Abstract
Background
Locoregional recurrence (LR) in colon cancer is uncommon but often incurable, while the factors associated with it are unclear. The purpose of this study was to identify patterns and predictors of LR after curative resection for colon cancer.
Methods
All patients who underwent colon cancer resection with curative intent between 1994 and 2008 at a tertiary referral center were identified from a prospectively maintained institutional database. The association of LR with clinicopathologic and treatment characteristics was determined using univariable and multivariable analyses.
Results
A total of 1397 patients were included with a median follow-up of 7.8 years; 635 (45%) were female, and the median age was 69 years. LR was detected in 61 (4.4%) patients. Median time to LR was 21 months. On multivariable analysis, the independent predictors of LR were disease stage [hazard ratio (HR) for Stage II 4.6, 95% confidence interval (CI) 1.05–19.9, HR for Stage III 10.8, 95% CI 2.6–45.8], bowel obstruction (HR 3.8, 95% CI 1.9–7.4), margin involvement (HR 4.1, 95% CI 1.9–8.6), lymphovascular invasion (HR 1.9, 95% CI 1.06–3.5), and local tumor invasion (fixation to another structure, perforation, or presence of associated fistula, HR 2.2, 95% CI 1.1–4.5). Adjuvant chemotherapy was not associated with reduced LR in patients with either Stage II or Stage III tumors.
Conclusions
Adherence to oncologic surgical principles in colon cancer resection results in low rates of LR, which is associated with tumor-dependent factors. Recognition of these factors can help to determine appropriate postoperative surveillance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
An estimated 93,090 new cases of colon cancer were diagnosed in the United States in 2015.1 For those presenting with resectable disease, surgery offers the only chance for cure. Even though distant recurrence remains the most common cause of cancer-related mortality for these patients, locoregional recurrence (LR) is estimated to occur in 4–11.5% of cases, with severe impact on survival and morbidity.2–4 Over the past few decades, LR has been the focus of intensive research in rectal cancer, as differences in long-term survival between patients with colon and rectal primary tumors were traditionally attributed to dramatic differences in LR rates. Contrary to rectal cancer, only a few studies attempted to address LR of colon cancer, with no significant improvement in LR rates.2–7 In fact, with the widespread adoption of advances in the multidisciplinary treatment of rectal cancer, including the principles of total mesorectal excision (TME) and the administration of neoadjuvant chemoradiotherapy, there is now evidence suggesting that oncological outcomes in rectal cancer patients might be better than in patients with colon cancer.8–11
Colon cancer recurs locally because viable tumor cells remain in situ after the resection. This may be due to poor operative technique, where potentially removable cells are not removed or viable cells are implanted at the time of resection, or it may be due to aggressive biology, where viable cells have already escaped the limits of resectability. It is important to discover whether operative or tumor factors are primarily associated with LR to improve surgical techniques appropriately or treat and to survey tumors with aggressive biology.12 The purpose of our study was to measure the incidence of LR in a cohort of patients with colon cancer treated in a single academic referral center, identify the disease and treatment parameters associated with LR, and define high-risk groups that could benefit from more intensive adjuvant therapy and closer follow-up.
Methods
All patients who underwent curative surgical resection for Stage I-III adenocarcinoma of the colon between 1994 and 2008 were identified from a prospectively maintained institutional colorectal cancer database. Informed consent was obtained before enrolment into the database and a waiver of consent was received for database studies with anonymous data. The study was approved by the Cleveland Clinic Institutional Review Board.
Follow-up assessment included physical examination, serial evaluation of CEA levels, colonoscopy, and cross-sectional imaging, varying slightly among individual surgeons while remaining within accepted standards of practice.13 For patients whose postoperative cancer surveillance took place at outside institutions, follow-up assessment was by phone interviews while clinical documentation was obtained whenever possible. Any recurrence of disease (local or distant) or death was recorded.
Tumors included in the present study were primary adenocarcinomas of the large bowel that arose anywhere between the cecum and rectosigmoid junction, defined as 15 cm proximal to the anal verge. The sites of the tumors were categorized anatomically as cecum, ascending colon, hepatic flexure, transverse colon, splenic flexure, descending, and sigmoid colon. Those who underwent palliative surgery (R2 resection) were excluded. Data on patient demographics, year of surgery, individual surgeon, laparoscopic versus open approach, tumor location, histologic grade, presence of lymphovascular invasion (LVI), TNM stage according to the American Joint Committee on Cancer 7th edition manual, number of lymph nodes examined, local invasion of the tumor (defined as fixation to another structure, perforation, or presence of associated fistula), obstruction, and adjuvant chemotherapy administration were collected.
Operations were performed by surgeons trained in oncologically appropriate radical colectomy, including high vascular ligation, and en bloc resection of attached organs or the abdominal wall. Most surgeons were trained in the preliminary lymphovascular isolation technique described originally by Turnbull.14 LR was defined as any histological or clinical evidence of tumor regrowth near the primary site. The sites of LR were divided into four groups on the basis of established criteria: perianastomotic colon, mesenteric and paracolic lymph nodes, peritoneum (including serosal and omental implants), and retroperitoneum.15,16 Tumor recurrence at nonregional sites, such as liver or lung, was recorded as distant relapse. LR was recorded regardless of the presence of metastatic disease.
Statistical Analyses
Kaplan–Meier (KM) curves were used to demonstrate univariable associations between locoregional recurrence and categorical study variables. Continuous variables were dichotomized at their median values for the purpose of forming categories for the KM curves. Multivariable Cox Proportional Hazards models were generated with independent variables selected in a stepwise fashion based on Akaike’s information criterion (AIC). Statistical significance of results was defined as P < 0.05. Analyses were also carried out with subsets according to stage. R version 2.15.1 was used for the analyses (www.r-project.org).
Results
Patient and Treatment Characteristics
A total of 1,397 patients met the inclusion criteria, and their outcomes were further analyzed. The median age was 69 years, and 635 (45%) were female (Table 1). Median follow-up was 7.8 years [interquartile range (IQR) 45–135 months], and 1349 of 1397 (96.6%) patients were followed for a minimum of 5 years or until death. Resections were performed by 20 different colorectal surgeons, although 84% were performed by 8 surgeons. A laparoscopic approach was undertaken in 15% of the resections. Histology confirmed microscopically clear resection margins in 1344 (96%) patients . Among those resections with positive resection margin, 95% was radial margin involvement in T3/4 tumors. The median number of lymph nodes per patient was 24, and 87% of resections had at least 12 lymph nodes evaluated. Data regarding adjuvant chemotherapy were available for 1194 patients, of whom 388 (32.5%) received adjuvant chemotherapy following surgery. Among patients with Stage III disease, 73.8% were treated with adjuvant chemotherapy (Table 2).
Patterns of Locoregional Recurrence
LR was detected in 61 (4.4%) patients. The median time to LR was 21 (IQR 12–36) months. Isolated LR occurred in 36 (2.6% of all patients, 59% of LR) patients, whereas LR occurred synchronously with distant metastases in 25 (41%). The patterns of local recurrence are described in Table 3; the majority presented as locoregional peritoneal implants (46%). The median survival after the index operation was 2.9 years for patients with LR, whereas for those who did not develop LR it was 11.1 years. There was no statistically significant difference in survival between patients who developed isolated LR compared with those who developed LR synchronously with distant metastases (P = 0.54) or between the specific types of LR, although the low incidence of LR does not allow definitive conclusions. The median survival after diagnosis of LR was only 9 (IQR 4–27) months. Ultimately, 29 (48%) patients with LR underwent surgery for their recurrent disease, which was palliative in 17 cases.
Predictors of Locoregional Recurrence
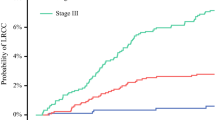
Factors associated with a statistically significant increase in LR rate on univariable analysis included tumor size, pathological stage, T and N classification, moderate or poor differentiation, LVI, positive resectional margin, lymph node harvest of less than 24 nodes, local tumor invasion (defined as fixation to another structure, perforation, or presence of associated fistula), and associated bowel obstruction (Table 1). The impact of stage on cumulative LR rates is presented in Fig. 1. There was no significant association between the risk of LR and the operating surgeon (P = 0.1), year of operation (before or after 2001, P = 0.49), or the use of a laparoscopic approach (P = 0.72).
On multivariable analysis, independent factors significantly associated with an increased LR rate were pathological stage, local tumor invasion, associated bowel obstruction, lymphovascular invasion, and positive margin (Table 4). Local recurrence developed in 40 (12.7%) of the 316 patients who had at least one of the following: N2 disease, local tumor invasion, associated bowel obstruction, or positive margin . By contrast, local recurrence developed in 21 (1.9%) of the remaining 1,081 patients who had none of these factors.
Adjuvant chemotherapy did not correlate with LR. Among patients with both Stage II and Stage III primary tumors, the administration of adjuvant chemotherapy was not associated with any statistically significant difference in LR (Table 2).
Discussion
Our findings demonstrated that LR in colon cancer treated at a specialized center is relatively uncommon, affecting approximately 4.4% of patients. However, its impact on survival is profound, as half of these patients survived less than 9 months following the diagnosis of LR. Surgery-related factors, including the use of minimally invasive approaches, whether the surgery was performed during the second half of the study period (after 2001), the number of harvested lymph nodes, and the individual surgeon, did not independently affect LR. Whereas adjuvant chemotherapy has been shown to improve overall survival in stage III colon cancer, our study did not demonstrate any advantageous effects of chemotherapy on LR specifically, regardless of the index cancer stage. Rather, tumor-related factors seemed to dictate LR rates. As such, pathological stage was an independent predictor of locoregional recurrence [hazard ratio (HR): 4.6 for Stage II; 10.8 for Stage III]. In addition, patients with local tumor invasion (fixation to another structure, perforation, or associated fistula) or with associated obstruction were found to be independently associated with LR (HR of 2.2 and 4.6 respectively). Finally, resections with microscopically positive margins also exhibited a greater rate of LR independent of other parameters. In total, two-thirds of LR occurred in patients with either N2 disease, local tumor invasion, associated bowel obstruction or positive margin, a group comprising only 23% (315/1397) of the entire cohort.
Our findings on the association between tumor characteristics and LR highlight the importance of the underlying disease biology and are in agreement with recent reports from other institutions.2–4,6 Disease stage has been consistently confirmed as a predictor of LR; advanced tumors have a higher likelihood of harboring locoregional micrometastases that may remain after radical surgery and thus result in LR. Even though not as clearly linked in the literature, the fact that locally invasive and or obstructed tumors are associated with greater LR rates is not surprising. Tumors that are locally invasive or that progress to the point of obstruction likely represent biologically more aggressive tumors. Additionally, patients presenting with obstructing tumors are more likely to undergo urgent or emergent operations under suboptimal conditions. These findings are also consistent with current NCCN guidelines, which identify colon adenocarcinomas presenting with obstruction as high-risk and recommend adjuvant chemotherapy administration even in the absence of nodal involvement.17 It should be noted, however, that in our cohort chemotherapy administration did not result in any reduction of LR rates.
Recently, the surgical treatment of colon cancer has received renewed attention. Some centers have suggested that a more radical and anatomical type of resection referred to as complete mesocolic excision (CME) can lead to improved outcomes.18–20 These techniques are in some aspects similar to the Turnbull’s “no touch isolation” technique.14 Studies have shown that these more extensive resections result in superior pathologic specimens with longer vascular pedicles, larger mesenteric surface areas, and higher lymph node yields.20,21 However, it is still controversial whether improvements in such surrogate endpoints evinced from specimen examinations are consistently associated with improved long-term oncologic outcomes in the same way that the adoption of TME is associated with reduced locoregional relapse in rectal cancer. It also is notable that a reduced incidence of LR has historically been the tangible endpoint assessing optimization of both surgical and (neo)adjuvant treatments in rectal cancer, while the same pattern is not detectable in colon cancer, where LR remains an uncommon occurrence as the present study indicates. Further highlighting the limitations of extrapolating rectal cancer principles to colon cancer, surgery for rectal cancer has been associated with considerable variability among individual surgeons and centers, which was not the case in our cohort.22–25 If LR rates in colon cancer are to be primarily attributed to surgical technique, future studies will need to demonstrate that there is considerable surgeon variability with regards to LR after colon cancer resection, similar to rectal cancer.5,26,27
Our study has certain limitations. Some patients developed LR during postoperative surveillance at an outside institution. Despite attempts at follow-up telephone interviews for all patients, it is possible that some LR occurrences may have been missed. However, with median follow-up greater than 7 years, and almost 97% of patients followed for a minimum of 5 years or until death, it is unlikely that many LR cases were not captured. In addition, not all recurrences were histologically confirmed, reflecting the current patient care paradigm, diagnosis was sometimes clinical and relied on a combination of physical exam, CEA levels, and appearance on serial CT or PET scans. In an effort to include all locoregional recurrences in our study, we used a liberal definition of LR to include essentially all intra-abdominal recurrences in nonparenchymal organs. This definition may lead to an overestimation of the true incidence of LR, possibly including distant peritoneal or omental metastatic implants. Nevertheless, we decided that an inclusive rather than selective definition better serves the purpose of this study and facilitates appropriate classification of recurrences. Furthermore, even though we made an effort to study as many potential predictors of LR as possible, our analyses were limited by what was routinely reported by our GI pathologists for the majority of the study period. Therefore, the evaluation of certain pathologic factors (including perineural invasion, tumor deposits, etc.) and genetic factors (including microsatellite status, RAS and RAF mutations, etc.) was not technically possible. Finally, our study did not evaluate the completeness of mesocolic resection as a predictor of LR. Unlike rectal cancer specimens, it is currently not our pathologists’ standard practice to record the quality of colonic specimens by measuring the length of the vascular pedicle and examining the mesocolon for intactness as described by West.21 A large, prospective study with detailed analysis of the oncologic quality of colonic specimens and long-term outcomes is needed to examine whether pathologic specimen quality is a predictor for LR and survival.28
Based on our results, the majority of LR occurred in patients with locally aggressive disease, obstruction or multiple positive lymph nodes. This population is therefore relatively easy to identify and could receive closer surveillance to potentially detect LR at an earlier time when salvage surgery remains feasible. In addition, understanding the patterns and timing of recurrences is important for determining appropriate surveillance schedules. The median time between surgery and detection of LR in this study was 21 months, emphasizing the importance of close surveillance during the first 3 postoperative years. While more intense follow-up has been found to be associated with improved outcomes and an increased rate of surgical treatment of recurrence, the optimal timing and choice of diagnostic tests has yet to be determined.12,29
Conclusions
Our data demonstrated that in patients with colon cancer treated at a specialized center with adherence to oncologic principles, the incidence of locoregional recurrence is relatively low. However, due to the dismal outcomes of patients who develop LR, this remains a significant problem. While treatment factors appear to have limited impact on LR, characteristics of the underlying disease allow appropriate risk-stratification of colon cancer patients, identifying those that may benefit from close surveillance.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29.
Read TE, Mutch MG, Chang BW, et al. Locoregional recurrence and survival after curative resection of adenocarcinoma of the colon. J Am Coll Surg. 2002;195(1):33–40.
Sjovall A, Granath F, Cedermark B, Glimelius B, Holm T. Loco-regional recurrence from colon cancer: a population-based study. Ann Surg Oncol. 2007;14(2):432–40.
Elferink MA, Visser O, Wiggers T, et al. Prognostic factors for locoregional recurrences in colon cancer. Ann Surg Oncol. 2012;19(7):2203–11.
Dorrance HR, Docherty GM, O’Dwyer PJ. Effect of surgeon specialty interest on patient outcome after potentially curative colorectal cancer surgery. Dis Colon Rectum. 2000;43(4):492–8.
Harris GJ, Church JM, Senagore AJ, et al. Factors affecting local recurrence of colonic adenocarcinoma. Dis Colon Rectum. 2002;45(8):1029–34.
Manfredi S, Bouvier AM, Lepage C, Hatem C, Dancourt V, Faivre J. Incidence and patterns of recurrence after resection for cure of colonic cancer in a well defined population. Br J Surg. 2006;93(9):1115–22.
Heald RJ, Moran BJ, Ryall RD, Sexton R, MacFarlane JK. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978-1997. Arch Surg. 1998;133(8):894–9.
Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345(9):638–46.
Birgisson H, Talback M, Gunnarsson U, Pahlman L, Glimelius B. Improved survival in cancer of the colon and rectum in Sweden. Eur J Surg Oncol. 2005;31(8):845–53.
Lemmens V, van Steenbergen L, Janssen-Heijnen M, Martijn H, Rutten H, Coebergh JW. Trends in colorectal cancer in the south of the Netherlands 1975–2007: rectal cancer survival levels with colon cancer survival. Acta Oncol. 2010;49(6):784–96.
Jeffery M, Hickey BE, Hider PN. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev. 2007. doi:10.1002/14651858.CD002200.pub2.
Meyerhardt JA, Mangu PB, Flynn PJ, et al. Follow-up care, surveillance protocol, and secondary prevention measures for survivors of colorectal cancer: American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol. 2013;31(35):4465–70.
Turnbull RB Jr, Kyle K, Watson FR, Spratt J. Cancer of the colon: the influence of the no-touch isolation technic on survival rates. Ann Surg. 1967;166(3):420–7.
Bowne WB, Lee B, Wong WD, et al. Operative salvage for locoregional recurrent colon cancer after curative resection: an analysis of 100 cases. Dis Colon Rectum. 2005;48(5):897–909.
Galandiuk S, Wieand HS, Moertel CG, et al. Patterns of recurrence after curative resection of carcinoma of the colon and rectum. Surg Gynecol Obstet. 1992;174(1):27–32.
Network NCC. Clinical Practice Guidelines in Oncology (NCCN Guidelines): Colon Cancer, Version 3.2014. Vol 20142014.
Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation–technical notes and outcome. Colorectal Dis. 2009;11(4):354–64; discussion 364-5.
Kessler H, Hohenberger W. Extended lymphadenectomy in colon cancer is crucial. World J Surg. 2013;37(8):1789–98.
Kobayashi H, West NP, Takahashi K, et al. Quality of surgery for stage III colon cancer: comparison between England, Germany, and Japan. Ann Surg Oncol. 2014;21 Suppl 3:S398–404.
West NP, Hohenberger W, Weber K, Perrakis A, Finan PJ, Quirke P. Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J Clin Oncol. 2010;28(2):272–8.
Abbas MA, Chang GJ, Read TE, et al. Optimizing rectal cancer management: analysis of current evidence. Dis Colon Rectum. 2014;57(2):252–9.
Martling A, Holm T, Rutqvist LE, et al. Impact of a surgical training programme on rectal cancer outcomes in Stockholm. Br J Surg. 2005;92(2):225–9.
Nedrebo BS, Soreide K, Eriksen MT, et al. Survival effect of implementing national treatment strategies for curatively resected colonic and rectal cancer. Br J Surg. 2011;98(5):716–23.
Stocchi L, Nelson H, Sargent DJ, et al. Impact of surgical and pathologic variables in rectal cancer: a United States community and cooperative group report. J Clin Oncol. 2001;19(18):3895–902.
Madoff RD. Defining quality in colon cancer surgery. J Clin Oncol. 2012;30(15):1738–40.
Kessler H, Mansmann U, Hermanek Jr P, Riedl S, Hermanek P. Does the Surgeon Affect Outcome in Colon Carcinoma? Semin Colon Rectal Surg. 1998;9(4):233–40.
West NP, Morris EJ, Rotimi O, Cairns A, Finan PJ, Quirke P. Pathology grading of colon cancer surgical resection and its association with survival: a retrospective observational study. Lancet Oncol. 2008;9(9):857–65.
Primrose JN, Perera R, Gray A, et al. Effect of 3 to 5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer: the FACS randomized clinical trial. JAMA. 2014;311(3):263–70.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liska, D., Stocchi, L., Karagkounis, G. et al. Incidence, Patterns, and Predictors of Locoregional Recurrence in Colon Cancer. Ann Surg Oncol 24, 1093–1099 (2017). https://doi.org/10.1245/s10434-016-5643-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-016-5643-z