Abstract
Great Lake is the largest of 14 lakes situated in Lurë National Park, in the eastern part of the Lurë Mountains, Albania. To characterize lake ecological status, epiphytic and epilithic diatom samples were collected from the lakeshore in summer 2005, 2013, and 2017. However, in 2017 no macrophytes were present, such that only epilithic diatom samples could be collected. After laboratory analysis, 52 diatom taxa were identified in July 2005, 67 in June 2013, 111 in August 2013, and 126 in August 2017. The genera Diatoma, Epithemia, Fragilaria, and Surirella were not recorded in 2017, contrary to previous years. We applied two diatom indices to asses the ecological status of the Great Lake, IPS (Indice de Polluo–sensibilité), and TDIL (Trophic Diatom Index for lakes). The IPS index indicated very good water quality in all years, while the TDIL indicated moderate water quality in 2005 and 2013 and good water quality in 2017. Because more species have indicator values for calculating the IPS than the TDIL (valve number used to calculate the IPS was above 95%, while for TDIL was below 50% of 400 counted valves), the IPS may be a more promising tool for bioindication of lakes in Albania according to the demands of the Water Framework Directive.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diatoms are a widespread group of organisms and many taxa have a cosmopolitan distribution (Round et al. 1990). They can colonize different microhabitats in all types of water, from small streams to the sea, and from clean to polluted water (Kociolek and Spaulding 2000; Sanal and Demir 2018). A feature that sets them apart from other organisms is their highly resistant and ornamented silica cell wall, called the frustule. The stable structure of the frustule enables identification to the species level, but according to Mann (1990), diatom identification should not be underestimated due to high morphological variability. Some diatoms live in highly polluted waters, while some are characteristic of clean water, which makes them very useful in assessing the ecological status of surface waters. For this reason, many diatom water quality indices were developed, based on strong species-environment relationships (Kelly et al. 2008).
Since 1949, diatoms have been used as indicators for water quality (Sanal and Demir 2018). Since 2000 the Water Framework Directive (WFD, European Parliament 2000) demands that all water bodies in Europe achieve good ecological quality, and phytobenthos is one of the mandatory quality elements for monitoring standing and flowing waters. Most countries in Europe have chosen to use diatoms for monitoring because they have been shown to represent the entire phytobenthos well (Kelly et al. 2009). Today, diatoms are routinely used for monitoring and assessment of water quality in running waters (Kelly 2013), but methods specifically for lakes are still scarce. Many countries use the IPS index (Coste in Cemagref 1982), which was primarily developed for rivers, also in lakes (Bolla et al. 2010; Cheshmedjiev et al. 2010; Kahlert and Gottschalk 2014). The reason for the widespread use of the IPS, including the calibration of other indices, is its large taxonomical and ecological database, describing ecological preferences of many diatom species (Kelly 2013; Teittinen et al. 2015). However, specific indices for lakes were developed in some countries, e.g. Hungary - Trophic Diatom Index for Lakes TDIL (Stenger-Kovács et al. 2007), Poland - Trophic Index for Lakes TIJ (Picinska-Faltynowicz and Blachuta 2008), Ireland and UK - a version of the TDI optimized for lakes LTDI (Bennion et al. 2014). Of the above indices, only the TDIL can be calculated using the software OMNIDIA. A disadvantage with the TDIL is that it is based on ecological characteristics for 127 taxa only (Stenger-Kovács et al. 2007).
The Albanian National Monitoring System for lakes does not include biological parameters, and no officially accepted diatom index is in place. To assess the ecological status of rivers in Albania, Rott saprobic and trophic diatom indices (SI and TI, include the reference to Rott) are mainly used (Miho et al. 2006; Kupe et al. 2011). These indices were recently also applied in some lakes in Albania (Alikaj et al. 2019).
In Lurë National Park, which has an area of 1280 ha, 14 glacial lakes are located, of which the Great Lake is the largest (Golloshi et al. 2014). In the past years, increased logging activities, as well as littering were observed in the area, which may affect the lake littoral and the organisms that live in the lakes (Golloshi et al. 2014; Cena 2016). According to the available literature, only floristic studies of several lakes in the Lura area were done without even specifying to which exact lakes it refers (Miho and Lange-Bertalot 2001; Shumka and Miho 2006).
The aims of our studies were: (1) to analyze the benthic diatom communities (epiphytic and epilithic) in Great Lake and (2) to assess the ecological status of this lake using diatom indices.
Material and methods
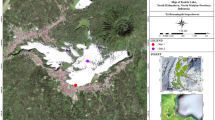
Great Lake (Liqeni i Madh in Albanian) is a glacial, high mountain lake situated in the eastern part of Lurë Mountain in the Lurë National Park, at an altitude of 1722 m a.s.l. Samples were collected in July 2005, June and August 2013, and August 2017. A high anthropogenic impact on the lake is reflected by cutting trees around the lake and the disturbance of the coast (Fig. 1). During the first fieldwork, July 2005, a large number of macrophytes caused collection epiphytic samples to form the diatom checklist. Eight years later, in June epilithic sample was collected (macrophytes were not found due to snow cover), while in August we collected epiphytic samples. In August 2017 no macrophytes were found, which is the reason why we collected epilithic samples only. In 2005, samples were collected from a single site only, as well as in 2013. In 2017, samples were collected from 6 localities (LM1–LM6) around the lake (Fig. 2).
All samples were collected from around 0.5 m water depth. Epiphytic samples were collected by squeezing out or scraping off the periphyton from the dominant macrophyte species, while epilithic samples were sampled by scrubbing the surface of stones with a toothbrush. Samples were immediately fixed with formaldehyde to a final concentration of 4%. The organic content of the raw material was removed from the samples with two different methods: H2O2 method (EN 13946:2003) (samples from 2005 and 2013) and using hot HCl according to Taylor et al. (2007) (samples from 2017). Permanent slides of the cleaned material were mounted with Naphrax®. Light microscope observations and micrographs were made using a Motic BA310 optic microscope and with digital camera CMOS 1/2” 3MP (at the University of Tirana, Faculty of Natural Sciences, Tirana) and Zeiss AxioImagerM.1 microscope with DIC optics and AxioVision 4.9 software (at the University of Belgrade, Faculty of Biology, Serbia). Taxon-specific relative abundances were calculated after counting 400 valves on the slide.
With the software OMNIDIA 6.0. IPS (Index Polluosensitivity Specific, Coste in Cemagref 1982), TDIL (Trophic Diatom Index for Lakes, Stenger-Kovács et al. 2007) were calculated. Class boundaries for IPS and TDIL and ecological status of the lake were determined by Prygiel and Coste (2000):
-
1˗4.99 - very poor water quality,
-
5˗8.99 - poor water quality,
-
9˗12.99 - medium water quality,
-
13˗16.99 - good water quality,
-
17˗20 - very good water quality.
Results
Diatom species composition
We generally found a higher species richness in epilithic than epiphytic samples, and in samples collected in August compared to June/July. Also, diatom richness increased from 2005 to 2017 (Fig. 3). Dominant taxa were different between the years. In the epiphytic community collected in July 2005, Achnanthidium minutissimum (Kützing) Czarnecki (13.01%), Diatoma vulgaris Bory (13.84%) and Meridion circulare (Greville) C.Agardh (11.76%) were dominant. In contrast, only A. minutissimum (22.26%) dominated the epiphytic community in 2013. In the epilithic community collected in June, 2013 A. minutissimum was dominant, with a similar relative abundance as in the epiphytic community collected in August in the same year (21.48%). In Aug 2017 species richness was higher than previously observed, and different taxa dominated. At different sampling localities around the lake, dominant taxa were different: LM1 Cyclotella cretica var. cyclopuncta (Håkansson & J.R.Carter) R.Schmidt (29.38%), LM2 and LM3 Encyonopsis microcephala (Grunow) Krammer (20.74%, 21.05%, respectively), LM4 Cyclotella cretica var. cyclopuncta (17.41%) and Encyonopsis krammeri E.Reichardt (18.91%), LM5 and LM6 A. minutisimum (27.79%, 30.47%, respectively).
The genera Diatoma, Epithemia, Fragilaria and Surirella were not recorded in 2017, contrary to previous years. Diatoma vulgaris was recorded in the epiphytic community in 2005, as well as Fragilaria capucina Desmazières sensu lato. In 2013, F. capucina sensu lato was found in low abundance in both the epiphytic and the epilithic community (0.6–0.9%). Epithemia smithii Carruthers, Surirella spiralis Kützing and S. terricola Lange-Bertalot & E.Alles were recorded only in August 2013 (all species 0.1%). Figures 4, 5, 6 and 7 show some of the diatom taxa collected in August 2017 and the list of identified taxa with their percentage representation in the diatom communities in Great Lake is given as Supplementary Material.
Light microscopy (LM) micrographs. 1 Cymbopleura lata (Grunow ex Cleve) Krammer; 2 Cymbella lange-bertalotii Krammer; 3 C. cymbiformis C.Agardh; 4, 5 C. vulgata Krammer; 6 C. excisiformis var. nonprotracta Krammer; 7, 8 Encyonopsis subminuta Krammer & E.Reichardt; 9 E. microcephala; 10 E. perborealis Krammer; 11 Cymbella hustedtii Krasske; 12, 13 C. neoleptoceros Krammer; 14, 15 Cymbopleura florentina var. brevis Krammer; 16, 17 C. naviculiformis (Auerswald ex Heiberg) Krammer; 18, 19 C. amphicephala (Nägeli ex Kützing) Krammer; 20, 21 C. frequens Krammer. Scale bar = 10 μm
Light microscopy (LM) micrographs. 1 Amphora paracopulata Levkov & Edlund; 2. A. ovalis (Kützing) Kützing; 3, 4 A. copulata (Kützing) Schoeman & R.E.M.Archibald; 5, 6 Cyclotella cretica var. cyclopuncta; 7, 8 Cavinula jaernefeltii (Hustedt) D.G.Mann & A.J.Stickle; 9 C. cocconeiformis (W.Gregory ex Greville) D.G.Mann & A.J.Stickle; 10 Staurosirella martyi (Héribaud-Joseph) E.A.Morales & K.M.Manoylov; 11–13 S. pinnata (Ehrenberg) D.M.Williams & Round; 14, 15 Delicata delicatula (Kützing) Krammer; 16 Encyonema cespitosum Kützing; 17, 18 E. silesiacum (Bleisch) D.G.Mann; 19 E. subelginense Krammer; 20 Kurtkrammeria aequalis (W.Sm.) Krammer; 21 Epithemia smithii. Scale bar = 10 μm
Light microscopy (LM) micrographs. 1 Pinnularia nodosa (Ehrenberg) W.Smith, 2 P. subcommutata var. nonfasciata Krammer; 3 P. microstauron (Ehrenberg) Cleve; 4 Caloneis alpestris (Grunow) Cleve; 5 C. silicula (Ehrenberg) Cleve; 6, 7 C. bacillum (Grunow) Cleve; 8 Tryblionella angustata W.Smith; 9, 10 Nitzschia hantzschiana Rabenhorst; 11, 12 N. denticula Grunow; 13, 14 N. bryophila (Hustedt) Hustedt; 15 N. alpina Hustedt; 16, 17 Denticula tenuis Kützing; 18 Neidium hercynicum Ant.Mayer; 19 Reimeria uniseriata Sala, Guerrero and Ferrario; 20, 21 Sellaphora stroemii (Hustedt) H.Kobayasi; 22 S. pupula (Kützing) Mereschkovsky; 23, 24 Brachysira zellensis (Grunow) Round & D.G.Mann; 25 B. vitrea (Grunow) R.Ross; 26, 27 B. neoexilis Lange-Bertalot; 28, 29 Stauroneis smithii Grunow. Scale bar = 10 μm
Light microscopy (LM) micrographs. 1, 2 Gomphonema brebissonii Kützing; 3, 4 G. lateripunctatum E.Reichardt & Lange-Bertalot; 5, 6 G. truncatum Ehrenberg; 7, 8 G. elegantissimum E.Reichardt & Lange-Bertalot; 9, 10 G. auritum A.Braun ex Kützing; 11–13 Navicula cryptotenelloides Lange-Bertalot; 14 Gomphonema minusculum Krasske; 15 G. drutelingense E.Reichardt; 16, 17 Diploneis oculata (Brébisson) Cleve; 18 D. krammeri Lange-Bertalot & E.Reichardt; 19 Navicula radiosa Kützing; 20, 21 N. densilineolata (Lange-Bertalot) Lange-Bertalot; 22 N. aff. Trophicatrix Lange-Bertalot; 23, 24 N. subalpina E.Reichardt; 25, 26 N. cryptocephala Kützing; 27, 28 N. wygaschii Lange-Bertalot. Scale bar = 10 μm
Diatom indices
To assess the ecological status of the Great Lake we selected one saprobic (IPS-Indice de Polluo-sensibilité) and one trophic diatom index (TDIL-Trophic Diatom Index for lakes). The values of the IPS indicated very good water quality in all years at all sites, while the TDIL indicated moderate water quality in 2005 and 2013 and good water quality in 2017 (Fig. 8). Valve number used to calculate the IPS was above 95%, while for TDIL was below 50% of 400 counted valves.
Discussion
Detailed diatom research of lakes in the Lurë area started in 1993 and 460 taxa were recorded (Miho and Lange-Bertalot 2001). They described 3 new taxa: Cymbopleura albanica Krammer & Miho, C. lata var. lura Miho & Krammer and C. lura Miho & Krammer. Of these three species, we recorded only C. albanica during our investigations in August 2013.
The Great Lake is a high mountain lake, with a short vegetation time due to snow cover. This probably also was the reason why we found a higher diatom species richness in August than in June and July. We also noticed a higher species richness in epilithic than in epiphytic communities (Fig. 3). Some studies have shown that diatoms do not have clear habitat preferences and that species overlap between different habitats (Winter and Duthie 2000; Cetin 2008). In general, physicochemical differences between stone and macrophyte habitats, or different grazing by invertebrates can lead to variation in diatom communities between different substrates (Stevenson and Hashim 1989).
Achnanthidium minutissimum is one of the most common diatom species in different types of freshwater (Blanco et al. 2004; Wojtal and Sobczyk 2006; Potapova and Hamilton 2007; Lange-Bertalot et al. 2017), and this species was dominant in 2005 and 2013 and at two localities (LM5 and LM6) in 2017. This species has a very wide ecological amplitude, although it seems to be absent from water with very low electrolyte content (Lange-Bertalot et al. 2017). Diatoma vulgaris and Meridion circulare were dominant together with A. minutissimum in the epiphytic community in June 2005. Both species can be found in standing freshwater in epilithic and epiphytic communities but are more commonly found in running waters (Lange-Bertalot et al. 2017). However, D. vulgaris, as well as M. circulare, were recorded in different lakes before, but mainly in epilithic communities (e.g. Ohrid Lake, North Macedonia and Albania (Miho et al. 2004), Jeziorak Mały Lake, Poland (Zębek et al. 2012), Asartepe Dam Lake, Turkey (Atici and Obali 2010)). Cyclotella cretica var. cyclopuncta and Encyonopsis microcephala were two of the most dominant taxa in August 2017. These species are characteristic of standing freshwater. Cyclotella cretica var. cyclopuncta was described from Kournas Lake, Crete (John and Economon-Amilli 1990), but also found in Lake Ohrid, Albania (Miho et al. 2004), Malo and Veliko Jezero, Croatia (Wunsam et al. 1999), or Tatar Dam Reservoir, Turkey (Varol et al. 2018). Encyonopsis microcephala is not a rare species and may be locally abundant, but it is taxonomically still not distinguished from similar taxa (Lange-Bertalot et al. 2017). It was previously recorded for instance in Ohrid Lake, North Macedonia and Albania (Miho et al. 2004), in lakes in Spain in epilithic communities (Blanco et al. 2004), in benthos in Işıklı Lake, Turkey (Barinova et al. 2014), in the Vrutci Reservoir, Serbia (Trbojević et al. 2019).
In August 2017 no macrophytes were found in the lake littoral, which is the reason why we could not sample epiphytic diatoms. We believe that this was due to water level fluctuations, possibly caused by the use of lake water for hydropower generation. Large water level fluctuations in lakes generally lead to a degradation of the lake littoral and negatively affect macrophyte biomass and taxonomic richness (Carmignani and Roy 2017). The loss of microhabitat for the development of epiphytic diatom communities may have caused the absence of the genera Diatoma, Epithemia, Fragilaria and Surirella. According to Lange-Bertalot et al. (2017), Epithemia is preferentially an epiphytic genus. Epithemia smithii is reported as widespread in Europe, often in epiphytic communities, in lakes and rivers (Krammer and Lange-Bertalot 1988). Diatoma vulgaris was described by Bory (1824), found attached on Conferva glomerata (a green algae currently known as Cladophora glomerata). Most of the species in the genus Fragilaria are growing attached to a common pad of mucilage adhering to the substrate (Lange-Bertalot et al. 2017). The genus Surirella mostly can be found in the benthos. Surirella terricola is characteristic of soils (Lange-Bertalot et al. 2017) and it probably reached the lake from the surrounding soil. In our samples, we observed it in very low abundance (0.1%).
Despite differences between the diatom communities in rivers and lakes, the IPS index, which was developed for rivers in Belgium, is sometimes used for ecological assessment of lakes, e.g. in Sweden (Kahlert and Gottschalk 2014), Bulgaria (Cheshmedjiev et al. 2010), and Hungary (Bolla et al. 2010). The diatom index for lakes – TDIL was developed in 2007 for Hungarian lakes (reference TDIL). However, it includes much fewer indicator species than the IPS. For example, only about 40% of the diatom species found in Lake Balaton were assigned indicator values in the TDIL, while 75% were assigned IPS indicator values (Bolla et al. 2010). Because indices which are based on only a small proportion of the species occurring in a lake are uncertain, Bolla et al. (2010) recommend using the IPS index in Lake Balaton. In our samples, 95% of the valve number was included in the IPS, while less than 50% were included in the TDIL. The IPS seems therefore to be a promising tool for ecological status evaluation of lakes in Albania.
Despite good lake water quality, the Great Lake is in danger of ecological degradation. The absence of macrophytes in the lake observed in 2017 is a warning signal which should not go unnoticed. The pictures in Fig. 1 shows how the lake littoral and the surrounding area changed during the last years, and that natural forest was removed from the surroundings. Also, this lake is used as a reservoir for a local hydropower station influencing water level fluctuations. If no measures are taken, this may lead to a lack of habitats for various organisms and a loss of biodiversity.
References
Alikaj M, Brahushi F, Kupe L (2019) Ecological status assessment using diatom indices of water ecosystems in Gjirokastra district, Albania. Fresenius Environ Bull 28(2):857–862
Atici T, Obali EO (2010) The diatoms of Asartepe dam Lake (Ankara), with environmental and some physicochemical properties. Turk J Bot 34(6):541–548. https://doi.org/10.3906/bot-0912-271
Barinova S, Solak CN, Erdoğan O, Romanov R (2014) Algae and zooplankton in ecological assessment of the Işıklı Lake, Turkey. Aquat Biol Res 2(2):23–35. https://doi.org/10.12966/abr.05.02.2014
Bennion H, Kelly MG, Juggins S, Yallop ML, Burgess A, Jamieson J, Krokowski J (2014) Assessment of ecological status in UK lakes using benthic diatoms. Freshw Sci 33(2):639–654. https://doi.org/10.1086/675447
Blanco S, Ector L, Bécares E (2004) Epiphytic diatoms as water quality indicators in Spanish shallow lakes. Vie Milieu 54(2–3):71–80
Bolla B, Borics G, Kiss KT, Reskóné NM, Várbíró G, Ács É (2010) Recommendations for ecological status assessment of Lake Balaton (largest shallow lake of Central Europe), based on benthic diatom communities. Vie Milieu 60(3):197–208
Carmignani RR, Roy AH (2017) Ecological impacts of winter water level drawdowns on lake littoral zones: a review. Aquat Sci 79:803–824. https://doi.org/10.1007/s00027-017-0549-9
Cemagref (1982) Etude des méthodes biologiques quantitatives d’appréciation de la qualité des eaux. Rapport Division Qualité des Eaux Lyon - Agence financière de Bassin Rhône-Méditerranée-Corse, Pierre-Bénite, pp 1–218
Cena F (2016) Protected areas in the district of dobra and assessment of their tourist. Int J Acad Res Reflect 4(4):13–21
Cetin AK (2008) Epilithic, epipelic, and epiphytic diatoms in the Göksu stream: community relationships and habitat preferences. J Freshw Ecol 23(1):143–149. https://doi.org/10.1080/02705060.2008.9664565
Cheshmedjiev S, Mladenov R, Belkinova D, Gecheva G, Dimitrova-Dyulgerova I, Ivanov P, Mihov S (2010) Development of classification system and biological reference conditions for Bulgarian rivers and lakes according to the water framework directive. Biotechnol Biotechnol Equip 24:155–163. https://doi.org/10.1080/13102818.2010.10817832
Bory de Saint-Vincent JBGM (1824) Diatome. Diatoma. In: Audouin I et al. (eds) Dictionnaire Classique d'Histoire Naturelle. CRA-D., Vol. 5. Rey et Gravier; Baudouin Frères, Paris, pp 1–461
EN 13946:2003: Water quality. Guidance standard for the routine sampling and pretreatment of benthic diatoms from rivers. ISBN 0580419606; Pages 18. http://standards.mackido.com/en/enstandards24_view_3175.html
European Parliament (2000) Directive 2000/60/EC of the European Parliament and of the council establishing a framework for community action in the field of water policy. Off J Eur Communities L327:1–73
Golloshi A, Lika A, Hysko M (2014) Monitoring the water microbial parameters of some lakes of Lura Park. Albanian J Agric Sci, suppl. Special edition 155–157
John J, Economon-Amilli A (1990) Cyclotella cretica, a new species of diatom from the island of Crete, Greece. Diatom Res 5(1):43–50
Kahlert M, Gottschalk S (2014) Differences in benthic diatom assemblages between streams and lakes in Sweden and implications for ecological assessment. Freshw Sci 33(2):655–669. https://doi.org/10.1086/675727
Kelly M (2013) Data rich, information poor? Phytobenthos assessment and the water framework directive. Eur J Phycol 48(4):437–450. https://doi.org/10.1080/09670262.2013.852694
Kelly MG, King L, Jones RI, Barker PA, Jamieson BJ (2008) Validation of diatoms as proxies for phytobenthos when assessing ecological status in lakes. Hydrobiologia 610(1):125–129. https://doi.org/10.1007/s10750-008-9427-8
Kelly M, King L, Ní Chatháin B (2009) The conceptual basis of ecological-status assessments using diatoms. Biol Environ 109(3):175–189. https://doi.org/10.3318/BIOE.2009.109.3.175
Kociolek JP, Spaulding SA (2000) Freshwater diatom biogeography. Nova Hedwigia 71:223–241
Krammer K, Lange-Bertalot H (1988) Bacillariophyceae. 2. Teil. Bacillariaceae, Epithemiaceae, Surirellaceae. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (eds) Sübwasserflora von Mitteleuropa, 2/2. G. Fischer Verlag, Jena, p 596
Kupe L, Miho A, Çullhaj A (2011) Evaluation of trophic and Saprobic diatom index in Albanian Rivers. J Int Environ Appl Sci 6(5):692–698
Lange-Bertalot H, Hofmann G, Werum M, Cantonati M (2017) Freshwater benthic diatoms of Central Europe: over 800 common species used in ecological assessment. Koeltz Botanical Books, Schmitten-Oberreifenberg
Mann DG (1990) The species concept in diatoms. Phycologia 38:437–495
Miho A, Lange-Bertalot H (2001) Data on microflora of Albanian waters: diatoms from glacial lakes. Studime Biologjike Special Issue 5(6):234–236 [In Albanian]
Miho A, Tase D, Lange-Bertalot H (2004) Overview on diatoms from Ohrid Lake. Proceedings of BALWOIS pp 1–9
Miho A, Çullaj A, Lazo V, Hasko A, Kupe L, Bachofen R, Brandl H, Schanz F (2006) Assessment of water quality of some Albanian rivers using diatom-based monitoring. Alban J Nat Sci 19:94–105
Picinska-Faltynowicz J, Blachuta J (2008) Zasady poboru i opracowania prób fitobentosu okrzemkowego z rzek i jezior. Przewodnik metodyczny, Warsaw
Potapova M, Hamilton PB (2007) Morphological and ecological variation within the Achnanthidium minutissimum (Bacillariophyceae) species complex. J Phycol 43:561–575. https://doi.org/10.1111/j.1529-8817.2007.00332.x
Prygiel J, Coste M (2000) Guide méthodologique pour la mise en oeuvre de l’Indice Biologique Diatomées NF T 90-354. Agences de l’Eau, Ministere de l’Aménagement du Territoire et de l’Environnement, Cemagref
Round FE, Crawford RM, Mann DG (1990) The diatoms. Biology and Morphology of the Genera. Cambridge University press, Cambridge
Sanal M, Demir N (2018) Use of the epiphytic diatoms to estimate the ecological status of Lake Mogan. Appl Ecol Environ Res 16(3):3529–3543. https://doi.org/10.15666/aeer/1603_35293543
Shumka S., Miho A (2006) Assessment of the water quality and trends at the Drini cascade system based on plankton data. International Conference on Water Observation and Information System. BALWOIS 2006
Stenger-Kovács CS, Buczkó K, Hajnal É, Padisák J (2007) Epiphytic, littoral diatoms as bioindicators of shallow lake trophic status: trophic diatom index for lakes (TDIL) developed in Hungary. Hydrobiologia 589:141–154. https://doi.org/10.1007/s10750-007-0729-z
Stevenson RJ, Hashim S (1989) Variation in diatom community structure among habitats in sandy streams. J Phycol 25:678–686
Taylor JC, Harding WR, Archibald CGM (2007) WRC Report TT 281/07: A methods manual for the collection, preparation and analysis of diatom samples version 1.0. Water Research Commission, The Republic of South Africa
Teittinen A, Taka M, Ruth O, Soininen J (2015) Variation in stream diatom communities in relation to water quality and catchment variables in a boreal, urbanized region. Sci Total Environ 530:279–289. https://doi.org/10.1016/j.scitotenv.2015.05.101
Trbojević IS, Predojević DD, Subakov-Simić GV, Krizmanić JŽ (2019) Periphytic diatoms in the presence of a cyanobacterial bloom: a case study of the Vrutci reservoir in Serbia. Arch Biol Sci 71(2):215–223. https://doi.org/10.2298/ABS181120003T
Varol M, Blanco S, Alpaslan K, Karakaya G (2018) New records and rare taxa for the freshwater algae of Turkey from the Tatar dam reservoir (Elazığ). Turk J Bot 42(4):533–542 https://doi.org/10.3906/bot-1710-55
Winter JG, Duthie HC (2000) Stream epilithic, epipelic and epiphytic diatoms: habitat fidelity and use in biomonitoring. Aquat Ecol 34(4):345–353 https://doi.org/10.1023/A:1011461727835
Wojtal A, Sobczyk Ł (2006) Composition and structure of epilithic diatom assemblages on stones of different size in a small calcareous stream (S Poland). Algol Stud 119:105–124. https://doi.org/10.1127/1864-1318/2006/0119-0105
Wunsam S, Schmidt R, Müller J (1999) Holocene lake development of two Dalmatian lagoons (Malo and Veliko Jezero, isle of Mljet) in respect to changes in Adriatic Sea level and climate. Palaeogeogr Palaeoclimatol Palaeoecol 146(1–4):251–281. https://doi.org/10.1016/S0031-0182(98)00147-3
Zębek E, Bonar A, Szymańska U (2012) Periphytic diatom communities in the littoral zone of the urban lake Jeziorak Mały (Masurian Lake District, Poland). Ekológia (Bratislava) 31(1):105–123. https://doi.org/10.4149/ekol201201105
Acknowledgments
We thank Dag Hjermann, NIVA, for help with Fig. 2. The project was funded by the Norwegian Ministry of Foreign Affairs, Project title: “Assessment of ecological status according to the Water Framework Directive – intercalibration among W-Balkan countries” (No RER-14/0008).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm the absence of any conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 60 kb)
Rights and permissions
About this article
Cite this article
Vidaković, D., Krizmanić, J., Ndoj, E. et al. Changes in the diatom community in the great lake (Lurë National Park, Albania) from 2005 to 2017 and first steps towards assessment the water quality. Biologia 75, 1815–1824 (2020). https://doi.org/10.2478/s11756-020-00538-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11756-020-00538-3