Abstract
Background
Minimally invasive esophagectomy (MIE) has been increasingly performed for locally advanced esophageal cancer in place of open transthoracic esophagectomy (OE). This study explored the significance of MIE for esophageal squamous cell carcinoma (ESCC), focusing mainly on the depth of primary esophageal tumors.
Methods
This study retrospectively assessed short- and long-term outcomes of patients who underwent esophagectomy for ESCC from 2005 through 2021. The inverse probability of the treatment-weighting (IPTW) method was used to compare the outcomes between OE and MIE. The outcomes also were evaluated in the subgroups stratified by cT category.
Results
Among 1117 patients, 447 (40%) underwent OE and 670 (60%) underwent MIE. After IPTW adjustment, the incidence of any postoperative complications was significantly higher in the OE group than in the MIE group (60.8% vs 53.7%; p = 0.032), whereas the R0 resection rate was significantly higher in the MIE group (98.6% vs 92.7%; p < 0.001). The MIE group showed better 3 year overall and cancer-specific survival than the OE group (p < 0.001). The incidence of locoregional recurrence within the surgical field was significantly more frequent in the OE group (p < 0.001). In the subgroup analysis stratified by cT category, the R0 resection rate was significantly higher and the incidence of locoregional recurrence was lower in the MIE group among the patients with cT3–4 tumors. In the patients with cT1–2 tumors, MIE showed no significant benefit over OE.
Conclusions
For the patients with cT3–4 tumors, MIE showed fewer postoperative complications, better locoregional control, and better prognosis than OE. Compared with OE, MIE is beneficial, especially for locally advanced ESCC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Esophageal cancer is the sixth leading cause of cancer-related mortality worldwide.1 Esophageal squamous cell carcinoma (ESCC), which accounts for more than 80% of esophageal cancer in Asia, typically presents at an advanced stage, necessitating aggressive therapeutic interventions. Surgery remains the mainstay of definitive treatment despite the advances in multimodal treatment strategy.2,3
Open esophagectomy (OE), a long-standing standard for esophagectomy, is associated with high rates of postoperative morbidity and a prolonged recovery period.4 The recent advent of minimally invasive esophagectomy (MIE) techniques has changed the treatment landscape of esophageal cancer. Several studies have demonstrated the advantages of MIE over OE, including reduced blood loss, shorter hospital stays, and fewer respiratory complications.5,6 These benefits are likely attributable to the reduced surgical trauma and better visualization of the surgical field offered by minimally invasive techniques.7,8,9
However, OE has been the preferred choice for locally advanced ESCC, with a penetration depth of cT3 or more to achieve complete resection and avoid injury to the neighboring organs, such as the trachea, bronchus, and aorta. To date, favorable results of MIE have been reported mainly in early-stage cancer, but not so much in locally advanced ESCC.10,11 The adoption of MIE to treat locally advanced ESCC was cautious, with concerns of technical difficulty and adequate oncologic outcomes. However, based on accumulated experiences, MIE has been increasingly implemented even for locally advanced cancers. This study aimed to elucidate the significance of MIE for ESCC, especially in cases with locally advanced ESCC.
Patients and Methods
Data Collection
We retrospectively reviewed patients who underwent radical esophagectomy for ESCC at the Cancer Institute Hospital of the Japanese Foundation for Cancer Research (JFCR) between January 2005 and December 2021. The study excluded patients with synchronous cancer in the other organs and those who underwent palliative resection or total pharyngolaryngectomy.
In this study, OE was defined as the combination of the open right-side transthoracic and open abdominal approaches, whereas MIE was defined as the combination of the right thoracoscopic and laparoscopic approaches. The study also excluded patients who underwent hybrid MIE, including thoracoscopy plus laparotomy and thoracotomy plus laparoscopy.
The tumor stage was clinically evaluated based on endoscopy and computed tomography (CT) images before the initiation of treatment and classified using the tumor-node-metastasis (TNM) staging system of the Union for International Cancer Control-American Joint Committee on Cancer (8th edition).12 Postoperative complications within the first 30 days after surgery were evaluated according to the Clavien-Dindo (CD) classification.13 Overall survival (OS) was calculated from the day of surgery to the day of death or last follow-up visit. Cancer-specific survival (CSS) was calculated from the day of surgery to the day of cancer-related death or last follow-up visit.
This study was approved by the Institutional Review Board of the JFCR (2023-GB-065). All procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or an equivalent was obtained from all the patients.
Treatment for Esophageal Cancer, Esophagectomy, and Postoperative Follow-up Evaluation
The treatment strategy for each patient was decided by a multidisciplinary tumor board based on the Japanese Guidelines for the Treatment of Esophageal Carcinoma14 and included surgery alone for cStage I cancers, neoadjuvant chemotherapy (NAC) followed by surgery for cStage II and III cancers, and definitive chemoradiotherapy (CRT) for T4 cancers or in case of patient refusal for surgery. Salvage surgery was considered for persistent or relapsed cancers after definitive CRT. For borderline resectable T3 cancers with suspected tumor invasion to the adjacent organs but indefinite T4 disease, induction CRT or chemotherapy was administered, and surgery was performed only when a curative resection was considered possible.15
Regarding the types of esophagectomies, we performed both the McKeown and the Ivor-Lewis esophagectomies. In our institutions, the McKeown esophagectomy was the standard, whereas the Ivor-Lewis esophagectomy was occasionally selected considering the tumor location, histologic type, and comorbidities. We could perform both types of esophagectomies in MIE or OE.
Patients were followed up every 3 or 4 months for at least 1 year, then every 6 months thereafter. Follow-up evaluation included physical examination, blood tests, and CT imaging.
Statistical Analysis
Continuous variables are presented as median (range), and between-group differences were assessed for statistical significance using the Mann–Whitney U test. Categorical variables are presented as frequency (percentage) and were analyzed using Fisher’s exact test.
An inverse probability of treatment-weighting (IPTW) based on a propensity score (PS) was performed to balance the OE and MIE groups with respect to patient characteristics.16 The PS was estimated using logistic regression analysis based on the following variables: age, sex, body mass index (BMI), American Society of Anesthesiologists Physical Status (ASA-PS), type of preoperative therapy, location of the primary tumor, and clinical T and N categories. Subgroup analysis was performed by stratifying patients according to the cT category (cT1–T2 and cT3–T4), and IPTW adjustments were performed. Notably, the PS was recalculated in the subgroup analysis.
The Kaplan–Meier method was used to generate survival curves. The Cox proportional hazards model was used to estimate the hazard ratios (HRs) with 95% confidence intervals (CIs). All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient Characteristics
The characteristics of the study population are summarized in Table 1. Among the 1117 eligible patients, 447 (40%) underwent OE and 670 (60%) underwent MIE. Before IPTW adjustment, the MIE group had significantly higher proportions of males (p = 0.006) and patients who received chemotherapy (p = 0.04), whereas the OE group had a significantly greater proportion of patients with nodal involvement (p < 0.001). No significant between-group differences were observed with respect to age, BMI, ASA-PS, cT category (cT1–T2 vs cT3–T4), or primary tumor location. After IPTW adjustment, the study included 382 patients in the OE group and 651 patients in the MIE group, and the patient characteristics were well-balanced between the two groups.
Surgical Outcomes and Survival Analysis in the Inverse Probability of Treatment Weighting‑Adjusted Cohort
The surgical outcomes are presented in Table 2. The operation time in the OE group was significantly shorter than in the MIE group (p < 0.001), whereas the intraoperative blood loss was significantly less in the MIE group than in the OE group (p < 0.001). The incidence of any postoperative complications CD grade ≥2 was significantly higher in the OE group than in the MIE group (60.8% vs 53.7%; p = 0.032). The incidence of recurrent laryngeal nerve (RLN) palsy, anastomotic leakage, and postoperative pneumonia CD grade ≥2 was comparable in the two groups. Surgical-site infection CD grade ≥2 occurred more frequently in the OE group than in the MIE group (11.6% vs 6.6%; p = 0.007). Also, the incidences of chylothorax and pleural effusion tended to be lower in the MIE group than in the OE group. The incidence of severe complications (CD grade ≥3) and the mortality within 30 days postoperatively also was comparable in the two groups. The of hospital stay after MIE was significantly shorter than after OE (p < 0.001). The R0 resection rate was significantly higher in the MIE group (98.6% vs 92.7%; p < 0.001).
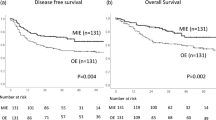
The survival curves are shown in Fig. 1. The MIE group had better 3 year OS (HR of MIE vs OE, 0.54; 95% CI, 0.43–0.68; p < 0.001) and 3 year CSS (HR of MIE vs OE: 0.51; 95% CI, 0.39–0.67; p < 0.001) than the OE group.
Regarding the first site of recurrence, locoregional recurrence, including local and lymph node recurrence within surgical fields, occurred significantly less frequently in the MIE group (HR, 0.48; 95% CI, 0.35–0.67; p < 0.001). In contrast, there was no significant between-group difference regarding the incidence of distant metastasis (p = 0.591).
Subgroup Analysis Stratified by CT Category
Additionally, we created the IPTW-adjusted cT1–T2 cohort (n = 654) and cT3–T4 cohort (n = 382). The patient characteristics were well-balanced between the two groups (Tables S1 and S2).
Surgical outcomes are described in Table 3. In both subgroups, the operation time was significantly shorter in the OE group, whereas intraoperative blood loss was significantly less in the MIE group. The incidence of any postoperative complications was comparable between the groups in the IPTW-adjusted cT1–T2 cohort (p = 0.554). However, in the IPTW-adjusted cT3–T4 cohort, the incidence of any postoperative complications CD grade ≥2 was higher in the OE group than in the MIE group, although the difference was not statistically significant (p = 0.063). Although the R0 resection rate did not differ significantly in the IPTW-adjusted cT1–T2 cohort (99.8% vs 98.2%; p = 0.117), the R0 resection rate in the IPTW-adjusted cT3–T4 cohort was significantly higher in the MIE group than in the OE group (96.5% vs 84.6%; p<0.001).
Survival analyses of the subgroups are shown in Figs. 2 and 3. In both subgroups, MIE provided a longer 3 year OS than OE. The 3 year CSS also was significantly longer in the MIE group than in the OE in the IPTW-adjusted cT3–T4 cohort (HR of MIE vs OE, 0.65; 95% CI, 0.47–0.92; p = 0.014). Meanwhile, no significant differences in the 3 year CSS were observed in the IPTW-adjusted cT1–T2 cohort (HR of MIE vs OE, 0.60; 95% CI, 0.35–1.04; p = 0.085). In addition, locoregional recurrence occurred more frequently in the OE cohort than in the MIE patients of the IPTW-adjusted cT3–T4 cohort (HR of MIE vs OE, 0.44; 95% CI, 0.26–0.74; p < 0.001). In contrast, the IPTW-adjusted cT1–T2 cohort showed no significant between-group difference regarding the incidence of locoregional recurrence (p = 0.317).
Comparison of survival curves and cumulative recurrence rates between OE and MIE subgroups with cT1–T2 ESCC in the IPTW-adjusted cohort. OE, open esophagectomy; MIE, minimally invasive esophagectomy; ESCC, esophageal squamous cell carcinoma; IPTW, inverse probability of weighting treatment; HR, hazard ratio; CI, confidence interval
Comparison of survival curves and cumulative recurrence rates between OE and MIE subgroups with cT3–T4 ESCC in the IPTW-adjusted cohort. OE, open esophagectomy; MIE, minimally invasive esophagectomy; ESCC, esophageal squamous cell carcinoma; IPTW, inverse probability of weighting treatment; HR, hazard ratio; CI, confidence interval
Discussion
This study examined the safety and oncologic advantages of MIE over OE for ESCC using real-world data from a single high-volume center. The results demonstrated significantly fewer postoperative complications after MIE than after OE. Moreover, MIE achieved better locoregional control and resulted in a better prognosis than OE. Finally, the benefit differed by cT category and was more pronounced in more advanced cancers. Our results suggest that advanced cases with cT3–T4 tumors, including borderline resectable cancers, may benefit from MIE rather than OE.
Although some studies have yielded inconclusive results,17,18,19 several studies have reported the short-term benefits of MIE over OE, especially with respect to reduced postoperative complications.5,6,20,21 Some studies have shown that MIE is associated with a significantly lower incidence of pulmonary complications than OE.6,17 In contrast, nationwide real-world data analyses in Japan found a significantly higher incidence of RLN palsy after MIE than after OE, suggesting that mediastinal lymph node dissection in MIE might be technically more challenging than in OE.17,20,21,22
In the current study, we showed comparable results regardomg the incidence of pneumonia and RLN palsy between the two groups, although the incidence of overall postoperative complications and surgical-site infection occurred less frequently in the MIE group than in the OE group. In modern esophagectomy, multidisciplinary perioperative care is mandatory,23 which might minimize the difference in pneumonia incidence between the groups. A recent randomized controlled trial conducted by the Japan Clinical Oncology Group Study (JCOG 1409 trial) comparing OE and MIE for locally advanced clinical stages I to III esophageal cancer could not demonstrate a significant difference in pneumonia incidence between the groups, although MIE could maintain patient respiratory function.24
Regarding long-term outcomes, in the current study, the OS and CSS in the MIE group were better than in the OE group. However, previous studies have not yielded conclusive results in this regard.20,25,26,27,28 The inconsistent results in terms of survival outcomes may be attributable to several factors such as differences in sample size, perioperative management, method of neoadjuvant therapy, tumor location, and tumor histology. The aforementioned JCOG 1409 trial demonstrated non-inferiority of MIE compared with OE in terms of 3 year OS (72.9% vs 61.9%) with an HR of 0.68.24 In that trial, the R0 resection rate was 95.3% in the MIE group and 90.0% in the OE group, similar to the rates in the current study (98.6% vs 92.7%). An improved R0 resection rate is known to improve the prognosis of patients who have undergone esophagectomy.29,30
The current study suggests that MIE may achieve better locoregional control than OE.31,32 High-resolution imaging and a magnified view are considered beneficial in dissection of the appropriate surgical layer, visualization of the lymphatic chain, and avoidance of lymph node remnants in the surgical field.33 These advantages of MIE can facilitate precise lymph node dissection in the narrow cervicothoracic border region, which is a frequent site of lymph node metastasis from thoracic ESCC.34 Meanwhile, the systemic spread of tumors might be more influenced by the biologic behavior of the tumor itself than by the surgical approach, which could be the reason why there was no significant difference in the incidence of distant metastasis.
Moreover, in the current study, the oncologic benefits of MIE were more pronounced in advanced cases with cT3–T4 tumors than in those with cT1–T2 tumors. The R0 resection rate was significantly higher in the MIE group, whereas the incidence of locoregional recurrence was more frequent in the OE group. However, for the patients with cT1–T2 tumors, MIE showed no significant benefit over OE regarding the R0 resection rate or locoregional control. The advantages of MIE mentioned earlier could contribute to local control in patients with T3–4 tumors, and therefore, MIE can be recommended even for cases with locally advanced ESCC.
Some limitations of this study should be considered when the results are interpreted. First, this was a single-center, retrospective study with a relatively long study period.
Second, OE was the standard in our hospital before 2013, and MIE has been introduced since 2013. Moreover, during the induction phase, MIE was more likely to be performed for patients with early-stage cancer. In addition, the management of patients tends to improve with each passing year. Although we adjusted for various variables, including preoperative therapy, these historical changes are likely to have influenced the results. In fact, the rates for preoperative CRT and more advanced tumors were higher in the OE group even after IPTW adjustment. In addition, for these patients, induction CRT was mainly administered. Therefore, we could not completely eliminate the selection bias. However, the exclusion of patients with preoperative CRT did not lead to major changes in the values of the HRs or the main conclusions of this study (Fig. S1). Notably, the current study included only the patients before the standard strategy change based on the JCOG 1109 trial results, which demonstrated the superiority of the triplet chemotherapy regimen.35
Third, we did not assess surgical quality. However, all operations were performed by board-certified esophageal surgeons, ensuring a certain level of surgical skill. Despite the aforementioned limitations, IPTW adjustment was performed to improve the comparability between the OE and MIE groups.
In conclusion, MIE was associated with fewer postoperative complications, better locoregional control, and a better prognosis than OE. In addition, the oncologic benefit of MIE was more pronounced in the patients with locally advanced tumors. Therefore, MIE may be recommended for patients with locally advanced ESCC at institutions with sufficient surgical proficiency.
References
Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–84.
Shapiro J, van Lanschot JJB, Hulshof MCCM, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–8.
Burdall OC, Boddy AP, Fullick J, et al. A comparative study of survival after minimally invasive and open oesophagectomy. Surg Endosc. 2015;29:431–7.
Yoshida N, Yamamoto H, Baba H, et al. Can minimally invasive esophagectomy replace open esophagectomy for esophageal cancer? Latest analysis of 24,233 esophagectomies from the Japanese national clinical database. Ann Surg. 2020;272:118–24.
Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet. 2012;379:1887–92.
Kikuchi H, Takeuchi H. Future perspectives of surgery for esophageal cancer. Ann Thorac Cardiovasc Surg. 2018;24:219–22.
Okamura A, Takeuchi H, Matsuda S, et al. Factors affecting cytokine change after esophagectomy for esophageal cancer. Ann Surg Oncol. 2015;22:3130–5.
Yamashita K, Watanabe M, Mine S, et al. Minimally invasive esophagectomy attenuates the postoperative inflammatory response and improves survival compared with open esophagectomy in patients with esophageal cancer: a propensity score-matched analysis. Surg Endosc. 2018;32:4443–50.
Kubo K, Kanematsu K, Kurita D, et al. Feasibility of conversion thoracoscopic esophagectomy after induction therapy for locally advanced unresectable esophageal squamous cell carcinoma. Jpn J Clin Oncol. 2021;51:1225–31.
Zhang X, Yang Y, Ye B, et al. Minimally invasive esophagectomy is a safe surgical treatment for locally advanced pathologic T3 esophageal squamous cell carcinoma. J Thorac Dis. 2017;9:2982–91.
Bertero L, Massa F, Metovic J, et al. Eighth edition of the UICC classification of malignant tumours: an overview of the changes in the pathological TNM classification criteria-what has changed and why? Virchows Arch. 2018;472:519–31.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Kitagawa Y, Ishihara R, Ishikawa H, et al. Esophageal cancer practice guidelines 2022 edited by the Japan esophageal society: part 1. Esophagus. 2023;20:343–72.
Suzuki T, Okamura A, Watanabe M, et al. Neoadjuvant chemoradiotherapy with cisplatin plus fluorouracil for borderline resectable esophageal squamous cell carcinoma. Ann Surg Oncol. 2020;27:1510–7.
Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661–79.
Takeuchi H, Miyata H, Ozawa S, et al. Comparison of short-term outcomes between open and minimally invasive esophagectomy for esophageal cancer using a nationwide database in Japan. Ann Surg Oncol. 2017;24:1821–7.
Sakamoto T, Fujiogi M, Matsui H, Fushimi K, Yasunaga H. Comparing perioperative mortality and morbidity of minimally invasive esophagectomy versus open esophagectomy for esophageal cancer: a nationwide retrospective analysis. Ann Surg. 2021;274:324–30.
Seesing MFJ, Gisbertz SS, Goense L, et al. A propensity score-matched analysis of open versus minimally invasive transthoracic esophagectomy in the Netherlands. Ann Surg. 2017;266:839–46.
Wang H, Shen Y, Feng M, et al. Outcomes, quality of life, and survival after esophagectomy for squamous cell carcinoma: a propensity score-matched comparison of operative approaches. J Thorac Cardiovasc Surg. 2015;149:1006–14 (discussion 14–5).
Dyas AR, Stuart CM, Bronsert MR, et al. Minimally invasive surgery is associated with decreased postoperative complications after esophagectomy. J Thorac Cardiovasc Surg. 2023;166:268–78.
Takeuchi H, Kawakubo H, Kitagawa Y. Current status of minimally invasive esophagectomy for patients with esophageal cancer. Gen Thorac Cardiovasc Surg. 2013;61:513–21.
Low DE, Allum W, De Manzoni G, Ferri L, Immanuel A, Kuppusamy M, et al. Guidelines for perioperative care in esophagectomy: enhanced recovery after surgery (ERAS®) society recommendations. World J Surg. 2019;43:299–330.
Takeuchi H, Ando M, Tsubosa Y, et al. A randomized controlled phase III trial comparing thoracoscopic esophagectomy and open esophagectomy for thoracic esophageal cancer: JCOG1409 (MONET trial). J Clin Oncol. 2024;42:249.
Gottlieb-Vedi E, Kauppila JH, Mattsson F, et al. Long-term survival in esophageal cancer after minimally invasive esophagectomy compared to open esophagectomy. Ann Surg. 2022;276:e744–8.
Sihvo E, Helminen O, Gunn J, et al. Long-term outcomes following minimally invasive and open esophagectomy in Finland: a population-based study. Eur J Surg Oncol. 2019;45:1099–104.
Straatman J, van der Wielen N, Cuesta MA, et al. Minimally invasive versus open esophageal resection: three-year follow-up of the previously reported randomized controlled trial: the TIME trial. Ann Surg. 2017;266:232–6.
Weksler B, Sullivan JL. Survival after esophagectomy: a propensity-matched study of different surgical approaches. Ann Thorac Surg. 2017;104:1138–46.
Ohkura Y, Ueno M, Iizuka T, Udagawa H. Prognostic factors and appropriate lymph node dissection in salvage esophagectomy for locally advanced T4 esophageal cancer. Ann Surg Oncol. 2019;26:209–16.
Markar SR, Gronnier C, Duhamel A, et al. Significance of microscopically incomplete resection margin after esophagectomy for esophageal cancer. Ann Surg. 2016;263:712–8.
Thomson IG, Smithers BM, Gotley DC, et al. Thoracoscopic-assisted esophagectomy for esophageal cancer: analysis of patterns and prognostic factors for recurrence. Ann Surg. 2010;252:281–91.
Hsu PK, Huang CS, Wu YC, Chou TY, Hsu WH. Open versus thoracoscopic esophagectomy in patients with esophageal squamous cell carcinoma. World J Surg. 2014;38:402–9.
Takeno S, Takahashi Y, Moroga T, et al. Retrospective study using the propensity score to clarify the oncologic feasibility of thoracoscopic esophagectomy in patients with esophageal cancer. World J Surg. 2013;37:1673–80.
Kanemura T, Makino T, Miyazaki Y, et al. Distribution patterns of metastases in recurrent laryngeal nerve lymph nodes in patients with squamous cell esophageal cancer. Dis Esophagus. 2017;30:1–7.
Kato K, Ito Y, Daiko H, et al. A randomized controlled phase III trial comparing two chemotherapy regimen and chemoradiotherapy regimen as neoadjuvant treatment for locally advanced esophageal cancer, JCOG1109 NExT study. J Clin Oncol. 2022;40:238.
Acknowledgments
The authors sincerely appreciate Jun Okui, MD, PhD, MPH (Department of Preventive Medicine and Public Health, School of Medicine, Keio University, Tokyo, Japan) for his valuable assistance with statistical interpretation in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
DISCLOSURE
There are no conflicts of interest.
ETHICAL APPROVAL
All procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or an equivalent was obtained from all patients
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10434_2024_15596_MOESM2_ESM.tif
Comparison of survival curves and cumulative recurrence rates of patients excluding those who recieved preoperative chemoradiotherapy in the IPTW-adjusted cohort between OE and MIE
Supplementary file2 (TIF 689 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Terayama, M., Okamura, A., Kuriyama, K. et al. Minimally Invasive Esophagectomy Provides Better Short- and Long-Term Outcomes Than Open Esophagectomy in Locally Advanced Esophageal Cancer. Ann Surg Oncol 31, 5748–5756 (2024). https://doi.org/10.1245/s10434-024-15596-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-024-15596-z