Abstract
Medicines' management or pharmaceutical care in paediatric patients is particularly demanding, mainly because the majority of available drugs have been developed for use in adults. As a result, in children, drugs are often unlicensed or used off-label, suitable formulations or appropriate strengths are lacking, and drugs have to be extemporaneously prepared, liquids and injections diluted, and tablets split. These factors increase the likelihood of medication errors and may lead to a reduction in drug effect. Age-specific changes in pharmacokinetics and pharmacodynamics further complicate drug therapy in children. All these challenges provide unique opportunities for pharmacists to improve the quality of care for paediatric patients.
We conducted a systematic literature review examining whether the interventions of hospital pharmacists improve drug therapy in children. Several medical and pharmaceutical databases were searched systematically to identify articles investigating hospital pharmacists' interventions that were intended to improve drug therapy in children. Inclusion criteria were English language, primary research papers and studies in which clinical pharmacists contributed directly to patient care. Exclusion criteria were reviews, editorials, questionnaire studies, modelling studies, letters and studies only available in abstract form.
This systematic search identified 18 articles documenting the role of a clinical hospital pharmacist in paediatric care. These articles were divided into the following groups based on study type: (i) studies documenting interventions made by pharmacists and their role in inpatients; (ii) articles presenting the outcomes of a satellite pharmacy; and (iii) articles examining pharmacist involvement in paediatric outpatient clinics. No randomised study comparing pharmacist interventions with standard care was found.
In conclusion, although it was difficult to compare the various studies identified because of the different settings, design, duration, size, methodology and definition, all these studies highlighted the importance of hospital pharmacists to medicines' management in paediatric patients. On the basis of this review, we can conclude that pharmacist reviewing of medication charts is very important in identifying medication errors; hence, it is likely to be the most effective method of improving drug therapy in children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The majority of marketed medicines have been developed for use in adults. As a result, most medicines are not licensed in children; suitable paediatric formulations and appropriate strengths are also lacking. Conroy et al.[1] reported that over two-thirds of 624 children admitted to wards in five European hospitals were prescribed drugs that were unlicensed in children or the use of which was off-label in children.
Nurses and parents may be required to subdivide tablets, open capsules or dilute injections in order to administer the correct dosage. Such practices can potentially lead to a reduction in drug effect and/or toxicity.[2] Moreover, this increases the likelihood of a 10-fold medication error in children; for drugs with a narrow therapeutic index, a 10-fold dosage increase may lead to serious morbidity or mortality.[3] Fortescue et al.[4] conducted a prospective cohort study in 1020 patients who were admitted to two academic medical centres in the US during a 6-week period in April and May 1999. They modelled the data and concluded that ward-based clinical pharmacists might have prevented 81% of potentially harmful errors.
Human growth is not a linear process; age-associated changes in body composition and organ function are dynamic and can be discordant during the first decade of life. Thus, simplified dose administration approaches are not adequate for individualising drug dosages across the span of childhood.[5] These challenges provide unique opportunities for pharmacists to reduce medication-related problems and improve the quality of care for paediatric patients. In one US study, the authors concluded that hospital pharmacists play a crucial role in preventing harm, and minimising unnecessary costs and potential liability that may result from drug errors.[6]
We conducted a systematic literature review examining whether the interventions of hospital pharmacists improve drug therapy in children.
Literature Search and Review
Search Methodology
The following databases were searched: EMBASE (1980 – 2004 week 19), Ovid MEDLINE® (1966 –April week 5 2004), Ovid MEDLINE® In-Process & Other Non-indexed Citations (May 12, 2004), International Pharmaceutical Abstracts (1970 – April 2004), Ovid old MEDLINE® (1951–1965) and Pharmline (1978–2004). The following keywords were used: ‘chart review’, ‘clinical’, ‘consultation’, ‘counselling’, ‘drug monitoring’, ‘drug review’, ‘intervention’, ‘interventions’, ‘medication review’, ‘medicines management’, ‘pharmaceutical care’, ‘prescription review’, ‘ward’. These were combined with the following using ‘AND’: ‘pharmacy’, ‘pharmacist(s)’. The result of this search was limited further by combining with the following keywords using ‘AND’: ‘pediatric(s)’, ‘paediatric(s’), ‘child’, ‘children’, ‘infant(s)’, ‘adolescent(s)’, ‘teenager(s)’, ‘neonate(s)’, ‘neonatal’.
The following inclusion criteria were used: English language, primary research paper, and clinical pharmacists contributed directly to patient care. Exclusion criteria included: reviews, editorials, questionnaire studies, modelling studies, letters and studies only available in abstract form. The reference lists of the selected papers were also reviewed in order to identify additional relevant studies.
Review Procedure
From a previous systematic review in paediatric pharmacy research[3] we had anticipated that the studies would be heterogeneous as a result of different practices in different countries, a lack of standardised methodologies and outcome measures. As such, we did not attempt to analyse the data statistically. Instead, results were summarised in tabular form according to the characteristics of each study (see table I).
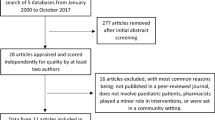
A total of 1902 references were identified. After a preliminary review of titles and abstracts, 1799 articles were excluded. The reasons for exclusion were: community pharmacy studies, abstracts of meetings, or personal opinions of individuals about paediatric pharmacy. This left 103 references for full review, 83 of which were subsequently excluded because they were: descriptions of the role of a pharmacist, hospital pharmacy practice guidelines, questionnaire studies, modelling studies, or personal opinions of individuals about paediatric pharmacy. Three studies were unobtainable. The reference lists of the remaining 17 articles were scrutinised and a further two articles were identified; however, both were rejected after full review as one was a description of the role of a pharmacist[24] and the other was an observational study conducted by a pharmacist, determining the medication errors made by nurses.[7] Finally, one reference was obtained through a personal contact with an expert in paediatric medication error research. A total of 18 studies were included in this review.
Literature Search Results
An analysis of the final set of 18 articles can be found in table I. These articles were divided into the following groups based on study type:
-
1.
Fourteen of the studies conducted documented interventions made by pharmacists. Ten of these were conducted in the US, three in Canada and one in the UK.
-
2.
One study from the US examined whether the quality of medication therapy in paediatric patients was improved if pharmacist involvement in direct patient care was increased via a satellite pharmacy.
-
3.
Another three studies examined the results of pharmacist involvement in paediatric outpatient clinics. One was conducted in South Africa, one was conducted in the US and one in Canada.
Almost all studies reported positive outcomes, such as reduction in medication errors and medication-related problems. Some also reported a reduction in total drug cost.
Pharmacists’ Interventions in Paediatric Inpatients
The earliest study looking at pharmacists’ interventions was conducted in 1971 in the US by Munzenberger et al.[7] This study identified the pharmacist’s role as monitoring patient charts, providing admission drug histories, providing discharge consultations, and providing drug information to medical and nursing staff. Pharmacists’ involvement in the above areas led to improved paediatric medical care and provided a valuable service to doctors and nurses working in the unit during the study. However, the authors stressed that in order for pharmacists to provide these services fully they need to be readily available on the ward at all times, an aspiration that has still not been met 30 years on.
A study by Folli et al.[10] involving two paediatric hospitals in the US identified a total of 281 and 198 errant orders at the two institutions, respectively, over 6 months. No harm to patients because of errors occurred during the study and the frequency of errant orders declined as physician training status increased. Within both hospitals, 27 errors were potentially lethal, which the authors feel justifies the additional cost of a clinical pharmacist. However, the authors point out that the true specificity of error detection cannot be determined from the data as the number of potentially errant orders identified by the pharmacist but not changed by the physician were not recorded.
Again in the US, Blum et al.[6] repeated the study by Folli et al.[10] over 3 months with similar results. This study again shows the importance of a clinical paediatric pharmacist in detecting and preventing medication errors.
A study by Koren et al.[11] in Canada recorded a number of dose administration errors, particularly 10-fold errors, many of which could have led to serious morbidity or mortality. As with Folli et al.,[10] these findings present a strong case for the role of a clinical paediatric pharmacist, as “prevention of man-made morbidity and mortality should always be a goal of patient care”.[10]
Another study in Canada, by Strong and Tsang,[12] found 361 interventions over 2 weeks; however, interventions resulting from drug information questions were not included. The physician acceptance rate (percentage of pharmacists’ interventions accepted by physicians) was found to be 95.8%. 190 out of 361 interventions had an impact on patient care; of these, 93% of interventions were found to have a positive effect. Eighty-two randomly selected interventions were assessed and 8.5% were classified as life-saving. The authors also calculated a cost avoidance of $679 (1991 value) over 2 weeks, which represents $17 654 annually. However, this is likely to be an underestimate as no control group was included in the study, so it was not possible to accurately estimate how an intervention influenced cost in terms of duration of treatments, length of hospital stay, costs avoided as a result of allergy notification, and adverse drug reaction (ADR) identification. Nevertheless, the study demonstrated that ‘pharmacists’ interventions which represent only a proportion of a pharmacist’s responsibilities, improve the quality of patient care and result in cost avoidance”.[12]
In the US, Lal et al.[13] documented 504 clinical interventions and services accepted over a 6-month period. Pharmacists’ interventions were found to have decreased hospital stay from 4.38 to 4.26 days (a decrease of 2.7%) and led to a cost saving of $7227.83 during the study period. This study also demonstrated improved pharmacy relationships with nurses and doctors, with a 50% reduction in the number of complaints filed with the pharmacy by ward staff. As with the Strong and Tsang study,[12] a possible limitation is an underestimation of the cost saving.
A total of 610 interventions were identified over 4 months in a US study by Falck et al.[14] Over this time the pharmacist spent 227 hours devoted to pharmacy activities in the paediatric intensive care unit (PICU), which represents 2.7 interventions per hour. Limitations included inability to evaluate correlations between pharmacist time spent in the PICU and patient length of stay, doses dispensed and interventions completed. “These measurements could justify the need for consistent as opposed to sporadic pharmacist involvement with the care team”,[14] the same conclusion reached by Munzenberger et al.[7]
An interesting US study by Chan and Kotzin[15] compared pharmacists’ clinical intervention trends between paediatric and adult inpatients. Over 4 years the study documented 706 interventions in paediatric compared with 379 in adult patients. The mean time spent by a pharmacist per intervention was 35.4 minutes for paediatric and 31.1 minutes for adult patients. The incidence of interventions was 75.3 per 10 000 orders written for children compared with 4.8 per 10 000 orders written for adults. The incidence of drug-related problems for children was 165.0 per 10 000 orders written compared with only 8.7 per 10 000 orders written for adults. Overall, a higher incidence of interventions was reported in children than adults. Limitations of the study included the fact that it was performed retrospectively, and that it lacked sufficient data to perform cost and explicit quality of care analysis. Another limitation was that there was a change in the clinical pharmacy staffing during the study period, which may have affected the number of interventions recorded because of variation in experience. Overall the study highlights the value of a paediatric clinical pharmacist.
A 24-week (79-day) study in the US by Krupicka et al.[16] documented 172 interventions for 77 patients, equivalent to 35 recommendations per 100 patient-days. The average time spent by the pharmacist in the PICU was 0.73 hours/day. Patients with recommended interventions on admission had a longer intensive care unit and hospital stay, and the pharmacist spent more time on these patients. There was a $1977 (1997 value) cost saving during the study, which is equivalent to $9135 annually. A limitation of the study was that there was no control group, so benefits had to be assumed rather than proven causal. In addition, a patient’s clinical course was not factored into potential savings as a result of interventions, and there was no direct evidence of a positive or lasting impact of medical staff education.
The only study conducted in the UK was by Guy et al.[17] During the 4 weeks of the study, 363 interventions were recorded: 190 interventions were detected by pharmacists, 80% of which were accepted by medical staff. 60% of interventions resulted in prescriptions being amended; advice was acknowledged in 6% of cases, while 0.5% of detected errors were regarded as life threatening. The majority of interventions were resolved within 5 minutes. Limitations of the study include the fact that only one pharmacist and nurse were available in the active phase of the study to promote and manage the project. Staff shortages in pharmacy may have resulted in incomplete data capture and, in addition, not all returns were fully completed. Thus, the chosen time period may have affected the results.
Virani and Crown[18] recorded 48 interventions over 4 weeks in a Canadian study, of which the physicians accepted 98%. Forty-four interventions were analysed, of which 86% were judged to have a positive effect. Total drug cost per patient-day decreased by 14% in the 12 months after having a clinical pharmacist on the ward. The total drug cost was decreased by 21%, which represents a cost saving of $5485.80 during the study period. The small number of patients and interventions during the study may have limited the results. The small number of patients was further compounded by a reduced number of admissions during the study period. The cost analysis was retrospective, which meant it was not possible to determine the extent to which a single factor was responsible for the observed changes. However, the high percentage of accepted interventions in this study demonstrates how a clinical pharmacist positively influences patient outcomes in the paediatric population.
A recent study by Condren et al.[19] in the US documented a total of 4605 interventions for 3978 patients over 12 months. Ninety-one percent of recommendations were accepted by the physician. A total of 223 adverse drug events or medication errors were prevented or detected during the study period, which resulted in an estimated cost saving of $458 516 (2002 value). However, data used to derive cost savings of interventions were based on adult patients, so may be misleading as no validated data were available to guide the economic analysis of interventions in the paediatric population. There is also uncertainty about the outcome of interventions: for example, it is unknown whether the interventions resulted in shorter hospital stays or an overall decrease in healthcare costs. Nevertheless, this study justifies the role of pharmacists within the paediatric medical team through a reduction in medication errors.
Two studies, both conducted in the US, specifically compared the use of a standardised total parenteral nutrition (TPN) formulation with a pharmacist-assisted individualised programme of TPN in paediatrics. Mutchie et al.[8] found that pharmacist monitoring of TPN reduced the pharmacy’s mean cost per course of TPN to $44.10 (year not stated) less than the standard TPN. Additionally, a significantly greater mean weight gain (17 g/day) in the individualised group than the standardised group (4 g/day) was seen. Dice et al.[9] reported that pharmacist monitoring of an individualised programme of TPN in neonates provided a greater mean daily weight gain, allowed a greater amount of nutrients to be provided, and was cost effective compared with the standardised solution without pharmacist monitoring. A limitation of the study was that the cost of wasted solutions was not considered.
Methodological Limitations
As the majority of authors do not state what they mean by ‘intervention’, it is difficult to compare the intervention rates of the various studies. Moreover, the studies used different methods and in a variety of settings. The length of studies also varied from 2 weeks to 4 years.
One limitation that all these studies share is the method of intervention reporting. In all the studies, interventions were self-reported by the intervening pharmacist. This may lead to bias and also under-reporting of interventions due to time constraints or omission of activities the pharmacist does not consider important. As Hatoum et al.[25] suggested in a study in adults, “clinical pharmacists do not state all the daily interventions made, but their most favourable ones”.
Nevertheless, despite their shortcomings, all studies found the pharmacist to have a positive impact on the medical care of paediatric patients.
Contribution of a Satellite Pharmacy
The only study to examine whether the quality of medication therapy improved if pharmacist involvement in direct patient care was increased via a satellite pharmacy was by Gibson et al.[20] in the US. These authors found that there was no statistically significant improvement in the quality of medication therapy due to increased pharmacist involvement in drug therapy. However, the authors concluded that “results from this study do not mean that this decentralised pharmacy service is ineffective in influencing drug therapy. It simply means we did not obtain substantial evidence of its effectiveness”.[20] Indeed, the study had a number of shortcomings. For example, the approach to data collection only allowed measurement of therapy given; it did not consider therapy that should have been given. Also, the study involved the first 9 months after initiation of the system; it may be that at this early stage it was operating at less than maximum effectiveness.
Pharmacists’ Contribution to Outpatient Clinics
Three studies looked at how pharmacists’ participation improved services in outpatient clinics. All three studies are quite different, and examine the pharmacist’s contribution from different angles.
A study by Summers et al.[21] in a South African specialist paediatric neurology clinic found there was an increase in the number of patients seen per session with pharmacist involvement. The results also showed that polypharmacy, dose administration frequency and average dose per day were reduced with the pharmacist involvement, while disease control, i.e. seizure frequency, was no worse.
A Canadian study by Taylor et al.[22] in a paediatric haematology/oncology clinic identified 165 drug-related problems in 31 bone marrow transplant and 27 oncology patients. Ninety nine percent of the drug-related problems were identified by the pharmacist. The mean number of drug-related problems identified per patient was 4.8 in bone marrow transplant patients and 0.6 in oncology patients. The pharmacist made 177 interventions, 81% of which were accepted by the physician and/or the patient/parent. The review panel deemed 83.5% of the subset of interventions to have had a positive impact on the patient. A limitation of the study was the absence of detailed patient outcomes; this may have made the impact assessment more difficult. Reviewer bias may have been present, as one of the pharmacist reviewers was involved in the structure development of the study and one of the physician reviewers interacted extensively with the pharmacist during the study.
A study in the US by Moore and Shelton[23] in a paediatric asthma clinic identified important areas of pharmacist activities. These included: (i) device technique instruction; (ii) asthma diary implementation; (iii) influenza vaccination prompting; and (iv) step-down therapy identification through medication reassessment and extensive asthma education.
Limitations of the Review
It was difficult to compare the various studies identified, because of the different settings, design, duration, size, methodology and definition. Furthermore, the review covered studies conducted over three decades, so some early studies in 1970s are probably no longer relevant to current practice. No randomised study comparing the pharmacist interventions with usual care have been found; therefore, it is difficult to assess the true effectiveness of pharmacist intervention in improving paediatric drug therapy. The economic analysis of these studies was of poor quality and is insufficient to assist healthcare provider/payer to make definitive decisions. Almost all studies reported positive results and we cannot exclude the ‘publication biases’. Moreover, the pharmacy practice researcher might have chosen the areas that are likely to be successful for research such as detection of medication errors; therefore, they are more likely to report positive results.
Medication Errors and Pharmacists’ Role
On the basis of this literature review and other published literature on medication errors, we can conclude that pharmacists reviewing medication charts is very important in identifying medication-related problems; hence, it is likely to be the most effective factor in improving drug therapy in children. This is particularly highlighted by Koren et al.,[11] who reported that clinical pharmacists identified and corrected nine instances of 10-fold errors and six instances of 2- to 3-fold errors in 2 months. Six of these errors could have resulted in serious morbidity or mortality. If the reader is interested in learning more about paediatric medication errors, we recommend the literature reviews conducted by Wong et al.[3] and Ghaleb and Wong.[26]
Conclusions
Although it was difficult to compare the various studies identified because of the different settings, design, duration, size, methodology and definition, all these studies highlighted the importance of hospital pharmacists to medicines management in paediatric patients. On the basis of this review, we can conclude that pharmacists reviewing medication charts is very important in identifying medication errors; hence, it is likely to be the most effective method of improving drug therapy in children.
Acknowledgements
Professor Wong’s post is funded by a UK Department of Health Public Health Career Scientist Award. No direct sources of funding were used in the preparation of this article. The authors have no conflicts of interest that are directly relevant to the content of this review.
References
Conroy S, Choonara I, Impicciatore P, et al. Survey of unlicensed and off label drug use in paediatric wards in European countries. BMJ 2000; 320 (7227): 79–82
Baber N, Pritchard D. Dose estimation for children. Br J Clin Pharmacol 2003; 56: 489–93
Wong ICK, Ghaleb MA, Franklin-Dean B, et al. Incidence and nature of dosing errors in paediatric medications. Drug Saf 2004; 27: 661–70
Fortescue EB, Kaushal R, Landrigan CP, et al. Prioritizing strategies for preventing medication errors and adverse drug events in paediatric inpatients. Paediatrics 2003; 111: 722–9
Kearns GL, Abdel-Rahman SM, Alander W, et al. Developmental pharmacology: drug disposition, action and therapy in infants and children. N Engl J Med 2003; 349: 1157–67
Blum KV, Abel SR, Urbanski CJ, et al. Medication error prevention by pharmacists. Am J Hosp Pharm 1988; 45: 1902–3
Munzenberger P, Emmanuel S, Heins M. The role of a pharmacist on the paediatric unit of a general hospital. Am J Hosp Pharm 1972; 29: 755–60
Mutchie KD, Smith KA, MacKay MW, et al. Pharmacist monitoring of parenteral nutrition: clinical and cost effectiveness. Am J Hosp Pharm 1979; 36: 785–7
Dice JE, Burckart GJ, Woo JT, et al. Standardized versus pharmacist-monitored individualized parenteral nutrition in low-birth-weight infants. Am J Hosp Pharm 1981; 38: 1487–9
Folli HL, Poole RL, Benitz WE, et al. Medication error prevention by clinical pharmacists in two children's hospitals. Paediatrics 1987; 79: 718–22
Koren G, Reich A, Hales B. Use of clinical pharmacists to prevent medication errors in children. J Pharm Technol 1991; 7: 219–21
Strong DK, Tsang WY. Focus and impact of pharmacists' interventions. Can J Hosp Pharm 1993; 46: 101–8
Lal LS, Anassi EO, McCants E. Documentation of the first steps of paediatric pharmaceutical care in a county hospital. Hosp Pharm 1995; 30: 1107–12
Falck KA, Darsey EH, Naughton MJ. Pharmacy interventions in a multidisciplinary paediatric intensive care unit. J Paediatr Pharm Pract 1997; 2: 162–7
Chan DS, Kotzin DA. Adult vs paediatric clinical intervention trends: a four year, retrospective report. J Paediatr Pharm Pract 1998; 3: 144–9
Krupicka MI, Bratton SL, Sonnenthal K, et al. Impact of a paediatric clinical pharmacist in the paediatric intensive care unit. Crit Care Med 2002; 30: 919–21
Guy J, Persaud J, Davies E, et al. Drug errors: what role do nurses and pharmacists have in minimizing the risk? J Child Health Care 2003; 7: 277–90
Virani A, Crown N. The impact of a clinical pharmacist on patient and economic outcomes in a child and adolescent mental health unit. Can J Hosp Pharm 2003; 56: 158–62
Condren ME, Haase MR, Luedtke SA, et al. Clinical activities of an academic paediatric pharmacy team. Ann Pharmacother 2004; 38: 574–8
Gibson JT, Vance AL, Newton DS. Influence on medication therapy of increased patient services by pharmacists in a paediatric hospital. Am J Hosp Pharm 1975; 35: 495–500
Summers B, Summers RS, Rom S. The effect of a specialist clinic with pharmacist involvement on the management of epilepsy in paediatric patients. J Clin Hosp Pharm 1986; 11: 207–14
Taylor TL, Dupuis LL, Nicksy D, et al. Clinical pharmacy services in a paediatric hematology/oncology clinic: a description and assessment. Can J Hosp Pharm 1999; 52: 18–23
Moore SD, Shelton V. Assessment of children referred to a pharmacist-managed paediatric asthma clinic. J Paediatr Pharm Pract 2000; 5: 235–40
Tisdale JE. Justifying a paediatric critical-care satellite pharmacy by medication-error reporting. Am J Hosp Pharm 1986; 43: 368–71
Hatoum HT, Hutchinson RA, Witte KW, et al. Evaluation of the contribution of clinical pharmacists: inpatient care and cost reduction. Drug Intell Clin Pharm 1988; 22: 252–9
Ghaleb MA, Wong ICK. Medication errors in children [online]. Arch Dis Childhood Ed Pract91 Available from URL: http://www.ep.bmjjournals.com/cgi/content/full/91/1/ep20 [Accessed 2006 Oct 18]: ep20
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sanghera, N., Chan, PY., Khaki, Z.F. et al. Interventions of Hospital Pharmacists in Improving Drug Therapy in Children. Drug-Safety 29, 1031–1047 (2006). https://doi.org/10.2165/00002018-200629110-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002018-200629110-00003