Abstract
Background
The survival benefit of palliative gastrectomy in patients with peritoneal metastasis as a single incurable factor remains unclear.
Methods
A total of 148 gastric cancer patients with peritoneal metastasis underwent gastrectomy or chemotherapy at the Shizuoka Cancer Center between September 2002 and December 2008 and were included in this study. The effects of gastrectomy and chemotherapy on their long-term outcome were investigated. Multivariate analysis was also performed to identify independent prognostic factors.
Results
Gastrectomy was performed in 82 patients and subsequent chemotherapy was administered to 55. Chemotherapy was selected as an initial treatment for 66 patients. Median survival time (MST) was identical between patients with and without gastrectomy (13.1 vs. 12.0 months; P = 0.410). Conversely, MST was significantly longer in patients who received chemotherapy (13.7 months) than those who did not (7.1 months; P = 0.048). According to the results of multivariate analysis, chemotherapy (hazards ratio [HR] = 0.476; 95 % CI = 0.288–0.787) was selected as an independent prognostic factor, while gastrectomy was not.
Conclusions
The results of the present study did not show a survival benefit of palliative gastrectomy in selected patients with peritoneal metastasis. Instead, chemotherapy has to be considered as an initial treatment for these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is diagnosed frequently and is the second leading cause of cancer-related deaths in Japan [1]. Although the long-term outcome of early gastric cancer is good, that of advanced gastric cancer is dismal, particularly when combined with other incurable factors [2–4]. Recent advances in chemotherapy have improved the survival rate of gastric cancer patients with incurable factors. However, survival rates remain limited and there is still room for improvement in the survival rate [5, 6].
The incurable factors observed frequently in patients with advanced gastric cancer are peritoneal, liver, and distant lymph node metastases [7, 8]. Better survival rates were reported in Japan following gastrectomy plus metastasectomy if the incurable factors were liver or para-aortic lymph node metastases and if the surgery was curative [9–12]. In contrast, curative resections are difficult in patients with widespread peritoneal metastasis, which is the most frequently observed incurable factor [13–16]. Although a few surgeons have reported the efficacy of performing a peritonectomy, this concept has not been accepted widely, even in Japan [17].
Previously, a number of authors investigated the feasibility of palliative gastrectomy in patients with incurable factors [14, 18–24]. However, each study included patients with a range of incurable factors; therefore, the effect of gastrectomy in selected patients with peritoneal metastasis remains unclear. The aim of the present study was to clarify the effects of gastrectomy on gastric cancer patients with peritoneal metastasis. The appropriate treatment strategy in patients with localized peritoneal metastasis was also investigated.
Materials and methods
Patients
Between September 2002 and December 2008, 279 gastric cancer patients with peritoneal metastasis underwent gastrectomy or chemotherapy at the Shizuoka Cancer Center, Japan. Of these, 131 patients had incurable factors other than peritoneal metastasis so the remaining 148 patients with no other obvious incurable factors were included in this study. Pathological examination of biopsy specimens from the stomach revealed adenocarcinoma in all patients. Patients who had received any previous treatment for gastric cancer were not included in the present study. Peritoneal metastasis was diagnosed histopathologically in patients who underwent laparotomy (106 patients) or was diagnosed clinically using computed tomography in patients who did not undergo laparotomy (42 patients).
The patients’ characteristics and surgical and pathological findings were collected retrospectively from our prospectively recorded database and individual patient records. The patients’ clinicopathological characteristics were analyzed, and survival curves were compared according to the treatment modalities administered (gastrectomy and chemotherapy). Multivariate analysis was also conducted to identify independent prognostic factors.
This study followed ethical guidelines for human subjects and was approved by the institutional review board of the Shizuoka Cancer Center.
Pretreatment examinations
Computed tomography (CT) with contrast medium was performed as a routine pretreatment examination in all patients except those with poor renal function or with an allergy to the contrast medium. Patients were regarded as having clinically evident peritoneal metastasis (cP+) if the CT findings showed obvious peritoneal metastasis which included massive ascites, cirrhosal implants of the intra-abdominal area or on the small or large bowel, remarkably increased visceral fat density, and omental metastasis. If CT did not show any obvious peritoneal metastasis, patients were regarded as not having clinically evident peritoneal metastasis (cP−).
Macroscopic type was classified according to the Japanese Gastric Cancer Association (JGCA) classification system [25]. Histological type was also classified according to the JGCA classification system, in which tubular and papillary adenocarcinoma are defined as differentiated adenocarcinoma, while poorly differentiated adenocarcinoma, signet-ring cell carcinoma, and mucinous adenocarcinoma are defined as undifferentiated adenocarcinoma.
The degree of peritoneal metastasis was classified in patients who underwent laparotomy as follows: P0, no implants to the peritoneum; P1, cancerous implants to the region directly adjacent to the stomach peritoneum (above the transverse colon), including the greater omentum; P2, several scattered metastases to the distant peritoneum and ovarian metastasis alone; and P3, numerous metastases to the distant peritoneum [26].
Indications for gastrectomy
In patients with P1, gastrectomy was performed if macroscopic curative resection was expected. Gastrectomy was also selected as an initial treatment in patients with tumor-associated symptoms such as bleeding or gastric outlet obstruction even if curative resection could not be expected. If patients had P2 or P3 peritoneal metastasis and they did not have tumor-associated symptoms, gastrectomy would not be performed in principle.
Statistics
All continuous data are presented as the median (range). Survival rates were calculated using the Kaplan–Meier method, and the log-rank test was used to compare the groups. In this study, overall survival time was defined as time from initial treatment (surgery or chemotherapy) to any death, including noncancer-related death.
Independent prognostic factors were identified using the Cox proportional hazards model. In the analysis, each patient’s age (<60 or ≥60 years old), sex, clinically evident peritoneal metastasis (cP− or cP+), gastrectomy (performed or not performed), chemotherapy (received or not received), Eastern Cooperative Oncology Group (ECOG) performance status (0, 1 or 2, 3), macroscopic type (type 4 or other), and histology (differentiated or undifferentiated) were included as covariates. The Bonferroni test was used during multiple comparisons. A P value <0.05 was considered significant. All statistical analyses were conducted using R version 2.13.1.
Results
The patient characteristics are indicated in Table 1. Macroscopic type 3 tumors were observed in 43 % of the patients and type 4 tumors were observed in 39 %. Tumors were undifferentiated in three-fourths of the patients. The pretreatment ECOG performance status was generally good (≤1) and was 2 or higher in 10 % of patients. Gastrectomy was performed in 82 patients and subsequent chemotherapy was administered to 55 of these patients. Chemotherapy was selected as an initial treatment in 66 patients. We also compared the background data between patients according to the treatment provided. There were no differences between any two groups with respect to sex, ECOG performance status, histology, and macroscopic type. The median age was significantly different between the groups, with patients who received gastrectomy only the oldest followed by patients who received both gastrectomy and chemotherapy. The incidence of clinically evident peritoneal metastasis was significantly higher in patients who underwent chemotherapy only than in those who underwent gastrectomy only or both gastrectomy and chemotherapy.
Table 2 lists the treatments provided. Of the 82 patients who underwent gastrectomy, total gastrectomy was performed more frequently (67 %) than distal gastrectomy (33 %). S1-based chemotherapy was the most frequently selected treatment regimen in this study. Of 121 patients who received chemotherapy, second-line chemotherapy was given in 64 % of patients and third-line chemotherapy was administered in 35 % of patients.
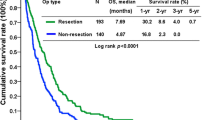
Figure 1 shows the overall survival curve of all patients. Of the 148 patients, 137 were followed until their death. Median follow-up period of survivors was 29.7 months. One-year and three-year overall survival rates were 53.9 and 18.1 %, respectively. Figure 2a shows the overall survival curves of patients with and without gastrectomy. The median survival time (MST) of patients with gastrectomy was 13.1 months (n = 82) and that without gastrectomy was 12.0 months (n = 66; P = 0.410). Overall survival curves of patients who did or did not receive chemotherapy are shown in Fig. 2b. MST was significantly longer in patients who received chemotherapy (13.7 months; n = 121) than in those who did not (7.1 months; n = 27; P = 0.048).
a Survival curves of patients with or without gastrectomy. There is no difference in MST between patients with gastrectomy (13.1 months; n = 82) and those without gastrectomy (12.0 months; n = 66; P = 0.410). b Survival curves of patients who received or did not receive chemotherapy. MST was significantly longer for patients who received chemotherapy (13.7 months; n = 121) than for those who did not (7.1 months; n = 27; P = 0.048)
Table 3 shows the results of the Cox proportional hazards model. Chemotherapy [hazards ratio (HR) = 0.476; 95 % CI = 0.288–0.787], ECOG performance status 0 or 1(HR = 0.278; 95 % CI = 0.156–0.495), and macroscopic tumor types other than type 4 (HR = 0.566; 95 % CI = 0.377–0.848) were selected as independent prognostic factors, while gastrectomy was not selected.
Investigation of 40 patients with localized peritoneal metastasis (P1)
The degree of peritoneal metastasis was confirmed by laparotomy in 106 of the 148 patients: it was P1 in 40 patients, P2 in 12 patients, and P3 in 54 patients. Survival analysis was conducted in 40 patients with P1 peritoneal metastasis. R0 resection according to 6th edition of the TNM classification was performed in 18 patients and the MST for these patients (26.4 months) was longer than that of the 16 patients who underwent R1 or R2 gastrectomy (Fig. 3, 12.3 months; P < 0.001) [27].
Survival curves of 40 patients with localized peritoneal metastasis confirmed by laparotomy. MST was significantly longer in 18 patients who underwent R0 gastrectomy (26.4 months) than in 16 patients who underwent R1 or R2 gastrectomy (12.3 months; P < 0.001). MST for 18 patients with R0 gastrectomy was also longer than that for six patients who received chemotherapy as an initial treatment (12.5 months), although this was not statistically significant (P = 0.414)
Discussion
Recent advances in chemotherapy regimens have improved the survival rates of gastric cancer patients with incurable factors. Koizumi et al. [5] reported an MST of 13 months in patients with advanced gastric cancer who were treated with S1 and cisplatin, and Bang et al. [6] reported a 13.8 month median overall survival time in patients with HER2-positive advanced gastric cancer who were treated with trastuzumab plus chemotherapy. However, to date, the effects of chemotherapy are limited and the 5 year survival rate of patients with unresectable gastric cancer remains grim [5, 6].
The feasibility of palliative gastrectomy in patients with unresectable gastric cancer is under debate [14, 18–24]. Many studies have examined a variety of patients with gastric cancer; however, the type and the number of incurable factors differed among patients. To the best of our knowledge, the present study is the first report that investigates a similar group of patients who all had peritoneal metastasis but did not have other obvious incurable factors. Therefore, we were able to identify the appropriate treatment strategy for patients with peritoneal metastasis with less bias than the previous studies.
The present study showed that there was no survival benefit associated with palliative gastrectomy. Instead, we recommend chemotherapy, as long as patients do not have tumor-associated symptoms. Sarela et al. [13, 14], and Kahlke et al. [20] also did not recommend palliative gastrectomy if patients did not have tumor-associated symptoms because it did not affect the patient’s survival time. In contrast, Kim et al. [19] and Li et al. [23] recommended palliative gastrectomy, and Lin et al. [28] recommended palliative gastrectomy with subsequent chemotherapy to improve the survival rate of patients.
Multivariate analysis identified pretreatment ECOG performance status, macroscopic tumor type, and chemotherapy as independent prognostic factors. Macroscopic tumor type 4 is a widely accepted prognostic factor, and the incidence of peritoneal metastasis associated with type 4 tumors is higher than with other macroscopic tumor types [3, 4, 22]. Poor ECOG performance status is also a well-known independent prognostic factor in advanced malignancies [13, 16, 20]. Sarela et al. [13] reported that poor ECOG performance status is an independent prognostic factor in patients with peritoneal metastasis, as found in our study.
We also investigated the efficacy of R0 surgery in patients with localized peritoneal metastasis and found that the survival rate was better in patients who were able to undergo curative resection than those who were not. Ouchi et al. [18] segregated patients according to the degree of peritoneal metastasis (P1 vs. P2 or P3) because they believed that the tumor load must also be taken into account. Moreover, Hioki et al. [29] reported a better outcome in patients with localized peritoneal metastasis following gastrectomy than in those with widespread peritoneal metastasis, and emphasized that patients with a good performance status and localized peritoneal metastasis should be considered appropriate surgical candidates. Based on the results from these reports it may be plausible to distinguish whether patients have localized or widespread peritoneal metastases in order to establish the appropriate treatment strategy for these patients.
However, it has been reported that the accuracy of computed tomography for diagnosing peritoneal metastasis is limited, and the degree of peritoneal metastasis would not be diagnosed without laparotomy [30]. Recently, the feasibility of diagnostic laparoscopy, which is less invasive than laparotomy and more sensitive for finding peritoneal metastasis than computed tomography, was reported [31, 32]. In our institute, we also perform this procedure in patients in whom a high incidence of peritoneal metastasis was estimated. However, we began diagnostic laparoscopy in the middle of 2008 so most of the patients in the present series did not receive diagnostic laparoscopy before treatment.
There are limitations associated with this retrospective study. These include a possible bias in the selection of treatment strategies, including chemotherapeutic regimens and indication for gastrectomy, and the possibility that patient backgrounds differ between groups. In fact, patient age and the incidence of clinically evident peritoneal metastasis were different between groups. Therefore, we conducted multivariate analysis including these factors as covariates. To overcome these problems and to obtain conclusive results, a well-designed prospective trial is necessary. Groups in Japan and Korea are currently collaborating on an international randomized controlled trial investigating the efficacy of gastrectomy in gastric cancer patients with a single incurable factor. Therefore, we must await the results of this study, although the patients being investigated in the prospective study are not identical to those included in the present study [33].
In the present study, we used overall survival to evaluate the efficacy of each treatment. We could not evaluate patient quality of life after treatment, the burden of care, and cost because it was difficult to collect these data retrospectively. However, these factors should also be taken into account, particularly in patients with incurable disease [34]. If poor quality of life and increased burden of care were observed in patients who had undergone gastrectomy, they would further reinforce the arguments against gastrectomy in patients having peritoneal metastasis.
In conclusion, the results of the present study did not show a survival benefit with palliative gastrectomy in patients with peritoneal metastasis. Instead, chemotherapy has to be considered an initial treatment for these patients. We still have to await the result of randomized controlled trial being performed in the East to address this specific issue.
References
Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55(2):74–108
Kakeji Y, Maehara Y, Tomoda M et al (1998) Long-term survival of patients with stage IV gastric carcinoma. Cancer 82(12):2307–2311
Isobe Y, Nashimoto A, Akazawa K et al (2011) Gastric cancer treatment in Japan: 2008 annual report of the JGCA nationwide registry. Gastric Cancer 14(4):301–316
Maruyama K, Kaminishi M, Hayashi K et al (2006) Gastric cancer treated in 1991 in Japan: data analysis of nationwide registry. Gastric Cancer 9(2):51–66
Koizumi W, Narahara H, Hara T et al (2008) S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 9(3):215–221
Bang YJ, Van Cutsem E, Feyereislova A et al (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376(9742):687–697
Maehara Y, Hasuda S, Koga T et al (2000) Postoperative outcome and sites of recurrence in patients following curative resection of gastric cancer. Br J Surg 87(3):353–357
Sakuramoto S, Sasako M, Yamaguchi T et al (2007) Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 357(18):1810–1820
Koga R, Yamamoto J, Ohyama S et al (2007) Liver resection for metastatic gastric cancer: experience with 42 patients including eight long-term survivors. Jpn J Clin Oncol 37(11):836–842
Sakamoto Y, Ohyama S, Yamamoto J et al (2003) Surgical resection of liver metastases of gastric cancer: an analysis of a 17-year experience with 22 patients. Surgery 133(5):507–511
Shirabe K, Wakiyama S, Gion T et al (2006) Hepatic resection for the treatment of liver metastases in gastric carcinoma: review of the literature. HPB (Oxford) 8(2):89–92
Tokunaga M, Ohyama S, Hiki N et al (2010) Can super extended lymph node dissection be justified for gastric cancer with pathologically positive para-aortic lymph nodes? Ann Surg Oncol 17(8):2031–2036
Sarela AI, Miner TJ, Karpeh MS et al (2006) Clinical outcomes with laparoscopic stage M1, unresected gastric adenocarcinoma. Ann Surg 243(2):189–195
Sarela AI, Yelluri S (2007) Gastric adenocarcinoma with distant metastasis: is gastrectomy necessary? Arch Surg 142(2):143–149 discussion 149
Hartgrink HH, Putter H, Klein Kranenbarg E et al (2002) Value of palliative resection in gastric cancer. Br J Surg 89(11):1438–1443
Kim KH, Lee KW, Baek SK et al (2011) Survival benefit of gastrectomy +/− metastasectomy in patients with metastatic gastric cancer receiving chemotherapy. Gastric Cancer 14(2):130–138
Yonemura Y, Kawamura T, Bandou E et al (2005) Treatment of peritoneal dissemination from gastric cancer by peritonectomy and chemohyperthermic peritoneal perfusion. Br J Surg 92(3):370–375
Ouchi K, Sugawara T, Ono H et al (1998) Therapeutic significance of palliative operations for gastric cancer for survival and quality of life. J Surg Oncol 69(1):41–44
Kim DY, JooJK Park YK et al (2008) Is palliative resection necessary for gastric carcinoma patients? Langenbecks Arch Surg 393(1):31–35
Kahlke V, Bestmann B, Schmid A et al (2004) Palliation of metastatic gastric cancer: impact of preoperative symptoms and the type of operation on survival and quality of life. World J Surg 28(4):369–375. doi:10.1007/s00268-003-7119-0
Doglietto GB, Pacelli F, Caprino P et al (2000) Surgery: independent prognostic factor in curable and far advanced gastric cancer. World J Surg 24(4):459–463. doi:10.1007/s002689910073 discussion 464
Yook JH, Oh ST, Kim BS (2005) Clinicopathological analysis of Borrmann type IV gastric cancer. Cancer Res Treat 37(2):87–91
Li C, Yan M, Chen J et al (2010) Survival benefit of non-curative gastrectomy for gastric cancer patients with synchronous distant metastasis. J Gastrointest Surg 14(2):282–288
Chang YR, Han DS, Kong SH et al (2012) The value of palliative gastrectomy in gastric cancer with distant metastasis. Ann Surg Oncol 19(4):1231–1239
Japanese Gastric Cancer Association (1998) Japanese Classification of Gastric Carcinoma—2nd English Edition. Gastric Cancer 1(1):10–24
Japanese Research Society for Gastric Cancer (1995) Japanese classification of gastric carcinoma, 1st English edn. Kanehara & Co, Tokyo
Sobin L, Wittekind D (eds) (2002) TNM classification of malignant tumors, vol 6. Wiley, New York
Lin SZ, Tong HF, You T et al (2008) Palliative gastrectomy and chemotherapy for stage IV gastric cancer. J Cancer Res Clin Oncol 134(2):187–192
Hioki M, Gotohda N, Konishi M et al (2010) Predictive factors improving survival after gastrectomy in gastric cancer patients with peritoneal carcinomatosis. World J Surg 34(3):555–562. doi:10.1007/s00268-010-0396-5
Kim SJ, Kim HH, Kim YH et al (2009) Peritoneal metastasis: detection with 16- or 64-detector row CT in patients undergoing surgery for gastric cancer. Radiology 253:407–415
Shim JH, Yoo HM, Lee HH et al (2011) Use of laparoscopy as an alternative to computed tomography (CT) and positron emission tomography (PET) scans for the detection of recurrence in patients with gastric cancer: a pilot study. Surg Endosc 25:3338–3344
Tsuchida K, Yoshikawa T, Tsuburaya A et al (2011) Indications for staging laparoscopy in clinical T4M0 gastric cancer. World J Surg 35:2703–2709. doi:10.1007/s00268-011-1290-5
Fujitani K, Yang HK, Kurokawa Y et al (2008) Randomized controlled trial comparing gastrectomy plus chemotherapy with chemotherapy alone in advanced gastric cancer with a single non-curable factor: Japan Clinical Oncology Group Study JCOG 0705 and Korea Gastric Cancer Association Study KGCA01. Jpn J Clin Oncol 38(7):504–506
Russell RC, Treasure T (2012) Counting the cost of cancer surgery for advanced and metastatic disease. Br J Surg 99:449–450
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tokunaga, M., Terashima, M., Tanizawa, Y. et al. Survival Benefit of Palliative Gastrectomy in Gastric Cancer Patients with Peritoneal Metastasis. World J Surg 36, 2637–2643 (2012). https://doi.org/10.1007/s00268-012-1721-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-012-1721-y