Abstract
Polymers with stable radical groups are promising materials for organic electronic devices due to their unique redox activity. Block copolymers with one redox active block could be used in nanostructured devices for electronic applications. We report on the synthesis and characterization of such multifunctional block copolymers in which phase separation on the 10 nm (half pitch) scale is achieved by using fluorinated blocks. Fluorination of one block increases the degree of phase separation and leads to smaller accessible domain sizes. Block copolymers with 60%, 80% and 90% of a stable radical containing block and either fluorinated or non-fluorinated second blocks were made by atom transfer radical polymerization, and their microstructure formation as a function of fluorine content is described after solvent vapor or thermal annealing. Electrical characterization of such a partly fluorinated block copolymer shows their potential for electronic devices.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Almost 50 years ago, Griffith et al. described the synthesis of polymers with stable radical groups (stable radical polymers).[1] Their oxidation or reduction is reversible and proceeds through a fast one-electron transfer reaction, which makes them promising materials as active species in the cathode or anode material of batteries.[2–5] Stable radicals bound to polymer backbones have been reported to increase conductivity and open potential applications in metal-free batteries.[3–5]
Polymers made from 2,2,6,6-tetxamethyl-1-piperidinyloxy-4-yl methacrylate are the most widely investigated of all stable radical polymers. Most of the more recent research on stable radical polymers focuses on polymerization techniques with limited control over molecular weight dispersity.[2,6] Only within the last few years, polymer formation by living anionic[1,7–10] or controlled polymerization techniques of such polymers such as ring-opening metathesis polymerizations,[11,12] reversible addition-fragmentation chain-transfer polymerizations,[13–17] nitroxide mediated polymerizations,[15] or atom transfer radical polymerizations (ATRP)[15,18–24] was reported. A limited number of articles have reported block copolymers with a block that contains stable radical groups,[12,14,16,23] and they only show disordered phase-separated block copolymer structures.
Block copolymer phase separation only occurs if the interaction parameter between the blocks, χ, is sufficiently big, the degree of polymerization is high and neither block is very short.[25] To achieve small structures, increasing χ is the most convenient strategy.[26] Compared with their hydrocarbon analogue block copolymers, selective fluorination of one block may lead to increased χ.[27] Especially in thin films, however, film preparation often leads to kinetically trapped non-equilibrium structures, and thermodynamic equilibrium is only achieved after annealing techniques.
In this contribution, we combine the effects of fluorination of one block of a block copolymer with evaluation of electronic properties by incorporating stable radical groups in the other block. We will describe the synthesis, and phase separation and compare microstructure formation after advanced annealing techniques of both fluorinated and non-fluorinated block copolymers. We subsequently characterize the electric properties of such multifunctional block copolymers.
Experimental part
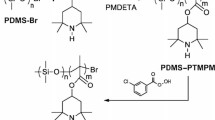
Block copolymers were synthesized by ATRP. In a Schlenk flask, 107.5 µL (1 eq) ethyl 2-bromoisobutyrate, 584.7 mg (2 eq) 4,4′-dinonyl-2,2′-dipyridyl (dNbpy) and 19.62 g (120 eq) 2,2,6,6-tetramethyl-4-piperidinyl methacrylate (TMPMA) were dissolved in 35.3 mL toluene under argon atmosphere and cleaned by five freeze-pump-thaw cycles. With a degassed syringe, the solution was transferred into a second argon-flushed Schlenk flask equipped with a stir bar and 52.0 mg CuBr (0.5 eq) and degassed by two more freeze-pump-thaw cycles. The reaction was carried out in an oil bath under argon at 70 °C for 6 h and stopped by removal of the CuBr by filtration through neutral alumina. The homopolymer was cleaned by repeated precipitation in excess cold hexanes and dried in vacuum at elevated temperature. The molecular weight was determined by 1H Nuclear Magnetic Resonance (NMR) spectroscopy.
For synthesizing block copolymers, a part of the homo-polymer was dissolved in anisole under argon in a Schlenk flask, together with 1.05 eq pentamethyldiethylenetriamine (PMDETA) and 115 eq of the second monomer, and degassed by three to four freeze-pump-thaw cycles. The solution was transferred to a second argon-flushed Schlenk flask equipped with a stir bar and 1 eq CuBr and degassed by two more freeze-pump-thaw cycles. The reaction was carried out in an oil bath under argon at 70 °C for a previously determined time, until the desired block ratio was achieved. The reaction was stopped by removal of the CuBr by filtration through neutral alumina. The polymer was precipitated in a large excess of cold hexanes and dried in vacuum at elevated temperature. The block ratio was confirmed by 1H NMR spectroscopy.
Block copolymers with stable radical blocks were gained by oxidizing the PTMPMAblockto poly(2,2,6,6-tetramethylpiperidinyloxy-4-yl methacrylate) (PTMA). The polymer was dissolved in methylene chloride and stirred at 0 °C under argon. Metachloroperbenzoic acid (mCPBA) (1.1 eq respective to the number of 2,2,6,6-tetramethylpiperidin groups) in methylene chloride was added dropwise within 1 h. After stirring at 0 °C for another hour, the solution was allowed to warm up while stirring overnight. The polymer was gained by precipitation in large excess of water and purified by redissolution in tetrahydrofuran (THF), stirring with excess cyclohexene, and consecutively precipitation in hexanes and water.
1H NMR spectroscopy was measured in chloroform at an Varian Inova 400 NMR. For electron paramagnetic resonance (EPR) spectroscopy, we measured samples in chloroform using a Bruker Elexsys E500 Continuous Wave EPR spectrometer. Atomic force microscopy (AFM) images were taken at a Veeco Icon AFM with Olympus AC160TS probes.
Thin film annealing was performed at elevated temperature in a vacuum oven or in solvent vapor in a custom-built annealing station: nitrogen gas was led at a constant flow rate of 50 mL/min through a closed stainless steel chamber in which the samples were placed over 5 mL of acetone at room temperature.
Results and discussion
We fabricated block copolymers with stable radical blocks by ATRP according to Scheme 1, which exemplarily shows the reaction scheme for a block copolymer from TMPMA and 2,2,2-trifluoroethyl methacrylate (3FEMA) monomers. Block copolymers with stable radical groups were gained by oxidizing the resulting block copolymers.
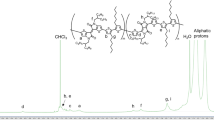
We performed screening experiments first to adjust the polymerization times for the desired block lengths and block ratios. 1H NMR spectroscopy was an easy and reliable way to determine block lengths and -ratios as shown in Fig. 1. The proton signals next to the ester groups (approximately between 3.5 ppm ≤ δ ≤ 5.25 ppm) are well separated from other proton signals and close enough together to use the integrated intensities to calculate the degrees of polymerization. The ratio between peak B at δ≈ 5.1 ppm [1 H, OCH(CH2)2] and peak A at δ ≈ 4.1 ppm [2 H, OCH2CH3, magnification around this chemical shift shown in Fig. 1(b)] yields the number average degree of polymerization of the PTMPMA block and the respective Mn.Mn of the second block could be calculated using the integrated intensities of peak C at δ ≈ 4.3 ppm (2 H, OCH2CF3) and peak B. In contrast to NMR measurements, size exclusion chromatography and dynamic light scattering experiments resulted in unsatisfactory polymer analysis due to the complex solubility properties of polymers with fluorinated and stable radical groups. We note that the presence of remaining PTMPMA homopolymer in the block copolymer cannot be excluded, but large quantities are very unlikely since no macrophase separation is observed in AFM imaging (see below).
Instead of 3FEMA, we also used other methacrylates and copolymerized blocks of three different fluorinated monomers and two different non-fluorinated monomers with PTMPMA macroinitiators. For each fluorinated block copolymer, we made a non-fluorinated one with similar block ratio. Fluorinated polymers were made from 3FEMA, 2,2,3,3,3-pentafluoropropyl methacrylate (5FPMA) and 2,2,3,4,4,4-hexafluorobutyl methacrylate (6FBMA) monomers, and similar non-fluorinated polymers comprised blocks from methyl methacrylate (MMA) and butyl methacrylate (BMA). Table I summarizes the different polymers, their average molecular weight and the block ratios. Block copolymers comprising ~60%, ~80% and ~90% of PTMA by weight were synthesized. Volume fractions depend on the processing method and swelling behavior of the individual blocks in solvents.
In general, a low degree of polymerization, combined with asymmetric blocks prevents microstructure formation in block copolymers. The more symmetrical polymers PTMPMA-b-PMMA and PTMPMA-b-P3FEMA, which also have a higher degree of polymerization compared with the other polymers in Table I, and the corresponding polymers after oxidation are hence the most likely to form phase separated structures.
After film formation by spin-coating or similar methods, block copolymers are not in thermodynamic equilibrium, and solubility of the blocks greatly influences their structure due to different volume fractions of the individual blocks in solution. The lower interaction parameter between both blocks in solution also often leads to no clear phase separation and disordered morphologies. Annealing steps at elevated temperature or in solvent vapor are hence usually employed to yield ordered morphologies. We chose two different techniques, annealing in vacuum at 140 °C for 48 h and annealing in acetone vapor atmosphere at room temperature, to facilitate structure formation.
AFM imaging is a straightforward and reliable method to investigate not only the topography but also the material properties of surfaces. In AFM phase images, phase separation in block copolymers with two similar blocks regarding their chemical composition and electron density can be easily investigated even in the case of only slightly different mechanical properties of the blocks.
Figure 2 shows AFM topography and phase images of thin PTMPMA-b-PMMA films. The samples were produced by spin-coating from CH2Cl2 and subsequent thermal [Figs. 2(a) and 2(b)] or solvent vapor annealing [Figs. 2(c) and 2(d)]. Topography images [Figs. 2(a) and 2(c)] show a flat surface with a roughness of less than 0.5 nm. Phase imaging shows two distinct chemical compounds at the surface but no ordered structure, neither after thermal nor solvent vapor annealing. No macrophase separation is observable. The low phase contrast and disordered morphology further indicate a low degree of phase separation and partial mixing of the two methacrylates.
Figure 3 shows AFM images of the fluorinated block copolymer PTMPMA-b-P3FEMA with comparable composition and molecular weight of the individual blocks. The samples were treated the same like the ones shown in Fig. 2: after spin-coating from CH2Cl2, the samples were annealed in vacuum at 140 °C for 48 h [Figs. 3(a) and 3(b)] together with the films from Figs. 2(a) and 2(b) or annealed in acetone vapor for 3 h [Figs. 3(c) and 3(d)] together with the ones depicted in Figs. 2(c) and 2(d). After either annealing technique, flat samples with a roughness of less than 1 nm resulted. Most pronounced in phase imaging, however, circular structures with a spacing of approximately 20 nm (~10nm half pitch) are clearly visible. Dark spots are roughly hexagonally arranged but not long-range ordered. Patterns like this may indicate a perforated lamellar or a cylindrical block copolymer morphology. Because the hexagonally perforated lamellar structure is only stable in strongly phase separated block copolymer films, and dark lines occur next to the circular structures in Figs. 3(b) and 3(d), which is characteristic for cylindrical morphologies, we conclude that the fluorinated block copolymer forms cylinders.
PTMPMA-b-PMMA and PTMPMA-b-P3FEMA have almost identical molecular weight and block ratios. The same annealing techniques lead to a clearly phase separated morphology in films of the latter while the former shows disordered structures. The chemical composition of both is similar with the short fluorinated side groups in PTMPMA-b-P3FEMA being the main difference. This indicates that by introducing fluorinated groups to our complex block copolymers, we can increase the degree of phase separation and form small ordered structures of PTMPMA. Longer fluorinated chains are expected to lead to an even more pronounced phase separation. The polymers PTMPMA-b-P5FPMA and PTMPMA-b-P6FBMA, however, did not phase separate in thin films after similar annealing techniques, just like the non-fluorinated polymers PTMPMA-b-PBMA-1 and PTMPMA-b-PBMA-2. In all of these polymers, the block ratio is much more asymmetric, and the total molecular weight is much smaller, which may cause mixing of the blocks and prevent phase separation. This indicates that the composition of the presented block copolymers is in the border region close to the order-disorder spinodal. Upon increasing the degree of phase separation by introducing fluorinated groups, decreasing domain sizes compared with conventional block copolymers or, as deducible from Figs. 2 and 3, ordered structures are accessible.
Besides fluorinated groups, the presented block copolymers comprise stable radicals, another kind of functional group. We used EPR spectroscopy to demonstrate successful oxidation of the PTMPMA blocks to PTMA.[28] Figure 4 shows EPR spectra of PTMPMA-b-P3FEMA and PTMA-b-P3FEMA (block copolymer before and after oxidation, red filled circles and black hollow circles, respectively). The intensity of the PTMA-b-P3FEMA signal relative to the PTMPMA-b-P3FEMA signal (Fig. 4) demonstrates the formationofstable radical groups. Additionally, the lack of a hyperfine triplet from the nitrogen in the 2,2,6,6-tetramethylpiperidinyloxyl (TEMPO) groups indicates exchange narrowing between stable radical groups, demonstrating that they are coupled to each other. Coupled stable radical groups indicate the general suitability for fabricating coin cells as we show in the supporting information (Supplementary Figure SI-1).
The use of fluorinated blocks and possible phase separated structures may help in coin cells from future polymer materials to decrease the amount of binders and conducting additives due to a better interaction with the electrolyte and the formation of conducting pathways. This is the focus of ongoing research and will be covered in future publications. Phase separated structures and decreased structure sizes by using fluorinated blocks together with PTMA may make it possible to form small ordered conductive pathways in polymer cathodes. The nonradical block may not only help in terms of better interaction with the electrolyte but also to increase stability due to confinement of the radical sites. By using different non-radical blocks, we may even be able to direct the swelling and interaction of the cathode polymer material with the electrolyte solution and hence optimize discharging processes.
Conclusion
We synthesized multifunctional diblock copolymers in which we incorporated fluorinated and stable radical groups by ATRP. Polymers comprised 60%, 80% and 90% of PTMA as the stable radical block and fluorinated and non-fluorinated methacrylate second blocks with side chain lengths between C1 and C4. When comparing block copolymers with and without fluorinated blocks of similar composition and molecular weight, phase separation occurred only in the one with fluorinated groups after thermal or solvent vapor annealing steps. Low molecular weight and asymmetric composition as well as non-fluorinated second blocks impede phase separation in other block copolymers, indicating advanced phase separation properties and the potential of structure size miniaturization. EPR characterization of the most promising polymer showed the occurrence of coupled stable radical groups. The introduction of fluorinated blocks together with stable radical containing polymers hence opens new possibilities for synthesizing small regular patterns with conductive pathways on a substrate.
References
O.H. Griffith, J.F.W. Keana, S. Rottschaefer, and T.A. Warlick: Preparation and magnetic resonance of nitroxide polymers. J. Am. Chem. Soc. 89, 5072 (1967).
K. Nakahara, S. Iwasa, M. Satoh, Y. Morioka, J. Iriyama, M. Suguro, and E. Hasegawa: Rechargeable batteries with organic radical cathodes. Chem. Phys. Lett. 359, 351 (2002).
K. Oyaizu and H. Nishide: Radical polymers for organic electronic devices: a radical departure from conjugated polymers?Adv. Mater. 8555, 2339 (2009).
K. Nakahara, K. Oyaizu, and H. Nishide: Organic radical battery approaching practical use. Chem. Lett. 40, 222 (2011).
T. Janoschka, M.D. Hager, and U.S. Schubert: Powering up the future: radical polymers for battery applications. Adv. Mater. 24, 6397 (2012).
H-Q. Li, Y. Zou, and Y-Y. Xia: A study of nitroxide polyradical/activated carbon composite as the positive electrode material for electrochemical hybrid capacitor. Electrochim. Acta 52, 2153 (2007).
M. Kamachi, M. Tamaki, Y. Morishima, S-i Nozakura, W. Mori, and M. Kishita: Electron exchange phenomena of polymers containing nitroxyl radicals. Polym. J. 14, 363 (1982).
F. MacCorquodale, J.A. Crayston, J.C. Walton, and D.J. Worsfold: Synthesis and electrochemical characterization of poly(TEMPO-Acrylate). Tetrahedron Lett. 31, 771 (1990).
Y. Yonekuta, K. Susuki, K. Oyaizu, K. Honda, and H. Nishide: Battery-inspired, nonvolatile, and rewritable memory architecture: a radical polymer-based organic device. J. Am. Chem. Soc. 129, 14128 (2007).
T. Sukegawa, H. Omata, I. Masuko, K. Oyaizu, and H. Nishide: Anionic polymerization of 4-methacryloyloxy-TEMPO using an MMA- capped initiator. ACS Macro Lett. 3, 240 (2014).
K. Oyaizu, Y. Ando, H. Konishi, and H. Nishide: Nernstian adsorbate-like bulk layer of organic radical polymers for high-density charge storage purposes. J. Am. Chem. Soc. 130, 14459 (2008).
T. Suga, M. Sakata, K. Aoki, and H. Nishide: Synthesis of pendant radical- and ion-containing block copolymers via ring-opening metathesis polymerization for organic resistive memory. ACS Macro Lett. 3, 703 (2014).
L. Rostro, A.G. Baradwaj, and B.W. Boudouris: Controlled radical polymerization and quantification of solid state electrical conductivities of macromolecules bearing pendant stable radical groups. ACS Appl. Mater. Interfaces 5, 9896 (2013).
X. Zhuang, C. Xiao, K. Oyaizu, N. Chikushi, X. Chen, and H. Nishide: Synthesis of amphiphilic block copolymers bearing stable nitroxyl radicals. J. Polym. Sci. Part A Polym. Chem. 48, 5404 (2010).
T. Janoschka, A. Teichler, A. Krieg, M.D. Hager, and U.S. Schubert: Polymerization of free secondary amine bearing monomers by RAFT polymerization and other controlled radical techniques. J. Polym. Sci. Part A Polym. Chem. 50, 1394 (2012).
T. Uemukai, T. Hioki, and M. Ishifune: Thermoresponsive and redox behaviors of poly(N-isopropylacrylamide)-based block copolymers having TEMPO groups as their side chains. Int. J. Polym. Sci. 2013, 196145 (2013).
K. Saito, K. Hirose, T. Okayasu, H. Nishide, T. Milton, and W. Hearn: TEMPO radical polymer grafted silicas as solid state catalysts for the oxidation of alcohols. RSC Adv. 3, 9752 (2013).
Y. Wang, M. Hung, and C. Lin: Patterned nitroxide polymer brushes for thin-film cathodes in organic radical batteries. Chem. Commun. 47, 1249 (2011).
H. Lin, C. Li, and J. Lee: Nitroxide polymer brushes grafted onto silica nanoparticles as cathodes for organic radical batteries. J. Power Sources 196, 8098 (2011).
C-H. Lin, W-J. Chou, and J-T. Lee: Three-dimensionally ordered macroporous nitroxide polymer brush electrodes prepared by surface-initiated atom transfer polymerization for organic radical batteries. Macromol. Rapid Commun. 33, 107 (2012).
C. Lin, C. Chau, and J. Lee: Polymer Chemistry Synthesis and characterization of polythiophene grafted with a nitroxide radical polymer via atom transfer radical polymerization. Polym. Chem. 1467 (2012).
M-K. Hung, Y-H. Wang, C-H. Lin, H-C. Lin, and J-T. Lee: Synthesis and electrochemical behaviour of nitroxide polymer brush thin-film electrodes for organic radical batteries. J. Mater. Chem. 22, 1570 (2012).
G. Hauffman, J. Rolland, J. Bourgeois, and A. Vlad: Synthesis of nitroxide-containing block copolymers for the formation of organic cathodes. J. Polym. Sci. Part A Polym. Chem. 51, 101 (2013).
F. Behrends, H. Wagner, A. Studer, O. Niehaus, R. Pöttgen, and H. Eckert: Polynitroxides from Alkoxyamine Monomers: Structural and Kinetic Investigations by Solid State NMR. Macromolecules 46, 2553 (2013).
M.W. Matsen, and F.S. Bates: Unifying weak- and strong-segregation block copolymer theories. Macromolecules 29, 1091 (1996).
A. Nunns, J. Gwyther, and I. Manners: Inorganic block copolymer lithography. Polymer (Guildf). 54, 1269 (2013).
M.A. Hillmyer, and T.P. Lodge: Synthesis and self-assembly of fluorinated block copolymers. J. Polym. Sci. Part A Polym. Chem. 40, 1 (2002).
H. Nishide, S. Iwasa, Y-J. Pu, T. Suga, K. Nakahara, and M. Satoh: Organic radical battery: nitroxide polymers as a cathode-active material. Electrochim. Acta 50, 827 (2004).
Acknowledgments
C. L. acknowledges financial support by the Deutsche Forschungsgemeinschaft (German Research Foundation, Forschungsstipendium Li 2526/2-1). A. M. acknowledges support by a seed grant from the Cornell Center for Materials Research, an NSF MRSEC program (DMR-1120296). AFM measurements were performed at the Cornell NanoScale Facility, a member of the National Nanotechnology Infrastructure Network, which is supported by the National Science Foundation (grant ECCS-0335765). NMR spectroscopy was measured at the Cornell University NMR Facility. EPR spectroscopy was performed at ACERT, which is supported by the National Institute of General Medical Sciences of the National Institutes of Health (Award Number P41GM103521). We composed coin cells in the laboratories of Lynden A. Archer and thank Sampson Lau for assistance and help with the measurements of the charge-discharge behavior. We thank Boris Dzikovski for help with EPR measurements.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Supplementary materials
Supplementary materials
For supplementary material for this article, please visit http://dx.doi.org/10.1557/mrc.2015.50
Rights and permissions
About this article
Cite this article
Liedel, C., Moehle, A., Fuchs, G.D. et al. Block copolymers with stable radical and fluorinated groups by ATRP. MRS Communications 5, 441–446 (2015). https://doi.org/10.1557/mrc.2015.50
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/mrc.2015.50