Abstract
Background
Angiostrongylus cantonensis is a metastrongylid nematode that has a heteroxenous cycle, where snails act as intermediate hosts and the rodents Rattus rattus and Rattus novergicus are the definitive hosts. However, humans may act as accidental hosts presenting an atypical form of parasitism. This fact has motivated research to better understand systems of relationships involving A. cantonensis, targeting the control of species of gastropods that act as intermediary hosts.
Methods
For this, six groups were formed: three control groups (uninfected) and three infected groups, exposed to approximately 1200 L1 larvae of A. cantonensis. At the end of each week (1, 2, and 3 weeks), snails were dissected without anesthesia and the gonad–digestive gland (DGG) complex was separated for determination of oxygen consumption through high-resolution titration-injection respirometer (Oroboros, Oxygraph; Innsbruck, Austria).
Results
The results indicate suppression of mitochondrial oxidative metabolism of the host and compromised in different mitochondrial respiratory states. This effect, mainly observed in the group exposed to 1 week of infection, showed a decrease of approximately 38% (2.78 ± 0.37 pmol O2/mg of tissue; P < 0.05), 41% (2.76 ± 0.34 pmol O2/mg of tissue; P < 0.05) e 46% (2.91 ± 0.36 pmol O2/mg of tissue; P < 0.05) in the basal oxygen consumption after sequential addition (P + M), succinate and (ADP) in the respiratory medium, differing significantly from the control group.
Conclusion
The results presented indicate that the prepatent infection by this metastrongylid impairs the aerobic oxidative metabolism of its host, causing a reduction in basal oxygen consumption. This effect, observed at the start of development of the parasites, indicates that this stage is the most critical for the success of the infection, and can be explained by a reduction of the mitochondrial density of the tissue analyzed, or also by suppression of enzyme centers related to the oxidative reactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Angiostrongylus cantonensis is a metastrongylid nematode that has a heteroxenous cycle, where snails act as intermediate hosts and the rodents Rattus rattus and Rattus norvegicus are the definitive hosts [27]. Although rodents are the natural hosts of this nematode, humans can accidentally become infected, causing a serious pathological process called neural angiostrongyliasis [20]. In recent years, cases of this disease have been notified in various countries, including Brazil, where it is classified as an emerging disease [18].
According to Caldeira et al. [4], one of the factors contributing directly to the rapid spread of the disease is the parasite’s low specificity in relation to intermediate host. Epidemiological studies have confirmed the involvement of different land snail species as natural hosts of A. cantonensis [15, 18], including the participation of the planorbid Biomphalaria glabrata [10, 23, 24]. This is cause for concern because studies have noted the excellent adaptation of this planorbid species to tropical regions like Brazil [19], heightening the risk of transmission of diseases, including human eosinophilic meningoencephalitis [8].
This situation has motivated studies to better understand the A. cantonensis–B. glabrata relationship, to allow identifying the different pathways by which the parasite acts on the host’s metabolism and how the host reacts to the parasite’s influence [23,24,25,26]. The results of these studies demonstrate that infection by A. cantonensis induces significant changes in the carbohydrate metabolism of B. glabrata, characterized by a decrease in the glucose content in the hemolymph and of polysaccharide reserves stored in the digestive gland and cephalopedal mass, besides variations in the protein, lipid and mineral profiles. Those alterations occur along with the superposition of important enzyme centers, such as aminotransferases and lactate dehydrogenase, indicating that infection by A. cantonensis results in an increase in the rate of deamination of amino acids as well as activation of their anaerobic metabolism. However, the precise mechanism through which infection by A. cantonensis affects the oxidative metabolism of the intermediate host has not yet been characterized.
Previous studies have demonstrated that experimental infection by Schistosoma mansoni in B. alexandrina not only reduces the oxygen consumption rate, but also compromises the host’s aerobic and anaerobic metabolism, inhibiting oxidation of the intermediaries of the tricarboxylic acid cycle and stimulating the production of lactic acid and other final products of anaerobic metabolism in infected snails [11, 17]. This condition, also observed in other host–parasite systems, allows the host snail to maintain its redox balance, while at the same time assuring the production of energy [21].
Therefore, with the aim of complementing the data previously published by Tunholi-Alves et al. [23, 24], which demonstrate the participation of fermentation pathways in the redox balance of the host, here we report the effect of prepatent infection by A. cantonensis on some protein complexes that integrate the mitochondrial respiratory chain of B. glabrata, focusing on the host’s digestive gland (DGG), because it is the main site for development of the nematode.
Materials and Methods
Maintenance of the Snails and Formation of Groups
The snails were kept in aquariums containing 1500 ml of dechlorinated water, to which 0.5 g of CaCO3 was added, in the Laboratório Laboratório de Biologia e Parasitologia de Mamíferos Silvestres Reservatórios—LABPMR (IOC/FIOCRUZ). The water was replaced once a week. Six groups were formed: three control groups (uninfected) and three infected groups. Each aquarium contained 5 snails, reared in the laboratory from hatching, to be certain of their age and that the snails were free of infection by other parasites. The entire experiment was conducted in duplicate, using a total of 60 snails, 30 snails formed the control groups (uninfected) and 30 snails formed the infected groups. The aquariums were kept in a room with controlled temperature of 25 °C throughout the experiment. The study was made in 3 weeks, period that corresponds to the prepatent development of A. cantonensis in B. glabrata [25].
The snails were fed with dehydrated lettuce (Lactuca sativa L.) ad libitum. The lettuce leaves were changed every day to prevent their fermentation, at which time the number of dead snails was also noted.
Parasites
Third-stage larvae (L3) of A. cantonensis, obtained from specimens of A. fulica collected in the municipality of Olinda, Pernambuco, Brazil in 2008, in the area surrounding the home of a patient diagnosed with eosinophilic meningoencephalitis, were inoculated in Rattus norvegicus in the Laboratório of Patologia do Instituto Oswaldo Cruz (Fiocruz), where the cycle was maintained. The first-stage larva (L1) utilized in this study were obtained from this experimental cycle maintained in the Laboratory de Biologia e Parasitologia de Mamíferos Silvestres Reservatórios—LABPMR (IOC) (Fiocruz).
Infection of the Snails
The feces of parasitized R. norvegicus were collected and used to obtain the larvae by the technique of Baermann, employed to separate and decant the L1 larvae [28]. After processing the fecal samples, specimens of B. glabrata (8–12 mm) at 90 days of age on average were exposed individually to approximately 1.200 L1 larvae on plates with 24 holes. After 48 h, the snails from each group were individually examined under a stereomicroscope to detected larvae (L1 stage) in the plates [22]. The absence of larvae in the plates ensured the infectivity and susceptibility of snails under laboratory conditions. Posteriorly, snails were removed from the plates and transferred to the aquariums for formation of the experimental groups. The susceptibility of B. glabrata during the experimentally infection by A. cantonensis has been showed as 100% infection rate [24].
The snails were grouped according to their infection stage (1, 2, and 3 weeks post exposure). In each period analyzed, 5 infected and 5 uninfected snails (control) were dissected.
Dissection of the Snails to Collect Tissues
Each week snails from each experimental group (n = 5) were dissected without anesthesia and the gonad–digestive gland (DGG) complex was separated for analysis. Mechanical permeabilization of the tissue was performed as described by [12], adapted by our group for this snail’s DDG complex.
Oxygen Consumption in the Permeabilized DGG Measured by High-Resolution Respirometry
Oxygen consumption was measured with a high-resolution titration-injection respirometer (Oroboros, Oxygraph; Innsbruck, Austria) [7]. The tissues were incubated in 2.0 ml of a MiR05 solution, and the chamber was closed immediately before the start of the experiments. The basal respiration was evaluated after addition of the mitochondrial substrates (5 mmol l−1 of pyruvate and 5 mmol/l of malate for complex I and 10 mmol l−1 of succinate for complex II). The phosphorylation respiration rate (state 3) was measured with the addition of 0.5 mmol l−1 of ADP. In turn, state 4º (proton leak) and state 3u (uncoupled state) were reached with the addition of 1 μg/ml of oligomycin and 0.4 μmol l−1 of FCCP (fluorocarbonyl-cyanide phenylhydrazone), respectively. Pulses of 2.5 μM–5 μM were added to the experiment to check mitochondrial permeability (outer membrane). Rotenone and antimycin (1 μM and 5 μM, respectively) were used to reveal non-mitochondrial respiration. The use of these drugs was adapted from Kuznetsov et al. [12] and Lemieux et al. [13].
Histological Analyses
Three snails from each experimental group (control and infected) were dissected and transferred to Duboscq-Brasil fixative [6]. The soft tissues were processed according to routine histological techniques [9]. The sections (5 µm) were stained using hematoxylin and eosin and observed under a Zeiss Axioplan light microscope; images were captured with an MRc5 AxioCam digital camera and processed with the Axiovision software.
Statistical Analyses
The results obtained were expressed as mean ± standard deviation and the Tukey test and ANOVA were used to compare the means. (P < 0.05) (InStat, GraphPad, v.4.00, Prism, GraphPad, v.3.02, Prism, Inc.).
Results
The values of the variables of interest observed in the control group did not differ significantly in the 3 weeks of the experiment; so, we grouped them in an average value, called time zero, or week zero, of infection.
In the present study, significant changes in mitochondrial system of Biomphalaria glabrata experimentally infected by A. cantonensis were shown. The results indicate suppression of mitochondrial oxidative metabolism of the host and compromised in different mitochondrial respiratory states. This effect, mainly observed in the group exposed to 1 week of infection, showed a decrease of approximately 38% (2.78 ± 0.37 pmol O2/mg of tissue; P < 0.05), 41% (2.76 ± 0.34 pmol O2/mg of tissue; P < 0.05) e 46% (2.91 ± 0.36 pmol O2/mg of tissue; P < 0.05) in the basal oxygen consumption after sequential addition (P + M), succinate and (ADP) in the respiratory medium, differing significantly from the control group (Fig. 1a). Similar condition was observed for mitochondrial state 3u (uncoupled state). In this circumstance, the addition of the FCCP in the respiratory medium induced a significant decrease in oxygen consumption in infected snails, differing in relation to the average of the control group (7.39 ± 0.69 pmol O2/mg of tissue; P < 0.05).
Mitochondrial physiology of B. glabrata after the first (a), second (b) and third (c) week of infection by Angiostrongylus cantonensis. Relationship established between the consumption of oxygen, expressed by O2 pmol/mg tissue after sequential additions of malate (5 mM) and pyruvate (5 mM), Succinate (10 mM), ADP (0.5 mM), cytochrome c (2.5–5 μM), oligomycin (1 μg/ml), FCCP (0.4 μM), rotenone (1 μM) and antimycin (5 μM). Note: for cytochrome c, experiments were very little or no responsive to this drug. *Means differ significantly (mean ± SE). Control group (n = 6 snails); infected group (n = 10 snails)
In snails exposed to 2 weeks of infection, changes in mitochondrial respiratory status were demonstrated only to the state 3u (uncoupled state). In this case, infection by A. cantonensis induced a decrease of 63% (2.9 ± 0.57 pmol O2/mg of tissue; P < 0.05) in oxygen consumption after addition of FCCP in the respiratory medium, in relation to the control group (7.87 ± 0.84 pmol O2/mg of tissue; P < 0.05) (Fig. 1b).
In contrast, a contrary variation profile occurred in the later periods of infection, suggesting a mitochondrial biogenesis process. In all analyzed mitochondrial states, infection by this metastrongylid did not result in significant decreases in oxygen consumption in relation to the control snail (Fig. 1c). This trend of mitochondrial plasticity was maintained when we compared the different profiles among the infected groups. In this case, snails exposed to 3 weeks of infection presented a significant increase in basal oxygen consumption after sequential addition of succinate, ADP, FCCP and rotenone, differing from the other groups exposed to 1 and 2 weeks of infection (Fig. 2).
Influence of the pre-patent development of A. cantonensis on mitochondrial physiology of B. glabrata. Relationship established between the consumption of oxygen, expressed by O2 pmol/mg of tissue after sequential additions of malate (5 mM) and pyruvate (5 mM), Succinate (10 mM), ADP (0.5 mM), cytochrome c (2.5–5 μM), oligomycin (1 μg/ml), FCCP (0.4 μM), rotenone (1 μM) and antimycin (5 μM). *Means differ significantly (mean ± SE) (n = 10 snails)
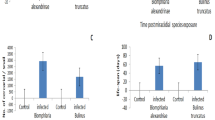
The histochemical results revealed the presence of larvae stages in the DGG (A) and muffle (B–C) of the A. cantonensis-infected snails, showing severe cellular disorganization, characterized by the formation of a hemocyte infiltrate and by the presence and proliferation of collagen fibers and fibrous connective tissue, resulting in the loss of organ function. In the control snails, the absence of larval stages of the parasite was observed in sections of digestive gland tissue, with their integrity and functioning reserved (Fig. 3).
Histological section of the digestive gland (a) and muffle (b, c) of B. glabrata infected by A. cantonensis stained with hematoxylin–eosin showing a region characterized by intense cellular disorganization induced by an inflammatory infiltrate. Scale bar = 20 µm. d Uninfected snails section showing the region functional digestive gland. Scale bar = 40 µm
Discussion
Under physiological conditions, snails’ oxidative metabolism is predominantly based on aerobic pathways, in which the Krebs cycle assumes a central role in the process of energy generation [21, 22]. However, when subjected to physiological stress conditions, like those induced by infection by helminth larvae, these organisms can redirect their metabolic flow, resulting in activation of enzyme centers related to fermentation pathways [1]. This process was recently documented by Tunholi-Alves et al. [23, 24] studying the B. glabrata/A.cantonensis interface, who observed the transition of the host’s oxidative metabolism in response to the ontogenetic development of the nematode. Despite the findings of that study, the possible changes resulting from infection by A. cantonensis on the mitochondrial physiology of B. glabrata have not yet been determined, limiting the understanding of this relationship.
The data presented in this study indicate that prepatent infection by A. cantonensis induces substantial alterations in the mitochondrial physiology of B. glabrata, characterized by suppression of the aerobic oxidative metabolism and consequent reduction of the basal oxygen consumption rate in relation to the control group. These alterations were more pronounced in the initial stage of the parasite development (first and second weeks), with partial reestablishment of the original rate in the third week of infection. The results demonstrate impairment in respiratory states 2 (basal) and 3 (phosphorylation) of B. glabrata at the start of infection, causing a lesser response after addition of succinate and ADP, respectively. Similar conditions were reported by Mohamed and Ishak [17], studying the B. alexandrina/S. mansoni interface. According to them, the reduction of the oxygen consumption rate in the mitochondrial system of infected snails can be partly explained by the direct action of the larvae in different stages in the host’s tissue, resulting in relevant tissue changes, especially in the gonad–digestive gland complex, or can also be mediated by the action of the parasite’s secretion and excretion products, which inhibit the oxide reduction reactions that integrate the Krebs cycle, and consequently mitochondrial phosphorylation process. In this circumstance, the maintenance of the host’s redox balance is seriously compromised, favoring the activation of complementary mechanisms [16], as has been described in other studies involving the relation between snails and helminth larvae [21].
Studying the interrelationship of B. glabrata/A.cantonensis [26], demonstrated significant tissue changes in the host’s digestive gland as a consequence of parasitic development. According to the authors, the occurrence of tissue lesions in visceral organs of B. glabrata was demonstrated by a strong inflammatory reaction together with significant increase in the activities of l-aspartate: 2 oxoglutarate aminotransferase (AST) and l-alanine: 2 oxoglutarate aminotransferase (ALT) in the infected snails’ hemolymph. This process was still accompanied by areas of metastatic calcification as a complementary reaction during encapsulation of A. cantonensis and by diffuse proliferation of hemocytes and expansion of the extracellular matrix, leading to the formation of areas of fibrosis. These changes, which result in the loss of structural and functional viability of the cells that make up this organ, contribute to reduction of the mitochondrial density in the digestive gland of infected B. glabrata snails, favoring diminished oxygen consumption.
In this study, we observed significant changes in the 3U mitochondrial state (uncoupled state) after the addition of FCCP (carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone). This compound is characterized by its liposoluble nature, acting to dissipate the electrochemical gradient generated during oxidative phosphorylation through the internal mitochondrial membrane. This result in a loss of respiratory control that leads to an increase in the consumption of oxygen not coupled to ATP synthesis. Therefore, the lower oxygen consumption, and hence the lower respiration rate, of snails infected by A. cantonensis (1 and 2 weeks), in the presence of the uncoupling agent, suggests a reduction in the number of protein complexes that integrate the respiratory chain, or a smaller number of mitochondria, as a consequence of the destructive action of the parasite on the host’s tissue. Another explanation is the reduction in the speed of the oxidation reactions of the tricarboxylic acid cycle in infected snails in relation to uninfected ones. As a consequence, a decrease in the rate or production and reoxidation of NADH and FADH2 is expected, directly impairing the conversion of electromotive force into proton-motive force. These results complement those presented by Tunholi-Alves et al. [23, 24] and suggest that under such conditions, maintenance of the host’s redox balance depends directly on the fermentation pathways, in which lactate dehydrogenase assumes a central role in the process.
Interestingly, a contrary variation profile occurred in the group 3 weeks after exposure to L1 larvae. At this stage of parasitic development, infection does not induce significant changes in the aerobic oxidative metabolism studied, suggesting a mitochondrial biogenesis process [3, 14]. Such results can be explained by the dynamics of parasite development, since in the third week of infection, the second-stage larvae have abandoned the gonad–digestive gland complex, and through the hemolymph system have migrated toward the ducts of the mucus-producing glands located in the cephalopedal mass, completing their development. Another possibility is the participation of mechanisms involved in the plasticity process of the mitochondrial function [5]. This effect was documented by Bishop et al. [2] studying the effect of starvation on the mitochondrial system in gastropod Helix aspersa. According to the authors, the reduction in the aerobic oxidative metabolism of metabolically depressed snails is due to the temporary down-regulation of enzyme centers related with the Krebs cycle (citrate synthase) and the electron transport chain (cytochrome and oxidase), which is reestablished with the return of the metabolic capacity. Hence, the increase in the oxidative capacity presented by the snails 3 weeks after the start of infection in relation to those in the initial phase of parasite development can also be related to intrinsic mitochondrial factors, whose activity is normalized together with the migration of the larval stages to the final development site.
This article reports for the first time the effect of infection by A. cantonensis on the mitochondrial physiology of B. glabrata. The results presented indicate that the prepatent infection by this metastrongylid impairs the aerobic oxidative metabolism of its host, causing a reduction in basal oxygen consumption. This effect, observed at the start of development of the parasites, indicates that this stage is the most critical for the success of the infection, and can be explained by a reduction of the mitochondrial density of the tissue analyzed, or also by suppression of enzyme centers related to the oxidative reactions. These findings complement those reported by Tunholi-Alves et al. [23, 24] and emphasize the importance of the fermentation pathways involved in the reestablishment of the host’s redox balance.
References
Bezerra JCB, Becker W, Zelck UE (1997) A comparative study of the organic acid content of the hemolymph of Schistosoma mansoni-resistant and susceptible strains of Biomphalaria glabrata. Mem Inst Oswaldo Cruz 92:421–425
Bishop T, Ocloo A, Brand MD (2002) Structure and function of mitochondria in hepatopancreas cells from metabolically depressed snails. Physiol Biochem Zool 75:134–144
Bremer K, Monk CT, Gurd BJ, Moyes CD (2012) Transcriptional regulation of temperature-induced remodeling of muscle bioenergetics in goldfish. Am J Physiol 303:150–158
Caldeira RL, Mendonça CLG, Goveia CO, Lenzi HL, Graeff-Teixeira C, Lima WS, Mota EM, Pecora IL, Medeiros AMZ, Carvalho OS (2007) First Record of molluscs naturally infected with Angiostrongylus cantonensis (Chen, 1935) (Nematoda: Metastrongylidae) in Brazil. Mem Inst Oswaldo Cruz 102:887–889
Dos-Santos RS, Galina A, Seixas W (2012) Cold acclimation increases mitochondrial oxidative capacity without inducing mitochondrial uncoupling in goldfish white skeletal muscle. BiO 2:82–87
Fernandes MC (1949) Métodos Escolhidos de Técnicas Microscópicas. Imprensa Nacional, Rio de Janeiro
Gnaiger E (2001) Bioenergetics at low oxygen: dependence of respiration and phosphorylation on oxygen and adenosine diphosphate supply. Respir Physiol 128:277–297
Graeff-Teixeira C, Silva ACA, Yoshimura K (2009) Update on eosinophili meningoencephalitis and its clinical relevance. Clin Microbiol 22:322–348
Humason GL (1979) Animal tissue techniques. Freeman, San Francisco
Ibrahim MM (2007) Prevalence and intensity of Angiostrongylus cantonensis in freshwater snails in relation to some ecological and biological factors. Parasite 14:61–70
Ishak MM, Mohamed AM, Shraf AA (1975) Carbohydrate metabolism in uninfected and trematode-infected snails Biomphalaria alexandrina and Bulinus truncatus. Comp Biochem Physiol B 53:499–505
Kuznetsov AV, Strobl D, Ruttmann E, Konigsrainer A, Margreiter R, Gnaiger E (2002) Evaluation of mitochondrial respiratory function in small biopsies of liver. Anal Biochem 305:186–194
Lemieux H, Semsroth S, Antretter H, Höfer D, Gnaiger E (2011) Mitochondrial respiratory control and early defects of oxidative phosphorylation in the failing human heart. Int J Biochem Cell Biol 43:1729–1738
Lemoine CM, Genge CE, Moyes CD (2008) Role of the PGC-1 family in the metabolic adaptation of goldfish to diet and temperature. J Exp Biol 211:1448–1455
Maldonado A, Simões RO, Oliveira AP, Mota EM, Fernandez MA, Pereira ZM, Monteiro SS, Torres EJ, Thieng SC (2010) First report of Angiostrongylus cantonensis (Nematoda: Metastrongylidae) in Achatina fulica (Mollusca: Gastropoda) from Southeast and South Brazil. Mem Inst Oswaldo Cruz 105:1–4
Mantawy MM, Mohamed NZ, Arfa AF, Aly HF (2013) Carboxylic acids and their metabolic enzymes as new novel biomarkers of susceptible, resistant strains of Biomphalaria alexandrina and snails infected with Schistosoma mansoni. Int J Sci Eng Technol 04:1039–1047
Mohamed AM, Ishak MM (1982) Comparative effects of schistosome infection and starvation on the respiratory transport chain of the snails Biomphalaria alexandrina and Bulinus truncatus. Comp Biochem Physiol B 71:289–292
Morassutti AL, Thiengo SC, Fernandez M, Sawanyawisuth K, Graeff-Teixeira C (2014) Eosinophilic meningitis caused by Angiostrongylus cantonensis: an emergent disease in Brazil. Mem Inst Oswaldo Cruz 109:399–407
Paraense WL (1975) Estado atual da sistemática dos planorbídeos brasileiros. Arquivos do Museu Nacional 55:105–111
Thiengo SC, Maldonado A, Mota EM, Torres EJ, Caldeira R, Carvalho OS, Oliveira AP, Simões RO, Fernandez MA, Lanfredi RM (2010) The giant African snail Achatina fulica as natural intermediate host of Angiostrongylus cantonensis in Pernambuco, northeast Brazil. Acta Trop 115:194–199
Tunholi VM, Tunholi-Alves VM, Lustrino D, Castro N, Sant’Ana L, Garcia J, Maldonado AJ, Santos MAJ, Rodrigues MLA, Pinheiro J (2013) Aerobic to anaerobic transition in Biomphalaria glabrata (Say, 1818) infected with different miracidial doses of Echinostoma paraensei (Lie and Basch, 1967) by high performance liquid chromatography. Exp Parasitol 133:403–410
Tunholi VM, Tunholi-Alves VM, Santos AT, Garcia J, Maldonado AJ, da-Silva WS, Pinheiro J (2016) Evaluation of the mitochondrial system in the gonad-digestive gland complex of Biomphalaria glabrata (Mollusca, Gastropoda) after infection by Echinostoma paraensei (Trematoda, Echinostomatidae). J Invertebr Pathol 136:136–141
Tunholi-Alves VM, Tunholi VM, Castro RN, Sant’ana L, Santos-Amaral L, Garcia J, Oliveira AM, Maldonado A, Thiengo SC, Pinheiro J (2014) Activation of anaerobic metabolism in Biomphalaria glabrata (Mollusca:Gastropoda) experimentally infected by Angiostrongylus cantonensis (Nematoda, Metastrongylidae) by high-performance liquid chromatography. Parasitol Int 63:64–68
Tunholi-Alves VM, Tunholi VM, Garcia J, Costa-Neto SF, Maldonado A, Santos MAJ, Thiengo SC, Pinheiro J (2014) Changes in the calcium metabolism of Biomphalaria glabrata experimentally infected with Angiostrongylus cantonensis. J Helminthol 88:160–165
Tunholi-Alves VM, Tunholi VM, Lustrino D, Amaral LS, Thiengo SC, Pinheiro J (2011) Changes in the reproductive biology of Biomphalaria glabrata experimentally infected with the nematode Angiostrongylus cantonensis. J Invertebr Pathol 108:220–223
Tunholi-Alves VM, Tunholi VM, Pinheiro J, Thiengo SC (2012) Effects of infection by larvae of Angiostrongylus cantonensis (Nematoda, Metastrongylidae) on the metabolism of the experimental intermediate host Biomphalaria glabrata. Exp Parasitol 131:143–147
Wang QP, De-Hua L, Xing-Quan Z, Xiao-Guang C, Zhao-Rong L (2008) Human angiostrongyliasis. Lancet Infect Dis 8:621–630
Willcox HP, Coura JR (1989) Nova concepção para o método de Baermann Moraes—Coutinho na pesquisa de larvas de nematódeos. Mem Inst Oswaldo Cruz 84:539–565
Acknowledgements
This study was supported in part by Conselho Nacional para o Desenvolvimento Científico e Tecnológico (CNPq) and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tunholi-Alves, V.M., Tunholi, V.M., Amaral, L.S. et al. Alterations in the Mitochondrial Physiology of Biomphalaria glabrata (Mollusca: Gastropoda) After Experimental Infection by Angiostrongylus cantonensis (Nematoda: Metastrongylidae). Acta Parasit. 64, 693–699 (2019). https://doi.org/10.2478/s11686-019-00039-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11686-019-00039-7