Abstract
Background and Objective
Increasing numbers of women in childbearing years are treated with antidepressants. Concerns regarding fetal exposure to medication has led to large studies on drug effects on birth outcome and on the risk of congenital anomalies. The risk of adverse effects due to paroxetine use during pregnancy has been associated with the extent of exposure. Nevertheless, few studies have covered dosing aspects in order to minimize fetal antidepressant exposure while limiting the risk of treatment failure. Essential pharmacokinetic data in pregnancy are lacking, even regarding paroxetine, one of the most commonly used antidepressants. We examined the changes of maternal paroxetine concentrations during pregnancy in relation to cytochrome P450 (CYP) 2D6 genotype.
Method
An observational cohort study was conducted in 74 pregnant women aged from 25 to 45 years treated with paroxetine during pregnancy. Blood samples and information on dosing, weight, smoking and mood were provided at 16–20, 27–31 and 36–40 weeks of pregnancy. Samples were analysed for paroxetine plasma concentrations and CYP2D6 genotype.
Results
Women who were genotyped as extensive metabolizers (EMs) or ultra-rapid metabolizers (UMs) for CYP2D6 (EM n = 43; UM n=1) showed steadily decreasing plasma paroxetine concentrations during the course of pregnancy, with a decrease of 0.3 µg/L (95% CI −0.58, −0.07) for each week of pregnancy. In contrast, plasma paroxetine concentrations of intermediate metabolizers (IMs [n = 25]) and poor metabolizers (PMs [n = 5]) increased during pregnancy, resulting in an increase of 0.82 µg/L (95% CI 0.42, 1.22) for each week of pregnancy. Weight gain, maternal age or smoking did not influence plasma drug concentrations. Decreasing plasma concentrations in EMs are in accordance with induced CYP2D6 activity during pregnancy. Accumulation of paroxetine in women with impaired CYP2D6 metabolism may be explained by competition with an endogenous substrate. In EMs/UMs the depressive symptoms increased significantly during the course of pregnancy, while in the IM/PM group these did not change.
Conclusions
Differences in CYP2D6 genotype may have divergent effects on maternal plasma paroxetine concentrations during pregnancy, with therapeutic consequences. Accumulation of paroxetine in a considerable group of pregnant women will lead to unintended increased exposure of paroxetine to the unborn child. Knowledge about a patient’s CYP2D6 genotype is indispensable when prescribing paroxetine in pregnancy [trialregister.nl Identifier ISRCTN25383361].
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Antidepressant use during pregnancy is increasing.[1–3] Although the safety of gestational antidepressant use is still under debate, approximately 2% of all pregnant women in the Netherlands use antidepressant drugs. Paroxetine is the most frequently used antidepressant.[4] Antidepressants are prescribed for various psychiatric and somatic illnesses but predominantly for depression and anxiety disorders. The lack of knowledge concerning risks and benefits, optimal dosing and pharmacokinetic behaviour is in contrast to the extensive use of antidepressants during pregnancy.[5,6] Changes in activity of metabolizing enzymes during pregnancy, differences in enzyme activity due to genetic constitution and the fact that paroxetine metabolism is easily saturable are of concern. There are few data available indicating increased clearance of selective serotonin reuptake inhibitors (SSRIs) during pregnancy, but none of them concerned paroxetine.[6–9]
Paroxetine is inactivated through oxidative pathways catalysed by the cytochrome P450 (CYP) 2D6 enzyme system in the liver.[10–17] The pharmacokinetic characteristics of paroxetine show high interindividual variability due to the existence of genetic CYP2D6 polymorphism, nonlinear kinetics and interactions with inhibitors of CYP2D6 isoenzyme.[18] According to their CYP2D6 genotype, individuals are divided into four major phenotypes: poor metabolizers (PMs), intermediate metabolizers (IMs), extensive metabolizers (EMs) and ultra-rapid metabolizers (UMs). PMs, representing 4–10% of Caucasian populations, are individuals with the absence of a functional enzyme that has been associated with the accumulation of various drugs.[19–23] An IM is defined as being heterozygous for a defect allele and EMs are carrying two functional alleles, representing 30–40% and 50–70%, respectively, of the Dutch population.[24] UMs are carrying more than two functional alleles and represent 1–10% of the Dutch population.
Pregnancy influences pharmacokinetics of drugs by changing CYP2D6 activity, plasma volume, hepatic blood flow and plasma protein binding.[25] Low plasma concentrations of SSRIs in the third trimester of pregnancy compared with postpartum concentrations have been attributed to increased CYP2D6 activity.[6,9] Since the exact onset and magnitude of change in plasma concentrations is unknown, it is difficult to anticipate the resulting therapeutic failure.[6] However, in the light of poor neonatal adaptation, which commonly occurs after exposure in the third trimester, decreasing exposure to paroxetine may also be beneficial to the fetus.[26]
The aim of our study was to evaluate the effect of pregnancy on maternal plasma paroxetine concentrations and the influence of CYP2D6 genotypes on pharmacokinetic variation in particular.
Methods
Study Design and Subject Eligibility
In this study we investigated paroxetine plasma concentrations during pregnancy in women who were treated with paroxetine for depression or anxiety disorders. Subjects were enrolled in an ongoing observational cohort study on the effects of antidepressants during pregnancy on mother and child [the OAZE study; trialregister.nl Identifier ISRCTN25383361]. The study protocol was approved by the Central Committee on Research Involving Human Subjects (CCMO; the Hague, the Netherlands) and the local medical ethical committees of the 12 collaborating centres. Recruitment started in July 2003 and ended in July 2007. Informed consent was obtained from each woman before inclusion in the study.
Participants were considered eligible if information on medication use, co-medication, smoking habits, alcohol (ethanol) use and CYP2D6 genotype could be provided (n = 74). Decisions about treatment were made by the women’s own healthcare providers and were independent of participation in our study. Blood sample collection did not continue if paroxetine treatment had been stopped near the end of pregnancy. Effect changes were estimated with the Edinburgh Postnatal Depression Scale, a 10-item questionnaire validated for pregnancy.[27]
Blood Sample Collection and Drug Analysis
Maternal venous blood samples (4.5 mL) were collected at appointment time during routine visits at 16–20, 27–31 and 36–40 weeks of pregnancy. Time between drug intake and sample collection varied depending on the dosing regimen: evening or morning dosing. The blood cells and plasma were separated and stored at −20°C until analysis.
Plasma concentrations of paroxetine were analysed using a modified straight phase high-performance liquid chromatography with ultraviolet detection (HPLC-UV) or, if plasma sample volumes were smaller than 500 µL or concentrations were ≤1.0 µg/L, liquid chromatography-tandem mass spectrometry (LC-MS/MS).[28,29] The overall intra- and inter-assay coefficients of variation were <15% over the ranges 5 µg/L (lower limit of quantification on 500 µL sample) to 150 µg/L using HPLC-UV and 1 µg/L (lower limit of quantification on 100 µL) to 350 µg/L using LC-MS/MS.
Determination of Cytochrome P450 2D6 Genotypes and Phenotypes
Genotype analyses for the CYP2D6*3, *4, *5, *6, *9, *10, and *41 alleles on isolated DNA obtained from the blood sample drawn at the first visit were performed by polymerase chain reaction using the method described by Schenk et al.[30] Alleles with none of the mutations were classified as CYP2D6*1.
Subjects were defined as EMs if they were homozygous for coding alleles (CYP2D6*1) and as UMs in the case of gene duplications. Subjects were defined as IMs or PMs if they were heterozygous or homozygous for non-functional alleles (CYP2D6*3, *4, *5, *6), respectively. If subjects were heterozygous or homozygous for decreased activity alleles (CYP2D6*9, *10 or *41) they were categorized as EMs or IMs, respectively. Considering the prevalence of other non-functional alleles in the Dutch population of less than 10%, the chance of misclassification was small.
Factors that may have an effect on the CYP2D6 phenotype were all considered, i.e. concomitant use of drugs metabolized by the same enzymatic mechanism as paroxetine, substrates for the CYP2D6 enzyme system (e.g. bupropion) or known inhibitors of paroxetine metabolism (e.g. cimetidine), maternal weight gain, cigarette smoking and alcohol use.
Data Analysis
Paroxetine plasma concentrations were examined over time. If the plasma concentrations were below the limit of quantification, the value 0.5 µg/L was used in calculations.[9] A linear mixed-effects model was used to study the effect of genotype and gestational age on paroxetine plasma concentrations and to test the influence of weight gain, smoking, co-medication and age.
A p-value of <0.05 was regarded as statistically significant. All available patient data were included. To assess the model’s goodness of fit we created plots of observed plasma concentrations versus fitted plasma concentrations and examined residuals. Normality of distribution of effects in the final model and normality of residuals was tested with the Kolmogorov-Smirnov test. In order to compare paroxetine concentrations in the different periods, they were adjusted for daily dose by multiplying plasma concentrations by a factor representing the daily defined dose (20 mg) divided by the actual dose, thereby ignoring possible dose-dependent pharmacokinetics. The influence of pregnancy and of genotype on Edinburgh Postnatal Depression Scale (EPDS) scores across pregnancy were also analysed using a mixed-effects model. Analyses were performed with SPSS for Windows, version 15.0 (SPSS Inc., Chicago, IL, USA) and R, version 2.6.2 with library ‘nlme’.
Results
Participants
All participants (n = 74) were using paroxetine, at least since early pregnancy, when entering the study at 16 weeks. The main patient characteristics are summarised in table I. The mean ± SD maternal age was 32.5 ± 4.9 (range 23–45) years. Potential confounders did not differ significantly among the different stages of pregnancy, except for maternal weight and smoking.
Seventeen subjects smoked during pregnancy (23%); one of them also used alcohol.
We determined 43 EMs (58%), 1 UM (1%), 25 IMs (34%) and 5 PMs (7%) [table I].
Plasma Concentrations
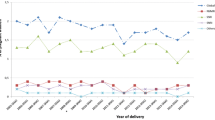
The 74 participants provided a total of 190 plasma paroxetine concentrations. Longitudinal results are presented in figure 1. Of 20 women, plasma concentrations from the first period were not available because of late entry into the study. In eight cases, sampling in the second period was omitted. Four third trimester samples were missing: two because of preterm delivery, one because medication was stopped a few weeks before delivery, and one that was lost to follow-up.
Paroxetine plasma concentration normalized to 20 mg by dividing the observed plasma concentration by the dose and multiplying by the standard dose of 20 mg: (a) extensive metabolizers (EMs) [n = 43] and ultra-rapid metabolizers (UMs) [n = 1]; and (b) intermediate metabolizers (IMs) [n = 25] and poor metabolizers (PMs) [n = 5].
Modelling of Changes in Plasma Concentrations over Time and Relationship with Genotype and Other Patient Characteristics
We ran several models incorporating the characteristics listed in table I, except for gravida and parity, and observed the log-likelihood, Akaike information criterion and p-values of the coefficients, and thus obtained the best model. The final model incorporated genotype, gestational age and dose, and the interactions of genotype with gestational age and dose.
Finally, the observed paroxetine plasma concentration (C) in the second and third trimester of pregnancy is described by equation 1:
where I denotes the intercept, genotype = ‘EM’ or ‘IM/PM’ and D is the daily dose.
The effect of bodyweight on plasma concentrations was not significant, and nor were the effects of smoking, co-medication or maternal age. Random effects and residuals were normally distributed. We found no significant nonlinearity in the relationship between dose and paroxetine plasma concentrations (table II). The model shows that in EMs, paroxetine plasma concentrations decrease as pregnancy proceeds (−0.3 µg/L per week; p = 0.014). However, in IMs and PMs, plasma concentrations increased during the course of pregnancy (effect of IM/PM genotype on plasma concentrations: 0.87 − 0.3 = 0.57 µg/L per week; p < 0.001). Scatter plots of population-predicted and individual-predicted paroxetine concentrations are shown in figure 2. The percentage of predicted values within the range of ±10 µg/L of the observed concentrations was 79.5%, and 96.8% were within the range of ±20 µg/L of the observed concentrations.
Although we expected a nonlinear relationship between dose and concentration, we did not find a deviation from the linear relationship in the higher dose range. Results of patients using haloperidol (one EM) and mirtazapine (one IM) did not deviate from mean results in their groups. As an example, for a woman on a daily dose of paroxetine 30 mg, the model-predicted plasma concentration at 38 weeks would be 1.7 − 0.33 × 38 + 1.15 × 30 = 23.7 µg/L if she was genotyped EM. The same women if genotyped IM would have a 3-fold higher plasma concentration: (1.7 − 0.33 × 38 + 1.15 × 30) − 32.1 + 0.82 × 38 + 1.59 × 30 = 70.4 µg/L. In figure 3 we present the results of dose-corrected plasma concentrations of subjects with complete samplings. Differences between the periods were analysed using the Wilcoxon rank test.
Dose-normalized paroxetine plasma concentrations during pregnancy, using longitudinal data from women with complete samplings: extensive metabolizers (EMs) [n = 32], intermediate metabolizers (IMs) [n = 13] and poor metabolizers (PMs) [n = 2]. Boxes represent 50% of the population and median, whiskers represent values within 1.5 times box width, circles indicate values between 1.5 and 3 times box width and asterisks represent extreme values more than 3 times box width. p-Values for differences between first and last period for each subgroup were <0.01 for EMs and 0.05 for PMs. The p-value for the difference between the first and second period for IMs was 0.04.
Clinical effects are expressed by EPDS test results, which we obtained from all subjects (n = 74) who responded at least once to the questionnaires. Using a linear mixed-effects model to test the effect of genotype and the influence of gestational age, we found that depression, with an intercept of 4.8 (95% CI 2.1, 7.5), worsened by 0.08 points (95% CI 0.001, 0.15; p = 0.05) on the EPDS scale for each proceeding week of pregnancy. According to the model, the subjects in the group IM/PM showed 2.8 points (95% CI 0.5, 5.0; p = 0.02) higher EPDS scores than the EM group.
The prevalence of EPDS scores exceeding 12 (indicating depression) in the whole study group increased from 15% in the first period to 21% in the second period and 29% in the last weeks of pregnancy. Differences in the prevalence of high EPDS scores between EMs and IM/PMs were not statistically significant.
Discussion
We found that PMs and IMs have higher paroxetine plasma concentrations than subjects genotyped as EM or UM. Plasma concentrations of EMs tend to decline during pregnancy. This is in accordance with the findings of Tracy et al.[31] who found a temporal increase in CYP2D6 activity during pregnancy in EMs using dextromethorphan/dextrorphan urinary ratios. Our research extends their study by differentiating between EMs and IMs or PMs. Our results demonstrate that a drug biotransformed by CYP2D6 will not necessarily show a decrease in plasma concentrations during pregnancy. On the contrary: paroxetine concentrations of PMs and IMs increased. In 1997, Wadelius et al.[32] found almost doubled dextromethorphan/metabolite ratios in CYP2D6 PMs during pregnancy, compared with postpartum ratios. As CYP2D6 PMs do not express functional CYP2D6, the metabolism in these subjects depends exclusively on other enzymes, which have decreasing activity during pregnancy. These findings, however, have never been linked to antidepressants. In IMs, both processes may middle out the effect.
We hypothesize that during pregnancy, CYP2D6 activity is induced by rising concentrations of endogenous substrates. As steroid hormones and chorionic gonadotropin do not induce CYP2D6, the nature of this substrate is yet unknown. According to observations by Dickmann et al.,[33] high concentrations of substrate indeed catalyse CYP2D6 enzyme activity. In EMs this leads to decreasing concentrations of exogenous substrates, e.g. paroxetine. However, in PMs or IMs the induction may not hold pace with the increased supply of substrate. In those patients the endogenous substrate may act as a competitive inhibitor for the metabolism of paroxetine and paroxetine concentrations will rise.
Our model gives an estimate of the probable level of plasma concentrations and the direction of change in the course of pregnancy from the 16th week till the end of pregnancy in the dose range of 10–40 mg for the different CYP2D6 genotypes.
Although some aspects of our findings remain to be studied and no clear-cut relationship between the paroxetine concentration and the antidepressant effect has been provided, the results are useful when evaluating the necessity of antidepressant drug therapy during pregnancy.[34]
Generally, depressive symptoms may worsen during the course of pregnancy.[35] Low to undetectable plasma concentrations of antidepressants may contribute to this effect and to therapeutic failure. An increase in mean EPDS scores, as observed in the EM group, was not seen in the IM/PM group.
The accumulation of paroxetine in IMs or PMs is of concern, since it becomes more and more clear that adverse effects on the child, such as low birth weight, poor neonatal adaptation and cardiac teratology, are related to the extent of antidepressant exposure.[36–38] Our study adds to the work of DeVane et al.[39] They strongly advocate individualized drug treatment using therapeutic drug monitoring during pregnancy in order to achieve optimal treatment while minimizing exposure to the fetus.
One of the limitations of our study was that, because the blood sampling had to take place during regular visit times, the time between drug intake and blood sampling varied between subjects, although not so much within subjects. By standardizing the sampling time, some of the observed variability may be reduced. Moreover, we did not have data on some of the other variables that may cause variation of blood values measured during pregnancy, such as protein levels and hemodilution.
Conclusions
Decreasing as well as increasing plasma concentrations in the course of pregnancy demonstrate that the effect of pregnancy on paroxetine pharmacokinetics is far from uniform. Decreased plasma concentrations in EMs can lead to therapeutic failure and relapse, although increasing paroxetine concentrations in IMs and PMs do not substantially contribute to the mother’s well-being and they may result in unintended and unwanted exposure to the unborn child. Our results show that knowledge about a patient’s CYP2D6 genotype is indispensable when prescribing paroxetine in pregnancy. Studies with comparable outlines are needed before our findings can be extrapolated to other antidepressants such as tricyclic antidepressants and SSRIs metabolized by CYP2D6.
References
Andrade SE, Raebel MA, Brown J, et al. Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol 2008; 198(2): 194.el–5
Hallberg P, Sjoblom V. The use of selective serotonin reuptake inhibitors during pregnancy and breast-feeding: a review and clinical aspects. J Clin Psychopharmacol 2005; 25(1): 59–73
Bakker MK, Kolling P, van den Berg PB, et al. Increase in use of selective serotonin reuptake inhibitors in pregnancy during the last decade, a population-based cohort study from the Netherlands. Br J Clin Pharmacol 2008; 65(4): 600–6
Ververs T, Kaasenbrood H, Visser G, et al. Prevalence and patterns of antidepressant drug use during pregnancy. Eur J Clin Pharmacol 2006; 62(10): 863–70
Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA 2006; 295(5): 499–507
Sit DK, Perel JM, Helsel JC, et al. Changes in antidepressant metabolism and dosing across pregnancy and early postpartum. J Clin Psychiatry 2008; 69(4): 652–8
Hostetter A, Stowe ZN, Strader Jr JR, et al. Dose of selective serotonin uptake inhibitors across pregnancy: clinical implications. Depress Anxiety 2000; 11(2): 51–7
Kim J, Riggs KW, Misri S, et al. Stereoselective disposition of fluoxetine and norfluoxetine during pregnancy and breast-feeding. Br J Clin Pharmacol 2006; 61(2): 155–63
Heikkinen T, Ekblad U, Palo P, et al. Pharmacokinetics of fluoxetine and norfluoxetine in pregnancy and lactation. Clin Pharmacol Ther 2003; 73(4): 330–7
Carrillo JA, Dahl ML, Svensson JO, et al. Disposition of fluvoxamine in humans is determined by the polymorphic CYP2D6 and also by the CYP1A2 activity. Clin Pharmacol Ther 1996; 60(2): 183–90
Haffen E, Vandel P, Broly F, et al. Citalopram: an interaction study with clomipramine in a patient heterozygous for CYP2D6 genotype. Pharmacopsychiatry 1999; 32: 232–4
Sindrup SH, Brøsen K, Hansen MG, et al. Pharmacokinetics of citalopram in relation to the sparteine and the mephenytoin oxidation polymorphisms. Ther Drug Monit 1993; 15: 11–7
Kobayashi K, Ishizuka T, Shimada N, et al. Sertraline N-demethylation is catalyzed by multiple isoforms of human cytochrome P-450 in vitro. Drug Metab Dispos 1999; 27: 763–6
DeVane CL, Liston HL, Markowitz JS. Clinical pharmacokinetics of sertraline. Clin Pharmacokinet 2002; 41: 1247–66
Obach RS, Cox LM, Tremaine LM. Sertraline is metabolized by multiple cytochrome P450 enzymes, monoamine oxidases, and glucuronyl transferases in human: an in vitro study. Drug Metab Dispos 2005; 33: 262–70
Kirchheiner J, Brøsen K, Dahl ML, et al. CYP2D6 and CYP2C19 genotype based dose recommendations for antidepressants: a first step towards subpopulation-specific dosages. Acta Psychiatr Scand 2001; 104: 173–92
Xu ZH, Wang W, Zhao XJ, et al. Evidence for involvement of polymorphic CYP2C19 and 2C9 in the N-demethylation of sertraline in human liver microsomes. Br J Clin Pharmacol 1999; 48: 416–23
Rasmussen BB, Brøsen K. Is therapeutic drug monitoring a case for optimizing clinical outcome and avoiding interactions of the selective serotonin reuptake inhibitors? Drug Monit 2002; 22: 143–54
Bradford LD. CYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendants. Pharmacogenomics 2002; 3(2): 229–43
Hamelin BA, Turgeon J, Vallee F, et al. The disposition of fluoxetine but not sertraline is altered in poor metabolizers of debrisoquin. Clin Pharmacol Ther 1996; 60(5): 512–21
Grasmader K, Verwohlt PL, Rietschel M, et al. Impact of polymorphisms of cytochrome-P450 isoenzymes 2C9, 2C19 and 2D6 on plasma concentrations and clinical effects of antidepressants in a naturalistic clinical setting. Eur J Clin Pharmacol 2004; 60(5): 329–36
Sindrup SH, Brosen K, Gram LF. Pharmacokinetics of the selective serotonin reuptake inhibitor paroxetine: nonlinearity and relation to the sparteine oxidation polymorphism. Clin Pharmacol Ther 1992; 51(3): 288–95
Spigset O, Granberg K, Hagg S, et al. Relationship between fluvoxamine pharmacokinetics and CYP2D6/CYP2C19 phenotype polymorphisms. Eur J Clin Pharmacol 1997; 52(2): 129–33
Tamminga WJ, Wemer J, Oosterhuis B, et al. The prevalence of CYP2D6 and CYP2C19 genotypes in a population of healthy Dutch volunteers. Eur J Clin Pharmacol 2001; 57(10): 717–22
Anderson GD. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet 2005; 44(10): 989–1008
Moses-Kolko EL, Bogen D, Perel J, et al. Neonatal signs after late in utero exposure to serotonin reuptake inhibitors: literature review and implications for clinical applications. JAMA 2005; 293(19): 2372–83
Cox JL, Chapman G, Murray D, et al. Validation of the Edinburgh Postnatal Depression Scale (EPDS) in non-postnatal women. J Affect Disord 1996; 39(3): 185–9
Blakesley JD, Howse CG, Spencer-Peet J, et al. An improved method for measurement of common tricyclic antidepressant drugs and their metabolites using normal phase HPLC. Ann Clin Biochem 1986; 23(Pt 5): 552–8
Sauvage FL, Gaulier JM, Lachatre G, et al. A fully automated turbulentflow liquid chromatography-tandem mass spectrometry technique for monitoring antidepressants in human serum. Ther Drug Monit 2006; 28(1): 123–30
Schenk PW, van Fessem MA, Verploegh-Van Rij S, et al. Association of graded allele-specific changes in CYP2D6 function with imipramine dose requirement in a large group of depressed patients. Mol Psychiatry 2008; 13(6): 597–605
Tracy TS, Venkataramanan R, Glover DD, et al. Temporal changes in drug metabolism (CYP1A2, CYP2D6 and CYP3A activity) during pregnancy. Am J Obstet Gynecol 2005; 192(2): 633–9
Wadelius MM, Darj E, Frenne G, et al. Induction of CYP2D6 in pregnancy. Clin Pharmacol Ther 1997 Oct; 624: 400–7
Dickmann LJ, Tay S, Senn TD, et al. Changes in maternal liver CYP2C and CYP2D expression and activity during rat pregnancy. Biochem Pharmacol 2008; 75(8): 1677–87
Tasker TC, Kaye CM, Zussman BD, et al. Paroxetine plasma levels: lack of correlation with efficacy or adverse events. Acta Psychiatr Scand Suppl 1989; 350: 152–5
Evans J, Heron J, Francomb H, et al. Cohort study of depressed mood during pregnancy and after childbirth. BMJ 2001; 323(7307): 257–60
Berard A, Ramos E, Rey E, et al. First trimester exposure to paroxetine and risk of cardiac malformations in infants: the importance of dosage. Birth Defects Res B Dev Reprod Toxicol 2007; 80(1): 18–27
Oberlander TF, Warburton W, Misri S, et al. Effects of timing and duration of gestational exposure to serotonin reuptake inhibitor antidepressants: population-based study. Br J Psychiatry 2008; 192(5): 338–43
Noorlander CW, Ververs FF, Nikkels PG, et al. Modulation of serotonin transporter function during fetal development causes dilated heart cardiomyopathy and lifelong behavioral abnormalities. PLoS ONE 2008; 3(7): e2782
DeVane CL, Stowe ZN, Donovan JL, et al. Therapeutic drug monitoring of psychoactive drugs during pregnancy in the genomic era: challenges and opportunities. J Psychopharmacol 2006; 20 (4 Suppl.): 54–9
Acknowledgements
We thank all the participating women and the staff of the participating medical centres who made this study possible. We also thank the technicians at the laboratory of Apotheek Haagse Ziekenhuizen in the Hague, the Department of Clinical Pharmacy and Clinical Chemistry of the University Medical Center Utrecht and the Erasmus Medical Center (Rotterdam, the Netherlands) for analysis, and pharmacy students Jeroen Hassink, Hetty Rijksen and Yasmin Al Khalil of the Utrecht University for technical assistance. We thank Dr Daan Touw and Dr Ron van Schaik for critical review of the draft of this article and for their valuable suggestions.
The study was supported in part by an unrestricted educational grant from Pfizer Inc. (New York, NY, USA) and Eli Lilly BV (Houten, the Netherlands) and by grants from the Brain Foundation of the Netherlands (the Hague, the Netherlands), the Foundation for the Advancement of Appropriate Drug Usage in the Central Region of the Netherlands (Utrecht, the Netherlands) and the Arijan Porsius Foundation for Evidence Based Pharmacotherapy (Utrecht, the Netherlands). The work was performed independently of the funders. The authors have no conflicts of interest that are directly relevant to the contents of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ververs, F.F.T., Voorbij, H.A.M., Zwarts, P. et al. Effect of Cytochrome P450 2D6 Genotype on Maternal Paroxetine Plasma Concentrations during Pregnancy. Clin Pharmacokinet 48, 677–683 (2009). https://doi.org/10.2165/11318050-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11318050-000000000-00000