Abstract
Objective
The aim of this study was to determine the extent and patterns of antidepressant use before, during and after pregnancy in a large population in The Netherlands.
Methods
Health care records and prescription data from one of the largest Dutch health insurance companies were analysed. The study cohort consisted of 29,005 women who had live births in the period between January 2000 and July 2003. Antidepressant drug use during a specified period was defined as there being a record of a prescription during that period.
Results
During the first trimester of pregnancy 2% of all pregnant women of the study cohort were found to have taken antidepressants; in the second and third trimesters, this figure had dropped to 1.8% of all pregnancies. Antidepressant use before as well as during pregnancy was almost twofold higher in women over 35 years of age than in those under 35 years. Almost 60% of the women who used antidepressants before pregnancy stopped taking them in the first trimester, and a smaller number stopped thereafter. Of all women using antidepressants during pregnancy, one third started this medication during gestation. In the 3 months following delivery, the prevalence of antidepressant use was the same as before pregnancy (2.9%). There was no shift to benzodiazepines in the group of women who stopped taking antidepressants during pregnancy. Although paroxetine and fluoxetine were the most frequently used antidepressants among the study group, all modern antidepressants were used.
Conclusion
A considerable number of women are being exposed to antidepressants throughout pregnancy up until delivery. One consequence of this is that their newborns need special care and supervision during the first days of life. However, women who stop taking the medication may risk a relapse of their illness, and this may also have a negative effect on the child.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There has been a steady increase in antidepressant use in recent years, especially by women of childbearing potential between 20 and 40 years of age [1–3]. This increase can be explained – at least in part – by the broadening therapeutic indications for which antidepressants are now being prescribed: in addition to depression, anxiety disorders, premenstrual syndrome, pain and eating disorders are being treated with antidepressants [1, 2]. A woman is twice as likely to experience mood and anxiety disorders at some time during her life than a man. The lifetime prevalence of major depression in women is 17%. Social phobia, post-traumatic stress syndrome and panic disorder each have a life time prevalence of 2–3%.
The prevalence of depression during pregnancy is estimated to be 10–16% [4, 5]. Despite the high degree of worldwide utilization and experience with antidepressants, there is still uncertainty and debate concerning the safety of antidepressant use during pregnancy. Although several studies have shown no increased risk of congenital malformations as a result of antidepressant use during pregnancy, associations have recently been made between paroxetine or clomipramine use during pregnancy and an elevated rate of cardiovascular malformations in the newborn [6, 7]. Even more recent are the concerns that have been expressed about persistent pulmonary hypertension in the newborn following maternal use of selective serotonin re-uptake inhibitors during the second half of pregnancy [8]. Experimental data on the safety of most antidepressant drugs during pregnancy are far from complete. An increased risk of preterm birth and lower birth weight have been demonstrated, but adverse effects on the child’s neurodevelopment are unknown [9, 10]. Another potential complication of the use of antidepressants during pregnancy up until delivery is the occurrence of postnatal withdrawal effects [11, 12]. It is therefore necessary to closely monitor women who use antidepressants during pregnancy as well as their offspring to gain more insight into the frequency and severity of potential negative effects of these drugs [8, 9].
Drug utilization studies have shown varying prevalences of gestational use of psychotropic drugs in different study settings and countries [13–18]. The prevalence of prescriptions for antidepressant drugs during pregnancy in a German sickness fund population was 0.2%, whereas a study in the United States showed a total prevalence during pregnancy of 2.8% [17, 18]. All studies consistently reported a decline in antidepressant drug use during pregnancy compared to the period before pregnancy. None of the studies reported patterns of use of all of the individual antidepressants during the three different trimesters of pregnancy.
Most of the studies conducted to data measured antidepressant drug use during pregnancy by means of either interview data or pharmacy dispensing data. While data compiled on the basis of interviews have the problem of recall bias, they may reveal non-compliance with intentionally prescribed drugs [19]. Dispensing data based on pharmaceutical records have the advantage of not being subject to recall bias, but suffer from a severe sensitivity problem since the recording of pregnancy is dependent on whether or not a patient reports her pregnant status to the pharmacy. Although algorithms have been developed to identify pregnant women from a population-based drug-dispensing database, the main limitation is that not all parents are identified, which may result in misclassification. Moreover, since the gestational age is not known, the conception date can only be estimated [20].
Health care insurance data have the advantage of combining drug-dispensing information with information on birth and delivery. Delivery can be confirmed by recorded obstetric help [17], although the date of conception is not available. In the study reported here we extracted prescriptions during specific periods before and after delivery from the database of a national health care insurance company in The Netherlands. The aim of our study was to determine the prevalence and patterns of antidepressant drug use before, during and after pregnancy in a Dutch population.
Materials and methods
Setting
The study cohort consisted of all persons having a health care insurance policy with the VGZ Health Insurance Company in The Netherlands during the entire period between January 2000 and July 2003.
VGZ is one of the largest health care insurance companies in The Netherlands, with more than 2 million policyholders living predominantly in the centre and south of the country. Their client population can be considered to be representative of the whole country, with a slight overrepresentation of people who depend on social health care. VGZ provides health insurance for the cost of pharmacy prescriptions, general practitioners, prenatal care, hospital admissions and other medical facilities. The computerized health-care-utilization data contain information on the dispensed drugs and dispensing date. Each person has a unique, anonymous identification number.
Study population
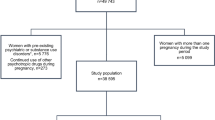
Using data extracted from the database of VGZ, we identified all children born between January 2000 and July 2003. We then selected all females between the ages of 15 and 50 living at the same address as the child. If there was no or more than one potential mother within a single household, the data were discarded. To ensure that the assumed mother had been pregnant and had delivered, the records were checked for obstetric help (hospital or at home) provided on the day of the child’s birth. In this manner we identified 29,005 pregnancies in 26,719 women.
Data analysis
We investigated patterns of antidepressant use before, during and after pregnancy in this study group. Prescriptions for antidepressants were identified by anatomical therapeutic classification (ATC) codes starting with N06A. To indicate the different relevant time periods, we defined the beginning of pregnancy as 270 days (9 months) before delivery, although the length of a pregnancy from an estimated date of conception is 265 days and may be even shorter in antidepressant-exposed pregnancies [21]. The start of the follow-up was set at 450 days (15 months) before delivery. Drug use was studied during two periods of 90 days each (3 months) before pregnancy, during three 90-day trimesters during the assumed pregnancy and during the first 90 days after delivery [18]. Women were considered to be using antidepressants if there had been at least one prescription for an antidepressant drug dispensed during a specific time frame. Prevalence was calculated for each time frame by dividing the number of users by the number of pregnancies. Prevalences were calculated for the whole group as well as for different age categories and for individual antidepressants. In addition, three different mutually exclusive patterns of antidepressant use were studied:
-
1)
Antidepressant use before pregnancy as well as during pregnancy;
-
2)
Antidepressant use before pregnancy but no gestational use;
-
3)
No use of antidepressants before pregnancy, but start of antidepressant use during pregnancy.
In all groups we also looked for recorded benzodiazepine use as a co-medication. Antidepressants were further classified according to their risk factor, using the Swedish and Australian risk classification systems [13, 22].
We performed database management and internal quality and validation procedures using Microsoft Access 2000. SPSS for Windows (ver. 11; SPSS, Chicago, Ill.) was used for the statistical analysis, which includes logistic regression. Prevalence of antidepressant use in the different age categories was compared to the prevalence in the largest age group: 25–30 years. Differences in prevalence of specific drug use in the different periods before, during and after pregnancy was tested.
Results
The Dutch study cohort
We studied 29,005 deliveries during the 3.5-year observation period. Of these, 92% of the women had one delivery, 8% (n=2252) had two deliveries and 0.06% (n=17) had three deliveries. There were 511 twins (1.8%) and 15 triplets (0.05%). Mean maternal age at delivery was 29.8 (±4.4) years; median age was 30.0 years. These findings are consistent with the mean age at delivery of 30.9 years in the overall Dutch birth registry data during the same period [23].
Antidepressant use before, during and after pregnancy
Table 1 shows the prevalence of antidepressant use during the different 3-month time frames before conception, during pregnancy and after delivery. Before pregnancy, 2.9% of women were using antidepressants; during the first trimester this dropped to 2% and decreased still further to 1.8% during the second and third trimesters. During the post- delivery period, 2.9% of the women were using antidepressants. During all of the 3-month periods, women over the age of 35 consistently used antidepressants more frequently than younger women (Table 1).
Of the 1075 women who used antidepressants during the 6-month period immediately preceding the pregnancy, almost 60% stopped during the first trimester and another 19% stopped during the second trimester. However, approximately 10% started using their medication again during pregnancy. At the end of gestation, 25% of this population was still using antidepressants (Table 2). Unfortunately, we were not able to distinguish between prescriptions that were meant for immediate use and those for predetermined postnatal use.
The incidence of new antidepressant use during pregnancy (i.e. no use during the 6 months preceding the pregnancy) was 0.5% for each trimester.
Co-medication with benzodiazepines
Of the women who used antidepressants before the pregnancy, 33% also used benzodiazepines. The use of benzodiazepines declined dramatically in these women during pregnancy, and only 3.4% of this group used a benzodiazepine during the third trimester. Of the 495 women who stopped using antidepressants during pregnancy, 17% still had prescriptions for a benzodiazepine during the first trimester, 3.2% during the second trimester and 2.8% during the third trimester.
Antidepressant use and pregnancy risk category
No significant difference in prevalence was seen between use during pregnancy of antidepressants belonging to the different risk categories according to either the Swedish classification system or the Australian classification system. The two classification systems view the risk associated with taking modern antidepressants differently. The most frequently prescribed products are presented in Table 3. Paroxetine was by far the most frequently prescribed drug before and during pregnancy, followed by fluoxetine. We did not find a significant shift towards one specific antidepressant during pregnancy. We calculated the percentage of prevalence of each drug compared to the total number of pregnancies registered with antidepressant use in each period. Table 3 shows that all different antidepressants are used to a similar extent with an over representation of paroxetine and fluoxetine.
Discussion
We evaluated health care records in order to gain insight into the extent and patterns of antidepressant use. Of the pregnant women included in this Dutch study population, about 2% used antidepressant drugs. The majority stopped before or sometime during pregnancy, but new use was also recorded. All available antidepressants were prescribed, despite the lack of safety data.
Health care insurance data can provide insight into the prevalence of drug use in large populations, such as children, the elderly and pregnant women [14, 17]. A limitation of all studies on drug use during pregnancy is the necessary assumption that conception took place 9 months before delivery in order to be able to classify the pregnancy into trimesters [21]. Additional limitations to the method employed in this study are the inclusion of only live births and the fact that dispensing of the drug does not always mean the drug was actually taken. We were unable to rule out the possibility that drugs dispensed just before pregnancy were still being used in the first trimester of pregnancy or, on the other hand, that the use of drugs dispensed in the first trimester may have been stopped shortly after the pregnancy became apparent. There may have been some overlap in the other time frames as well. We made the assumption that a prescription in the third trimester implies use during this trimester, but it may also have been intended for use after delivery.
The prevalence of antidepressant use before, during and after pregnancy in this Dutch population is more or less comparable with that in an American population [18]. In the latter group, Andrade et al. found exposure rates for antidepressants of 2.2%, 1.3% and 1.4% during the first, second and third trimesters respectively. In a German study, prescription rates for antidepressants before pregnancy were only 1% and declined to 0.2% during pregnancy [17]. This difference may be due to the regulations in Germany, where the sickness fund may not cover all antidepressants, or may be caused by the use of herbal medicines, which is more common in Germany.
Based on our data, we estimated that 75% of the 1075 women who were on antidepressants before pregnancy succeeded in stopping antidepressant use during pregnancy. Only 9% of this population restarted during the second trimester and 12% restarted during the third trimester. This is much lower than the incidence rates described by Cohen et al., who found a 52% reintroduction rate of antidepressant use [24]. On the other hand, our population had many new users who started antidepressants during pregnancy. The overall incidence of antidepressant use during pregnancy was higher in women over 35 years of age, especially preconceptionally. The background for this is unknown, but could be related to work, prior infertility problems, multiple pregnancies and larger families, among others.
In The Netherlands the policy is to taper or stop antidepressants to avoid fetal exposure during pregnancy. In cases of serious relapse or risk of a major depression, tricyclic antidepressants or fluoxetine are the drugs of first choice based on the experience of the clinician with these compounds. For all of the other modern antidepressants research data are insufficient to make a solid risk-benefit evaluation. Antidepressants are dispensed with a warning that safe use in pregnancy is not established and it is known that antidepressant use during the third trimester is related to potentially severe neonatal withdrawal symptoms (including convulsions and respiratory distress), although overall incidence is unknown [11]. Current policy in The Netherlands is to admit these infants to a neonatal medium-care unit for about 48 h [25]. This implies that at the present time 2% of all newborns are admitted because of maternal use of these drugs. The 3% women using antidepressants after delivery will experience a certain degree of uncertainty regarding the safety of breast feeding [26].
Although one third of the study group used benzodiazepines (risk category C) before pregnancy, only a few continued to use them throughout pregnancy. Fortunately, we did not see a shift towards benzodiazepines in the group of women who stopped taking antidepressants during pregnancy.
Paroxetine and fluoxetine were the drugs most commonly used before and during pregnancy. The former accounted for almost half of the antidepressant use among the study group, which is in line with use in the general population in The Netherlands [3]. Our study showed that all modern antidepressants were used during pregnancy, despite the fact that evidence on safety varies considerably. For example,, we did not see a profound decline during pregnancy of the use of new drugs like venlafaxine, sertraline, citalopram and mirtazapine. Nor did we see a shift towards fluoxetine, an antidepressant for which the most documented evidence on use during pregnancy is available [27, 28]. Therefore, we conclude that physicians presume that the teratologic risk of all antidepressants is the same, based on their similarity in therapeutic effect. By doing so, they disregard considerable differences in chemical structure, metabolic pathways and side effects that might induce different teratologic effects, as has been shown in comparative studies on the increased relative risk in congenital heart disease after fetal exposure to paroxetine [6, 7]. While waiting for a definite answer on paroxetine, one should try to avoid paroxetine in pregnancy. A possible shift towards herbal drugs or other self medication drugs such as St. John’s wort could not be extracted from our data. Although the written instructions of preparations containing St. John’s wort recommend that it not be used during pregnancy due to limited data that may –or may not – confirm safe use, it still may be used during pregnancy by some women.
Conclusion
Although evidence on the safety of antidepressants during pregnancy is far from complete, these drugs are prescribed frequently. The reasons for this are manifold and possibly include the assessment of the physician that the adverse effects of depression, anxiety and stress may be considered to be more harmful to mother and child than the elevated teratologic risk or the unknown effect of the antidepressant on the development of the child later in life. We found that most women tried to stop using antidepressant medication once they were pregnant; however, they were still being prescribed for 1.8% of all pregnant women in the third trimester of pregnancy. This is in part due to the fact that a significant number of women who had not used antidepressants before pregnancy started doing so during gestation. This result of this usage is the need for extra neonatal care in order to anticipate withdrawal effects.
The prevalence of antidepressant use shortly after delivery returned to the level of the period before pregnancy. This results provides a good estimate of the size of the group of women who should be receiving counseling and information on safe drug use during lactation.
The use of benzodiazepines as co-medication declined dramatically in the group of women who had a history of antidepressant use before pregnancy, indicating that the user had not substituted another drug of this group for the one she had been taking, thereby reducing possible effects on the child.
Our study revealed that many diverse modern antidepressants are being prescribed at similar levels, even though most lack sufficient data to confirm safe use during pregnancy. This is an alarming development.
References
Hansen DG, Sondergaard J, Vach W, Gram LF, Rosholm JU, Kragst J (2003) Antidepressant drug use in general practice: inter-practice variation and association with practice characteristics. Eur J Clin Pharmacol 59:143–149
De las Cuevas C, Sanz E (2004) Do therapeutic indications of antidepressants change from one year to another? Pharmacoepidemiol Dr S 13:309–314
Foundation for Pharmaceutical Statistics, Farmacie in cijfers. [Pharmacy in figures. Drug use of antidepressants is increasing] 2005 Pharmaceutisch Weekblad 140:767 or http://www.sfk.nl/publicaties/farmacie_in_cijfers/2005
Kallen B, Otterblad Olausson P (2006) Antidepressant drugs during pregnancy and infant congenital heart defect. Reprod Toxicol 21:221–222
Williams M, Wooltorton E (2005) Paroxetine(Paxil) and congenital malformations. Can Med Assoc J 173:1320–1321
Casper RC, Fleisher BE, Lee-Ancajas JC, Gilles A, Gaylor E, DeBattista A, Hoyme HE (2003) Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. J Pediatr 142:402–408
Zeskind PS, Stephens LE (2004) Maternal Selective serotonin reuptake inhibitor use during pregnancy and newborn neurobehavior. Pediatrics 113:368–375
Chambers CD, Hernandez-Diaz S, Van Marter LJ, Werler MM, Louik C, Jones KL, Mitchell AA (2006) Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med 354:579–587
Nonacs R, Cohen LS (2003) Assessment and treatment of depression during pregnancy: an update. Psychiatr Clin N Am 26:547–562
Sanz EJ, De-las-Cuevas D, Kiuru A, Bate A, Edwards R (2005) Selective serotonin reuptake inhibitors in pregnant women and neonatal withdrawal syndrome: a database analysis. Lancet 365:482–487
Olesen C, Sorensen HT, de Jong-van den Berg L, Olsen J, Steffensen FH (1999) Prescribing during pregnancy and lactation with reference to the Swedish classification system. A population-based study among Danish women. The Euromap Group. Acta Obstet Gynecol Scand 78:686–692
Beyens MN, Guy C, Ratrema M, Ollagnier M (2003) Prescription of drugs to pregnant women in France: the HIMAGE study. Therapie 58:505–511
Schirm E, Meijer WM, Tobi H, de Jong-van den Berg LT (2004) Drug use by pregnant women and comparable non-pregnant women in the Netherlands with reference to the Australian classification system. Eur J Obstet Gynecol Reprod Biol 114:182–188
Headley J, Northstone K, Simmons H, Golding J; ALSPAC Study Team (2004) Medication use during pregnancy: data from the Avon longitudinal study of parents and children. Eur J Clin Pharmacol 60:355–361
Egen-Lappe V, Hasford J (2004) Drug prescription in pregnancy: analysis of a large statutory sickness fund population. Eur Clin Pharmacol 60:659–666
Andrade SE, Gurwitz JH, Davis RL, Chan KA, Finkelstein JA, Fortman K, McPhillips H, Raebel MA, Roblin D, Smith DH, Yood MU, Morse AN, Platt R (2004) Prescription drug use in pregnancy. Am J Obstet Gynecol 191:398–407
Czeizel AE, Petik D, Vargha P (2003) Validation studies of drug exposures in pregnant women. Pharmacoepidemiol Dr S 12:409–416
Schirm E, Tobi H, de Jong-van den Berg LTW (2004) Identifying parents in pharmacy data: a tool for the continuous monitoring of drug exposure to unborn children. J Clin Epidemiol 57:737–741
Mittendorf R, Williams MA, Berkey CS, Cotter PF (1990) The length of uncomplicated human gestation. Obstet Gynecol 75:929–932
Australian Drug Evaluation Committee. Medicines in Pregnancy Working Party, Prescribing Medicines in Pregnancy. Australian categorisation of drugs, 4th ed. [cited 3 September 2004]. Available from URL: http://www.health.gov.au/tga/docs/html/medpreg.htm
Statistics Netherlands (CBS) Voorburg, the Netherlands. Available from URL: http://www.cbs.nl/enGB/default.htm
Nulman I, Rovet J, Stewart DE, Wolpin J, Pace-Asciak P, Shuhaiber S, Koren G (2002) Child development following exposure to tricyclic antidepressants or fluoxetine throughout fetal life: a prospective, controlled study. Am J Psychiatry 159:1889–1895
Nordeng H, Spigset O (2005) Treatment with selective serotonin reuptake inhibitors in the third trimester of pregnancy. Drug Safety 28:565–581
Cohen LS, Altshuler LL, Stowe ZN, Faraone SV (2004) Reintroduction of antidepressant therapy across pregnancy in women who previously discontinued treatment. A preliminary retrospective study. Psychother Psychosom 73:255–258
Jaiswal S, Coombs RC, Isbister GK (2003) Paroxetine withdrawal in a neonate with historical and aboratory confirmation. Eur J Pediatr 162:723–724
Gentile S (2005) SSRIs in pregnancy and lactation: emphasis on neurodevelopmental outcome. CNS Drugs 19:623–633
Hines RN, Adams J, Buck GM, Faber W, Holson JF, Jacobson SW, Keszler M, McMartin K, Segraves RT, Singer LT, Sipes IG, Williams PL (2004) NTP-CERHR Expert Panel Report on the Reproductive and Developmental Toxicity of Fluoxetine. Birth Defects Res Part B71:193–280
Kallen B (2004) Fluoxetine use in early pregnancy. Birth Defects Res Part B 71:395–396
Acknowledgement
We are grateful to the VGZ Health Insurance Company for providing the health care and medication records we used in our study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mr. H. Kaasenbrood died on October 14, 2005.
Rights and permissions
About this article
Cite this article
Ververs, T., Kaasenbrood, H., Visser, G. et al. Prevalence and patterns of antidepressant drug use during pregnancy. Eur J Clin Pharmacol 62, 863–870 (2006). https://doi.org/10.1007/s00228-006-0177-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-006-0177-0