Abstract
Due to the increase in utilization of chemotherapies and antibodies, drug hypersensitivity reactions have increased dramatically worldwide, preventing the use of first-line therapies and impacting patients’ survival and quality of life. Some of the more frequently used medications in cancer include taxanes for ovarian, lung, breast, and prostate cancers. Monoclonal antibodies are used in the treatment of neoplastic, autoimmune, and inflammatory diseases, and their clinical applications are becoming broader. Monoclonal antibody targets include CD20, HER-2, EGFR, IL-6 receptor, TNF-α, CD30, VEGF-A, IgE, and more, and examples of immune-mediated and inflammatory diseases that respond to monoclonal antibodies include rheumatoid arthritis, Crohn’s disease, ulcerative colitis, juvenile idiopathic arthritis, psoriasis and psoriatic arthritis, Wegener’s granulomatosis, microscopic polyangiitis, ankylosing spondylitis, plaque psoriasis, and asthma. Neoplastic diseases include non-Hodgkin’s lymphoma, chronic lymphocytic leukemia, and colorectal, breast, gastric, and lung cancer. The clinical presentation of drug hypersensitivity reactions ranges from mild cutaneous reactions to life-threatening symptoms including anaphylaxis. Rapid drug desensitization (RDD) has become a groundbreaking approach to the management of immediate drug hypersensitivity reactions IgE and non-IgE mediated. It is the only effective procedure that enables sensitized patients to receive the full treatment dose safely, thus representing an important advance in the patients’ treatment and prognosis. The aim of this review is to provide an update on hypersensitivity reactions to commonly used monoclonal and taxanes, their clinical presentations, diagnosis, and the use of RDD for their management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adverse drug reactions are defined by WHO as "any noxious, unintended, and undesired effect of a drug that occurs at doses used for prevention, diagnosis, or treatment" and are estimated to occur in 15 to 30 % of hospitalized patients, with 0.1 % of deaths reported for clinic patients and 0.01 % for surgical patients [1, 2]. The incidence of ADR is 5 % in adult ambulatory patients. Drug hypersensitivity reactions (DHR) correspond to 10 to 15 % of all adverse reactions [1]. In this context, DHR affect more than 7 % of the general population representing an important public health problem [3].
The last international consensus on drug allergy suggests that the term "allergy" to be restricted to the reactions in which it was possible to establish an immunological mechanism, either via in vivo or in vitro testing. If is not possible to demonstrate, priority should be given the term "DHR" (Fig. 1) [4].
DHR is an immunologically mediated reaction; they can be an acute as immediate or delayed. Immediate hypersensitivity reaction (HSR) was defined as an adverse reaction during or under 1 h of the infusion and with symptoms suggestive of mast cell/basophil degranulation, although in patient with pre-medications, the reactions can be delayed to more than 1 h. The definition of delayed HSR is an adverse reaction with onset of greater than 1 h to 1 week after the infusion and with symptoms suggestive of either a cell-mediated HSR (e.g., a maculopapular rash) or a mast cell/basophil-mediated HSR (e.g., flushing with onset <48 h after the infusion). The severity of immediate HSR was graded by Brown, as described in Table 1 [5–7].
Some patients can experience reactions such as chills, fever, nausea, and malaise. These symptoms have been attributed to the release of proinflammatory cytokines, interleukin-6 and TNF-α, also known as cytokine storm [8].
Patients with chronic inflammatory and cancer diseases are increasingly exposed to new monoclonal antibodies (mAbs) and chemotherapy drugs, respectively, with sensitization potential. In the last 15–20 years, clinical and basic research has provided evidence that patients with type I and type IV reactions can be safely reexposed to their allergy through desensitization protocols (Table 2) [8].
The aim of this review is to provide up to date information on the presentation, diagnoses, and management of HSR to taxane chemotherapy agents and biological agents including drug challenges and desensitization protocols.
Taxanes
In the USA, the third leading cause of fatal drug-induced anaphylaxis is antineoplastics [9], and among the most frequently implicated antineoplastics in these reactions are taxanes. Since their commercialization, the US Food and Drug Administration reported more than 300 fatalities [10–12].
In gynecology, taxanes are an integral part of the chemotherapy regimen used for lung, breast, and prostate cancers [13, 14].
Paclitaxel and docetaxel are the two main taxane molecules, and recently cabacitaxel and Abraxane (albumin-bound paclitaxel) have been added to the taxane family. Paclitaxel is a natural molecule that was originally isolated from the bark of the Pacific yew tree, and docetaxel is a semi-synthetic molecule derived from a taxoid precursor found in European yew tree needles. Cremophor is used to solubilize paclitaxel molecules, and polysorbate is used to solubilize 80 for docetaxel. These solvents can cause complement activation leading to anaphylatoxin production and mast cell activation [15].
In the initial studies with taxanes, DHR were very frequent and led to the use of premedication with antihistamine and corticosteroids. HSR occur in almost 10 % of patients and in 1 % are severe [16, 17]. Reactions occur during the patient’s first or second lifetime drug exposure in up to 40 % of the patients and include symptoms such as throat tightness, flushing, hypotension, and dyspnea. However, the same patients also report atypical symptoms such as severe chest and back and/or pelvic pain [12, 17].
Patients with severe cutaneous adverse drug reactions (e.g., blistering skin reactions/Stevens-Johnson syndrome/desquamative) are advised to avoid all taxanes.
Diagnosis of Taxane Hypersensitivity Reactions
Immunoglobulin E (IgE)-mediated HSR to the taxane molecule has been reported, generating interest in providing skin test evaluations for patients with DHR to taxanes [18, 19].
Brigham and Women’s Hospital (BWH) Drug Hypersensitivity and Desensitization Center and Dana-Farber Cancer Institute (DFCI) conducted a study with 164 patients treated for a taxane-related HSR from April 2011 to August 2014, the largest cohort of patients treated for taxane-induced HSR reported to date. Skin testing was performed in 145 patients: 103 (71 %) had positive results, 35 (24 %) had negative results, 6 (4 %) had equivocal results, and 1 (0.7 %) had results that converted from negative to positive [20].
Of 138 patients desensitized, 29 (21 %) had an immediate and 20 (14 %) had a delayed HSR with the procedure. Forty-nine patients were challenged, two (4 %) had a mild immediate HSR, and one (2 %) had a delayed HSR. Factors associated with an HSR during challenge were ovarian, fallopian, or peritoneal cancer and atopy (allergic asthma and/or rhinoconjunctivitis, food allergy, atopic dermatitis, or hymenoptera allergy) for immediate reactions, and for the delayed reactions, older age is the factor associated (Table 3). No patients had a severe immediate HSR with desensitization or challenge. Thirty-six (22 %) patients eventually resumed regular infusions. These patients were more likely to have negative skin test responses and to have experienced a delayed or mild immediate initial HSR [20].
This study showed that risk stratification based on skin testing and the severity of the initial HSR can safely guide DHR management and allow a significant number of patients to resume regular infusions (Fig. 2) [20].
Algorithm to taxane reintroduction in patients with HSR. In patients with an HSR with desensitization or challenge, premedication is generally adjusted for the next procedure, which is administered by using either the same or a longer protocol. Patients in whom the HSR does not recur are then treated with a shorter desensitization protocol, challenge, or regular infusion, according to the algorithm. See Table 1 for a description of the grading of immediate HSR. Severe cutaneous adverse drug reactions (SCARs) include Stevens-Johnson syndrome and desquamative/blistering skin reactions. [Adapted from Picard [20]]
Rapid Drug Desensitization to Taxanes
The DFCI/BWH Desensitization Program has generated a flexible 12-to-20 step protocol, which rendered mast cells unresponsive by delivering ×2 to ×2.5 doses of drug antigens at fixed time intervals starting at 1/1000 to 1/100 dilutions of the final concentration [12]. The challenge of rapid drug desensitization (RDD) is to gradually increase the dose of medication without reaching a threshold concentration that would trigger anaphylaxis, although mast cells/basophils may release some amount of mediators during RDD. Figure 3 illustrates the concept that each administered dose induces more cell inhibition and raises the threshold for clinical symptoms.
Patients with HSR grade I and II and skin test positive or grade II and skin test negative with comorbidities are desensitized with 12 steps (3 bags). An example of 12-step RDD for taxane is described in Table 4. Patients with HSR grade III or with comorbidities (e.g., uncontrolled asthma and/or significant impairment in FEV1, unstable or symptomatic coronary heart disease, and poor Eastern Cooperative Oncology Group performance status), beta-blocker users, or pregnant are indicated to follow a 16–20-step protocol on intensive care unit (Table 5) [8].
In one study where 77 desensitizations to paclitaxel and docetaxel in 17 patients were performed, 72 were without reactions. During the desensitization protocol, four patients had symptoms, such as palmar erythema, pruritus, mild abdominal pain, chest burning sensation, and mild flushing. All four patients successfully completed the planned infusions in their entirety. Three of the four patients had subsequent desensitizations without HSR. The fourth patient no longer received taxane because she opted to change therapeutic agent [21].
RDD is a safe and effective method of reintroducing taxanes in patients with past immediate HSR [12, 21, 22]. Yet this method is time-consuming and necessitates a 1:1 nursing ratio [10].
Monoclonal Antibodies
The applications of mAb drugs cover a wide range of diseases, such as the treatment of neoplastic, inflammatory, and autoimmune diseases [23, 24]. This drug class started in the 1970s, but mAb use became widespread in the past decade, leading to an increase in reported DHR and sometimes preventing the use of first-line therapies. Some of the most frequently used mAbs are presented in Table 6, including their targets, incidence of overall injection/infusion site reactions, and severe immediate HSR.
MAb immunogenicity depends on the presence of human content, varying from chimeric mouse-human, humanized, to a fully human mAb [25]. Even with fully human mAbs, such as adalimumab and ofatumumab, severe DHR can occur. First exposure to mAbs can lead to DHR, as it can be observed with cetuximab and trastuzumab, predominantly in the first three infusions, as with omalizumab, or after multiple exposures [23, 24].
In a significant number of patients, infusion-related reactions to mAbs can occur. Certain patients can manifest with nausea, chills, fever, and malaise [7, 25, 26]. For trastuzumab, typical first-time infusion reactions include chills and/or fever and occur in approximately 40 % of patients [27]. These are thought to be due to the release of proinflammatory cytokines (such as IL-6 and TNF-α) and do not tend to be severe, except for the findings of the anti-CD28 mAb TGN1412 phase 1 trial in which six volunteers who received the drug developed multiorgan failure as a result of a severe cytokine storm [28].
Grade 2–4 hypersensitivity reactions were reported in 27 % of 51 patients treated with cetuximab in a Florida Veterans Affairs facility [29]. This association was later explained by the role of a galactose-a-1,3-galactose IgE antibodies possibly generated by tick exposure (Amblyomma americanum—lone star tick), whose geographical distribution matched that of cases of anaphylaxis to meat and cetuximab hypersensitivity. The carbohydrate galactose-a-1,3-galactose is expressed on nonprimate mammalian proteins and present on the cetuximab heavy chain [30].
Immediate and delayed hypersensitivity reactions (skin lesions with CD4þ T cells and eosinophils infiltrate in the upper dermis) can occur secondary to the use of tocilizumab [31, 32].
Infusion-related reactions tend to occur in approximately 12 % patients, and the most common signs and/or symptoms include chills, nausea, dyspnea, pruritus, pyrexia, and cough. There have been reports on anaphylaxis associated with brentuximab, and desensitizations have been performed [33–36].
In addition, there have been reports of type I, III, and IV DHR related to mAb infusion. Patients can present with signs and symptoms typical of the type I HSR, including cutaneous, cardiovascular, respiratory, gastrointestinal, and/or neurological manifestations, while the drug is being infused or within the first hour after administration. Delayed DHR suggestive of type IV reactions have been reported, as well as reactions suggestive of type III reactions (serum sickness-like), with symptoms such as rash, myalgia, fever, polyarthralgias, pruritus, edema, and fatigue [32, 37]. Examples of the latter are DHR induced by infliximab (1 to 14 days after the infusion) and omalizumab (1 to 5 days after infusion) [38, 39].
mAbs whose application is subcutaneous might elicit injection site reactions. These include local redness, warmth, burning, stinging, itching, urticaria, pain, and induration, varying in frequency from 0.8 to 4.5 % with certolizumab to up to 45 % with omalizumab. Such reactions can start in the first hour of the injection and tend to resolve in the subsequent days [24].
When managing a DHR related to mAb, the infusion must be immediately stopped and it is strongly advised to obtain a tryptase level within 30 to 120 min of the reaction [40–42].
Tryptase is one of many mast cell-derived mediators, and it can be measured in peripheral blood. Rise in serum tryptase during an anaphylactic event may peak 15–60 min after the onset of symptoms and then decline with a half-life of about 2 h. Acute serum total tryptase level should be at least 20 % plus 2 ng/ml over the baseline level (of tryptase) to be indicative of mast cell activation [43].
Increased levels of tryptase will point out to a reaction with an underlying mast cell activation mechanism. Epinephrine is indicated in severe reactions involving hypotension and/or desaturation and should be promptly administered [44].
Diagnosis of Monoclonal Antibody Hypersensitivity Reactions
Skin testing with the offending agent can be done when an IgE-mediated reaction is suspected, but this specific investigation should wait 2 to 4 weeks to minimize the chances of false-negative results [40, 45]. The negative predictive value for most mAbs is not known. It was reported that out of 23 patients desensitized to trastuzumab, infliximab, or tituximab, only 13 patients had positive skin test [7].
If skin tests are negative, tryptase levels obtained during the reaction are within normal range and/or the clinical history is not suggestive of a true, IgE-mediated, allergic reaction, a graded challenge with the medication can be performed [40]. The challenge consists of providing the patient with 1/10 of the total dose of the offending drug under medical surveillance, and if no reactions occur, the patient can receive the rest of the dose. If the challenge is positive, the patient may be a candidate to desensitization; likewise, if the challenge is negative, the patient can resume regular infusions [46, 47].
Rapid Drug Desensitization for Monoclonal Antibodies
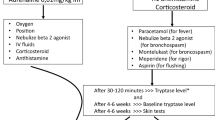
RDD is a novel therapeutic option for selected patients who present with DHR to mAbs [48]. The general algorithm for rapid drug desensitization should be applied for mAb HSR (Fig. 4) [8]. A standard desensitization protocol to mAbs has been developed with 3 intravenous dilution bags, 12 steps, and an approximate total duration of 6 h [49]. High-risk patients can be desensitized with additional dilutions and/or steps (16 or 20 steps). It enables the patient to receive the full treatment dose while protecting from anaphylaxis [7].
Propose algorithm for rapid drug desensitization. [Adapted from Giavina-Bianchi [8]]
Patients with type I DHR to mAbs are candidates for RDD, and immediate injection site and systemic reactions elicited by subcutaneous agents (such as adalimumab and etanercept) have also had successful desensitization protocols established (Tables 7, desensitization to adalimumab, and 8, desensitization to ofatumumab) [40, 50]. For mAbs that are administered subcutaneously, a six-step rapid desensitization protocol was developed. The initial dose is typically 1/10 of the target doses of the drug below the threshold of HSR, and the doses are doubled until it reaches the target dose in six steps with an interval of 30 min.
Until now, it has been successfully desensitized mAb HSR to rituximab, ofatumumab, obinutuzumab, trastuzumab, cetuximab, tocilizumab, infliximab, etanercept, adalimumab, golimumab, certolizumab, brentuximab, bevacizumab, and omalizumab.
Conclusion
Most patients with DHR are candidates for RDD, except for patients with SCARs. The success of rapid drug desensitization relies on categorization of the intensity and nature of the initial reaction, skin testing, and risk stratification, with adjustments based on the patient’s response.
The largest desensitization study worldwide reported that 370 highly allergic patients received 2177 successful desensitizations to 15 drugs, 3 of which (bevacizumab, tocilizumab, and gemcitabine) are unprecedented. Most importantly, carboplatin-desensitized patients had a nonstatistically significant lifespan advantage over nonallergic controls, indicating that the efficacy of carboplatin was not reduced in allergic patients and that RDD protocols are as effective as regular infusions [51].
RDD is safe, based on the results of the 2177 desensitizations, and 93 % had no or mild reactions, whereas 7 % had moderate to severe reactions, which did not preclude the completion of the treatment, and there were no deaths (Fig. 5) [51].
a Percentage and severity of breakthrough reactions occurring during 2177 desensitization courses to chemotherapy and monoclonal. b One hundred twenty reactions to rituximab. c Five hundred fifty reactions to paclitaxel. d One thousand sixty-nine reactions to carboplatin. [Adapted from Sloane [51]
Rapid drug desensitization is a groundbreaking procedure for the management of immediate drug hypersensitivity reactions. It protects patients against anaphylaxis, maintaining patients on first-line therapy, thus representing an important advance in patients’ treatment and prognosis.
Abbreviations
- ADR:
-
Adverse drug reaction
- DHR:
-
Drug hypersensitivity reactions
- HSR:
-
Hypersensitivity reactions
- IgE:
-
Immunoglobulin E
- mAbs:
-
Monoclonal antibodies
- RDD:
-
Rapid drug desensitization
References
Gomes ER, Demoly P (2005) Epidemiology of hypersensitivity drug reactions. Curr Opin Allergy Clin Immunol 5(4):309–316
Gruchalla RS (2003) 10. Drug allergy. J Allergy Clin Immunol 111(2 Suppl):S548–S559
Gomes E, Cardoso MF, Praça F, Gomes L, Mariño E, Demoly P (2004) Self-reported drug allergy in a general adult Portuguese population. Clin Exp Allergy J Br Soc Allergy Clin Immunol 34(10):1597–1601
Demoly P, Adkinson NF, Brockow K, Castells M, Chiriac AM, Greenberger PA et al (2014) International consensus on drug allergy. Allergy 69(4):420–437
Johansson SGO, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF et al (2004) Revised nomenclature for allergy for global use: report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol 113(5):832–836
Brown SGA (2004) Clinical features and severity grading of anaphylaxis. J Allergy Clin Immunol 114(2):371–376
Brennan PJ, Bouza TR, Hsu FI, Sloane DE, Castells MC (2009) Hypersensitivity reactions to mAbs: 105 desensitizations in 23 patients, from evaluation to treatment. J Allergy Clin Immunol 124(6):1259–1266
Pedro Giavina-Bianchi MVA (2015) Rapid desensitization in immediate hypersensitivity reaction to drugs. Curr Treat Options Allergy 1
Jerschow E, Lin RY, Scaperotti MM, McGinn AP (2014) Fatal anaphylaxis in the United States, 1999–2010: temporal patterns and demographic associations. J Allergy Clin Immunol 134(6):1318–28.e7
Banerji A, Lax T, Guyer A, Hurwitz S, Camargo CA, Long AA (2014) Management of hypersensitivity reactions to carboplatin and paclitaxel in an outpatient oncology infusion center: a 5-year review. J Allergy Clin Immunol Pract 2(4):428–433
Raisch DW, Campbell W, Garg V, Qureshi ZP, Bookstaver PB, Norris LB et al (2011) Description of anaphylactic reactions to paclitaxel and docetaxel reported to the FDA, with a focus on the role of premedication. Expert Opin Drug Saf 10(4):521–528
Castells MC, Tennant NM, Sloane DE, Hsu FI, Barrett NA, Hong DI et al (2008) Hypersensitivity reactions to chemotherapy: outcomes and safety of rapid desensitization in 413 cases. J Allergy Clin Immunol 122(3):574–580
Morgan RJ, Alvarez RD, Armstrong DK, Burger RA, Chen L, Copeland L et al (2013) Ovarian cancer, version 2.2013. J Natl Compr Cancer Netw JNCCN 11(10):1199–1209
Picard M, Castells MC (2015) Re-visiting hypersensitivity reactions to taxanes: a comprehensive review. Clin Rev Allergy Immunol 49(2):177–191
Weiszhár Z, Czúcz J, Révész C, Rosivall L, Szebeni J, Rozsnyay Z (2012) Complement activation by polyethoxylated pharmaceutical surfactants: Cremophor-EL, Tween-80 and Tween-20. Eur J Pharm Sci Off J Eur Fed Pharm Sci 45(4):492–498
Kwon JS, Elit L, Finn M, Hirte H, Mazurka J, Moens F et al (2002) A comparison of two prophylactic regimens for hypersensitivity reactions to paclitaxel. Gynecol Oncol 84(3):420–425
Markman M, Kennedy A, Webster K, Kulp B, Peterson G, Belinson J (2000) Paclitaxel-associated hypersensitivity reactions: experience of the gynecologic oncology program of the Cleveland Clinic Cancer Center. J Clin Oncol Off J Am Soc Clin Oncol 18(1):102–105
Prieto García A, Pineda de la Losa F (2010) Immunoglobulin E-mediated severe anaphylaxis to paclitaxel. J Investig Allergol Clin Immunol 20(2):170–171
Picard M, Pur L, Caiado J, Giavina-Bianchi P, Galvão V, Castells MC (2014) Added value of skin testing in hypersensitivity reactions to taxanes. J Allergy Clin Immunol 133(2):AB152
Picard M, Pur L, Caiado J, Giavina-Bianchi P, Galvão VR, Berlin ST, et al. (2015) Risk stratification and skin testing to guide re-exposure in taxane-induced hypersensitivity reactions. J Allergy Clin Immunol. Dec 23
Feldweg AM, Lee C-W, Matulonis UA, Castells M (2005) Rapid desensitization for hypersensitivity reactions to paclitaxel and docetaxel: a new standard protocol used in 77 successful treatments. Gynecol Oncol 96(3):824–829
Madrigal-Burgaleta R, Berges-Gimeno MP, Angel-Pereira D, Ferreiro-Monteagudo R, Guillen-Ponce C, Pueyo C et al (2013) Hypersensitivity and desensitization to antineoplastic agents: outcomes of 189 procedures with a new short protocol and novel diagnostic tools assessment. Allergy 68(7):853–861
Kotsovilis S, Andreakos E (2014) Therapeutic human monoclonal antibodies in inflammatory diseases. Methods Mol Biol Clifton NJ 1060:37–59
Li GN, Wang SP, Xue X, Qu XJ, Liu HP (2013) Monoclonal antibody-related drugs for cancer therapy. Drug Discov Ther 7(5):178–184
Lemery SJ, Zhang J, Rothmann MD, Yang J, Earp J, Zhao H et al (2010) U.S. Food and Drug Administration approval: ofatumumab for the treatment of patients with chronic lymphocytic leukemia refractory to fludarabine and alemtuzumab. Clin Cancer Res Off J Am Assoc Cancer Res 16(17):4331–4338
Quercia O, Emiliani F, Foschi FG, Stefanini GF (2011) Adalimumab desensitization after anaphylactic reaction. Ann Allergy Asthma Immunol Off Publ Am Coll Allergy Asthma Immunol 106(6):547–548
Thompson LM, Eckmann K, Boster BL, Hess KR, Michaud LB, Esteva FJ et al (2014) Incidence, risk factors, and management of infusion-related reactions in breast cancer patients receiving trastuzumab. Oncologist 19(3):228–234
Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD et al (2006) Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med 355(10):1018–1028
Commins SP, Platts-Mills TAE (2009) Allergenicity of carbohydrates and their role in anaphylactic events. Curr Allergy Asthma Rep 10(1):29–33
Commins SP, Satinover SM, Hosen J, Mozena J, Borish L, Lewis BD et al (2009) Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-alpha-1,3-galactose. J Allergy Clin Immunol 123(2):426–433
Sieper J, Porter-Brown B, Thompson L, Harari O, Dougados M (2014) Assessment of short-term symptomatic efficacy of tocilizumab in ankylosing spondylitis: results of randomised, placebo-controlled trials. Ann Rheum Dis 73(1):95–100
Yoshiki R, Nakamura M, Tokura Y (2010) Drug eruption induced by IL-6 receptor inhibitor tocilizumab. J Eur Acad Dermatol Venereol JEADV 24(4):495–496
ADCETRIS (2012) (brentuximab), package insert. Seattle Genetics, Inc., Wash, USA
DeVita MD, Evens AM, Rosen ST, Greenberger PA, Petrich AM (2014) Multiple successful desensitizations to brentuximab vedotin: a case report and literature review. J Natl Compr Cancer Netw JNCCN 12(4):465–471
Story SK, Petrov AA, Geskin LJ (2014) Successful desensitization to brentuximab vedotin after hypersensitivity reaction. J Drugs Dermatol JDD 13(6):749–751
O’Connell AE, Lee JP, Yee C, Kesselheim J, Dioun A (2014) Successful desensitization to brentuximab vedotin after anaphylaxis. Clin Lymphoma Myeloma Leuk 14(2):e73–e75
Cheifetz A, Mayer L (2005) Monoclonal antibodies, immunogenicity, and associated infusion reactions. Mt Sinai J Med N Y 72(4):250–256
Gamarra RM, McGraw SD, Drelichman VS, Maas LC (2006) Serum sickness-like reactions in patients receiving intravenous infliximab. J Emerg Med 30(1):41–44
Pilette C, Coppens N, Houssiau FA, Rodenstein DO (2007) Severe serum sickness-like syndrome after omalizumab therapy for asthma. J Allergy Clin Immunol 120(4):972–973
Galvão VR, Castells MC (2015) Hypersensitivity to biological agents-updated diagnosis, management, and treatment. J Allergy Clin Immunol Pract 3(2):175–185, quiz 186
Laroche D, Vergnaud MC, Sillard B, Soufarapis H, Bricard H (1991) Biochemical markers of anaphylactoid reactions to drugs. Comparison of plasma histamine and tryptase. Anesthesiology 75(6):945–949
Schwartz LB, Yunginger JW, Miller J, Bokhari R, Dull D (1989) Time course of appearance and disappearance of human mast cell tryptase in the circulation after anaphylaxis. J Clin Invest 83(5):1551–1555
Valent P, Akin C, Arock M, Brockow K, Butterfield JH, Carter MC et al (2012) Definitions, criteria and global classification of mast cell disorders with special reference to mast cell activation syndromes: a consensus proposal. Int Arch Allergy Immunol 157(3):215–225
Simons FER, Ardusso LRF, Bilò MB, El-Gamal YM, Ledford DK, Ring J et al (2011) World Allergy Organization anaphylaxis guidelines: summary. J Allergy Clin Immunol 127(3):587–93.e1–22
Brockow K, Romano A, Blanca M, Ring J, Pichler W, Demoly P (2002) General considerations for skin test procedures in the diagnosis of drug hypersensitivity. Allergy 57(1):45–51
Blatman KSH, Castells MC (2014) Desensitizations for chemotherapy and monoclonal antibodies: indications and outcomes. Curr Allergy Asthma Rep 14(8):1–8
Joint Task Force on Practice Parameters, American Academy of Allergy, Asthma and Immunology, American College of Allergy, Asthma and Immunology, Joint Council of Allergy, Asthma and Immunology (2010) Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol 105:259–273
Cernadas JR, Brockow K, Romano A, Aberer W, Torres MJ, Bircher A et al (2010) General considerations on rapid desensitization for drug hypersensitivity—a consensus statement. Allergy 65(11):1357–1366
del Carmen SM, Breslow R, Sloane D, Castells M (2012) Desensitization for hypersensitivity reactions to medications. Chem Immunol Allergy 97:217–233
Bavbek S, Ataman Ş, Bankova L, Castells M (2013) Injection site reaction to adalimumab: positive skin test and successful rapid desensitisation. Allergol Immunopathol (Madr) 41(3):204–206
Sloane D, Govindarajulu U, Harrow-Mortelliti J, Barry W, Hsu FI, Hong D, et al. (2016) Safety, costs, and efficacy of rapid drug desensitizations to chemotherapy and monoclonal antibodies. J Allergy Clin Immunol Pract [Internet]. 2016 Feb 16 [cited 2016 Feb 18];0(0). Available from: http://www.jaci-inpractice.org/article/S2213219816000118/abstract
RITUXAN (2011) (rituximab), package insert. Biogen Idec Inc., MA, USA, and Genentech Inc., CA, USA
Grillo-López AJ, White CA, Varns C, Shen D, Wei A, McClure A et al (1999) Overview of the clinical development of rituximab: first monoclonal antibody approved for the treatment of lymphoma. Semin Oncol 26(5 Suppl 14):66–73
ARZERRA (2011) (ofatumumab), package insert. GlaxoSmithKline NC, USA
GAZYVA (2015) Full prescribing information. Genentech USA, Inc., South San Francisco, CA
Obinutuzumab (2016) (GA101) plus chlorambucil (Clb) or rituximab (R) plus Clb versus Clb alone in patients with chronic lymphocytic leukemia (CLL) and preexisting medical conditions (comorbidities): final stage 1 results of the CLL11 (BO21004) phase III trial. J Clin Oncol [Internet]. [cited 2016 Feb 29]; Available from: http://meetinglibrary.asco.org/content/116249-132
Guan M, Zhou Y-P, Sun J-L, Chen S-C, Guan M, Zhou Y-P et al (2015) Adverse events of monoclonal antibodies used for cancer therapy. BioMed Res Int BioMed Res Int 2015:e428169
HERCEPTIN (2011) (trastuzumab), package insert. Genentech, Inc., CA, USA
Cook-Bruns N (2001) Retrospective analysis of the safety of Herceptin immunotherapy in metastatic breast cancer. Oncology 61(Suppl 2):58–66
ERBITUX (2012) (cetuximab), package insert. Celegene Corporation NJ, USA
Yamaguchi K, Watanabe T, Satoh T, Ishiguro M, Izawa M, Inoshiri S et al (2014) Severe infusion reactions to cetuximab occur within 1 h in patients with metastatic colorectal cancer: results of a nationwide, multicenter, prospective registry study of 2126 patients in Japan. Jpn J Clin Oncol 44(6):541–546
Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A et al (2004) Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351(4):337–345
Siena S, Glynne-Jones R, Adenis A, Thaler J, Preusser P, Aguilar EA et al (2010) Reduced incidence of infusion-related reactions in metastatic colorectal cancer during treatment with cetuximab plus irinotecan with combined corticosteroid and antihistamine premedication. Cancer 116(7):1827–1837
Sobrero AF, Maurel J, Fehrenbacher L, Scheithauer W, Abubakr YA, Lutz MP et al (2008) EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol 26(14):2311–2319
Keating K, Walko C, Stephenson B, O’Neil BH, Weiss J (2014) Incidence of cetuximab-related infusion reactions in oncology patients treated at the University of North Carolina Cancer Hospital. J Oncol Pharm Pract Off Publ Int Soc Oncol Pharm Pract 20(6):409–416
O’Neil BH, Allen R, Spigel DR, Stinchcombe TE, Moore DT, Berlin JD et al (2007) High incidence of cetuximab-related infusion reactions in Tennessee and North Carolina and the association with atopic history. J Clin Oncol 25(24):3644–3648
George TJ, Laplant KD, Walden EO, Davis AB, Riggs CE, Close JL et al (2010) Managing cetuximab hypersensitivity-infusion reactions: incidence, risk factors, prevention, and retreatment. J Support Oncol 8(2):72–77
ACTEMRA (2013) (tocilizumab), package insert. Genentech, Inc., CA, USA
REMICADE (2013) (infliximab), package insert. Janssen Biotech, Inc., PA, USA
ENBREL (2013) (etanercept), package insert. Immunex Corp., CA, USA
HUMIRA (2008) (adalimumab), package insert. Abbott Laboratories, Ill, USA
Judson MA, Baughman RP, Costabel U, Drent M, Gibson KF, Raghu G et al (2014) Safety and efficacy of ustekinumab or golimumab in patients with chronic sarcoidosis. Eur Respir J 44(5):1296–1307
Smolen JS, Kay J, Doyle MK, Landewé R, Matteson EL, Wollenhaupt J et al (2009) Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial. Lancet Lond Engl 374(9685):210–221
Keystone E, Heijde DVD, Mason D, Landewé R, Vollenhoven RV, Combe B et al (2008) Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum 58(11):3319–3329
Fleischmann R, Vencovsky J, van Vollenhoven RF, Borenstein D, Box J, Coteur G et al (2009) Efficacy and safety of certolizumab pegol monotherapy every 4 weeks in patients with rheumatoid arthritis failing previous disease-modifying antirheumatic therapy: the FAST4WARD study. Ann Rheum Dis 68(6):805–811
UpToDate (acessed on february 29, 2016) http://www.uptodate.com/contents/infusion-reactions-to-therapeutic-monoclonal-antibodies-used-for-cancer-therapy
XOLAIR (2010) (omalizumab), package insert. Genentech, Inc., CA, USA
Cox L, Platts-Mills TAE, Finegold I, Schwartz LB, Simons FER, Wallace DV (2007) American Academy of Allergy, Asthma & Immunology/American College of Allergy, Asthma and Immunology Joint Task Force Report on omalizumab-associated anaphylaxis. J Allergy Clin Immunol 120(6):1373–1377
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bonamichi-Santos, R., Castells, M. Diagnoses and Management of Drug Hypersensitivity and Anaphylaxis in Cancer and Chronic Inflammatory Diseases: Reactions to Taxanes and Monoclonal Antibodies. Clinic Rev Allerg Immunol 54, 375–385 (2018). https://doi.org/10.1007/s12016-016-8556-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-016-8556-5