Abstract
Objective
The aim of this study was to explore whether palliative gastrectomy is suitable for gastric cancer patients with peritoneal metastasis, and for patients in whom the type of peritoneal metastasis should be selected to receive palliative gastrectomy.
Methods
A total of 747 patients diagnosed with gastric adenocarcinoma with peritoneal metastasis at our centers between January 2000 and April 2014 were retrospectively analyzed. After propensity score matching, the clinicopathologic characteristics and clinical outcomes of patients with peritoneal dissemination were analyzed.
Results
After propensity score matching, the median overall survival (OS) of patients in the gastrectomy group was longer than that for patients in the non-gastrectomy group (11.87 vs. 9.27 months; p = 0.020). Patients who received first-line chemotherapy had a significantly longer median OS than those who did not (11.97 vs. 7.03 months; p < 0.001); among these patients, those undergoing more than eight periods of first-line chemotherapy benefited the most (p < 0.001). Subgroup analyses revealed that patients classified as P1 who were undergoing chemotherapy benefited from gastrectomy (p = 0.024), and patients without multisite metastasis also benefited from gastrectomy with regard to OS (p = 0.007). In the multivariate survival analysis, multisite distant metastasis was the independent poor prognostic factor (p < 0.001), while palliative gastrectomy (p = 0.006) and a period of first-line chemotherapy (p < 0.001) were good prognostic factors. Morbidity rates in the gastrectomy and non-gastrectomy groups were 10.4 and 1.0 %, respectively (p = 0.003); however, no difference in mortality was noted between the two groups (p = 0.590).
Conclusions
Palliative gastrectomy can prolong the survival of P1 patients without multisite distant metastasis when combined with more than five periods, and particularly more than eight periods, of first-line chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Despite its decreasing global incidence, gastric cancer is still the fourth most common cancer, and the third leading cause of cancer-related death, worldwide.1
With early diagnosis, curative gastrectomy combined with standardized D2 lymphadenectomy, and adjuvant chemotherapy, overall survival (OS) in patients with stages II and III gastric cancer is increasing.2–4 Unfortunately, most gastric cancer patients in China are diagnosed with advanced, or even metastatic, gastric cancer.5,6
The pattern of metastasis of gastric cancer includes hematogenous metastasis, distant lymph node metastasis, and peritoneal dissemination. Among these, peritoneal dissemination is the most frequent pattern and cause of death in patients with gastric cancer.7 Approximately 10–20 % of patients have confirmed peritoneal dissemination that was not diagnosed preoperatively.8 Although palliative chemotherapy, novel targeted agents, and hyperthermic intraperitoneal chemotherapy have been proven to improve the prognosis of gastric cancer patients, the long-term survival of gastric cancer patients with peritoneal dissemination remains unsatisfactory.9–12 Moreover, the therapeutic effect of palliative gastrectomy for gastric cancer patients with peritoneal dissemination remains controversial.5,7,13–20 Some studies have indicated that palliative gastrectomy not only relieves cancer-related symptoms but also improves survival without increasing morbidity and mortality.13,16 However, some investigators have reported that palliative gastrectomy cannot prolong the survival of gastric cancer patients with peritoneal metastasis.5,19 In any case, the results of the previous studies conducted to date are of limited significance because of a small sample size, selection bias, and confounding factors.
Using a large sample size of two high-volume institutions and the propensity score matching method to balance the selected bias, the aim of this study was to analyze the survival outcomes of palliative gastrectomy and explore whether palliative gastrectomy is suitable for gastric cancer patients with peritoneal metastasis, and for patients in whom the type of peritoneal metastasis should be selected to receive palliative gastrectomy.
Patients and Methods
Patients
Between January 2000 and April 2014, 747 patients were histologically proven and diagnosed with gastric adenocarcinoma with peritoneal metastasis at the Sun Yat-sen University Cancer Center and The Sixth Affiliated Hospital of Sun Yat-sen University. Among these patients, 345 underwent palliative gastrectomy, while 402 patients did not. We reviewed the clinicopathologic characteristics and clinical outcomes of all patients. Institutional Review Board approval was sought and obtained.
Classification of Peritoneal Seeding
The second and third English versions of the Japanese Classification of Gastric Carcinoma do not detail the classification of peritoneal dissemination; however, we believe that the classification of peritoneal seeding is of great importance. Therefore, according to the first English edition of the above publication, the degree of peritoneal metastasis is classified as follows: P0, no peritoneal seeding; P1, disseminating metastasis to the region directly adjacent to the peritoneum of the stomach (above the transverse colon, including the greater omentum); P2, several scattered metastases to the distant peritoneum and ovarian metastasis alone; and P3, numerous metastases to the distant peritoneum.21 Additionally, the degrees of peritoneal metastasis in patients who did not undergo surgery were determined by computed tomography (CT) or positron emission tomography/CT (PET/CT).
After analyzing the baseline clinicopathologic characteristics of 747 patients in this study, the covariates for propensity score matching were as follows: tumor size, ascites grading, classification of peritoneal metastasis, multisite distant metastasis, and the period of first-line chemotherapy. Next, the baseline clinicopathologic characteristics and outcome survival of patients after 1:1 propensity score matching were analyzed. All of the regular follow-up assessments after 1:1 propensity score matching were completed by November 2015, and the median follow-up was 8.9 months (range 0.1–49.7 months).
Statistical Analysis
Chi square tests were used to compare categorical variables, and non-parametric tests were used to compare continuous variables. OS was calculated from the diagnosis of peritoneal metastasis to death from any cause. Unadjusted Kaplan–Meier survival curves with log-rank testing were generated to compare the survival benefits. Prognostic factors were analyzed by searching the clinicopathological factors in univariate analysis, with all variables with a p value <0.05 in the univariate analysis entered into multivariate analysis using Cox proportional hazard regression models. The hazard ratio (HR) and 95 % confidence interval (CI) were used to estimate the role of each predictor of survival. A two-sided p value <0.05 was considered to be significant. All of the above statistical analyses were performed using SPSS software version 17.0 (SPSS, Inc., Chicago, IL, USA).
Propensity Score Matching Analysis
Because patients were not randomly allocated to the gastrectomy or non-gastrectomy groups, indicating selection bias, propensity score matching was used to control the selection bias and balance some covariates that may be associated with the outcome. The propensity score, which represents the conditional probability of receiving a therapy given a vector of covariates, is commonly built in observational studies to adjust for selection bias.22,23 In this study, we chose the 1:1 nearest neighbor matching for the propensity score. Propensity score matching was performed using Stata 13.0 (StataCorp LP, College Station, TX, USA).
Results
Patient Characteristics
This study included 747 gastric cancer patients with peritoneal dissemination, including 345 patients in the gastrectomy group and 402 patients in the non-gastrectomy group. The general clinicopathological characteristics of both groups are summarized in Table 1. As shown in Table 1, in the gastrectomy group, the tumors were smaller (p = 0.002), ascites accumulation was less (p < 0.001), peritoneal seeding was less severe (p < 0.001), multisite distant metastasis was less frequent (p < 0.001), and the period of first-line chemotherapy was increased (p < 0.001), indicating selection bias that may influence survival. Therefore, the covariates for propensity score matching were tumor size, ascites grading, classification of peritoneal metastasis, multisite distant metastasis, and the period of first-line chemotherapy. The covariates were balanced after 1:1 propensity score matching (Table 1).
Survival
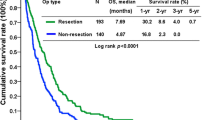
The median OS was 11.87 (95 % CI 9.95–13.77) months in the gastrectomy group and 9.27 (95 % CI 7.93–10.60) months in the non-gastrectomy group (Fig. 1), and the between-group median OS difference was significant (p = 0.020).
Patients receiving first-line chemotherapy had a significantly longer median OS of 11.97 (95 % CI 10.28–13.66) months compared with 7.03 (95 % CI 5.60–8.47) months in patients who did not receive first-line chemotherapy (p < 0.001) [Fig. 2]. Moreover, patients receiving more than eight periods of first-line chemotherapy had a significantly longer median OS of 24.77 (95 % CI 21.60–27.94) months compared with 13.40 (95 % CI 12.17–14.63) months in patients who received five to eight periods of first-line chemotherapy, 8.63 (95 % CI 7.33–9.94) months in patients who received one to four periods of first-line chemotherapy, and 7.03 (95 % CI 5.60–8.47) months in patients who did not receive first-line chemotherapy (p < 0.001) (Fig. S1).
In the subgroup analysis, patients in the gastrectomy group undergoing first-line chemotherapy who were classified as P1 had a significantly longer median OS than patients in the non-gastrectomy group [19.57 (95 % CI 6.67–32.47) months vs. 9.13 (95 % CI 6.78–11.49) months; p = 0.024] (Fig. 3a). The median OS of patients classified as P2 showed no significant difference between the groups [12.37 (95 % CI 10.26–14.48) months vs. 13.80 (95 % CI 12.05–15.55) months; p = 0.406] (Fig. 3b), and the median OS of patients classified as P3 also showed no significant difference between the groups [12.23 (95 % CI 4.48–19.99) months vs. 10.17 (95 % CI 7.30–13.03) months; p = 0.076] (Fig. 3c). Without first-line chemotherapy, the median OS showed no significant difference between the groups in patients classified as P1 [13.53 (95 % CI 9.68–17.38) months vs. 5.97 (95 % CI 0.34–11.59) months; p = 0.269] (Fig. 3d), patients classified as P2 [12.77 (95 % CI 3.67–21.87) months vs. 11.00 (95 % CI 4.51–17.49) months; p = 0.231] (Fig. 3e), and patients classified as P3 [5.23 (95 % CI 1.76–8.71) months vs. 5.73 (95 % CI 4.20–7.26) months; p = 0.299] (Fig. 3f).
Kaplan–Meier survival curves of the palliative gastrectomy and non-gastrectomy groups for gastric cancer patients with peritoneal dissemination stratified by first-line chemotherapy and classifications of peritoneal metastasis. a P1 with first-line chemotherapy (p = 0.024); b P2 with first-line chemotherapy (p = 0.406); c P3 with first-line chemotherapy (p = 0.076); d P1 without first-line chemotherapy (p = 0.269); e P2 without first-line chemotherapy (p = 0.231); f P3 without first-line chemotherapy (p = 0.299). p values were calculated using the log-rank test
With regard to patients with multisite distant metastasis, the median OS in the gastrectomy group was not different from that of the non-gastrectomy group [8.70 (95 % CI 7.34–10.06) months vs. 8.77 (95 % CI 5.54–11.99) months; p = 0.556] (Fig. S2a). However, patients without multisite distant metastasis in the gastrectomy group had a longer median OS than patients in the non-gastrectomy group, with a median OS of 15.23 (95 % CI 11.92–18.54) months and 9.80 (95 % CI 7.89–11.71) months, respectively (p = 0.007) (Fig. S2b).
Univariate and Multivariate Analysis for Overall Survival
Univariate survival analysis revealed that palliative gastrectomy (p = 0.020), multisite distant metastasis (p < 0.001), and the period of first-line chemotherapy (p < 0.001) were associated with OS (Table 2). Multivariate survival analysis demonstrated that multisite distant metastasis was an independent poor prognostic factor (p < 0.001), while palliative gastrectomy (p = 0.006) and the period of first-line chemotherapy (p < 0.001) were good prognostic factors (Table 2).
Morbidity and Mortality
The overall postoperative morbidity rate was significantly higher in the gastrectomy group than in the non-gastrectomy group [10.4 % (19/183) vs. 1.0 % (1/99), respectively; p = 0.003]. Complications in the gastrectomy group included eight cases of intestinal obstruction, five cases of pulmonary infection, two cases of anastomotic leakage, two cases of abdominal infection, one case of anastomotic bleeding, and one case of pancreatitis. The only complication in the non-gastrectomy group was duodenum stenosis. Overall postoperative mortality was 0.5 % (1/183) in the gastrectomy group and 2.0 % (2/97) in the gastrectomy group. No significant difference was observed between the groups (p = 0.590).
Discussion
Although the global incidence of gastric cancer is decreasing, most gastric cancer patients are still diagnosed at an advanced stage or even peritoneal metastasis.5 With a 5-year OS <2 %, peritoneal metastasis is considered the terminal period of gastric cancer.24 With the development of systemic chemotherapy, novel targeted drugs, hyperthermic intraperitoneal chemotherapy, and aggressive surgery, the survival of gastric patients with peritoneal dissemination has improved.9–12 However, most of the above treatments are debatable, including the role of palliative gastrectomy. Although the interim analysis of the REGATTA trial seemed to not favor palliative gastrectomy combined with chemotherapy, with a 2-year OS rate of 25.1 % compared with 31.7 % in the chemotherapy group (p = 0.68), detailed results concerning the subgroup of peritoneal metastasis were not shown.25
Some investigators have suggested that palliative gastrectomy should be indicated in patients with peritoneal dissemination,8,13 a theory that is based on the following: (i) gastrectomy can relieve cancer-related symptoms, such as tumor bleeding, obstruction, and perforation; (ii) resection of the primary tumor can reduce the amount of tumor stem cells, possibly increasing the sensitivity of palliative chemotherapy; and (iii) primary tumor removal can improve metabolism and immunity of the patients.14,26,27 However, the previous studies had obvious selection bias, such as a smaller tumor size in the gastrectomy group, confounding the results. Therefore, in our study, we used propensity score matching to minimize possible selection bias. After propensity score matching, our results showed that patients in the gastrectomy group had a longer survival time of 2.60 months (p = 0.020), indicating the benefit of palliative gastrectomy, in accordance with other studies.13–15
To select the appropriate patients for palliative gastrectomy, we performed subgroup analysis according to different clinicopathologic characteristics. In our subgroup analysis, P1 patients combined with chemotherapy had a significant longer OS in the gastrectomy group than in the non-gastrectomy group (p = 0.024). Yang et al. also reported that patients classified as P1/P2 alone might benefit from palliative gastrectomy,13 while Xia et al. found that resection could also provide a significant survival advantage to P1/P2 patients.14
Chang et al. demonstrated that, combined with chemotherapy, non-curative resection had a survival benefit; however, in patients with no chemotherapy, resection showed no benefit. In addition, no survival benefit was observed for the resection group when metastasis was confined to more than one site.28 Our results found that, without first-line chemotherapy, patients classified as P1 (p = 0.269), P2 (p = 0.231), and P3 (p = 0.299) in the gastrectomy group did not have an improved survival. Moreover, for patients with multisite distant metastasis, no difference in the median OS was observed between the gastrectomy and non-gastrectomy groups (8.70 vs. 8.77 months, respectively; p = 0.556). These results were also supported by other studies.15 We consider that the most important role of palliative gastrectomy is to relieve cancer-related symptoms, improve the metabolism of patients, and promote the efficacy of chemotherapy, but not to cure the patients. Therefore, it is reasonable that gastrectomy has no survival benefit without palliative chemotherapy. Additionally, if the burden of the tumor is very large, it is equitable that gastrectomy lacks an advantage due to the dismal outcome of gastrectomy with multisite distant metastasis.
In our study, multivariate analysis of survival showed that multisite distant metastasis was an independent poor prognostic factor, and palliative gastrectomy and the period of first-line chemotherapy were favorable prognostic factors. Tokunaga et al. also suggested that chemotherapy should be considered an initial treatment for patients with peritoneal metastasis.18 The results of Yang et al. revealed that, in multivariate analysis, palliative chemotherapy and resection were independently associated with good survival.13 Additionally, in our study, we found that patients receiving more than eight periods of chemotherapy had a median survival of 24.77 months, which was significant longer than in patients receiving less than eight periods of chemotherapy (p < 0.001). One to four periods of first-line chemotherapy did not show a survival benefit for patients compared with no chemotherapy (p = 0.245). Therefore, we recommend that first-line chemotherapy should be continued for more than five periods, or even more than eight periods.
In this study, the overall postoperative morbidity rate was higher in the gastrectomy group than in the non-gastrectomy group (10.4 vs. 1.0 %; p = 0.003), and overall postoperative mortality in the gastrectomy group was not significantly different from the non-gastrectomy group (0.5 vs. 2.0 %, respectively; p = 0.590). Previous studies have shown that the morbidity and mortality of palliative gastric resection ranged from 12 to 65 and 0 to 27 %, respectively.29,30 Sano et al. reported that the morbidity of standard D2 curative resection was 20.9 %.31 Therefore, we consider that morbidity and mortality in our study are acceptable, and palliative gastrectomy for patients with peritoneal metastasis is a safe procedure.
There are also some limitations to our study. First, as with all other retrospective surveys, this study was exposed to selection bias because of its retrospective nature. Second, for patients who had no surgery, we did not routinely perform laparoscopic exploration, which is regarded as the most helpful procedure to detect peritoneal dissemination with high sensitivity and specificity; however, the data in our study were from two centers. Additionally, we used propensity score matching and multivariate analysis to balance the selection bias, and employed a good study design with subgroups to explore the value of palliative gastrectomy for patients with peritoneal dissemination. In the future, large-scale and well-designed randomized controlled trials are required.
Conclusions
The present study indicated that palliative gastrectomy surgery can prolong the survival of P1 patients without multisite distant metastasis when combined with more than five periods of first-line chemotherapy, and particularly with more than eight periods of first-line chemotherapy. There are a number of other factors, such as assessing response to chemotherapy, that need to be explored.
References
Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
Songun I, Putter H, Kranenbarg EM, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439–49.
Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315–21.
Sasako M, Sakuramoto S, Katai H, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387–93.
Chen S, Li YF, Feng XY, et al. Significance of palliative gastrectomy for late-stage gastric cancer patients. J Surg Oncol. 2012;106:862–71.
Zhang XF, Huang CM, Lu HS, et al. Surgical treatment and prognosis of gastric cancer in 2,613 patients. World J Gastroenterol. 2004;10:3405–08.
Hioki M, Gotohda N, Konishi M, et al. Predictive factors improving survival after gastrectomy in gastric cancer patients with peritoneal carcinomatosis. World J Surg. 2010;34:555–62.
Sugarbaker PH, Yonemura Y. Clinical pathway for the management of resectable gastric cancer with peritoneal seeding: best palliation with a ray of hope for cure. Oncology. 2000;58(2):96–107.
Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97.
Koizumi W, Narahara H, Hara T, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–21.
Bernards N, Creemers GJ, Nieuwenhuijzen GA, et al. No improvement in median survival for patients with metastatic gastric cancer despite increased use of chemotherapy. Ann Oncol. 2013;24:3056–60.
Yang XJ, Huang CQ, Suo T, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol. 2011;18:1575–81.
Yang K, Liu K, Zhang WH, et al. The value of palliative gastrectomy for gastric cancer patients with intraoperatively proven peritoneal seeding. Medicine (Baltimore) 2015;94:e1051.
Xia X, Li C, Yan M, et al. Who will benefit from noncurative resection in patients with gastric cancer with single peritoneal metastasis? Am Surg. 2014;80:124–30.
He MM, Zhang DS, Wang F, et al. The role of non-curative surgery in incurable, asymptomatic advanced gastric cancer. PLoS One. 2013;8:e83921.
Lasithiotakis K, Antoniou SA, Antoniou GA, et al. Gastrectomy for stage IV gastric cancer. a systematic review and meta-analysis. Anticancer Res. 2014;34:2079–85.
Sun J, Song Y, Wang Z, et al. Clinical significance of palliative gastrectomy on the survival of patients with incurable advanced gastric cancer: a systematic review and meta-analysis. BMC Cancer. 2013;13:577.
Tokunaga M, Terashima M, Tanizawa Y, et al. Survival benefit of palliative gastrectomy in gastric cancer patients with peritoneal metastasis. World J Surg. 2012;36:2637–43.
Kokkola A, Louhimo J, Puolakkainen P. Does non-curative gastrectomy improve survival in patients with metastatic gastric cancer? J Surg Oncol. 2012;106:193–96.
Ouchi K, Sugawara T, Ono H, et al. Therapeutic significance of palliative operations for gastric cancer for survival and quality of life. J Surg Oncol. 1998;69:41–44.
Japanese Research Society for Gastric Cancer (1995) Japanese classification of gastric carcinoma, 1st English edn. Kanehara & Co., Ltd, Tokyo.
Joffe MM, Rosenbaum PR. Invited commentary: propensity scores. Am J Epidemiol. 1999;150:327–33.
Garrido MM, Kelley AS, Paris J, et al. Methods for constructing and assessing propensity scores. Health Serv Res. 2014;49:1701–20.
Bando E, Yonemura Y, Takeshita Y, et al. Intraoperative lavage for cytological examination in 1297 patients with gastric carcinoma. Am J Surg. 1999;178:256–62.
Fujitani K, Yang HK, Kurokawa Y, et al. Randomized controlled trial comparing gastrectomy plus chemotherapy with chemotherapy alone in advanced gastric cancer with a single non-curable factor: Japan Clinical Oncology Group Study JCOG 0705 and Korea Gastric Cancer Association Study KGCA01. Jpn J Clin Oncol. 2008;38:504–506.
Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol. 2013;14:e218–28.
Dittmar Y, Rauchfuss F, Goetz M, et al. Non-curative gastric resection for patients with stage 4 gastric cancer: a single center experience and current review of literature. Langenbecks Arch Surg. 2012;397:745–53.
Chang YR, Han DS, Kong SH, et al. The value of palliative gastrectomy in gastric cancer with distant metastasis. Ann Surg Oncol. 2012;19:1231–39.
Hartgrink HH, Putter H, Klein Kranenbarg E, et al. Value of palliative resection in gastric cancer. Br J Surg. 2002;89:1438–43.
Medina-Franco H, Contreras-Saldivar A, Ramos-De La Medina A, et al. Surgery for stage IV gastric cancer. Am J Surg. 2004;187:543–46.
Sano T, Sasako M, Yamamoto S, et al. Gastric cancer surgery: morbidity and mortality results from a prospective randomized controlled trial comparing D2 and extended para-aortic lymphadenectomy. Japan Clinical Oncology Group Study 9501. J Clin Oncol. 2004;22:2767–73.
Acknowledgments
The authors thank Dr. Zhiwei Zhou, Yuanfang Li, and Yupei Chen for their encouragement, support and valuable statistical help. This work was supported in part by a grant from the National Natural Science Foundation of China (81,302,144) and the Guangdong Science and Technology Department (No. 2012B0617000879).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosures
Run-Cong Nie, Shi Chen, Shu-Qiang Yuan, Xiao-Jiang Chen, Yong-Ming Chen, Bao-Yan Zhu, Hai-bo Qiu, Jun-Sheng Peng, and Ying-Bo Chen report no financial disclosures.
Additional information
Run-Cong Nie, Shi Chen, and Shu-Qiang Yuan have contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nie, RC., Chen, S., Yuan, SQ. et al. Significant Role of Palliative Gastrectomy in Selective Gastric Cancer Patients with Peritoneal Dissemination: A Propensity Score Matching Analysis. Ann Surg Oncol 23, 3956–3963 (2016). https://doi.org/10.1245/s10434-016-5223-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-016-5223-2