Abstract
Acute hypersensitivity reactions (HSRs) are an unpredictable and potentially catastrophic complication of treatment with chemotherapeutic agents. Reactions may affect any organ system in the body and range widely in severity from mild pruritus to systemic anaphylaxis. Certain classes of chemotherapeutic agents, such as the taxanes, platinum compounds, asparaginases, and epipodophyllotoxins are commonly associated with HSRs. The clinical characteristics of these high risk agents with respect to HSRs are discussed in this review.
Protocols to prevent or reduce the severity of these reactions have been developed, but despite these attempts, HSRs will still happen. Should a reaction occur, it is imperative that it be recognised quickly in order to minimise exposure to the inciting agent and implement appropriate therapeutic and supportive measures. When a patient becomes sensitised to a chemotherapeutic agent, avoidance of re-exposure is the mainstay of future prevention. For sensitised patients who have derived clinically meaningful benefit from a particular agent, however, continuation of treatment with the agent is desirable. Options may include attempting a trial of desensitisation or treatment with a related compound. Virtually all patients demonstrating HSRs to paclitaxel and docetaxel are able to successfully tolerate re-treatment following discontinuation and administration of diphenhydramine and hydrocortisone. Re-treatment has generally been less successful with platinum compounds, with recurrent HSRs occurring in up to 50% of patients following desensitisation protocols. Patients sensitised to asparaginase are often able to tolerate the alternative preparations, Erwinia carotovora asparaginase or polyethylene glycol-modified Escherichia coli asparaginase. There is very little experience with re-treatment following sensitisation to the epipodophyllotoxins. As re-treatment may have serious consequences, careful consideration of the risks and benefits of these strategies is imperative when deciding among these options.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Hypersensitivity reactions (HSRs) have been reported for nearly all chemotherapeutic agents in use. Clinical manifestations of HSRs range from mildly uncomfortable cutaneous symptoms to respiratory arrest, cardiac collapse and even death. Devising strategies for prevention is of paramount importance. However, when a patient does become sensitised to a chemotherapeutic agent, physicians are then faced with the decision of whether to discontinue therapy or to risk rechallenging the patient with the agent. For potentially life-saving or clinically effective palliative therapies, identifying strategies that safely allow continuation of the agent is highly desirable.
This review discusses the various immune mechanisms that mediate HSRs and provides general recommendations for their prophylaxis and treatment. It also details the incidence, clinical manifestations, and specific strategies for prevention or retreatment of the chemotherapeutic agents most commonly associated with HSRs: the taxanes, platinum compounds, asparaginases and epipodophyllotoxins.
1. Hypersensitivity Reactions (HSRs)
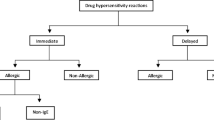
An HSR to a chemotherapeutic agent may be defined as any immunological response to a drug that results in an adverse reaction. Four categories of HSRs are identified: immediate, immunoglobulin (Ig) E-mediated (type I), antibody-mediated (type II), immune complex-mediated (type III), and cell-mediated or delayed-type (type IV). Clinical examples of each type of HSR are provided in table I.
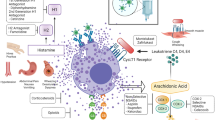
In most cases, the exact immune mechanism of HSR for a given chemotherapeutic agent is not well understood. However, it is thought that the majority of HSRs to chemotherapeutic agents are clinically most consistent with an immediate type I reaction. These reactions may be mediated by drug-specific IgE. Chemotherapeutic agents, their metabolites, or the vehicles in which they are dissolved may also induce mast cell or basophil degranulation directly, resulting in responses that are indistinguishable from IgE-mediated allergic reactions. Clinical manifestations of types II, III and IV reactions, although less common, have also been reported with the administration of many chemotherapeutic agents.
2. Prophylaxis and Treatment
HSRs are unpredictable. Therefore, precautions should always be exercised when administering any chemotherapeutic agent. Reliable intravenous access should be obtained prior to the administration of any agent. The drugs and equipment appropriate for resuscitation (i.e. a ‘crash cart’) should also be immediately available in the event of a severe HSR or anaphylaxis (table II).
Several protocols have been developed to prevent or reduce the severity of reactions in situations in which anaphylaxis is a risk. In general, these protocols involve pharmacological pretreatment of susceptible persons in an attempt to block or blunt the reaction. Premedication with histamine H1 and H2 receptor antagonists and corticosteroids has significantly reduced the incidence of anaphylactoid reactions to radiocontrast media[1] and pretreatment protocols similar to this are now standard for patients receiving taxanes (table III).[2,3]
Despite prophylaxis, HSRs still occur. In the event of an HSR, the primary intervention is to reduce the absorption of the antigen. For intravenously administered agents, the infusion should be discontinued as soon as the onset of an HSR is recognised. For intramuscular injections, infiltration of adrenaline (epinephrine) locally may slow down systemic absorption. If the injection is into a limb, a loose tourniquet may be applied temporarily to reduce venous outflow.
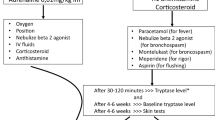
Attention should then turn to the ABCs of resuscitation: airway, breathing and circulation. For HSR manifestations involving the respiratory system, airway integrity should be established and oxygen therapy initiated. A β-agonist, such as orciprenaline (metaproterenol), may be administered via nebuliser for bronchospasm. Anaphylactoid reactions may also manifest as angioedema or laryngeal oedema. When present, adrenaline may be administered subcutaneously or racemic adrenaline delivered via nebuliser to improve airway patency. When symptoms of this severity develop, appropriate preparations for possible endotracheal intubation or cricothyrotomy should be initiated.
Cardiovascular manifestations of HSRs most often include tachycardia and hypotension. If hypotension is present, a bolus of intravenous crystalloid is administered to maintain systolic blood pressure ≥80 to 100mm Hg. Diphenhydramine is re-administered to antagonise the effects of histamine. Adrenaline administration will also improve hypotension. Pressors and antiarrhythmic medications may also be necessary, depending on the clinical situation.
To minimise the potential severity of the reaction, prompt intervention is of paramount importance. This requires vigilance and anticipation so that HSRs may be recognised early and interventions instituted immediately. Familiarity with the clinical characteristics of HSRs for a particular chemotherapeutic agent will enable improved anticipation and early intervention, should they occur. A few classes of chemotherapeutic agents are commonly associated with hypersensitivity and are reviewed in the following discussion.
3. The Taxanes: Paclitaxel and Docetaxel
Paclitaxel and its sister compound docetaxel are highly active drugs in the treatment of breast, ovarian, lung and other cancers. The tumouricidal activity of these agents is attributable to its ability to induce irreversible aggregation of cellular microtubules. The aqueous insolubility of paclitaxel requires the use of a vehicle consisting of 50% ‘Cremophor EL’ (polyoxyethylated castor oil) and 50% ethanol for intravenous administration, whereas docetaxel is diluted and solubilised with ‘Tween-80’ (polysorbate 80).
3.1 Incidence and Clinical Manifestations
HSR was among the most common treatment-limiting toxicity in the early phase I and II studies of the taxanes. Up to 42% of patients receiving paclitaxel experience an HSR, with serious reactions noted in up to 2% of patients.[3,4] HSRs occur in approximately 25 to 50% of patients receiving docetaxel.[2,3]
Up to 95% of HSRs to the taxanes occur during the first or second dose administered, with nearly 77% of reactions occurring with the first exposure to the agent.[3,5,6] Nearly 80% of patients develop symptoms within the first 10 minutes of drug infusion and many reactions occur with as little as 1mg of the agent delivered.[3,5] A minority of patients experience repeated episodes beyond the second course of treatment.[6] Clinical manifestations of HSR include dyspnoea/bronchospasm (81%), urticaria/flushing/rashes (74%), hypotension (41%) and angioedema (19%). In general, most severe/anaphylactic reactions to paclitaxel also occur with the first or second taxane exposure and are not preceded by a minor reaction. Minor reactions with the first or second exposure are usually not repeated with subsequent administration. However, a small subset of patients with mild reactions will continue to experience persistent mild reactions or develop a severe/anaphylactic reaction with subsequent exposure.[6]
The dose and schedule of paclitaxel and docetaxel affect both the efficacy and toxicity profile and schedule for the malignancies for which these agents are applied is evolving. The initial 24- and 96-hour infusion schedules of paclitaxel have been replaced by shorter, more convenient schedules.[3] Comparison of the early phase I data suggested that paclitaxel infusion rate influenced the incidence of HSR.[5] However, a prospective randomised comparison of 3- versus 24-hour paclitaxel infusion in patients treated for relapsed ovarian carcinoma later reported no difference in the incidence of allergic reaction observed with the different infusion rates.[4] In this study, a 42% incidence of mild HSR and 1.3% incidence of severe HSR were noted. Weekly and bi-weekly schedules to maximise the drug exposure of paclitaxel and docetaxel while limiting their treatment-limiting myelosuppression have more recently been introduced in phase I and phase II studies. These studies indicate an encouraging toxicity profile, reporting a 0 to 4% incidence of HSRs using varying prophylactic regimens.[7–10] Randomised comparisons of the incidence of HSR with weekly administration of paclitaxel or docetaxel to an every 3-week administration schedule have not been performed.
3.2 Immunological Mechanism
The immunological mechanism of paclitaxel-associated HSR is not known for certain. The clinical manifestations are consistent with a type I HSR. However, the occurrence of patients who experience HSRs with their first exposure to the agent suggests that the reactions may not be IgE-mediated. Rather, it is postulated that the agent induces mast cell degranulation directly.
In the case of paclitaxel, debate exists with regard to whether HSRs are attributable to paclitaxel or its excipient ‘Cremophor EL’. Because of the insolubility of paclitaxel, ‘Cremophor EL’, anon-ionic surfactant derived from castor oil, is used as a vehicle. Studies have demonstrated that intravenous administration of ‘Cremophor EL’ can induce histamine release and hypotension in dogs.[11] However, basophil histamine release tests in sensitised individuals revealed histamine release only with paclitaxel and not with ‘Cremophor EL’.[12] In addition, ‘Cremophor EL’ is not a vehicle for docetaxel, yet this agent is also associated with a high incidence of HSRs, suggesting that the taxane moiety is likely the more important aetiological factor.
3.3 Protocols for Prevention of HSRs
A large volume of data confirms that premedication with corticosteroids and H1 and H2 receptor antagonists, similar to that recommended for radiocontrast administration, successfully reduces the incidence and severity of both paclitaxel- and docetaxel-induced HSRs.[2,3] Prophylaxis in the early clinical trials with paclitaxel involved oral administration of 20mg of dexamethasone both the night before and the morning of paclitaxel delivery in addition to intravenous administration of H1 and H2 receptor antagonists 30 minutes prior to paclitaxel infusion.[5,13] Subsequently, a simplified regimen for HSR prophylaxis, designed to increase patient compliance, was evaluated in several studies.[14–16] Rather than 2 doses of self-administered oral corticosteroid, one 20mg dose of dexamethasone was administered intravenously concurrent with that of the histamine antagonists.[14–16] The studies demonstrated high compliance and comparable clinical efficacy compared with the more cumbersome regimens, with an overall incidence of HSRs of only 3.7 to 4.2% reported.[14,16]
Prophylaxis with corticosteroids and diphenhydramine also reduces the incidence of docetaxel-associated HSR and led to a significant reduction in treatment interruption attributable to this toxicity.[17] Although not considered to be a manifestation of hypersensitivity reaction, fluid retention and oedema of varying severity can be problematic with repeated administration of docetaxel.[18] Corticosteroid pretreatment effectively reduces both the incidence and severity of this cumulative toxicity and is also incorporated into the prophylaxis regimen of docetaxel for this reason.[19] The routine use of H1 and H2 receptor antagonists for the primary prevention of potential docetaxel-associated HSRs, however, is quite variable among studies[18,20–22]
To date, no standard prophylaxis regimen exists when these agents are given on a weekly or biweekly basis. Most studies reporting consist of either oral or intravenous corticosteroid prophylaxis and use of both H1 and H2 receptor antagonists is variable.[7–10] Weekly administration of high dose corticosteroids may lead to fluid retention, body-weight gain, facial hair growth and other undesirable adverse effects, and there is limited enthusiasm for their routine use in this setting. Lokich et al.[23] omitted prophylactic measures altogether in patients receiving weekly paclitaxel and docetaxel and noted only 1 mild HSR in 20 patients. It should be noted that at least half the patients in this study had previously successfully been treated with a taxane and would be considered to be at a substantially reduced risk compared with patients receiving their first or second exposure to the agents. While the safety of omitting prophylactic measures in all patients cannot be extrapolated from this study, patients who have previously tolerated these agents appear to have a low risk for HSR without prophylaxis with weekly administration of these agents.
Despite prophylaxis, HSRs to the taxanes will still occur. Since the majority of HSRs associated with paclitaxel and docetaxel infusion occur within the first few minutes of infusion, it is imperative that the treating nurse or physician be immediately present during the initial several minutes of paclitaxel infusion. With the appearance of any sign or symptom of HSR, the infusion is promptly discontinued and supportive measures as previously described are instituted.[6]
3.4 Strategies for Re-Treatment with the Taxanes in Sensitised Patients
Virtually all patients demonstrating HSRs to paclitaxel or docetaxel are able to successfully tolerate re-treatment. In fact, most patients are able to undergo re-treatment the same day as the initial HSR, using the strategy outlined in table IV[2,6,24]
With this protocol, nearly 90% of patients are able to complete the taxane infusion after a short delay, without the subsequent appearance of a second reaction.[6,22,25] Interestingly, one of the characteristics of a true HSR is that symptoms will reappear rapidly after re-exposure to the agent. It is unclear why patients are able to be successfully retreated with paclitaxel using essentially the same prophylactic regimen. It is possible that, like penicillin desensitisation, initial drug binding with IgE creates complexes which prevent further IgE binding and crosslinking with reinfusion of the agent.
Published experience regarding hypersensitivity and cross-reactivity with paclitaxel and docetaxel are limited. Four patients who experienced a paclitaxel HSR during their first or second course of chemotherapy subsequently received docetaxel without complication.[26] Since re-treatment with paclitaxel is possible in most patients who experience reactions that occur in the first or second course of a taxane, it is probable that patients in the report would also have tolerated paclitaxel if that agent had been reintroduced in these patients.
Uncommonly, patients will experience recurrent HSRs, despite the above protocol. For patients who experience a severe HSR or a second HSR after re-initiation of paclitaxel infusion, a formal desensitisation protocol based on a modification of the standard regimen for parenteral desensitisation to beta-lactam antibiotics[27,28] is recommended.[6,12] Using the protocol outlined in table V, 9 patients who experienced serious recurrent paclitaxel HSRs were successfully retreated with the agent.[6] Subsequent courses of paclitaxel did not necessarily require the desensitisation protocol.
Desensitisation protocol for paclitaxel-associated hypersensitivity reactions[6]
4. The Organoplatinums
Platinum compounds have demonstrated anti-tumour activity in a wide variety of cancers by producing interstrand DNA cross-links via its highly reactive platinum species. Cisplatin was the first platinum-containing compound to be approved by the US Food and Drug Administration for use as a chemotherapeutic agent. In 1989, the analogue carboplatin was introduced as an effective organoplatinum agent with an improved toxicity profile. These agents may be delivered by the intravenous, intraperitoneal or intravesical routes.
4.1 Incidence and Clinical Manifestations
Hypersensitivity to platinum compounds is well established and most notable among refinery workers inhaling complex salts of platinum.[29] After prolonged exposure to these agents, a small number of workers develop rhinitis, conjunctivitis, asthma, urticaria and contact dermatitis. Allergic reactions have also been reported in patients receiving platinum-containing chemotherapeutic agents. Both cisplatin and carboplatin are commonly associated with HSRs and their occurrence have been documented with all routes of chemotherapy administration, including intraperitoneal and intravesical.[30–32]
The reported incidence of HSR to platinum compounds varies greatly. An interesting feature of platinum HSR is that a rather prolonged period of exposure is characteristic before the development of sensitisation. In patients receiving carboplatin for the treatment of ovarian cancer, the initial episode of hypersensitivity develops only after a significant number of courses of the agent have been administered.[33] In a review of 205 patients receiving carboplatin, the median course number reported for the first HSR episode was 8 (range 6 to 21; table VI).[33] The incidence of HSRs in patients receiving 6 or fewer courses of carboplatin, on the other hand, was only 1% in this study. Multiple (6 or more) courses are also typical prior to the development of cisplatin HSR.[34] The dose of platinum delivered, however, does not seem to be a risk factor for HSRs.[33]
Hypersensitivity reactions (HSRs) in relation to carboplatin course number[33]
The severity of symptoms among patients experiencing an HSR to platinum compounds also varies greatly. Clinical manifestations are consistent with type I HSR. Unlike paclitaxel HSRs, greater than 50% of patients experiencing reactions to platinum compounds demonstrate at least moderately severe symptoms, with respiratory arrest, cardiac arrest and death being reported.[33,35,36] Also in contrast to paclitaxel-associated HSRs, the timing of the reaction with respect to drug infusion varies greatly. Only one-half of patients demonstrating an HSR with carboplatin experience the initial symptoms within the first several minutes after drug administration, with some patients experiencing mild symptoms up to 3 days after treatment.[33,35,36]
4.2 Immunological Mechanism
The immune mechanism responsible for HSRs to platinum-based chemotherapy is not well understood. Evidence among refinery workers inhaling complex salts of platinum suggests that allergy to platinum compounds is IgE-mediated. As stated, prior exposure is necessary before the development of sensitisation and, once sensitised, small amounts of platinum compound will elicit this reaction.[29]
The passive transfer of skin reactivity by the serum and demonstration of platinum-specific IgE by radioallergosorbent test in sensitised refinery workers provide evidence for an antibody-mediated phenomenon.[37]
4.3 Protocols for Prevention of HSR
No specific protocols for HSR prophylaxis exist for the organoplatinums. However, most antiemetic regimens for the organoplatinum agents involve the use of dexamethasone. Prophylaxis is recommended for patients who are receiving multiple courses of either agent.
Skin testing has also been applied for the early identification of patients receiving carboplatin at risk for HSRs.[38] An intradermal injection of carboplatin has been shown to identify patients who may safely be administered carboplatin chemotherapy with a 99% negative predictive value. As the incidence of HSRs in patients receiving 6 or fewer courses of carboplatin is negligible, skin tests need only be applied to patients receiving 7 or more courses of the agent. In this study, administering carboplatin only to patients with a negative skin test would reduce the rate of HSRs in this population from 27 to 3%[38] In patients manifesting a positive skin test, both patient and physician must evaluate risks and benefits of continuation of carboplatin therapy. Treatment may be discontinued in favour of an alternate second-line agent. However, if significant benefit is likely to accrue from continued carboplatin therapy, options may include a trial of desensitisation versus an attempt at crossover treatment with cisplatin.
4.4 Strategies for Re-Treatment with the Platinum Compounds in Sensitised Patients
Recurrent reactions, usually of increasing severity, occur with re-treatment in individuals sensitised to platinum compounds.[33] A few authors have reported successful re-treatment with crossover to an alternate platinum-based chemotherapy.[39,40] However, these reports are anecdotal and reports to the contrary suggest that cross-reactivity does occur, with death being reported as an outcome in one report.[35]
Although numerous desensitisation protocols have been suggested in the literature, these have not met with the uniform success of the paclitaxel desensitisation protocols. Prolonged infusion times and additional H1 and H2 receptor antagonists have not resulted in successful treatment in sensitised individuals.[41] Extended corticosteroid-based oral or intravenous desensitisation and an escalating dose infusion protocol similar to that used with paclitaxel hypersensitivity have been variably successful, with recurrent HSRs occurring in up to 50% of patients.[38,42–44] The decision to attempt desensitisation should be based on the perceived clinical benefit from continued platinum exposure in patients who likely have already received multiple courses of the agent.
5. Asparaginase
Asparaginase is a bacterial polypeptide protease derived from Escherichia coli that depletes sensitive tumour cells of asparagine and is a standard component of induction chemotherapy protocols for acute leukaemia. Its usual route of administration is intramuscular.
5.1 Incidence and Clinical Manifestations
Asparaginase has long been associated with potentially life-threatening HSRs. Overall rates of HSRs are estimated at 25 to 35% in patients receiving multiple courses of the agent.[45] Nearly half of sensitised patients demonstrate potentially life-threatening, anaphylactoid symptoms.[46] Anaphylactoid symptoms are more common with intravenous than with intramuscular administration and with intermittent (weekly or monthly) rather than continuous (daily) schedules.[46,47]
Most patients become sensitive to the agent only after exposure, with rates of HSR increasing substantially with repeat dosing and reinduction chemotherapy.[48] Reaction onset is typically within the first hour of intramuscular administration, however, delayed reactions of several hours have been noted.[49] The clinical manifestations of these reactions also suggest type I HSR. Symptoms such as fever, flushing, rash, urticaria, local oedema, wheezing and epigastric pain are common.
There is debate as to the prognostic significance of the development of HSRs to asparaginase from a treatment standpoint. Pharmacokinetic and pharmacodynamic studies in children demonstrate that high antibody levels correlated with rapid clearance of the enzyme and lower overall response rates.[50] However, data to the contrary also exist.[51,52]
5.2 Immunological Mechanism
There is substantial evidence that HSR to asparaginase is an IgE-mediated type I reaction.[53] As a bacterial peptide, asparaginase is tremendously antigenic and anti-asparaginase IgE antibody is detectable in most patients receiving the agent.[54]
5.3 Protocols for Prevention of HSRs
No standard regimen for prophylaxis exists. However, caution should be exercised in all patients receiving asparaginase therapy.[34] Intravenous access must be obtained in patients receiving this agent, even when it is administered by the intramuscular route. The standard premedication strategies described in table III may be instituted.
The appearance of anti-asparaginase antibody has been evaluated for its ability to predict patients at risk for HSRs. Although a statistically higher antibody titre is seen in sensitised individuals, asparaginase-specific antibody is also present in most patients who do not exhibit clinical hypersensitivity, thus limiting its prognostic utility.[53–55]
Although, intradermal skin testing with the agent has not been entirely reliable in predicting patients at risk for HSRs to E. coli asparaginase,[56] it is generally recommended that the test be performed prior to the initial administration of asparaginase and prior to subsequent doses when a week or more has passed between doses.[57]
5.4 Strategies for Re-Treatment with the Asparaginases in Sensitised Patients
In general, patients sensitised to E. coli asparaginase are unable to continue treatment with the agent. Although re-treatment may not be possible, 2 additional preparations of asparaginase have been developed as an alternative in sensitised individuals: Erwinia carotovora asparaginase and polyethylene glycol-modified E. coli asparaginase (PEG-asparaginase). PEG-asparaginase is E. coli asparaginase covalently bound to polyethylene glycol, allowing for an extended plasma half-life and reduced toxicity. PEG-asparaginase was given to 70 patients as part of a reinduction protocol, including 4 patients who experienced allergic reactions during induction chemotherapy.[58] No cases of HSR were seen, in contrast to a 30% baseline incidence normally seen with E. coli asparaginase at reinduction. PEG-asparaginase was also given to 5 children sensitised to E. coli or Erwinia asparaginase.[59] Four of 5 children were able to complete their intended treatments with PEG-asparaginase. Patients sensitised to E. coli asparaginase may also be successfully treated with Erwinia asparaginase. Although crossover reactions may occur in up to 23% of patients, none are reported to be life-threatening and greater than 90% of sensitised patients are able receive their intended asparaginase courses with this agent.[45]
Published experience with desensitisation protocols for asparaginase is considerably less than with paclitaxel or carboplatin. A protocol based on escalating intravenous dosage of the agent was successful in desensitising a 2-year-old girl with acute myelogenous leukaemia who developed allergy to asparaginase after her first course.[60] Likewise, 4 patients demonstrating HSRs to E. coli asparaginase intramuscular injection were successfully treated with the agents as a slow continuous infusion for 48 to 72 hours.[61]
6. The Epipodophyllotoxins
The epipodophyllotoxins, etoposide and teniposide, are antimitotic agents that appear to mediate single- and double-strand breaks in DNA via inhibitory effects on the nuclear enzyme topoisomerase II. They are useful for the treatment of testicular and ovarian germ cell tumours, small cell lung carcinomas, non-Hodgkin’s lymphomas, and many other malignancies. The agents are usually administered intravenously over 30 to 60 minutes. Etoposide may also be delivered by the oral route.
6.1 Incidence and Clinical Manifestations
Etoposide and teniposide are associated with a reported 6 to 41% incidence of HSRs and a 0.7 to 14% incidence of anaphylaxis.[62,63] These reactions are usually observed during or immediately after administration of the agent. Reactions may occur during the first exposure,[62] however, HSR risk increases with repeated exposure to the agents.[63] HSRs to oral etoposide have not been reported.
Clinical manifestations of these reactions include fever, chills, hypotension, dyspnoea and bronchospasm and are most consistent with type I HSRs. The majority of reactions reported are considered mild.
6.2 Immunological Mechanism
Debate regarding the mechanism of HSRs to the podophyllotoxins exists. The occurrence of patients who experience HSRs with their first exposure to the agent suggests that the reactions may not be antibody-mediated. Rather, infusion of the agents may elicit direct mast cell degranulation similar to that postulated for paclitaxel. Like paclitaxel, teniposide is also solubilised in ‘Cremophor EL’. Although ‘Cremophor EL’ may have the potential to precipitate HSRs, evidence suggests that the podophyllotoxins themselves are the causative agents in these reactions. Nolte et al.[64] evaluated histamine release from basophil leucocytes in sensitised individuals upon exposure to both teniposide and ‘Cremophor EL’ and demonstrated that teniposide degranulated basophils whereas ‘Cremophor EL’ did not. Further, IgE depletion of cells did not abolish the response to the teniposide challenge. In addition, ‘Cremophor EL’ is not a vehicle for etoposide and yet this agent is also associated with HSRs.
6.3 Protocols for Prevention of HSRs
No standard regimen for prophylaxis exists.
6.4 Strategies for Re-Treatment with the Epipodophyllotoxins in Sensitised Patients
Less than half of patients are successfully re-challenged with the drug.[62] Crossover to the alternate agent may be successful in sensitised individuals, however, subsequent reactions are reported.[63,65]
7. Conclusion
Acute HSR is a potentially severe complication of chemotherapy. For certain chemotherapeutic agents such as the taxanes, the organoplatinums, asparaginase, and the epipodophyllotoxins, HSRs may be among the most common treatment-related toxicities. The immunological mechanism of the HSRs are often poorly understood, although type I reactions are the most common. Strategies to prevent the occurrence or minimise the severity of these reactions have been developed and should be implemented if risk for HSR is significant. When a patient becomes sensitised to a chemotherapeutic agent, options include attempting re-treatment with the agent, a trial of desensitisation, treatment with a related compound, or discontinuation of the agent altogether. The risks and benefits of each strategy must be considered carefully when deciding among these options. Knowledge of the particular clinical characteristics with respect to HSRs of the chemotherapeutic agent in question is helpful when deciding among these options (table VII).
References
Greenberger PA, Patterson R, Simon R, et al. Pretreatment of high-risk patients requiring radiographic contrast media studies. J Allergy Clin Immunol 1981; 67: 185–7
Trudeau ME, Eisenhauer EA, Higgins BP, et al. Docetaxel in patients with metastatic breast cancer: a Phase II study of the National Cancer Institute of Canada-Clinical Trials Group. J Clin Oncol 1996; 14: 422–8
Verweij J, Clavel M, Chevalier B. Paclitaxel (Taxol) and docetaxel (Taxotere): not simply two of a kind. Ann Oncol 1994; 5: 495–505
Eisenhauer EA, ten Bokkel Huinink WW, Swenerton KD, et al. European-Canadian randomized trial of paclitaxel in relapsed ovarian cancer: high-dose versus low-dose and long versus short infusion. J Clin Oncol 1994; 12: 2654–66
Weiss RB, Donehower RC, Wiernik PH, et al. Hypersensitivity reactions from Taxol. J Clin Oncol 1990; 8: 1263–8
Markman M, Kennedy A, Webster K, et al. Paclitaxel-associated hypersensitivity reactions: experience of the gynecologic oncology program of the Cleveland Clinic Cancer Center. J Clin Oncol 2000; 18: 102–5
Akerly W, Glantz M, Choy H, et al. Phase I trial of weekly paclitaxel in advances lung cancer. J Clin Oncol 1998; 16: 153–8
Burris H. Weekly schedules of docetaxel. Semin Oncol 1998; 25Suppl. 13: 21–3
Tomiak E, Piccart MJ, Kerger J, et al. Phase I study of docetaxel administered as a 1-hous intravenous infusion on a weekly basis. J Clin Oncol 1994; 12: 1458–67
Trivedi c, Redman B, Flaherty LE, et al. Weekly 1-hour infusion of paclitaxel: clinical feasibility and efficacy in patients with hormone-refractory prostate carcinoma. Cancer 2000; 89: 431–6
Lorenz W, Reimann HJ, Schmal A, et al. Histamine release in dogs by Cremophor EL and its derivatives: oxethylated oleic acid is the most effective constituent. Agents Actions 1977; 7: 63–7
Essayan DM, Kagey-Sobotka A, Colarusso PJ, et al. Successful parenteral desensitization for paclitaxel. J Allergy Clin Immunol 1996; 97: 42–6
McGuire WP, Hoskins WJ, Brady MF, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med 1996; 334: 1–6
Markman M, Kennedy A, Webster K, et al. Simplified regimen for the prevention of paclitaxel-associated hypersensitivity reactions. J Clin Oncol 1997; 15(12): 3517
Bookman MA, Kloth DD, Kover PE, et al. Intravenous prophylaxis for paclitaxel-related hypersensitivity reactions. Semin Oncol 1997; 24(6 Suppl. 19): S19–13–S19–15
Langer CJ, Leighton JC, Comis RL, et al. Paclitaxel and carboplatin in combination in the treatment of advanced nonsmall cell lung cancer: a phase II toxicity, response, and survival analysis. J Clin Oncol 1995; 13: 1860–70
Trudeau ME, Eisenhauer EA, Higgins BP, et al. Docetaxel in patients with metastatic breast cancer: a phase II study of the National Cancer Institute of Canada-Clinical Trials Group. J Clin Oncol 1996; 14: 422–8
Verweij J, Clavel M, Chevalier B. Paclitaxel (Taxol) and docetaxel (Taxotere): not simply two of a kind. Ann Oncol 1994; 5(6): 495–505
Piccart MJ, Klijn J, Paridaens R, et al. Corticosteroids significantly delay the onset of docetaxel-induced fluid retention: final results of a randomized study of the European Organization for Research and Treatment of Cancer Investigational Drug Branch for Breast Cancer. J Clin Oncol 1997; 15(9): 3149–55
Takanow R. Docetaxel: a taxoid for the treatment of metastatic breast cancer. Am Soc Health Syst Pharm 1998; 55(17): 1777–91
Wanders J, Schrijvers D, Bruntsch U, et al. The EORTC-ECTG experience with acute hypersensitivity reactions in Taxotere studies. Proc ASOC 1993; 12: 73
Schrijvers D, Wanders J, Dirix L, et al. Coping with toxicities of docetaxel. Ann Oncol 1993; 4: 610–1
Lokich J. Phase I clinical trial of weekly combined paclitaxel plus docetaxel in patients with solid tumors. Cancer 2000; 89: 2309–14
Adachi I, Watanabe T, Takashima S, et al. A late phase II study of docetaxel in patients with advanced or recurrent breast cancer. Br J Cancer 1996; 73: 210–6
Pazdur R, Lassere Y, Soh LT, et al. Phase II trial of docetaxel in metastatic colorectal carcinoma. Ann Oncol 1994; 11945: 468
Lokich J, Anderson N. Paclitaxel hypersensitivity reactions: a role for docetaxel substitution. Ann Oncol 1998; 9: 573–4
Weiss ME, Adkinson NF. Immediate hypersensitivity reactions to penicillin and related antibiotics. Clin Allergy 1988; 18: 515–40
Stark BJ, Earl HS, Gross GN, et al. Acute and chronic desensitization of penicillin-allergic patients using oral penicillin. J All Clin Immunol 1987; 79: 523–32
Cleare MJ, Highes EG, Jacoby B, et al. Immediate (type I) allergic responses to platinum compounds. Clin Allergy 1976; 6: 183–95
Shukunami K, Kuorkawa T, Kawakami Y, et al. Hypersensitivity reactions to intraperitoneal administration of carboplatin in ovarian cancer: the first report of a case. Gyn Oncol 1999; 72(3): 431–2
Blumenreich MS, Needles B, Yagoda A, et al. Intravesical cisplatin for superficial bladder tumors. Cancer 1982; 50: 863–5
Denis L. Anaphylactoid reactions to repeated intravesical installation with cisplatin. Lancet 1983; I: 1378–9
Markman M, Kennedy A, Webster K, et al. Clinical features of hypersensitivity reactions to carboplatin. J Clin Oncol 1999; 17: 1141–5
Weiss RB. Hypersensitivity reactions. Semin Oncol 1992; 19(5): 458–77
Zweizig S, Roman LD, Muderspach LI. Death from anaphylaxis to cisplatin: a case report. Gynecol Oncol 1994; 53(1): 121–2
Saunders MP, Denton CP, O’Brien ME, et al. Hypersensitivity reactions to cisplatin and carboplatin: a report on six cases. Ann Oncol 1992; 3(7): 574–6
Cromwell O, Papys J, Parish WE, et al. Specific IgE antibodies to platinum salts in sensitized workers. Clin Allergy 1979; 9: 109–17
Zanotti KM, Kennedy AW, Belinson JL, et al. A simplified skin testing protocol for predicting hypersensitivity to carboplatin chemotherapy [abstract]. Gynecol Oncol 2000; 76(2): 241
Shlebak AA, Clark PI, Green JA. Hypersensitivity and cross-reactivity to cisplatin and analogues. Cancer Chemother Pharmacol 1995; 35(4): 349–51
Weidmann B, Mulleneisen N, Bojko P, et al. Hypersensitivity reactions to carboplatin: report of two patients, review of the literature, and discussion of diagnostic procedures and management. Cancer 1994; 73(8): 2218–22
Chang SM, Fryberger S, Crouse V, et al. Carboplatin hypersensitivity in children: a report of five patients with brain tumors. Cancer 1995; 75(5): 1171–5
Broome CB, Schiff RI, Friedman HS. Successful desensitization to carboplatin in patients with systemic hypersensitivity reactions. Med Pediatr Oncol 1996; 26(2): 105–10
Kook H, Kim HM, Choi SH, et al. Life-threatening carboplatin hypersensitivity during conditioning for autologous PBSC transplantation: successful rechallenge after desensitization. BMT 1998; 21(7): 727–9
Rose PG, Fusco N, Fluellen L, et al. Carboplatin hypersensitivity reactions in patients with ovarian and peritoneal carcinoma. Int J Gynecol Cancer 1998 8: 365–6
Billett AL, Carls A, Belber RD, et al. Allergic reactions to Erwinia asparaginase in children with acute lymphoblastic leukemia who had previous allergic reactions to Escherichia coli asparaginase. Cancer 1992; 70(1): 201–6
Evans WE, Tsiatis A, Rivera G, et al. Anaphylactoid reactions to Escherichia coli and Erwinia asparaginase in children with leukemia and lymphoma. Cancer 1982; 49: 1378–83
Nesbit ME, Chard R, Evans A, et al. Evaluations of intramuscular versus intravenous administration of L-asparaginase in childhood leukemia. Am J Pediatr Hematol Oncol 1979; 1: 9–13
Albo V, Miller D, Leiken S, et al. Toxicity experience with a second course of E. coli L-asparaginase therapy 3 years after induction course in children with acute lymphoblastic leukemia in continuous remission [abstract]. Proc Am Soc Clin Oncol 1983; 68
Spiegel RJ, Echelberger CK, Poplack DG. Delayed allergic reactions following intramuscular L-asparaginase. Med Pediatr Oncol 1980; 8(2): 123–5
Asselin BL. The three asparaginases. Comparative pharmacology and optimal use in childhood leukemia. Adv Exp Med Biol 1999; 457: 621–9
Woo MH, Hak LJ, Storm MC, et al. Hypersensitivity or development of antibodies to asparaginase does not impact treatment outcome of childhood acute lymphoblastic leukemia. J Clin Oncol 2000; 18(7): 1525–32
Larson RA, Fretzin MH, Dodge RK, et al. Hypersensitivity reactions to L-asparaginase do not impact on the remission duration of adults with acute lymphoblastic leukemia. Leukemia 1998; 12(5): 660–5
Korholz D, Wahn U, Jurgens H, et al. Allergic reactions in treatment with L-asparaginase. Significance of specific IgE antibodies Monatsschrift Kinderheilkunde 1990; 138(1): 23–5
Woo MH, Hak LJ, Storm MC, et al. Anti-asparaginase antibodies following E. coli asparaginase therapy in pediatric acute lymphoblastic leukemia. Leukemia 1998; 12(10): 1527–33
Capizzi RL, Bertino JR, Handschumacher RE. L-asparaginase. Annu Rev Med 1970; 21: 433–42
Land VH, Sutow WW, Fernbach DJ, et al. Toxicity of L-asparaginase in children with advanced leukemia. Cancer 1972; 40: 339–47
The United states Pharmacopeial Convention. USP dispensing information: Vol. I: drug information for the health care professional. Englewood (CO): Micromedex Inc., 2000
Muller HJ, Loning L, Horn A, et al. Pegylated asparaginase (Oncaspar) in children with acute lymphoblastic leukemia: drug monitoring in reinduction according to the ALL/NHLBFM 95 protocols. Br J Haematol 2000; 111(2): 379–84
Sikorska-Fic B, Makowska K, Rokicka-Milewska R. New possibilities of treatment with PEG-L-asparaginase in patients with acute lymphoblastic leukemia sensitized to L-asparaginase E. coli and erwinase. Wiadomosci Lekarskie 1998; 51Suppl. 4: 233–6
Bonno M, Kawasaki H, Hori H, et al. Rapid desensitization for L-asparaginase hypersensitivity. J Allergy Clin Immunol 1998; 101(4 Pt 1): 571–2
Rodriguez T, Baumgarten E, Fengler R, et al. Long-term infusion of L-asparaginase—an alternative to intramuscular injection? Klin Padiatr 1995; 207(4): 207–10
O’Dwyer PJ, King SA, Fortner CL, et al. Hypersensitivity reactions to teniposide (VM-26): an analysis. J Clin Oncol 1986; 4(8): 1262–9
Kellie SJ, Crist WM, Pui C, et al. Hypersensitivity reactions to epiphodophyllotoxins in children with acute lymphoblastic leukemia. Cancer 1991; 67: 1070–5
Nolte H, Carstensen H, Hertz H. VM-26 (teniposide)-induced hypersensitivity and degranulation of basophils in children. Am J Pediatr Hematol Oncol 1988; 10(4): 308–12
Carstensen H, Nolte H, Hertz H. Teniposide-induced hypersensitivity reactions in children. Lancet 1989; II: 55
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zanotti, K.M., Markman, M. Prevention and Management of Antineoplastic-Induced Hypersensitivity Reactions. Drug-Safety 24, 767–779 (2001). https://doi.org/10.2165/00002018-200124100-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002018-200124100-00005