Abstract
Clinical pharmacists provide beneficial services to adult patients, though their benefits for paediatric hospital patients are less defined. Five databases were searched using the MeSH terms ‘clinical pharmacist’, ‘paediatric/paediatric’, ‘hospital’, and ‘intervention’ for studies with paediatric patients conducted in hospital settings, and described pharmacist-initiated interventions, published between January 2000 and October 2017. The search strategy after full-text review identified 12 articles matching the eligibility criteria. Quality appraisal checklists from the Joanna Briggs Institute were used to appraise the eligible articles. Clinical pharmacist services had a positive impact on paediatric patient care. Medication errors intercepted by pharmacists included over- and under-dosing, missed doses, medication history gaps, allergies, and near-misses. Interventions to address these errors were positively received, and implemented by physicians, with an average acceptance rate of over 95%. Clinical pharmacist-initiated education resulted in improved medication understanding and adherence, improved patient satisfaction, and control of chronic medical conditions.
Conclusion: This review found that clinical pharmacists in paediatric wards may reduce drug-related problems and improve patient outcomes. The benefits of pharmacist involvement appear greatest when directly involved in ward rounds, due to being able to more rapidly identify medication errors during the prescribing phase, and provide real-time advice and recommendations to prescribers.
What is Known: • Complex paediatric conditions can require multiple pharmaceutical treatments, utilised in a safe manner to ensure good patient outcomes • The benefits of pharmacist interventions when using these treatments are well-documented in adult patients, though less so in paediatric patients | |
What is New: • Pharmacists are adept at identifying and managing medication errors for paediatric patients, including incorrect doses, missed doses, and gaps in medication history • Interventions recommended by pharmacists are generally well-accepted by prescribing physicians, especially when recommendations can be made during the prescribing phase of treatment |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Paediatric patients provide a unique set of challenges to their treating health professionals. This is in part due a limited capacity to communicate, particularly when suffering a traumatic illness, and differences in pharmacokinetic profiles compared to adults [4, 11]. Children represent approximately one-quarter of the global population, and although most experience a healthy childhood, a recent survey of children’s health estimated nearly half have at least once chronic health condition, and about 60% of children having received a prescription medication during the previous 12 months [6, 9]. The prevalence of complex paediatric conditions is also on the rise, including type 2 diabetes, asthma, hypertension, attention deficit and hyperactivity disorder, and depression [6]. These conditions usually require pharmaceutical interventions, with clinical pharmacists being responsible for providing direct, individualised pharmaceutical care to patients to ensure the optimal use of medications [17].
Clinical pharmacists in a multidisciplinary care team in adult care units play an integral part in ensuring the quality use of medicines, the reduction of medication errors, and enhance patient outcomes that lower costs [6, 32]. Reducing medication errors amongst the vulnerable paediatric population is of even greater significance, with previous research identifying paediatric patients as being at higher risk of errors compared to adults, and three times more likely for these errors to cause harm [16, 20, 39]. These errors, which can include the omission of medications, over-dosing and under-dosing, and administration errors, indicate that involvement of clinical pharmacists in paediatric condition management is essential for patient care [18]. Amongst adult hospital patients, pharmacists have been shown to improve medication adherence, knowledge, appropriateness of prescribed drugs, and reduced hospital stay [19]. However, not all hospitals employ paediatric clinical pharmacists, with fiscal scrutiny and changes in health care financing necessitating that healthcare professionals both outline and justify the medical and economical basis for their involvement in patient care [17].
This systematic review was conducted to evaluate whether paediatric clinical pharmacists afforded similar benefits to paediatric patients as for adult patients, and to what degree their interventions improved health outcomes for paediatric patients, and provided cost-savings to their respective institutions. The underpinning research question for this systematic review was ‘How do the professional activities of a clinical pharmacist impact on the treatment of paediatric hospital patients’?
Methods
This systematic review follows the recommendations by the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines [27].
Search strategy and study eligibility
Original-research, English-language articles published from 1st January 2000 to 1st October 2017 were eligible for inclusion, and identified through a systematic search of the PubMed, CINAHL, Google Scholar, AustHealth, and EMBASE databases. Searches were performed by a Townsville Hospital and Health Service librarian and the primary author. MeSH terms were clinical pharmacist, paediatric/paediatric, hospital, and intervention. Titles were read to identify potentially relevant articles, and we initially included any article that appeared to involve hospital patients of any age, and any health professional intervention. Abstracts were then read, with articles discussing paediatric patients and pharmacist involvement retained for full-text review. Articles were deemed eligible for inclusion if they recruited paediatric patients in a hospital environment, involved pharmacist-initiated interventions, and reported how these interventions may have influenced patient health. For this review, ‘paediatrics’ was considered as being between the ages of zero (birth) and 19 years old. Excluded articles were those describing interventions only partially managed by pharmacists, standard pharmacist interventions which were not linked to patient outcomes, only discussed older age groups, or had both paediatric and older patients but did not differentiate their results by age.
Data extraction and quality appraisal
Data extracted from eligible articles included author details, year published, country of participant origin, participant numbers, study design, frequency and methodology of interventions employed, and primary and secondary outcomes reported. The primary outcomes of interest were the types of pharmacist intervention employed, and their resulting health, and other outcomes relating to the care of paediatric patients. Data was grouped into the type of outcome reported, with health outcomes sub-grouped into: reduction in drug-related problems (DRPs), improved control of disease/condition, and reduction in medication-related errors and/or their severity. Study quality was assessed using validated checklists from the Joanna Briggs Institute (JBI). JBI checklists assess for study clarity, appropriateness of methodological design, analysis, presentation of results, and alignment of results and discussion to research objectives. Three JBI critical appraisal checklists were independently used by two authors for each article to assess study quality for eligible articles: analytical cross-sectional studies, cohort studies, and randomised controlled trials [28, 37]. Differences in scores were discussed until consensus, with articles considered as being of high quality if they scored ‘yes’ for at least 75% of the criteria, moderate if 50% or higher, and low if less than 50%.
Results
Study characteristics and quality appraisal
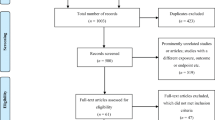
The search method initially identified 305 potential articles based on their titles, which was reduced to 28 after an initial abstract screening, with full-text screening leaving 12 articles matching the eligibility criteria. Common reasons for study ineligibility included pharmacist interventions not targeted at paediatric patients, articles not published in a peer-reviewed journal, interventions carried out by a multidisciplinary team, did not differentiate results between paediatric and adult patients, or involved pharmacist interventions in a community setting. Figure 1 illustrates the search strategy and article selection process.
The details of the 12 eligible articles are included in Table 1, all of which were conducted between 2000 and 2015, and evaluated pharmacist-initiated interventions aimed at improving paediatric patient outcomes. These studies were conducted across Africa, Europe, North America, and Asia. Of the 12 eligible articles, eight were prospective cross-sectional or cohort studies [1, 10, 13, 14, 21, 24, 33, 38], one was a retrospective study [25], and three were randomised controlled trials (RCTs) [2, 12, 40]. Common outcomes reported include the frequency, type, and acceptance rate of clinical pharmacist interventions, and their impact on the frequency and severity of DRPs, common medications implicated in DRPs and adverse events, paediatric patient compliance to prescribed medications, total patient health, and economic impact of pharmacist interventions.
Table 2 summarises the results from each of these studies, which cumulatively include over 35,000 paediatric patient admissions and describe 11,209 interventions for prescriptions and medication orders for these patients, with an average acceptance rate of 89.1% for studies reporting on intervention acceptance rates. Three main outcome themes emerged for these studies based on the interventions documented: error interventions, disease/condition improvement, and economic impacts. When excluding the low acceptance rate seen in Maat et al. (2013) [24], the average acceptance rate is 95.3%. Quality assessment of the eligible articles deemed eight articles as being of high quality, and the remaining four as moderate quality, with none as low quality. Common reasons for reductions in quality scores were an insufficient description of participants, a lack of detailed randomisation processes within RCTs, and confounding factors not adequately addressed. None of the articles eligible for inclusion were excluded on the basis of poor quality.
Error detection and interventions by pharmacists
The detection of errors and initiation of interventions by pharmacists were the most commonly reported outcome, though reporting rates per patient varied widely between studies, depending on the size of the facilities involved, and pharmacist numbers and workload. Antibiotics were the most common medications involved in DRPs, as described in six of the eligible articles, followed by drugs used for alimentary tract and metabolic disorders [1, 13, 14, 24, 25, 33]. Drug therapy changes were the most common recommendations by pharmacists in several studies, in response to off-label prescribing, medical conditions not receiving treatment according to accepted guidelines, and the prescribing of drug forms unsuitable for adolescents [10, 33, 38]. Incorrect dosing (under- and over-dose) was also a prominent issue identified in this review, with overdoses of between 1.5 and 10 times the maximum recommended dose being of significant concern for younger patients, increasing the risk of serious adverse events [13, 14, 24, 33, 38].
Studies which tracked pre- and post-pharmacist involvement in prescribing and clinical ward rounds found significant reductions in the frequency of errors made between one-fifth and one-third (p < .01) [1, 21]. The clinical significance of these interventions was reported in many studies, with errors made by physicians and intercepted by pharmacists being potentially life-threatening/fatal in 1.0–2.2% of cases, very/extremely significant in 2.9–29.7% of cases, and moderately significant in 38.0–64.7% of cases [1, 10, 13, 14, 33]. One of the studies found that free-text entries by prescribers were nearly five times more likely to have an error (p < .001) compared to standardised templates and electronic entries [40]. Other benefits of pharmacist involvement with paediatric patients described in individual studies included a significant reduction in missed doses of urgent and non-urgent medications (p = .03 and p < .001 respectively) [25], significant reductions in length of hospital stay (from 9.06 to 7.33 days, p = .02) and medication compliance rate (from 70.2 to 81.4%, p < .01) [40].
Medical condition improvement
Two RCTs discussed the impact of pharmacist interventions on specific disease states in paediatric patients; end-stage renal disease requiring haemodialysis [12], and iron-overloaded beta-thalassemia major [2]. Both studies found that non-compliance to therapy was a significant issue, which was greatly improved after interventions made by clinical pharmacists, particularly patient education. Unlike the other studies discussed, dosing issues in these RCTs (particularly overdosing) were less common, which is theorised to be due to increased prescriber familiarity to a smaller number of medications needed for these specific diseases. There were significant improvements in biomarkers (e.g. serum phosphate, parathyroid hormone, calcium, and serum ferritin) for both conditions in these studies (all p < .01), and significant increases in quality of life scores compared to the control groups (p < .001) [2, 12].
Economic impacts
For three of the four studies that discussed the financial aspects of clinical pharmacist interventions, all described financial savings from these interventions, as a result of the need for fewer or less expensive medications (reduction in total drug costs), or the prevention of adverse drug events and their associated costs [10, 33, 38]. One study in China found no significant difference in drug costs, or total costs related to patient care with a pharmacists’ involvement [40].
Discussion
Paediatric clinical pharmacists can provide significant benefits to paediatric patients through identifying a wide range of DRPs and recommending suitable interventions to reduce adverse events and non-compliance issues, improve condition control, and minimise drug expenditure. A previous systematic review (Ghaleb et al. 2006) found that dosing errors were the most common type of medication error, with antibiotics and sedatives most commonly associated with these errors [15].
In this review, we found that pharmacists were adept at identifying and managing these errors, with the acceptance rates of pharmacist-initiated interventions for paediatric patients generally being high, indicating physician confidence in pharmacists’ recommendations. Acceptance rates in studies not included in this review are similarly above 85% for both paediatric and adult patients [3, 8, 22, 26, 34, 35]. Having pharmacists present during clinical ward rounds allows them to provide real-time advice to physicians (rather than recommending changes after prescribing has occurred), which increases the likelihood that errors will be caught, and that interventions to amend these errors will be accepted [5, 20].
It must be noted that medication errors involve not only medications prescribed for inpatient use, but also those required short or long-term after discharge, and medications for chronic conditions unrelated to the presenting compliant [25]. These errors may be more likely when urgent health issues during initial presentation overshadow regular medication recording, particularly in the emergency department [25]. This may have significant adverse effects on patient health, particularly when low therapeutic index or immunosuppressant, anticonvulsant, or other medications requiring strict adherence are not correctly recorded during admission and continued throughout hospital stay [7, 25]. Medication reconciliation services performed by clinical pharmacists are shown to be an effective method for preventing errors during these critical stages of care [23, 29].
The vulnerability of paediatric patients to serious consequences arising from medication errors, combined with the error frequency reported in the studies in this review suggests the need for regular pharmacist involvement in drug treatments, to reduce the incidence and severity of errors, including missed doses [1, 10, 21, 25]. Dosing errors in particular are not only common, but can involve doses as high as 10 times the normal therapeutic range, representing a significant threat to patient safety [13, 14, 33]. One study reported that error rates decreased with an increase in the experience of the physician, suggesting that newly registered prescribers (and their patients) would benefit from a pharmacists’ assistance in medication ordering, particularly if free-text (as opposed to electronic) prescribing is relied upon [24, 40]. Two studies also found that younger participants in comparison to adolescents, were at a higher risk of errors, suggesting that pharmacist activities should be focused on younger patients [14, 24]. Pharmacists are trained to provide these interventions for patients, with the vast majority of interventions in the studies included in this review having a positive impact on patient health [13, 33]. In addition to physician experience and pharmacist involvement during prescribing, the use of specialised clinics appears to be an additional protective factor against medication errors for paediatric patients. Two of the RCTs in this review, which enrolled patients with a particular medical condition within a specialised unit, found fewer dosing errors than other studies, potentially due to a smaller number of commonly prescribed medications compared to in general medical or surgical units, and the awareness of staff when prescribing inherently high-risk drugs [2, 12, 13].
However, there are barriers to the involvement of pharmacists in medication prescribing and preventing errors, which may vary considerably between hospitals and health systems between different countries. Medication prescribing which occur at the bedside of the patient in a multidisciplinary setting offers a rapid and effective environment for identifying and resolving errors compared to orders which occur after ward rounds, where a pharmacist may not be involved, and the prescriber more difficult to contact [1, 21]. As many hospitals do not employ pharmacists to participate in ward rounds (including approximately half of those in the USA), delays in correcting medication errors would be more likely in these institutions [1, 31]. However, given the increased utilisation and benefits noted from pharmacist involvement, the number of hospitals including pharmacists in ward rounds has increased from approximately 30% in 2001 [30]. Pharmacist involvement in patient discharge procedures and patient education ensures a continuity of care, through improved patient satisfaction, reduced non-compliance to prescribed medications, and improved laboratory biomarkers [2, 12, 18, 36, 40].
In this review, a contributing factor to medication errors was the unavailability of medications adapted for use in children, with the wrong drug formulation being prescribed a common error in this review [24]. Whilst the availability of commercial formulations in a hospital formulary may be limited, the preparation of ‘tailored’ pharmaceutical preparations, particularly antibiotics as one of the most commonly prescribed medication classes and prone to errors, is a vital service which can be provided by pharmacists [1, 10, 13, 14, 30]. The availability of commercial medications is subject to market and other forces and is an ongoing issue, and pharmacists can communicate these availability issues to prescribers, nurses, and other relevant health professionals, to prevent errors relating to dosing and dosage forms.
The main strengths of this review are the large total number of admissions included, and the inclusion of health institutions across several countries, making the findings more robust. Limitations to consider when interpreting the results of this systematic review include having some studies with low participant numbers [2, 12, 28], or being conducted at a single site [1, 25, 38, 40], limiting their generalisability to other health institutions. There were also significant differences between studies on what constituted a reportable error, making it difficult to compare individual studies and extrapolating findings elsewhere difficult [1, 13]. This issue also prevented a meta-analytic study, which would have increased the strength of the findings.
Conclusion
Clinical pharmacists can significantly contribute to positive health outcomes for paediatric hospital patients through the identification and management of medication errors. These errors often involve antibiotics, and occur during prescribing on clinical ward rounds, and would benefit from the involvement of a clinical pharmacist to ensure the prompt and accurate provision of drug-related information to prescribers. Further research using standardised reporting of adverse events is required to allow a clear comparison between studies and a more accurate assessment of the broad range of benefits provided by clinical pharmacists.
Abbreviations
- DRP:
-
Drug-related problem
- ED:
-
Emergency department
- JBI:
-
Joanna Briggs Institute
- NICU:
-
Neonatal intensive care unit
- PICU:
-
Paediatric intensive care unit
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RCT:
-
Randomised controlled trial
References
Alagha HZ, Badary OA, Ibrahim HM, Sabri NA (2011) Reducing prescribing errors in the paediatric intensive care unit: an experience from Egypt. Acta Paediatr 100(10):e169–e174
Bahnasawy SM, El Wakeel LM, Beblawy NE, El-Hamamsy M (2017) Clinical pharmacist-provided services in iron-overloaded beta-thalassaemia major children: a new insight into patient care. Basic Clin Pharmacol Toxicol 120(4):354–359
Barber ND, Batty R, Ridout DA (1997) Predicting the rate of physician-accepted interventions by hospital pharmacists in the United States. Am J Health Syst Pharm 54(4):397–405
Batchelor HK, Marriott JF (2015) Paediatric pharmacokinetics: key considerations. Br J Clin Pharmacol 79(3):395–404
Bates DW et al (2003) Ten commandments for effective clinical decision support: making the practice of evidence-based medicine a reality. J Am Med Inform Assoc 10(6):523–530
Bhatt-Mehta V et al (2013) Recommendations for meeting the pediatric patient's need for a clinical pharmacist: a joint opinion of the pediatrics practice and research network of the American College of Clinical Pharmacy and the Pediatric Pharmacy Advocacy Group. Pharmacotherapy 33(2):243–251
Blix HS, Viktil KK, Moger TA, Reikvam A (2010) Drugs with narrow therapeutic index as indicators in the risk management of hospitalised patients. Pharm Pract (Granada) 8(1):50–55
Blum KV, Abel SR, Urbanski CJ, Pierce JM (1988) Medication error prevention by pharmacists. Am J Hosp Pharm 45(9):1902–1903
Central Intelligence Agency. The World Factbook. Retrieved 11th March 2018. Available from: https://www.cia.gov/library/publications/the-world-factbook/geos/xx.html
Condren ME, Haase MR, Luedtke SA, Gaylor AS (2004) Clinical activities of an academic pediatric pharmacy team. Ann Pharmacother 38(4):574–578
Da Silva FM, Jacob E, Nascimento LC (2010) Impact of childhood cancer on parents’ relationships: an integrative review. J Nurs Scholarship 42(3):250–261
El Borolossy R, El Wakeel L, El Hakim I, Badary O (2014) Implementation of clinical pharmacy services in a pediatric dialysis unit. Pediatr Nephrol 29(7):1259–1264
Fernández-Llamazares CM, Calleja-Hernandez MA, Manrique-Rodriguez S, Pérez-Sanz C, Duran-García E, Sanjurjo-Saez M (2012) Impact of clinical pharmacist interventions in reducing paediatric prescribing errors. Arch Dis Child 97(6):564–568
Fernández-Llamazares CM, Pozas M, Feal B, Cabañas MJ, Villaronga M, Hernández-Gago Y, Ruiz de Villegas M, Álvarez-del-Vayo C (2013) Profile of prescribing errors detected by clinical pharmacists in paediatric hospitals in Spain. Int J Clin Pharm 35(4):638–646
Ghaleb MA, Barber N, Franklin BD, Yeung VW, Khaki ZF, Wong IC (2006) Systematic review of medication errors in pediatric patients. Ann Pharmacother 40(10):1766–1776
Ghaleb MA, Barber N, Franklin BD, Wong ICK (2010) The incidence and nature of prescribing and medication administration errors in paediatric inpatients. Arch Dis Child 95(2):113–118
Graabaek T, Kjeldsen LJ (2013) Medication reviews by clinical pharmacists at hospitals lead to improved outcomes: a systematic review. Basic Clin Pharmacol Toxicol 112(6):359–373
Huynh C et al (2016) An evaluation of the epidemiology of medication discrepancies and clinical significance of medicines reconciliation in children admitted to hospital. Arch Dis Child 101(1):67–71
Kaboli PJ, Hoth AB, McClimon BJ (2006) Clinical pharmacists and inpatient medical care: a systematic review. Arch Intern Med 166(9):955–964
Kaushal R, Bates DW, Landrigan C, McKenna KJ, Clapp MD, Federico F, Goldmann DA (2001) Medication errors and adverse drug events in pediatric inpatients. JAMA 285(16):2114–2120
Kaushal R, Bates DW, Abramson EL, Soukup JR, Goldmann DA (2008) Unit-based clinical pharmacists’ prevention of serious medication errors in pediatric inpatients. Am J Health-Syst Ph 65(13):1254–1260
Krupicka MI, Bratton SL, Sonnenthal K, Goldstein B (2002) Impact of a pediatric clinical pharmacist in the pediatric intensive care unit. Crit Care Med 30(4):919–921
Kwan JL, Lo L, Sampson M, Shojania KG (2013) Medication reconciliation during transitions of care as a patient safety strategy: a systematic review. Ann Intern Med 158(5Pt2):397–403
Maat B, San AY, Bollen CW, van Vught AJ, Egberts TC, Rademaker CM (2013) Clinical pharmacy interventions in paediatric electronic prescriptions. Arch Dis Child 98(3):222–227
Marconi GP, Claudius I (2012) Impact of an emergency department pharmacy on medication omission and delay. Pediatr Emerg Care 28(1):30–33
Moffett BS, Mott AR, Nelson DP, Gurwitch KD (2008) Medication dosing and renal insufficiency in a pediatric cardiac intensive care unit: impact of pharmacist consultation. Pediatr Cardiol 29(4):744–748
Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097
Moola S, et al. Chapter 7: systematic reviews of etiology and risk. In: Aromataris E, Munn Z (editors). Joanna Briggs Institute Reviewer’s Manual. The Joanna Briggs institute, 2017
Mueller SK, Sponsler KC, Kripalani S, Schnipper JL (2012) Hospital-based medication reconciliation practices: a systematic review. Arch Intern Med 172(14):1057–1069
Pedersen CA, Schneider PJ (2001) Santell JP. ASHP national survey of pharmacy practice in hospital settings: prescribing and transcribing—2001. Am J Health-Syst Pharm 58(23):2251–2266
Pedersen CA, Schneider PJ (2014) Scheckelhoff DJ. ASHP national survey of pharmacy practice in hospital settings: prescribing and transcribing—2013. Am J Health-Syst Pharm 71(11):924–942
Pharmaceutical Society of Australia. Integrating pharmacists into primary care teams: Better health outcomes through cost-effective models of care. Submission to the 2015–16 Federal Budget. Accessed 7th Jan 2018. Available from: https://www.psa.org.au/news/submission-2015-16-federal-budget
Prot-Labarthe S, Di Paolo ER, Lavoie A, Quennery S, Bussières JF, Brion F, Bourdon O (2013) Pediatric drug-related problems: a multicenter study in four French-speaking countries. Int J Clin Pharm 35(2):251–259
Strong DK, Tsang GW (1993) Focus and impact of pharmacists’ interventions. Can J Hosp Pharm 46(3):101–108
Struck P, Pedersen KH, Moodley P, Rasmussen M (2007) A pilot study of pharmacist;initiated interventions in drug therapy in an Australian paediatric hospital. EJHP Sci 13(4):105–112
Swedlund MP, Schumacher JB, Young HN, Cox ED (2012) Effect of communication style and physician-family relationships on satisfaction with pediatric chronic disease care. Health Commun 27(5):498–505
Tufanaru C, Munn Z, Aromataris E, Campbell J, Hopp L. Chapter 3: systematic reviews of effectiveness. In: Aromataris E, Munn Z (editors). Joanna Briggs Institute Reviewer’s Manual. The Joanna Briggs institute, 2017
Virani A, Crown N (2005) The impact of a clinical pharmacist on patient and economic outcomes in a child and adolescent mental health unit. Can J Hosp Pharm 56(3):158–162
Wong IC, Ghaleb MA, Franklin BD, Barber N (2004) Incidence and nature of dosing errors in paediatric medications. Drug Saf 27(9):661–670
Zhang C, Zhang L, Huang L, Luo R, Wen J (2012) Clinical pharmacists on medical care of pediatric inpatients: a single-center randomized controlled trial. PLoS One 7(1):e30856
Acknowledgements
The authors would like to thank the following colleagues for their assistance with the review: M Stelmaschuk, J Hart-Davies, S Maltby, L Pell, K MacFarlane, S Leotta, J Ede, A Bond, and Townsville hospital librarian Bronia Renison. Literature search by Louisa D’Arrietta, Townsville Hospital Librarian, 10/10/2017. The Townsville Hospital, QLD, Australia.
Author information
Authors and Affiliations
Contributions
All authors were responsible for the development of the initial research plan and were involved in quality assessment of eligible articles. AD carried out an independent literature search and was responsible for drafting of the manuscript. KR and MT were responsible for assessing article eligibility and revising of the manuscript drafts. NR and SP assisted in drafting of earlier versions of the manuscript. TK assisted in the literature search, drafting of earlier versions of the manuscript, and review of the final manuscript version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Informed consent is not applicable in this study.
Additional information
Communicated by Piet Leroy
Rights and permissions
About this article
Cite this article
Drovandi, A., Robertson, K., Tucker, M. et al. A systematic review of clinical pharmacist interventions in paediatric hospital patients. Eur J Pediatr 177, 1139–1148 (2018). https://doi.org/10.1007/s00431-018-3187-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-018-3187-x