Abstract
Carbon monoxide (CO) produced by incomplete combustion of hydrocarbons, has many toxic effects on different organs, especially the heart and brain that have greater demands for oxygen. The present study aimed to evaluate the protective effects of granulocyte colony stimulating factor (G-CSF) on apoptosis after CO poisoning in rats. Male Wistar rats were exposed to CO 1500 or 3000 ppm for 60 min. Single and multiple doses of G-CSF (10, 50, and 100 μg/kg) were administered to animals. After CO poisoning, carboxyhemoglobin concentration was measured, apoptotic cells were evaluated by TUNEL assay and caspase 3 activity was determined by immunofluorescence. Blood levels of carboxyhemoglobin significantly increased following exposure to both 1500 and 3000 ppm concentrations of CO. However, carboxyhemoglobin levels were significantly higher following exposure to CO 3000 ppm compared to CO 1500 ppm (p < 0.05). Differences in caspase 3 activity between G-CSF and control groups were significant and G-CSF could decrease apoptosis following CO 3000 ppm poisoning (p < 0.001). TUNEL assay showed that in rats treat with 5 doses of G-CSF 100 μg/kg, apoptosis was significantly ameliorated compared to control rats and sham (rats that were not exposed to CO) group (p < 0.05). Concerning caspase 3 activity and apoptosis rate, the best results were found in rats exposed to 3000 ppm and treated with G-CSF 100 μg/kg. In this study, we confirmed that CO poisoning leads to cardiomyocytes apoptosis which could be significantly reduced by G-CSF treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carbon monoxide (CO) is an odorless, colorless, tasteless and nonirritating gas that is produced by incomplete combustion of fossil fuel (Weaver 2009). Following burning fossil fuels in defective furnaces and heating sources with poor ventilation, CO is produced (Tucciarone et al. 2009).
Throughout the world, CO poisoning causes many fatalities every year (Satran et al. 2005). The main mechanism of CO poisoning is hypoxia induced following elevation of blood levels of carboxyhemoglobin. The majority of CO-induced deaths happens in confined places specially during the cold season(s) of the year (Prockop and Chichkova 2007; Rosenthal 2006; Vanoli et al. 1989; Weaver 2009).
CO more markedly affects organs that have greater demands for oxygen like the brain, heart, kidney and lung (Satran et al. 2005). The heart is greatly affected by CO poisoning resulting in many deleterious effects such as apoptosis, necrosis, electrocardiogram (ECG) changes (e.g. ST segment depression, ST segment elevation, T wave abnormalities, atrial fibrillation, heart block and QT interval prolongation) (Hashemzaei et al. 2016a; Henry et al. 2006; Kalay et al. 2007; Marius-Nunez 1990; Penney 1990).
CO induces hypoxia at cellular level, binds oxygen and cytochrome-c oxidase and causes oxygen radicals formation and cellular apoptosis (Satran et al. 2005). CO toxic effects are mainly attributed to the occurrence of hypoxia following a decrease in oxygen release after left-shifting of oxyhemoglobin curve and a decrease in oxygen delivery to the tissues (Chiang and Tseng 2012; Ghorbani et al. 2017; Hasegawa et al. 2006; Rosenthal 2006; Satran et al. 2005) .
Granulocyte colony-stimulating factor (G-CSF) is a cytokine that mobilizes and differentiates stem cells towards granulocytes (Baldo et al. 2011). In recent years, it was cleared that G-CSF influences other tissues such as the heart and brain via its own receptors (Schneider et al. 2005). G-CSF can ameliorate hypoxic effects of ischemia/reperfusion and/or myocardial infarction through these receptors (Mohamadpour et al. 2012). Considering the protective effects of G-CSF on cardiac tissue following ischemia/reperfusion and/or myocardial infarction, it was hypothesized that it may reduce these effects in the heart after CO poisoning. So, we investigated whether G-CSF can decrease CO-induced apoptotic effects in cardiomyocytes in rats.
Materials and methods
Chemicals
Terminal Deoxynucleotidyl Transferase (TdT)-Mediated dUTP Nick-End Labeling (TUNEL) kit was obtained from Roche (Mannheim, Germany). Caspase-3/CPP32 colorimetric assay kit was obtained from BioVision, USA. Also, Pierce BCA Protein Assay Kit was used. Recombinant human G-CSF was purchased from Pooyesh Darou, Iran and ketamine/xylazine were obtained from Merck, Germany. Carboxyhemoglobin levels were measured by an ELISA assay kit (MyBioSource, CA). Carbon monoxide capsule with 99.999% purity, was obtained from Darman Gas (Tehran, Iran).
Animals
Twenty five Male Wistar rats (8–10 weeks; 200–250 g) were kept under standard conditions (at 25 °C with 12 h/12 h light/dark cycle) and they had free access to food and water. All animals were treated in accordance with the guidelines for the care and use of laboratory animals prepared by the Animal Research Ethic Committee of Mashhad University of Medical Sciences, Mashhad, Iran.
Experimental groups and study design
Animals were randomly divided in 5 groups (n = 5) placed in a 12 L airtight Plexiglas container with entrance and exit taps. CO/air mixture was flowed into the container. CO concentration was constantly monitored using a CO analyzer (TPI707 Carbon Monoxide Analyzer, Korea). According to our previous study, two different models of G-CSF treatment after intoxication (i.e. single and multiple (for 5 days on a daily basis) doses) with two concentrations of CO poisoning (1500 or 3000 ppm), were employed. At the end of CO exposure period (60 min), animals were exposed to normal air and G-CSF 10, 50 or 100 μg/kg were injected subcutaneously (SC) (Ghorbani et al. 2017).
Caspase3 activity calculation
According to the manufacturer’s instruction, caspase-3 activity was measured by Caspase-3/CPP32 Colorimetric Assay Kit (BioVision, USA) (Iwai-Kanai et al. 1999). First, hear tissues were harvested, homogenized and centrifuged at 10,000 g for 1 min at 4 °C, and finally the supernatants were collected and checked for caspase3 activity. Samples (100 μg) of the extracted protein (supernatant) were incubated with the reaction buffer and Ac-DEVD-p-nitroaniline (pNA) for 1 h at 37 °C. Enzyme-catalyzed release of pNA was measured at 405 nm using spectrophotometer (Awareness Technology Inc., USA). Before calculation of caspase3 activity, the amount of protein content in supernatant was measured by Bradford protein assay kit. The Total Protein Normalized Caspase Activity was measured by this equation:
% Control activity = (Sample Florescence/Total Sample Lysate Protein)/ (Mean of Media Control Florescence/ Mean of Total Media Control Lysate Protein).
In situ apoptosis assay
Myocardial tissue was collected after 5 days of G-CSF 100 μg/kg treatment following CO poisoning. The apoptotic cardiomyocytes were detected by a TUNEL assay using a cell death detection kit (Roche, Mannheim, Germany).
Based on the manufacturer’s instructions, the hearts were fixed in paraformaldehyde, embedded in paraffin, cut into 4-μm thick sections, and incubated with TUNEL reaction mixture containing TdT and fluorescein- dUTP. Before incubation of slices with TUNEL mixture, their permeability was enhanced by proteinase solution. The TUNEL signal was then detected by an anti-fluorescein antibody conjugated with alkaline phosphatase, a reporter enzyme, which catalytically generates a colored product. Three slides from each block and four fields in each slide, were evaluated for the percentage of apoptotic cells. From each field, one hundred cells were randomly counted at X200 magnification. Finally, apoptotic index (number of myocardial nuclei labeled by the TUNEL method/number of total myocardial nuclei) was calculated (Vanoli et al. 1989).
Statistical analysis
Data were analyzed using Statistical Package for the Social Sciences (SPSS) version 16 (SPSS, Inc.; Chicago, Illinois, USA). One-way analysis of variance (ANOVA) to compare continuous variables and Chi-square and Fisher’s Exact Test for categorical variables were used. A p < 0.05 was considered statistically significant.
Results
Carboxyhemoglobin level
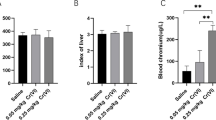
Carboxyhemoglobin concentrations were increased following exposure to both CO concentrations (i.e. 1500 and 3000 ppm) which confirmed the occurrence of CO poisoning at both CO levels. As shown in our past study, carboxyhemoglobin concentrations significantly varied between CO 3000 and 1500 ppm (60–76% vs. 19–46%, respectively) (Ghorbani et al. 2017).
Caspase 3 activity following CO poisoning
As shown in Fig. 1, administration of multiple doses of G-CSF 100 μg/kg/day for 5 days, significantly reduced caspase 3 activity in comparison to control group (p < 0.05). Among different groups that received multiple doses of G-CSF for 5 days, rats treated with G-CSF 100 μg/kg had more marked improvements compared to 10 and 50 μg/kg (Fig. 1). Therefore, rats that received G-CSF 100 μg/kg were further assessed for apoptosis rate using TUNEL assay.
Caspase 3 activity in rat cardiomyocytes following CO poisoning and administration of G-CSF 10, 50 and 100 μg/kg (CO + G-CSF 10, CO + G-CSF 50 and CO + G-CSF 100, respectively) for 5 days. Sham: sham animals (rats that were not exposed to CO) and negative control: CO-poisoned rats treated with normal saline. Data of different groups were compared to that of negative control group (*p < 0.0 5, **p < 0.01 and *** p < 0.001 represent significant differences between different groups and the control group; each bar represents mean ± SD; (n = 5))
TUNEL assay
The amount of apoptotic cells after CO poisoning in G-CSF 100 μg/kg group was decreased significantly (p < 0.05) as compared with the control group (Fig. 2).Treatment with multiple doses of G-CSF 100 μg/kg decreased apoptotic cells in cardiomyocytes after poisoning with CO 3000 ppm.
Discussion
Since is definite approach to prevent delayed effects of CO poisoning, in line with our previous studies (Tabrizian et al. 2017, 2018a, b; Shahsavand et al. 2012), here, we evaluated G-CSF effects on cardiomyocyte apoptosis following CO poisoning in rats. Our results demonstrate that at both 1500 or 3000 ppm CO concentrations, poisoning happened but CO 3000 ppm resulted in a more profound poisoning (Ghorbani et al. 2017; Hashemzaei et al. 2016b). G-CSF decreased apoptosis following CO poisoning.
CO poses its toxic effects through different mechanisms. Producing carboxyhemoglobin as a result of an interaction between CO and hemoglobin is the main player in CO intoxication. In our previous study, carboxyhemoglobin levels >20% indicated the occurrence of CO poisoning (Ghorbani et al. 2017). The results of this study are in accordance with those of previous experiments (Ghorbani et al. 2017; Weaver 2009). Carboxyhemoglobin levels >3% and > 10% in nonsmokers and smokers, respectively, confirm exposure to CO and start CO poisoning (Weaver 2009). It has been demonstrated that intoxication with CO 3000 ppm causes greater deleterious effects on cardimyocytes (Wolf and Green 1999).
Also, our result showed that G-CSF reduced caspase 3 activity in treatment groups in comparison to negative control group which was in line with previous studies. Caspase enzymes are the main enzymes involved in programmable cell death or apoptosis (Chen et al. 2012). Caspase 3 is one of the most active caspases that is activated in both internal and external pathways of cell apoptosis (Mohammad 2010; Wang et al. 2001; Wu et al. 2011). We also showed that G-CSF significantly reduced cardiovascular deleterious effects such as hemodynamic changes, ECG changes and necrosis following CO poisoning in rat cardiomoycytes (Earnshaw 1995). Our results showed that while CO induced apoptosis in the cardiomyocytes, G-CSF decreased the number of apoptotic cells in comparison to negative control group (Fig. 2).
DNA laddering is an irreversible process that occurs even before changes that happen in the cell membrane integrity and permeability, following apoptosis. Apoptosis begins with nuclear endonuclease activation (Maejima et al. 2005). This enzyme can break DNA between nucleosomes and it can be accurately detected by TUNEL assay (Iwanaga et al. 2004). A strong relationship between CO poisoning and cardiomyocytes apoptosis has been reported in the literature. Findings of the current study are consistent with those of Iwanaga and colleagues who found that G-CSF reduces TUNEL positive cells in mice with acute myocardial infarction (Baldo et al. 2008). In another study, it was revealed that G-CSF reduces apoptotic cell numbers after myocardial infarction in rats (Baldo et al. 2011). In the study of Baldo and colleagues, left coronary artery ligation induced myocardial infarction and G-CSF 50 μg/kg administered on days 1, 3 and 7 after surgical procedure, resulted in a reduction of apoptosis as shown by western blotting of Bax, Bcl2, and Bcl-XL (Baldo et al. 2011).
However, more research on this topic needs to be undertaken to clarify the association between anti-apoptotic effects of G-CSF and CO poisoning. Evaluation of G-CSF post-signaling and its probable interactions with apoptotic pathways is also of great importance.
Conclusion
In this study, it was found that CO poisoning causes apoptosis in rats’ cardiomyocytes which was significantly reduced by G-CSF. This reduction in apoptosis rate was at least in part due to decrement of caspase3 activity.
Abbreviations
- CO:
-
Carbon monoxide
- ECG:
-
Electrocardiogram
- G-CSF:
-
Granulocyte colony stimulating factor
- TUNEL:
-
Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labeling
- SC:
-
Subcutaneous
- ANOVA:
-
Analysis of variance
References
Baldo MP, Davel APC, Nicoletti-Carvalho JE, Bordin S, Rossoni LV, Mill JG (2008) Granulocyte colony-stimulating factor reduces mortality by suppressing ventricular arrhythmias in acute phase of myocardial infarction in rats. J Cardiovasc Pharmacol 52(4):375–380. https://doi.org/10.1097/FJC.0b013e31818a2bb0
Baldo MP, Davel AP, Damas-Souza DM, Nicoletti-Carvalho JE, Bordin S, Carvalho HF, Rodrigues SL, Rossoni LV, Mill JG (2011) The antiapoptotic effect of granulocyte colony-stimulating factor reduces infarct size and prevents heart failure development in rats. Cell Physiol Biochem 28(1):33–40. https://doi.org/10.1159/000331711
Chen Y-L, Loh S-H, Chen J-J, Tsai CS (2012) Urotensin II prevents cardiomyocyte apoptosis induced by doxorubicin via Akt and ERK. Eur J Pharmacol 5 680(1–3):88–94. https://doi.org/10.1016/j.ejphar.2012.01.034
Chiang CL, Tseng M-CM (2012) Safe use of electroconvulsive therapy in a highly suicidal survivor of carbon monoxide poisoning. Gen Hosp Psychiatry 34(1):103.e1–103.e3. https://doi.org/10.1016/j.genhosppsych.2011.08.017
Earnshaw WC (1995) Nuclear changes in apoptosis. Curr Opin Cell Biol 7(3):337–343
Ghorbani M, Mohammadpour AH, Abnous K, Movassaghi AR, Sarshoori JR, Shahsavand S, Hashemzaei M, Moallem SA (2017) G-CSF administration attenuates brain injury in rats following carbon monoxide poisoning via different mechanisms. Environ Toxicol 32(1):37–47. https://doi.org/10.1002/tox.22210
Hasegawa H, Takano H, Iwanaga K, Ohtsuka M, Qin Y, Niitsuma Y, Ueda K, Toyoda T, Tadokoro H, Komuro I (2006) Cardioprotective effects of granulocyte colony-stimulating factor in swine with chronic myocardial ischemia. J Am Coll Cardiol 21 47(4):842–849
Hashemzaei M, Barani AK, Iranshahi M, Rezaee R, Tsarouhas K, Tsatsakis AM, Wilks MF, Tabrizian K (2016a) Effects of resveratrol on carbon monoxide-induced cardiotoxicity in rats. Environ Toxicol Pharmacol 46:110–115. https://doi.org/10.1016/j.etap.2016.07.010
Hashemzaei M, Imen Shahidi M, Moallem SA, Abnous K, Ghorbani M, Mohamadpour AH (2016b) Modulation of JAK2, STAT3 and Akt1 proteins by granulocyte colony stimulating factor following carbon monoxide poisoning in male rat. Drug Chem Toxicol 39(4):375–379. https://doi.org/10.3109/01480545.2015.1123267
Henry CR, Satran D, Lindgren B, Adkinson C, Nicholson CI, Henry TD (2006) Myocardial injury and long-term mortality following moderate to severe carbon monoxide poisoning. JAMA 25 295(4):398–402
Iwai-Kanai E, Hasegawa K, Araki M, Kakita T, Morimoto T, Sasayama S (1999) α-And β-adrenergic pathways differentially regulate cell type–specific apoptosis in rat cardiac myocytes. Circulation 100:305–311
Iwanaga K, Takano H, Ohtsuka M, Hasegawa H, Zou Y, Qin Y, Odaka K, Hiroshima K, Tadokoro H, Komuro I (2004) Effects of G-CSF on cardiac remodeling after acute myocardial infarction in swine. Biochem Biophys Res Commun 24 325(4):1353–1359
Kalay N, Ozdogru I, Cetinkaya Y, Eryol NK, Dogan A, Gul I, Inanc T, Ikizceli I, Oguzhan A, Abaci A (2007) Cardiovascular effects of carbon monoxide poisoning. Am J Cardiol 1 99(3):322–324
Maejima Y, Adachi S, Morikawa K, Ito H, Isobe M (2005) Nitric oxide inhibits myocardial apoptosis by preventing caspase-3 activity via S-nitrosylation. J Mol Cell Cardiol 38(1):163–174
Marius-Nunez AL (1990) Myocardial infarction with normal coronary arteries after acute exposure to carbon monoxide. Chest 97(2):491–494
Mohamadpour AH, Moallem SA, Hashemzaei M, Abnous K, Tabatabaee Yazdi SA, Imenshahidi M (2012) Effects of granulocyte colony-stimulating factor on electrocardiogram changes after carbon monoxide poisoning in rats. Drug Chem Toxicol 35(4):353–360. https://doi.org/10.3109/01480545.2011.627863
Mohammad RA (2010) Use of granulocyte colony-stimulating factor in patients with severe sepsis or septic shock. Am J Health-Syst Pharm 67:1238–1245
Penney DG (1990) Acute carbon monoxide poisoning: animal models: a review. Toxicology 62:123–160
Prockop LD, Chichkova RI (2007) Carbon monoxide intoxication: an updated review. J Neurol Sci 15 262(1–2):122–130
Rosenthal LD (2006) Carbon monoxide poisoning. Immediate diagnosis and treatment are crucial to avoid complications. Am J Nurs 106(3):40–46
Satran D, Henry CR, Adkinson C, Nicholson CI, Bracha Y, Henry TD (2005) Cardiovascular manifestations of moderate to severe carbon monoxide poisoning. J Am Coll Cardiol 45(9):1513–1516
Schneider A, Kuhn HG, Schäbitz WR (2005) A role for G-CSF (granulocyte-colony stimulating factor) in the central nervous system. Cell Cycle 4(12):1753–1757
Shahsavand S, Mohammadpour AH, Rezaee R, Behravan E, Sakhtianchi R, Moallem SA (2012) Effect of erythropoietin on serum brain-derived biomarkers after carbon monoxide poisoning in rats. Iran J Basic Med Sci 15(2):752–758
Tabrizian K, Shahraki J, Bazzi M, Rezaee R, Jahantigh H, Hashemzaei M (2017) Neuro-protective effects of resveratrol on carbon monoxide-induced toxicity in male rats. Phytother Res 31(9):1310–1315. https://doi.org/10.1002/ptr.5855
Tabrizian K, Khodayari H, Rezaee R, Jahantigh H, Bagheri G, Tsarouhas K, Hashemzaei M (2018a) Magnesium sulfate protects the heart against carbon monoxide-induced cardiotoxicity in rats. Res Pharm Sci 13(1):65–72. https://doi.org/10.4103/1735-5362.220969
Tabrizian K, Shahriari Z, Rezaee R, Jahantigh H, Bagheri G, Tsarouhas K, Docea AO, Tsatsakis A, Hashemzaei M (2018b) Cardioprotective effects of insulin on carbon monoxide-induced toxicity in male rats. Hum Exp Toxicol 1:960327118788134. https://doi.org/10.1177/0960327118788134
Tucciarone M, Dileo PA, Castro ER, Guerrero M (2009) Myocardial infarction secondary to carbon monoxide poisoning: an uncommon presentation of a common condition. Case report and review of the literature. Am J Ther 16(5):462–465. https://doi.org/10.1097/MJT.0b013e31818c7204
Vanoli E, De Ferrari GM, Stramba-Badiale M, Farber JP, Schwartz PJ (1989) Carbon monoxide and lethal arrhythmias in conscious dogs with a healed myocardial infarction. Am Heart J 117(2):348–357
Wang GW, Zhou Z, Klein JB, Kang YJ (2001) Inhibition of hypoxia/reoxygenation-induced apoptosis in metallothionein-overexpressing cardiomyocytes. Am J Physiol Heart Circ Physiol 280(5):H2292–H2299
Weaver LK (2009) Carbon monoxide poisoning. N Engl J Med 19 360(12):1217–1225. https://doi.org/10.1056/NEJMcp0808891
Wolf BB, Green DR (1999) Suicidal tendencies: apoptotic cell death by caspase family proteinases. J Biol Chem 16 274(29):20049–20052
Wu Q, Li H, Wu Y, Shen W, Zeng L, Cheng H, He L (2011) Protective effects of muscone on ischemia–reperfusion injury in cardiac myocytes. J Ethnopharmacol 31 138(1):34–39. https://doi.org/10.1016/j.jep.2011.08.009
Acknowledgments
This report was a part of a PhD thesis supported by a grant from Mashhad University of Medical Sciences, Mashhad, Iran. The authors would like to thank pathology laboratory staff at Ghaem and Imam Reza Hospitals (Mashhad University of Medical Sciences, Mashhad, Iran) for their kind assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interests.
Rights and permissions
About this article
Cite this article
Hashemzaei, M., Mohammadpour, A., Imenshahidi, M. et al. Does granulocyte colony stimulating factor have protective effects against carbon monoxide-induced apoptosis?. Biologia 73, 1153–1157 (2018). https://doi.org/10.2478/s11756-018-0121-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11756-018-0121-7