv Peking infected with incompatible and compatible populations of H. glycines and found that expression of the gene encoding chalcone synthase (CHS) was more than 40-fold in the incompatible interaction at 3 and 9 days postinfection. However, there was no change in the compatible interaction, while one gene encoding chalcone isomerase (CHI) was elevated 4-, 6-, and 17-fold in the incompatible interaction.
Global analysis of gene expression changes in soybean after infection with H. glycines also showed that the CHS and CHI are upregulated in nematode-infected roots (Ithal et al. 2007). However, when CHS gene expression was inhibited by RNAi in Medicago trunculata, root-knot nematodes were still able to form gall although the galls formed were smaller compared to control plants (Wasson et al. 2009).
8 Coumarins
Coumarins are ubiquitously found in higher plants where they originate from the phenylpropanoid pathway, and they are involved actively against a wide range of microbes (Brooker et al. 2008). Coumarins are induced in response to fungal and insect attack (Olson and Roseland 1991; Rahman 2000) and may be involved in defense against pathogenic fungi and insects (Brooker et al. 2007; Razavi et al. 2010; Sharma et al. 2006). In plant-nematode interaction, coumarins (8-geranyloxypsoralen, imperatorin, and herclenin) lethal to B. xylophilus have been identified in the roots of Heracleum candicans Wall (Wang et al. 2008). Coumarins (osthole, columbianadin, bergapten, and xanthotoxin) isolated from roots of Angelica pubescens (Duhuo) were very toxic against B. xylophilus with a mortality rate of 95.25% in 72 h at 1.0 mg/mL (Guo et al. 2018). Coumarins from Ficus carica leaves (psoralen and bergapten) also exhibited a strong effect on B. xylophilus with a mortality rate of 91% within 72 h at 1.0 mg/mL. Furanocoumarin (xanthotoxin, psoralen, bergapten, and oxypeucedanin) from parsley exhibits significant nematicidal activity against M. incognita, M. hapla, and M. arenaria. Addition of fresh parsley paste to soil reduced the number of M. incognita females and plant galls on tomato roots (Caponi et al. 2015).
9 Tannins
Tannins are a group of water-soluble polyphenolic compounds that have the ability to precipitate proteins and other molecules such as polysaccharides, lipids, as well as metal ions (Schofield et al. 2001; Jakobek 2015). They are found in higher plants and are grouped into two classes, termed condensed (syn. Proanthocyanidins) and hydrolyzed tannins. Tannins are toxic to a wide range of fungi, bacteria, and yeast (Scalbert 1991). Studies on the effect of tannins on plant-parasitic nematodes are few. Tannins from chestnut significantly reduced egg hatching of the root-knot nematode M. javanica (Maistrello et al. 2010; D’Errico et al. 2018). Tannins in the extract of Fumaria parviflora have been shown to have strong nematicidal effects on J2 and eggs of M. incognita (Nax et al. 2013). Low concentration of tannic acid (less than 40 mg/L) increased hatching of H. glycines eggs. However, higher concentrations inhibit egg hatching (Chen et al. 1997).
The behavioral response of nematode species to tannic acid is variable. Hewlett et al. (1997) found that tannic acid was attractive for M. arenaria and M. incognita, whereas it was repellent for R. similis, and no effects were observed on H. glycines. The behavioral response of Meloidogyne J2s to tannic acid indicates that tannic acid may be used by the nematode as a chemical signal to locate roots of host plants. Soil treatments with tannic acid were found to control M. arenaria on squash (Mian and Rodriguez-Kabana 1982a, b).
10 Conclusion
The phenylpropanoid-derived compounds play essential roles in plant defense against pathogens. They have been identified in all defense reactions, including constitutive phytoanticipins, inducible phytoalexins, signaling molecules, and many other metabolites that are yet to be identified. Several benefits may result from the identification and characterization of phenylpropanoid-derived compounds such as nematode repellants, hatching stimulants or inhibitors, and nematoxicants. These compounds can be developed for use as nematicides themselves, or they can serve as model compounds for the development of chemical synthesized derivatives with enhanced activity. In addition, transcript profiling of key genes of the phenylpropanoid pathway during nematode infection could identify potential gene targets for nematode control strategies. Genes whose expression augments in response to nematode infection can be used as targets for knockout purposes. Overexpression of genes whose expression is downregulated during nematode can also be used as alternative method in nematode control strategies.
References
Alkharouf NW, Klink VP, Chouikha IB, Beard HS, MacDonald MH, Meyer S, Knap HT, Khan R, Matthews BF (2006) Time course microarray analyses reveals global changes in gene expression of susceptible Glycine max (soybean) roots during infection by Heterodera glycines (soybean cyst nematode). Planta 224:838–852
Barcala M, Garcia A, Cabrera J, Casson S, Lindsey K, Favery B, Garcia-Casado G, Solano R, Fenoll C, Escobar C (2010) Early transcriptomic events in microdissected Arabidopsis nematode-induced giant cells. Plant J 61:698–712
Bekal S, Niblack TL, Lambert KN (2003) A chorismate mutase from the soybean cyst nematode Heterodera glycines shows polymorphisms that correlate with virulence. Mol Plant-Microbe Interact 16:439–449
Bird DM, Kaloshian I (2003) Are roots special? Nematodes have their say. Physiol Mol Plant Pathol 62:115–123
Bohm BA (1998) Introduction to flavonoids. Harwood, Amsterdam
Braca A, Bader A, Siciliano T, Morelli I, De Tommasi N (2003) New pyrrolizidine alkaloids and glycosides from Anchusa strigosa. Planta Med 69:835–841
Breuske CH (1980) Phenylalanine ammonia lyase activity in tomato roots infected and resistant to the root-knot nematode, Meloidogyne incognita. Physiol Plant Pathol 16:409–414
Brooker NL, Kuzimichev Y, Laas J, Pavlis L (2007) Evaluation of coumarin derivatives as anti-fungal agents against soil-borne fungal pathogens. Commun Agric Appl Biol Sci 72:785–793
Brooker N, Windorski J, Blumi E (2008) Halogenated coumarins derivatives as novel seed protectants. Commun Agric Appl Biol Sci 73(2):81–89
Caponi P, Saba M, Oplos C, Aissani N, Maxia A, Menkissoglu-Spiroudi U, Casu L, Ntalli N (2015) Nematicidal activity of furanocoumarins from parsley against Meloidogyne spp. Pest Manag Sci 71(8):1099–1105
Chen S, Dickson DW, Hewlett TE (1997) Tannic acid effects on hatching of Heterodera glycines in vitro. J Nematol 29:742–745
Chin S, Behm C, Mathesius U (2018) Functions of flavonoids in plant-nematode interactions. Plan Theory 7(4):85
Christensen AB, Gregersen PL, Schroder J, Collinge DB (1998) A chalcone synthase with an unusual substrate preference is expressed in barley leaves in response to UV light and pathogen attack. Plant Mol Biol 37:849–857
Cook R, Tiller SA, Mizen KA, Edwards R (1995) Isoflavonoid metabolism in resistant and susceptible cultivars of white clover infected with the stem nematode Ditylenchus dipsaci. J Plant Physiol 146:348–354
D’Auria JC, Gershenzon J (2005) The secondary metabolism of Arabidopsis thaliana: growing like a weed. Curr Opin Plant Biol 8(3):308–316
D’Errico G, Lois Woo S, Lombardi N, Manganiello G, Roversi PF (2018) Activity of chestnut tannins against the southern root-knot nematode Meloidogyne incognita. Redia 101:53–59
Davis EL, Hussey RS, Baum TJ (2004) Getting to the roots of parasitism by nematodes. Trends Parasitol 20:134–141
Decraemer W, Hunt DJ (2006) Structure and classification. In: Perry RN, Moens M (eds) Plant nematology. CAB International, Wallingford, pp 3–32
Dhakshinamoorthy S, Mariama K, Elsen A, De Waele D (2014) Phenols and lignin are involved in the defence response of banana (Musa) plants to Radopholus similis infection. Nematology 16:565–576
Diaz Napal GN, Defago MT, Valladares GR, Palacios SM (2010) Response of Epilachna paenulata to two flavonoids, pinocembrin and quercetin, in a comparative study. J Chem Ecol 36(8):898–904
Doyle EA, Lambert KN (2003) Meloidogyne javanica chorismate mutase1 alters plant cell development. Mol Plant-Microbe Interact 16:123–131
Edens RM, Anand SC, Bolla RI (1995) Enzymes of the phenylpropanoid pathway in soybean infected with Meloidogyne incognita or Heterodera glycines. J Nematol 27(3):292–303
Faizi S, Fayyaz S, Bano S, Yawar Iqbal E, Lubna Siddiqi H, Naz A (2011) Isolation of nematicidal compounds from Tagetes patula L. yellow flowers: structure–activity relationship studies against cyst nematode Heterodera zeae infective stage larvae. J Agric Food Chem 59:9080–9093
Gao BL, Allen R, Maier T, Davis EL, Baum TJ, Hussey RS (2003) The parasitome of the phyto nematode Heterodera glycines. Mol Plant-Microbe Interact 16:720–726
Gheysen G, Mitchum MG (2009) Molecular insights in the susceptible plant response to nematode infection. Plant Cell 15:45–81
Gheysen G, Mitchum MG (2011) How nematodes manipulate plant development pathways for infection. Curr Opin Plant Biol 14:1–7
Giebel J (1973) Phenylalanine and tyrosine ammonia-lyase activities in potato roots and their significance in potato resistance to Heterodera Rostochiensis. Nematologica 19(1):3–6
Goddijn O, Lindsey K, van der Lee F, Klap J, Sijmons P (1993) Differential gene expression in nematode induced feeding structures of transgenic plants harbouring promoter-gus A fusion constructs. Plant J 4:863873
Golinowski W, Grundler FMW, Sobczak M (1996) Changes in the structure of Arabidopsis thaliana during female development of the plant-parasitic nematode Heterodera schachtii. Protoplasma 194:103–116
Goverse A, Overmars H, Engelbertink J, Schots A, Bakker J, Helder J (2000) Both induction and morphogenesis of cyst nematode feeding cells are mediated by auxin. Mol Plant Microbe Interact 13:1121–1129
Grundler FMW, Sobczak M, Golinowski W (1998) Formation of wall openings in root cells of Arabidopsis thaliana following infection by the plant-parasitic nematode Heterodera schachtii. Eur J Plant Pathol 104:545–551
Guo Q, Du G, Li Y, Liang C, Wang C, Zhang Y, Li R (2018) Nematotoxin coumarins from Angelica pubescens Maxim. f. biserrata Shan et Yuan roots and their physiological effect so Bursaphelenchus xylophilus. J Nematol 50(4):2018–2045
Harborne JB, Williams CA (2000) Advances in flavonoid research since 1992. Phytochemistry 55(6):481–504
Hassan S, Mathesius U (2012) The role of flavonoids in root-rhizosphere signaling: opportunities and challenges for improving plant-microbe interactions. J Exp Bot 63:3429–3444
Hewlett TE, Hewlett EM, Dickson DW (1997) Response of Meloidogyne spp., Heterodera glycines, and Radopholus similis to tannic acid. J Nematol 29:737–741
Hoffmann-Campo CB, Neto JA, de Oliveira MC, Oliveira LJ (2006) Detrimental effect of rutin on Anticarsia gemmatalis Pesqui. Agropec Bras 41:1453–1459
Huang JS, Barker KR (1991) Glyceollin I in soybean-cyst nematode interactions: spatial and temporal distribution in roots of resistant and susceptible soybeans. Plant Physiol 96:1302–1307
Huang G, Dong R, Allen R, Davis EL, Baum TJ, Hussey RS (2005) Two chorismate mutase genes from the root-knot nematode Meloidogyne incognita. Mol Plant Pathol 6:23–30
Hutangura P, Mathesius U, Jones MGK, Rolfe BG (1999) Auxin induction is a trigger for root gall formation caused by root-knot nematodes in white clover and is associated with the activation of the flavonoid pathway. Aust J Plant Physiol 26:221–231
Ibrahim H, Hosseini P, Alkharouf N, Hussein E, Gaml El-Din A, Aly M, Matthews BF (2011) Analysis of gene expression in soybean (Glycine max) roots in response to the root knot nematode Meloidogyne incognita using microarrays and KEGG pathways. BMC Genomics 12:220
Ithal N, Recknor J, Nettleton D, Hearne L, Maier T, Baum TJ, Mitchum MG (2007) Parallel genome-wide expression profiling of host and pathogen during soybean cyst nematode infection of soybean. Mol Plant Microbe Interact 20:293–305
Jakobek L (2015) Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem 175:556–567
Jammes F, Lecomte P, de Almeida-Engler J, Bitton F, Martin-Magniette ML, Renou JP, Abbad P, Favery B (2005) Genome-wide expression profiling of the host response to root-knot nematode infection in Arabidopsis. Plant J 44:447–458
Jones MGK (1981) Host cell responses to endoparasitic nematode attack: structure and function of giant cells and syncytia. Ann Appl Biol 97:353–372
Jones JT, Furlanetto C, Bakker E, Banks B, Blok V, Chen Q et al (2003) Characterization of a chorismate mutase from the potato cyst nematode Globodera pallida. Mol Plant Pathol 4:43–50
Jones JT, Furlanetto C, Phillips MS (2007) The role of flavonoid produced in response to cyst nematode infection of Arabidopsis thaliana. Nematology 9:671–677
Jones JT, Haegeman A, Danchin EG, Gaur HS, Helder J, Jones MG, Perry RN (2013) Top 10 plant-parasitic nematodes in molecular plant pathology. Mol Plant Pathol 14:946–961
Karczmarek A, Overmars H, Helder J, Goverse A (2004) Feeding cell development by cyst and root-knot nematodes involves a similar early, local and transient activation of a specific auxin-inducible promoter element. Mol Plant Pathol 5:343–346
Kennedy MJ, Niblack TL, Krishnan HB (1999) Infection by Heterodera glycines elevates isoflavonoid production and influences soybean nodulation. J Nematol 31:341–347
Kikuchi T, Eves-van den Akker S, Jones JT (2017) Genome evolution of plant-parasitic nematodes. Annu Rev Phytopathol 55:333–354
Klink VP, Overall CC, Alkharouf N, MacDonald MH, Matthews BF (2007a) A timecourse comparative microarray analysis of an incompatible and compatible response by Glycine max (soybean) to Heterodera glycines (soybean cyst nematode). Planta 226:1423–1447
Klink VP, Overall CC, Alkharouf N, MacDonald MH, Matthews BF (2007b) Laser capture microdissection (LCM) and comparative microarray expression analysis of syncytial cells isolated from incompatible and compatible soybean roots infected by soybean cyst nematode (Heterodera glycines). Planta 226:1389–1409
Kobayashi S, Goto-Yamamoto N, Hirochika H (2004) Retrotransposon-induced mutations in grape skin color. Science 304:982
Lambert KN, Allen KD, Sussex IM (1999) Cloning and characterization of an esophageal-gland specific chorismate mutase from the phytoparasitic nematode Meloidogyne javanica. Mol Plant-Microbe Interact 12:328–336
Maistrello L, Vaccari G, Sasanelli N (2010) Effect of chestnut tannins on the root-knot nematode Meloidogyne javanica. Helminthologia 47(1):48–75
McCarter JP (2009) Molecular approaches toward resistance to plant-parasitic nematodes. In: Berg RH, Taylor CG (eds) Cell biology of plant nematode parasitism. Springer, Berlin, pp 239–268
Melillo MT, Leonetti P, Bongiovanni M, Castagnone-Sereno P, Bleve-Zacheo T (2006) Modulation of reactive oxygen species activities and H2O2 accumulation during compatible and incompatible tomato-root-knot nematode interactions. New Phytol 170:501–512
Mian IH, Rodriguez-Kabana R (1982a) Organic amendments with high tannin and phenolic contents for control of Meloidogyne arenaria in infested soil. Nematropica 12:221–234
Mian IH, Rodriguez-Kabana R (1982b) Survey of the nematicidal properties of some organic materials available in Alabama as amendments to soil for control of Meloidogyne arenaria. Nematropica 12:205–220
Misra P, Pandey A, Tiwari M, Chandrashekar K, Sidhu OP, Asif MH, Chakrabarty D, Singh PK, Trivedi PK, Nath P et al (2010) Modulation of transcriptome and metabolome of tobacco by Arabidopsis transcription factor, AtMYB12, leads to insect resistance. Plant Physiol 152:2258–2268
Mitchum MG, Hussey RS, Baum TJ, Wang X, Elling AA, Wubben M, Davis E (2013) Nematode effector proteins: an emerging paradigm of parasitism. New Phytol 199:879–894
Nax I, Palomares-Rius JE, Blok V, Khan MR, Ali S (2013) In vitro and in planta nematicidal activity of Fumaria parviflora (Fumariaceae) against the southern root-knot nematode Meloidogyne incognita. Plant Pathol 62:943–952
Nicol JM, Turner SJ, Coyne DL, Nijs LD, Hockland S, Maafi ZT (2011) Current nematode threats to world agriculture. In: Genomics and molecular genetics of plant-nematode interactions. Springer, Dordrecht, pp 21–44
Olson MM, Roseland CR (1991) Induction of the coumarins scopoletin and ayapin in sunflower by insect-feeding stress and effects of coumarins on the feeding of sunflower beetle (Coleontara chrysomelidae). Environ Entomol 20:1166–1172
Popeijus H, Blok V, Cardle L, Bakker E, Phillips M, Helder J, Smant G, Jones J (2000) Analysis of genes expressed in second stage juveniles of the potato cyst nematodes Globodera rostochiensis and Globodera pallida using the expressed sequence tag approach. Nematology 2:567–574
Puthoff DP, Nettleton D, Rodermel SR, Baum TJ (2003) Arabidopsis gene expression changes during cyst nematode parasitism revealed by statistical analyses of microarray expression profiles. Plant J 33:911–921
Quentin M, Allasia V, Pegard A, Allais F, Ducrot PH, Favery B, Levis C, Martinet S, Masur C, Ponchet M, Roby D, Schlaich NL, Jouanin L, Keller H (2009) Imbalanced lignin biosynthesis promotes the sexual reproduction of homothallic oomycete pathogens. PLoS Pathog 5(1):e1000264. https://doi.org/10.1371/journal.ppat.1000264
Rahman AU (2000) Studies in natural product chemistry, vol 24. Elsevier, Amsterdam, pp 860–861
Razavi SM, Imanzadeh GH, Davari M (2010) Coumarins from Zosima absinthifolia seeds, with allelopatic effects. EurAsia J Biosci 4:17–22
Scalbert A (1991) Antimicrobial properties of tannins. Phytochemistry 30:3875–3883
Schofield P, Mbugua DM, Pell AN (2001) Analysis of condensed tannins: a review. Anim Feed Sci Technol 91:21–40
Sharma R, Negi DS, Shiu WK, Gibbons S (2006) Characterization of an insecticidal coumarin from Boenninghausenia albiflora. Phytother Res 20:607–609
Shirley BW (1996) Flavonoid biosynthesis: “new” functions for an “old” pathway. Trends Plant Sci 1:377–382
Siddique S, Matera C, Radakovic ZS, Hasan MS, Gutbrod P, Rozanska E, Sobczak M, Torres MA, Grundler FM (2014) Parasitic worms stimulate host NADPH oxidases to produce reactive oxygen species that limit plant cell death and promote infection. Sci Signal 7:320–333
Sijmons PC, Atkinson HJ, Wyss U (1994) Parasitic strategies of root nematodes and associated host cell responses. Annu Rev Phytopathol 32:235–259
Silverman P, Nuckles E, Ye XS, Kuc J, Raskin I (1993) Salicylic acid, ethylene, and pathogen resistance in tobacco. Mol Plant Microbe Interact 6:775–781
Simmonds MSJ (2003) Flavonoid-insect interactions: recent advances in our knowledge. Phytochemistry 64:21–30
Starr JL, Yang W, Yan Y, Crutcher F, Kolomiets K (2014) Expression of phenylalanine ammonia lyase gene in maize lines differing in susceptibility to Meloidogyne incognita. J Nematol 46:360–364
Szakasits D, Heinen P, Wieczorek K, Hofmann J, Wagner F, Kreil D, Sykacek P, Grundler FMW, Bohlmann H (2009) The transcriptome of syncytia induced by the cyst nematode Heterodera schachtii in Arabidopsis roots. Plant J 57:771–784
Thoison O, Sévenet T, Niemeyer HM, Russell GB (2004) Insect antifeedant compounds from Nothofagus dombeyi and N. pumilio. Phytochemistry 65:2173–2176
Tytgat T, De Meutter J, Gheysen G, Coomans A (2000) Sedentary endoparasitic nematodes as a model for other plant parasitic nematodes. Nematology 2:113–121
Uehara T, Sugiyama S, Matsuura H, Arie T, Masuta CR (2010) Resistant and susceptible responses in tomato to cyst nematode are differentially regulated by salicylic acid. Plant Cell Physiol 51:1524–1536
Vanholme B, Kast P, Haegeman A, Jacob J, Grunewald W, Gheysen G (2009) Structural and functional investigation of a secreted chorismate mutase from the plant-parasitic nematode Heterodera schachtii in the context of related enzymes from diverse origins. Mol Plant Pathol 10:189–200
Vasyukova NI, Pridvorova SM, Gerasimova NG, Chalenko GI, Ozeretskovskaya OL, Udalova ZV, Zinov’eva SV (2007) The involvement of phenylalanine ammonia-lyase and salicylic acid in the induction of resistance to tomato plants infested with gall nematode Meloidogyne incognita. Dokl Biol Sci 416:382–385
Vernooij B, Uknes S, Ward E, Ryals J (1994) Salicylic acid as a signal molecule in plant pathogen interactions. Curr Opin Cell Biol 6:275–279
Veronico P, Paciolla C, Pomar F, De Leonardis S, García-Ulloa A, Melillo MT (2018) Changes in lignin biosynthesis and monomer composition in response to benzothiadiazole and root-knot nematode Meloidogyne incognita infection in tomato. J Plant Physiol 230:40–50
Wang X-B, Li G-H, Li L, Zheng L-J, Huang R, Zhang K-Q (2008) Nematicidal coumarins from Heracleum candicans wall. Nat Prod Res 22:666–671
Wasson AP, Ramsay K, Jones MG, Mathesius U (2009) Differing requirements for flavonoids during the formation of lateral roots, nodules and root knot nematode galls in Medicago trun catula. New Phytol 183:167–179
Winkel-Shirley B (2001) Flavonoid biosynthesis: a colourful model for genetics, biochemistry, cell biology and biotechnology. Plant Physiol 126:485–493
Wuyts N, Lognay G, Swennen R, De Waele D (2006) Nematode infection and reproduction in transgenic and mutant Arabidopsis and tobacco with an altered phenylpropanoid metabolism. J Exp Bot 57:2825–2835
Wyss U, Grundler FMW (1992) Feeding behavior of sedentary plant parasitic nematodes. Eur J Plant Pathol 98:165–173
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Hamamouch, N., Adil, E. (2019). The Role of the Shikimate and the Phenylpropanoid Pathways in Root-Knot Nematode Infection. In: Cánovas, F., Lüttge, U., Leuschner, C., Risueño, MC. (eds) Progress in Botany Vol. 81. Progress in Botany, vol 81. Springer, Cham. https://doi.org/10.1007/124_2019_31
Download citation
DOI: https://doi.org/10.1007/124_2019_31
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-36326-0
Online ISBN: 978-3-030-36327-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)