Abstract
For the first time, alterations in the oxidative metabolism of Achatina fulica experimentally infected with different parasite loads of Angiostrongylus cantonensis were determined. For this, the hemolymph activities of lactate dehydrogenase (LDH) and hexokinase and the glucose concentrations in the hemolymph, as well as the polysaccharide reserves in the digestive gland and cephalopedal mass, were assessed. Additionally, the contents of some carboxylic acids in the hemolymph of infected and uninfected snails were determined by high-performance liquid chromatography (HPLC), permitting a better understanding of the alterations related to the host’s oxidative metabolism. As the main results, activation of oxidative pathways, such as the glycolytic pathway, was demonstrated in response to the increase in the activity of hexokinase. This tendency was confirmed by the decrease in the contents of glucose in the hemolymph of parasitized snails, indicating that the infection by A. cantonensis alters the host’s metabolism, and that these changes are strongly influenced by the parasite load. This metabolic scenario was accompanied by activation of the anaerobic fermentative metabolism, indicated not only by an increase in the activity of (LDH), but also by a reduction of the content of pyruvic acid and accumulation of lactic acid in the hemolymph of parasitized snails. In this circumstance, maintenance of the host’s redox balance occurs through activation of the fermentative pathways, and LDH plays a central role in this process. Together, the results indicate that A. cantonensis infection induces activation of the anaerobic metabolism of A. fulica, characterized not only by the accumulation of lactic acid, but also by a reduction in the pyruvic acid and oxalic acid contents in the hemolymph of the infected snails.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to systematic studies, the genus Angiostrongylus is composed of 21 species, which are widely distributed in the world, posing a threat to the health of wild and domestic animals (Robles et al. 2016). Of these, two stand out, A. costaricensis and A. cantonensis, due to their potential involvement in infection and pathological alterations in humans. The first species is a causal agent of abdominal angiostrongyliasis in the Americas, including Brazil, while the second is the main cause of eosinophilic meningoencephalitis in humans, a parasite-borne disease that causes significant alterations in the central nervous system due to the neurotropic behavior of the infective larvae (Martins et al. 2015).
To date, epidemiological studies have confirmed more than 2800 human cases of neural angiostrongyliasis, reported in about 30 countries, with the majority of cases having been notified in Southeast Asia and Pacific Islands (Cowie 2013; Wang et al. 2008). In the Americas, the first reports of infection were confirmed in the USA (New et al. 1995), followed by occurrences in other countries, such as Jamaica (Slom et al. 2002), Haiti (Raccurt et al. 2003), Cuba (Dorta-Contreras et al. 2011), and Brazil (Thiengo et al. 2010). The most recent data, presented by Morassutti et al. (2014), confirm the occurrence of 34 human cases of neural angiostrongyliasis in Brazil, where it is classified by the Ministry of Health as an emerging parasitic disease.
Biologically, A. cantonensis presents a heteroxenous life cycle, involving the sequential participation of two hosts, where terrestrial and aquatic snails act as intermediate hosts and rodents (mainly Rattus rattus and Rattus norvegicus) are the natural definitive hosts. According to Caldeira et al. (2007), the low specificity established between the nematode and its intermediate host is the main factor for the rapid dispersion of the parasite. Among the different species of mollusks that participate in this parasite’s development, particular attention has been focused on Achatina fulica, which has often been found to have a high prevalence of natural infection (Thiengo et al. 2010). This situation was demonstrated by Oliveira et al. (2014). According to the authors, the prevalence rate found was greater than 50%, but without a pattern of variation during the months studied. This information is in line with the results published by Maldonado et al. (2010), who also observed high prevalence of the nematode in specimens of A. fulica collected in the South and Southeast regions of Brazil. These findings are important, since Brazil and other regions of the world are experiencing an explosive invasion of this gastropod, a condition that is propitious for the rapid dispersion of this zoonosis (Lima et al. 2009; Lv et al. 2009).
Therefore, because of the importance attributed to gastropods in the transmission of A. cantonensis, studies of the parasite-host interaction have been conducted as part of a strategy to develop measures to control the snail and consequently the pathogens transmitted by it (Tunholi-Alves et al. 2012, 2014, 2015). According to these studies, infection by A. cantonensis induces significant metabolic alterations in Biomphalaria glabrata, a potential intermediate host, characterized by depletion of polysaccharide reserves, activation of anaerobic fermentative pathways, increased deamination rate of amino acids, accumulation of nitrogen degradation compounds, and an intense lipolytic process. Despite these findings, little is known about the alterations that occur during the development of A. cantonensis in A. fulica and the influence of different parasite loads on the snail’s metabolic status, an important parameter already studied in interaction models involving mollusks-trematodes (Bandstra et al. 2006; Tunholi et al. 2013, 2016). Therefore, this study was conducted to shed more light on the A. cantonensis-A. fulica interface and determine the impacts of different parasite loads on the metabolic profile of infected snails.
Material and methods
Maintenance of the snails and formation of groups
The snails used in this study were obtained from a colony kept in the Laboratório de Biologia e Parasitologia de Mamíferos Silvestres e Reservatórios, Instituto Oswaldo Cruz (Fiocruz), located in the city of Rio de Janeiro, Brazil. Nine experimental groups were formed: one control group (uninfected) and eight groups individually exposed to different quantities of larval nematodes (500, 1000, 1500, 2000, 2500, 5000, 10,000, and 15,000 L1 larvae). Each group contained ten snails, reared in the laboratory from hatching, to be certain of their age and that the snails were free of infection by other parasites. The entire experiment was conducted in duplicate, using a total of 180 snails, of which 20 snails constituted the control group and 160 snails the challenged groups. The terrariums were kept in a room with controlled temperature of 25 °C throughout the experiment. The snails were fed with fresh lettuce leaves (Lactuca sativa L.) ad libitum.
The terrariums were cleaned every other day, when the lettuce leaves were replaced to prevent their fermentation.
Parasites
The strain of A. cantonensis studied was isolated by collecting naturally infected A. fulica specimens in São Gonçalo (Rio de Janeiro state) (22° 49′ 37″ S; 43° 03′ 14″ W). The cycle was maintained in the Laboratório de Biologia e Parasitologia de Mamíferos Silvestres Reservatórios (IOC) FIOCRUZ—Rio de Janeiro by passages in R. norvegicus (Wistar) used as definitive host and Biomphalaria glabrata as intermediate host, with the permits for the use of animals obtained from Oswaldo Cruz Foundation (FIOCRUZ) Ethical Committee on Animal Use (permit number LW 24/10). The first-stage larvae (L1) utilized in this study were obtained from this experimental cycle maintained in the Laboratory of Patologia do Instituto Oswaldo Cruz (Fiocruz).
Infection of the snails
The feces of parasitized R. norvegicus were collected and used to obtain the larvae by the technique of Baermann, employed to separate and decant the L1 larvae (Willcox and Coura 1989). After processing the fecal samples, specimens of A. fulica with 90 days of age in average were exposed individually to different quantities mentioned above.
The determination of the different quantities of L1 larvae was performed according to Bonfim et al. (2014). Briefly, after decantation and separation of L1 larvae by the technique Baermann, a 100 μL aliquot was analyzed in magnifying glass to estimate the number of larvae. From this relationship, the parasite load was estimated.
The L1 larvae of A. cantonensis were spread on pieces of fresh lettuce leaves, which were in turn placed in Petri dishes with moistened filter paper at the bottom. The snails were added over the lettuce leaves. The Petri dishes were closed, and the snails were maintained in contact with the larvae overnight. After this, they were transferred to terrariums and maintained as described above.
Dissection and collection of the hemolymph and tissues
At the end of the 3-week experimental period, the snails from control and infected groups were dissected and the hemolymph was collected by cardiac puncture and the tissues separated (DGG and CM), stored in Eppendorf tubes and maintained at − 80 °C until the biochemical analyses. All the samples were kept in an ice bath during dissection. The choice of the study period (3 weeks) was based on Tunholi-Alves et al. (2011), period that corresponds to the prepatent development of A. cantonensis.
Determination of glucose concentration and LDH activity
The determination of the glucose was performed according to Tunholi et al. (2013). For this, 10 μL of sample was added to 1 mL of color reagent (0.05 M phosphate buffer solution, pH 7.45 ± 0.1; 0.03 mM aminoantipyrine and 15 mM of sodium p-hydroxybenzoate; 12 kU of glucose oxidase; and 0.8 kU peroxidase per liter). The product formed by oxidation of 4-aminoantipyrine was determined by spectrophotometry with maximum absorption at 510 nm, using a standard solution of glucose at a concentration of 100 mg/dL (Mello-Silva et al. 2010). The readings were expressed in milligram per deciliter.
The determination of LDH activity was conducted according Tunholi-Alves et al. (2014). The readings were taken in a spectrophotometer at 505 nm and the results were expressed in UI.
Determination of the glycogen concentration
The glycogen contents of the DGG and cephalopedal mass were determined according to the method 3.5 DNS (Sumner 1924; Pinheiro and Gomes 1994) and expressed as milligram glucose per gram tissue, wet weight. The samples used for biochemical analyses were obtained from ten snails/experimental group analyzed individually.
Chemicals
Standards of oxalic, succinic, pyruvic, and lactic acids were purchased from Sigma–Aldrich (Steinheim, Germany) in the highest purity grade available. Acetonitrile, sodium dihydrogen phosphate, and phosphoric acid were of analytical purity or for chromatographic use. Ultrapure water was obtained from a Milli-Q water purification system (Millipore, Bedford, MA, USA). Stock standard solutions were dissolved in mobile phase, phosphate buffer adjusted to pH 2.2 with phosphoric acid, and stored at 4 °C.
HPLC analysis
All HPLC experiments were carried out in a Shimadzu LC-20AT system equipped with photodiode array detector (PDA; SPD-M20A, Shimadzu, Japan) coupled to an LC Solution ChemStation data processing station. Separations were carried out with reversed phase column C18 (150 × 4.5 mM I.D., 5 μL, Allure ® Organic Acids, Restek) in isocratic conditions. The mobile phase consisted of 1% acetonitrile in 20 mol L−1 NaH2PO4 aqueous solution, adjusted to pH 2.2 with H3PO4. The temperature was set at 36 °C and the flow rate was 0.8 mL/min. The chromatograms were monitored at 210 nm and the injection volume was 20 μL. The identification of organic acids present in the samples was based on a comparison of UV spectra and retention times with those of the pure standard solutions. Quantification was performed on the basis of linear calibration plots of peak area against concentration. Calibration lines were constructed based on five concentration levels of standard solutions. The calibration graphs for oxalic, succinic, pyruvic, and lactic acids were linear (r = 0.99) in all cases. All experiments were performed in triplicate.
The hemolymph was vortexed and centrifuged for 10 min at 2520g. The supernatant was separated and undissolved particles were removed by filtration using 45-μm membrane filters. Aliquots of 20 μL were used for the chromatographic analysis.
Hexokinase activity
DGGs were homogenized in 1 mL extraction buffer containing 20 mM Tris–HCl, pH 7.5 and centrifuged at 10,000×g for 10 min. The supernatant was assayed for hexokinase (HK) activity in 20 mM Tris–HCl pH 7.5 containing 6 mM MgCl2, 1 mM ATP, 0.5 mM NAD+, 10 mM NaF, and the reaction started with 2 mM glucose. The glucose 6-phosphate formed was measured by adding an equal volume of 20 mM Tris–HCl pH 7.5, 6 mM MgCl2, 1 unit/mL glucose 6-phosphate dehydrogenase from Leuconostoc mesenteroides, and 0.3 mM β-NAD+. The production of β-NADH was read at 340 nm using a molar extinction coefficient of 6.22 M−1 as described by Galina and Da Silva (2000). The study was performed using six snails/experimental group analyzed individually.
Histological analyses
After 3 weeks of infection, four specimens of each group were dissected and transferred to Duboscq-Brasil fixative (Fernandes 1949). The soft tissues were processed according to routine histological techniques (Humason 1979). The sections (5 μm) were stained using hematoxylin and eosin and observed under a Zeiss Axioplan light microscope; images were captured with an MRc5 AxioCam digital camera.
Infectivity analysis
The cephalopodal masses of ten snails from each group infected with A. cantonensis, obtained 21 days after infection, were individually fragmented and digested in a 0.7% HCl solution for 6 h and subsequently subjected to the method of Baermann for L3 recovery. The larvae were counted with the aid of a stereoscopic microscope and a manual cell counter (Bonfim et al. 2014).
Statistical analyses
The results were expressed as mean ± standard deviation and submitted to one-way ANOVA and then the Tukey–Kramer test (P < 0.05%) to compare the means (InStat, GraphPad, v.4.00, Prism, GraphPad, v.3.02, Prism Inc.).
Results
The infection by A. cantonensis induced significant changes in the oxidative metabolism of A. fulica, with a load-dependent response. For the control group, due to the absence of variation in the parameters analyzed, the numerical values, expressed as (mean ± standard deviation), were grouped in a single value called zero load, to better reveal the variations between the parasitized groups.
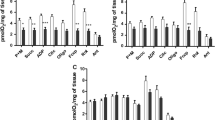
As a main result, a significant reduction in the hemolymph glucose content was observed only in the groups exposed to a load greater than or equal to 5.000 L1 larvae (0.22 ± 0.023 mg/dL), differing significantly from the control group. This represents a reduction of approximately 77.77% in relation to the uninfected snails (0.99 ± 0.061 mg/dL), where the lowest concentration was observed in the group submitted to a load of 15.000 L1 larvae (0.17 ± 0.054 mg/dL) (Fig. 1a). That variation was accompanied by activation of allosteric centers related to regulation of the glycolytic pathway, including hexokinase (HK), an enzyme involved in the first stage of oxidation of glucose molecules. The activity of HK was significantly greater in the groups submitted to a load higher than 2.500 L1 larvae (0.2 ± 0.015 U/mg of tissue), indicating the importance of the pathway to maintain the host’s energy status (Fig. 1b).
Glucose concentration (mg/dL) (a) in the hemolymph and hexokinase activity (U/mg tissue) (b) in the digestive gland of Achatina fulica experimentally infected by different parasite loads (500, 1000, 1500, 2000, 2500, 5000, 10, 000, and 15, 000 L1 larvae) of Angiostrongylus cantonensis. Values are expressed as (mean ± standard deviation). **/*** indicate means differ significantly in relation to the control group, P < 0.01; P < 0.05, respectively
This metabolic scenario was accompanied by activation of the fermentative anaerobic metabolism, characterized by an increase in the activity of lactate dehydrogenase (LDH). This increase was observed only in the experimental groups with the largest parasite loads, of 10.000 (4.65 ± 0.25 UI) and 15.000 (4.82 ± 0.35 UI) L1 larvae, differing significantly in relation to the control group (3.57 ± 0.42 UI) (Fig. 2a).
Lactate dehydrogenase activity (UI) (a) and contents of organic acids (oxalic (b), pyruvic (c), and lactic acid (d)) (mM) in the hemolymph of Achatina fulica infected by different parasite loads (500, 1000, 1500, 2000, 2500, 5000, 10, 000, and 15, 000 L1 larvae) of Angiostrongylus cantonensis. Values are expressed as (mean ± standard deviation). */**/*** indicate means differ significantly in relation to the control group, P < 0.05, P < 0.01, and P < 0.001
To better understand the impact of the infection on the oxidative metabolism of A. fulica, we also measured the concentrations of some carboxylic acids in the hemolymph, as important bioindicators of transition from aerobic to anaerobic pathways. The main finding was a significant decrease in the contents of oxalic acid in the groups exposed to 2.500 (36.89 ± 5.25 mM) and 5.000 (32.76 ± 4.35 mM) L1 of A. cantonensis, differing significantly from the average value of the control group (64.55 ± 5.6 mM) (Fig. 2b). This tendency was also observed for the pyruvic acid contents, where the lowest concentrations occurred in the groups exposed to the highest parasite loads. The infection by 15,000 L1 of A. cantonensis (20.78 ± 0.65 mM) resulted in a decline of approximately 62.79% in the pyruvic acid content, differing from the mean of the control group (55.85 ± 0.87 mM) (Fig. 2c). In contrast to these parameters, the exposure to infective larvae induced an accumulation of lactic acid in the hemolymph of A. fulica, where the highest values were found in the groups exposed to 10.000 (22.89 ± 0.72) and 15.000 larvae (25.9 ± 0.55 mM), increases of 422 and 513%, respectively, different from the average value of the uninfected snails (4.22 ± 0.55 mM) (Fig. 2d).
Variations in the level of polysaccharide reserves were found in both sites analyzed. There was a significant decrease in the glycogen stored in the digestive gland only of the snails exposed to a parasite load greater than 5.000 L1 larvae (4.13 ± 0.017 mg glucose/g tissue), representing a reduction of 37.70% in relation to the average of the control group (6.63 ± 0.31 mg glucose/g tissue) (Fig. 3a). The same tendency was observed for the level of glycogen stored in the cephalopedal mass, with the smallest values being in the snails exposed to the highest load, differing significantly from the uninfected mollusks (4.78 ± 0.28) (Fig. 3b).
Glycogen content in the digestive gland (a) and cephalopedal mass (b), expressed in milligram glucose per gram tissue, fresh weight, in Achatina fulica experimentally infected by different parasite loads (500, 1000, 1500, 2000, 2500, 5000, 10,000, and 15, 000 L1 larvae) of Angiostrongylus cantonensis. ** indicate means differ significantly in relation to the control group, P < 0.01
Variations in the parasite load of the different experimental groups were observed, with the number of L3 larvae recovered being proportional to the number of L1 larvae to which the snails were exposed (Fig. 4).
Histopathological changes were also observed indicating the presence of granulomatous reactions in different tissues, including mufla, kidneys, cephalopedal mass, and mantle, with increased cellularity, formation of vacuoles, and presence of degenerative processes (Fig. 5).
A perilarvar reaction (→) in the kidney (a), mufla (b), cephalopedal mass (c). Perilarval reaction between the kidney and the wall of the mantle cavity, with the carbohydrate concentrations evidenced by the presence of matrix elements, in the larvae (d). Presence of larvae in the digestive gland (e) and mufla (f)
Discussion
Control of the energy metabolism is an essential life process (Fraga et al. 2013). In pulmonate gastropods, the oxidative metabolism is principally maintained by metabolization of the carbohydrates that are stored in the form of glycogen in specific tissues, such as the digestive gland, cephalopedal mass, and mantle (Pinheiro et al. 2009). Under conditions of physiological stress, these reserves are drastically mobilized, allowing the formation of glucose units that will integrate the metabolic pathways in the cells, for production of adenosine triphosphate (ATP). This process occurs through activation of multiple reaction steps, where, besides glycogenolysis, the glycolytic pathway and Krebs cycle play central roles (Bezerra et al. 1997; Massa et al. 2007; Tunholi-Alves et al. 2014) (Fig. 1). This situation was demonstrated by El-ansary et al. (2000), in studying the impact of infection by Schistosoma mansoni on the carbohydrate metabolism of B. alexandrina. According to the authors, the reduction of the glucose level in the hemolymph occurred in parallel with a significant increase in the activity of hexokinase, pyruvate kinase, and glucose phosphate isomerase in snails after 2 weeks of infection in relation to the control group. That finding indicates that in infected snails, the increased demand for energy is supplied by acceleration of the glycolytic pathway, resulting in the formation of intermediate compounds that are essential to the host’s survival and the complete development of the parasite. In the present study, we only observed a significant reduction in the hemolymph glucose levels in the groups exposed to a parasite load greater than 2.500 L1 of A. cantonensis. According to Tunholi et al. (2013, 2016), the variations observed in the metabolism of infected mollusks result from the action of the developing larval stages of the parasite, characterized not only by tissue damages, but also due to the direct competition for nutrients and the secretion/excretion from the parasite’s own metabolism, which alters the composition of the internal medium. Therefore, because previous studies have confirmed the presence of enzymatic centers related to the glycolytic pathway in the larval stages of A. cantonensis (Shih and Chen 1982), our results indicate that glucose is used as a source of energy by the parasite. This condition was demonstrated by Wang et al. (2013), who characterized the transcriptomic profile of larval stages of A. cantonensis. According to the authors, a significant proportion of amino acid sequences were associated with the activation of metabolic pathways of the nematode, including those related to the metabolism of carbohydrates and oxidative pathways involved in the production of energy. Together, these findings confirm the ability of the nematode to capture glycidic sources from the host organism, including glucose, as a plastic and energy substrate for its development, contributing to the establishment of a hypoglycemic state in A. fulica.
With respect to the reduction in the content of glucose in the hemolymph, the infection by A. cantonensis increased the activity of HK, one of the allosteric centers involved in regulating the glycolytic pathway. Moraes et al. (2007), studying the metabolism of carbohydrates during embryogenesis of the tick Rhipicephalus Boophilus microplus, observed a relative increase in the catalytic action of this enzyme, indicating that glucose is the main substrate related to the cellularization process of that arthropod. In this circumstance, since the energy demand rises abruptly, polysaccharide reserves are mobilized from specific regions of the snail to assure maintenance of energy homeostasis. Therefore, the authors attributed the increased activity of HK as a response to glycogenolysis, to assure the phosphorylation of glucose molecules, and consequently, the synthesis of molecules with high energy content, including ATP and NADH, which are essential to support important cell functions. In this study, since the infection induced physiological alterations similar to those described by Moraes et al. (2007), the increased catalytic action of HK in the groups exposed to a load higher than 2.500 L1 larvae of A. cantonensis probably resulted from greater energy demand by the host during the parasitic development.
We also noted a significant depletion of polysaccharide reserves in the digestive gland and cephalopedal mass. The largest variations occurred in the group exposed to a parasite load of 15,000 L1 larvae. This situation, besides indicating the existence of precisely regulated homeostatic mechanisms among the tissues and hemolymph, indicates a load-dependent response pattern, corroborating the results previously published by Brockelman and Sithithavorn (1980). According to them, the infection by 2000 A. cantonensis L1 in A. fulica did not result in a decrease in the levels of glycogen in the host’s digestive gland, since the values fluctuated near those of the control group, similar to our results. Therefore, even though glycogenolysis exercises a role in reestablishment of normoglycemia in these organisms, we believe that the maintenance of basal levels of glucose initially occurs through redirecting metabolic pathways, especially from use of non-glycidic substrates like lipids (Alves et al. 2014; Tunholi-Alves et al. 2012, 2014) and proteins (Tunholi et al. 2011), where the mobilization of glycogen occurs belatedly, depending on what is needed for maintenance and the intensity of the stressing agent.
Studies of snail-parasite interactions have demonstrated that the alterations in the metabolism of carbohydrates are frequently accompanied by changes in the oxidative profile of these organisms (Abou Elseoud et al. 2010; Bezerra et al. 1997; Tunholi et al. 2013). Based on spectrometry and liquid chromatography, we demonstrated that the developing larval states induce the activation of fermentative pathways in the host, characterized by overlap of enzymatic centers, such as lactate dehydrogenase, at the same time, culminating in suppression of others, including cytochrome and oxidoreductase. This condition, reported both in models involving trematodes and nematodes (Tunholi-Alves et al. 2014), over the long-term results in an energy deficit of the host, since the quantity of ATP generated by the reduction of pyruvate into lactate is significantly lower compared to complete oxidation of the molecules in the Krebs cycle and phosphorylation chain. According to Tunholi et al. (2016), this process probably results from the inhibitory action of parasite antigens released in the snail’s hemolymph, inhibiting the catalytic activity of enzymes that compose the Krebs cycle and hence the electron transport chain. In this circumstance, maintenance of the host’s redox balance occurs anaerobically by the reduction of pyruvate to lactate via LDH, guaranteeing reoxidation of NADH+. This step is essential to the host’s survival, by allowing new glucose molecules to enter the glycolytic pathway, generating intermediate substances that will integrate important pathways in the cells.
We only noted the transition from aerobic to anaerobic metabolism in the snails exposed to parasite loads greater than or equal to 10.000 L1 larvae, indicating that the alterations observed in the oxidative metabolism of A. fulica is strongly influenced by the parasite load. The infection induced an increase in the activity of LDH, contributing to accumulation of lactate in the hemolymph and reduction of pyruvate, a product and substrate of the reaction. A contrary variation profile was found in the snails exposed to parasite loads higher than 2500 and lower than 10.000 L1 larvae. A significant reduction in the levels of oxalic acid in the hemolymph of A. fulica was observed, differing from the values of the control group. Two mechanisms can explain the occurrence of this metabolic pattern:
-
(I)
- Acceleration of the oxide reduction reactions that integrate the tricarboxylic acid cycle. As mentioned, both the Krebs cycle and electron transport chain play central roles in the oxidative metabolism of gastropods, by guaranteeing complete oxidation of glucose molecules (Tunholi-Alves et al. 2014). In this context, acetylated compounds such as acetyl-CoA play a key role in the cell metabolism, by acting as the main fuel to assure the functioning of these pathways. However, for its complete metabolization, a condensation step is first necessary. This process is catalyzed by specific enzymatic centers, with oxaloacetate molecules being responsible for the incorporation of these compounds. Therefore, the decrease in the levels of this acid found in the infected snails indicates greater consumption of acetylated compounds in response to the acceleration of the reactions that compose the Krebs cycle, guaranteeing production of the energy necessary for the host to survive and the nematodes to develop fully.
-
(II)
- Another possibility is the diversion of the oxaloacetate molecules to gluconeogenesis. This metabolic route assures the production of glucose from non-glycemic substrates, in an attempt by the host to reestablish its normoglycemia. That condition has been considered by several researchers, indicating the metabolic plasticity of the snail when faced with infection, since glucose is the principal substrate related to the snail’s energy metabolism.
To characterize the A. fulica-A. cantonensis interface better, we also conducted a histopathological study, as a tool to better understand the physiological alterations found in the host’s oxidative metabolism. The results demonstrated the existence of perilarval granulomatous reaction in different tissues, including mufla, kidneys, cephalopedal mass, and mantle, with increased cellularity, formation of vacuoles, and presence of degenerative processes. Additionally, the PAS staining revealed the presence of glycogen granules in the larval stages of the nematode. This result demonstrates the parasite’s ability to capture glucose monomers from the hemolymph of the intermediate host, contributing to the establishment of hypoglycemia in A. fulica.
The study reported here was the first to investigate the physiological changes in A. fulica exposed to different loads of A. cantonensis. The variations found indicate a load-dependent response, with activation of the fermentative pathway being observed in the groups with highest parasite loads. Furthermore, the infection induced activation of the glycolytic pathway, a metabolic situation characterized by a decline in the levels of glucose in the hemolymph and increase in the activity of HK. This physiological condition was accompanied by marked glycogenolysis due to the depletion of the reserves of polysaccharides in the digestive gland and cephalopedal mass, the main sites for parasite development.
References
Abou Elseoud SM, Abdel Fattah NS, Ezz HM, Abdel AH, Mossalem H, Elleboudy N (2010) Carboxylic acids as biomarkers of Biomphalaria alexandrina snails infected with Schistosoma mansoni. Korean J Parasitol 48:127–132
Alves H, Tunholi-Alves VM, Tunholi VM, Gôlo P, Bittencourt VREP, Pinheiro J (2014) Changes in the lipid profile of Bradybaena similaris (Férussac, 1821) (Gastropoda, Xanthonychidae) during the development of Eurytrema coelomaticum (Giard and Billet, 1892) (Digenea, Dicrocoeliidae). Exp Parasitol 144:52–56
Bandstra SR, Fried B, Sherma J (2006) High-performance thin-layer chromatographic analysis of neutral lipids and phospholipids in Biomphalaria glabrata patently infected with Echinostoma caproni. Parasitol Res 99:414–418
Bezerra JCB, Becker W, Zelck EU (1997) A comparative study of the organic acid content of the hemolymph of Schistosoma mansoni-resistant and susceptible strains of Biomphalaria glabrata. Mem Inst Oswaldo Cruz 92:421–425
Bonfim TC, Maldonado A, Tunholi VM, Tunholi-Alves VM, Faro M, Mota EM, da Silva TC, Pinheiro J, Garcia J (2014) Biochemical and histopathological alterations in Biomphalaria glabrata due to co-infection by Angiostrongylus cantonensis and Echinostoma paraensei. J Invertebr Pathol 115:80–85
Brockelman CR, Sithithavorn P (1980) Carbohydrate reserves and hemolymph sugars of the African giant snail, Achatina fulica in relation to parasitic infection and starvation. Zeitschrift fur Parasitenkunde 62:285–291
Caldeira RL, Mendonca CL, Goveia CO, Lenzi HL, Graeff-Teixeira C, Lima WS, Mota EM, Pecora IL, Medeiros AM, Carvalho Odos S (2007) First record of molluscs naturally infected with Angiostrongylus cantonensis (Chen, 1935) (Nematoda: Metastrongylidae) in Brazil. Mem Inst Oswaldo Cruz 102:887–889
Cowie RH (2013) Biology, systematics, life cycle, and distribution of Angiostrongylus cantonensis, the cause of rat lungworm disease. Hawaii J Med Public Health 72:6–9
Dorta-Contreras AJ, Padilla-Docal B, Moreira JM, Robles LM, Aroca JM, Alarcon F, Bu-Coifiu-Fanego R (2011) Neuroimmunological findings of Angiostrongylus cantonensis meningitis in Ecuadorian patients. Arq Neuropsiquiatr 69:466–469
El-ansary A, Sammour EM, Mohamed AM (2000) Susceptibility of Biomphalaria alexandrina to infection with Schistosoma mansoni: correlation with the activity levels of certain glycolytic enzymes. J Egypt Soc Parasitol 30:547–560
Fernandes MC (1949) Métodos Escolhidos de Técnicas Microscópicas, second edn. Imprensa Nacional, Rio de Janeiro
Fraga A, Ribeiro L, Lobato M, Santos V, Silva JR, Gomes H, Moraes JLC, Menezes JS, Logullo CJ, Campos E, Fonseca RN (2013) Glycogen and glucose metabolism are essential for early embryonic development of the red flour beetle Tribolium castaneum. PLoS One 8:e65125
Galina A, Da Silva WS (2000) Hexokinase activity alters sugar-nucleotide formation in maize root homogenates. Phytochemistry 53:29–37
Humason GL (1979) Animal Tissue Techniques, 4th edn. W.H. Freeman, San Francisco
Lima AR, Mesquita SD, Santos SS, Aquino ER, Rosa LR, Duarte FS, Teixeira AO, Costa ZR, Ferreira ML (2009) Alicata disease: neuroinfestation by Angiostrongylus cantonensis in Recife, Pernambuco, Brazil. Arq Neuropsiquiatr 67:1093–1096
Lv S, Zang Y, Liu H, Hu L, Yang K, Steinmann P, Chen Z, Wang LY, Utzinger J, Zhou XN (2009) Invasive snails and an emerging infectious disease: results from the first national survey on Angiostrongylus cantonensis in China. PLoS Neglect Trop Dis 3:e368
Maldonado Jr A, Simões RO, Oliveira APM, Motta EM, Fernandez MA, Pereira ZM, Monteiro SS, Torres EJL, Thiengo SC (2010) First report of Angiostrongylus cantonensis (Nematoda: Metastrongylidae) in Achatina fulica (Mollusca: Gastropoda) from Southeast and South Brazil. Mem Inst Oswaldo Cruz 105:938–941
Martins YC, Tanowitz HB, Kazacos KR (2015) Central nervous system manifestations of Angiostrongylus cantonensis infection. Acta Trop 141:46–53
Massa DR, Chejlava MJ, Fried B, Sherma J (2007) High performance column liquid chromatographic analysis of selected carboxylic acids in Biomphalaria glabrata patently infected with Schistosoma mansoni. Parasitol Res 101:925–928
Mello-Silva CC, Vilar MM, Vasconcellos MC, Pinheiro J, Rodrigues MLA (2010) Carbohydrate metabolism alterations in Biomphalaria glabrata infected with Schistosoma mansoni and exposed to Euphorbia splendens var. hislopii látex. Mem Inst Oswaldo Cruz 105:492–5
Moraes J, Galina A, Alvarenga PH, Rezende GL, Masuda A, Vaz IS, Logullo C (2007) Glucose metabolism during embryogenesis of the hard tick Boophilus microplus. Comp Biochem Physiol 146:528–533
Morassutti AL, Thiengo SC, Fernandez M, Sawanyawisuth K, Graeff-Teixeira C (2014) Eosinophilic meningitis caused by Angiostrongylus cantonensis: an emergent disease in Brazil. Mem Inst Oswaldo Cruz 109:399–407
New D, Little MD, Cross J (1995) Angiostrongylus cantonensis infection from eating raw snails. N Engl J Med 332:1105–1106
Oliveira APM, Gentile R, Maldonado A, Torres EL, Thiengo S (2014) Angiostrongylus cantonensis infection in molluscs in the municipality of São Gonçalo, a metropolitan area of Rio de Janeiro, Brazil: role of the invasive species Achatina fulica in parasite transmission dynamics. Mem Inst Oswaldo Cruz 110:739–744
Pinheiro J, Gomes EM (1994) A method for glycogen determination in molluscs. Braz Arch Biol Technol 37:569–576
Pinheiro J, Maldonado A, Lanfredi RM (2009) Physiological changes in Lymnaea columela (Say, 1818) (Mollusca, Gastropoda) in response to Echinostoma paraensei Lie and Basch, 1967 (Trematoda, Echinostomatidae) infection. Parasitol Res 106:55–59
Raccurt C, Blaise J, Durette-Desset M (2003) Présence d’Angiostrongylus cantonensis en Haiti. Tropical Med Int Health 8:423–426
Robles MR, Kinsella JM, Galliari C, Navone GT (2016) New host, geographic records, and histopathologic studies of Angiostrongylus spp (Nematoda: Angiostrongylidae) in rodents from Argentina with updated summary of records from rodent hosts and host specificity assessment. Mem Inst Oswaldo Cruz 111:181–191
Shih HH, Chen SN (1982) Glycolytic enzymes in juvenile and adult Angiostrongylus cantonensis. Southeast Asian J Trop Med Public Health 13:114–119
Slom TJ, Cortese MM, Gerber SI, Jones RC, Holtz TH, Lopez AS, Zambrano CH, Sufit RL, Sakolvaree Y, Chaicumpa W, Herwaldt BL, Johnson S (2002) An outbreak of eosinophilic meningitis caused by Angiostrongylus cantonensis in travelers returning from the Caribbean. N Engl J Med 346:668–675
Sumner JB (1924) The estimation of sugar in diabetic urine using dinitrosalicylic acid. J Biol Chem 62:287–290
Thiengo SC, Maldonado A, Mota EM, Torres EJ, Caldeira R, Carvalho OS, Oliveira AP, Simoes RO, Fernandez MA, Lanfredi RM (2010) The giant African snail Achatina fulica as natural intermediate host of Angiostrongylus cantonensis in Pernambuco, northeast Brazil. Acta Trop 115:194–199
Tunholi-Alves VM, Tunholi VM, Lustrino D, Amaral LS, Thiengo SC, Pinheiro J (2011) Changes in the reproductive biology of Biomphalaria glabrata experimentally infected with the nematode Angiostrongylus cantonensis. J Invertebr Pathol 108:220– 223
Tunholi VM, Lustrino D, Tunholi-Alves VM, Mello-Silva CCC, Maldonado A, Rodrigues MLA, Pinheiro J (2011) Biochemical profile of Biomphalaria glabrata (Mollusca: Gastropoda) after infection by Echinostoma paraensei (Trematoda: Echinostomatidae). Parasitol Res 109:885–891
Tunholi VM, Tunholi-Alves VM, Lustrino D, Castro N, Sant’Ana L, Garcia J, Maldonado AJ, Santos MAJ, Rodrigues MLA, Pinheiro J (2013) Aerobic to anaerobic transition in Biomphalaria glabrata (Say, 1818) infected with different miracidial doses of Echinostoma paraensei (Lie and Basch, 1967) by high performance liquid chromatography. Exp Parasitol 133:403–410
Tunholi VM, Tunholi-Alves VM, Santos AT, Garcia J, Maldonado AJ, da-Silva W, Rodrigues MLA, Pinheiro J (2016) Evaluation of the mitochondrial system in the gonad-digestive gland complex of Biomphalaria glabrata (Mollusca, Gastropoda) after infection by Echinostoma paraensei (Trematoda, Echinostomatidae). J Invertebr Pathol 136:136–141
Tunholi-Alves VM, Tunholi VM, Pinheiro J, Thiengo SC (2012) Effects of infection by larvae of Angiostrongylus cantonensis (Nematoda, Metastrongylidae) on the metabolism of the experimental intermediate host Biomphalaria glabrata. Exp Parasitol 131:143–147
Tunholi-Alves VM, Tunholi VM, Castro RN, Sant'Ana L, Santos-Amaral L, Oliveira AP, Garcia J, Thiengo SC, Pinheiro J, Maldonado A (2014) Activation of anaerobic metabolism in Biomphalaria glabrata (Mollusca: Gastropoda) experimentally infected by Angiostrongylus cantonensis (Nematoda, Metastrongylidae) by high-performance liquid chromatography. Parasitol Int 63:64–68
Tunholi-Alves VM, Tunholi VM, Santos-Amaral L, Mota EM, Maldonado A, Pinheiro J, Garcia J (2015) Biochemical profile of Achatina fulica (Mollusca: Gastropoda) after infection by different parasitic loads of Angiostrongylus cantonensis (Nematoda, Metastrongylidae). J Invertebr Pathol 124:1–5
Wang QP, Lai DH, Zhu XQ, Chen XG, Lun ZR (2008) Human angiostrongyliasis. Lancet Infect Dis 8:621–630
Wang LC, Chen KY, Chang SH, Chung LY, Gan RC, Cheng CJ, Tang P (2013) Transcriptome profiling of the fifth-stage larvae of Angiostrongylus cantonensis by next-generation sequencing. Parasitol Res 112:3193–3202
Willcox HP, Coura JR (1989) Nova concepção para o método de Baermann – Moraes – Coutinho na pesquisa de larvas de nematódeos. Memórias Inst Oswaldo Cruz 84:539–565
Acknowledgements
This study was supported in part by Conselho Nacional para o Desenvolvimento Científico e Tecnológico (CNPq) and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ).
Author information
Authors and Affiliations
Corresponding author
Additional information
Jairo Pinheiro is a Research Fellow, CNPq.
Rights and permissions
About this article
Cite this article
Tunholi-Alves, V.M., Tunholi, V.M., Garcia, J. et al. Unveiling the oxidative metabolism of Achatina fulica (Mollusca: Gastropoda) experimentally infected to Angiostrongylus cantonensis (Nematoda: Metastrongylidae). Parasitol Res 117, 1773–1781 (2018). https://doi.org/10.1007/s00436-018-5859-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-018-5859-x