Abstract
To estimate strength of a scopolamine transdermal delivery system (TDS) in vivo, using residual drug vs. pharmacokinetic analyses with the goal of scientifically supporting a single and robust method for use across the dosage form and ultimately facilitate the development of more consistent and clinically meaningful labeling. A two-arm, open-label, crossover pharmacokinetic study was completed in 26 volunteers. Serum samples were collected and residual scopolamine was extracted from worn TDS. Delivery extent and rate were estimated by (1) numeric deconvolution and (2) steady-state serum concentration determined from graphical and non-compartmental analyses. In residual drug analyses, mean ± SD scopolamine release rate was 0.015 ± 0.002 mg/h (11% RSD), vs. 0.016 ± 0.006 mg/h (35% RSD) from numeric deconvolution, 0.015 ± 0.005 mg/h (34% RSD) from graphical analysis, and 0.015 ± 0.007 mg/h (44% RSD) from non-compartmental analysis. In residual drug analyses, total drug released was 1.09 ± 0.11 mg (10% RSD), vs. 1.12 ± 0.40 mg (35% RSD) from numeric deconvolution, 1.07 ± 0.35 mg (33% RSD) from graphical analysis, and 1.07 ± 0.45 (42% RSD) from non-compartmental analysis. Extent and rate of scopolamine release were comparable by both approaches, but pharmacokinetic analysis demonstrated greater inter-subject variability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Transdermal delivery systems (TDS) are noninvasive options for patients, achieving therapeutic drug concentrations over an extended period of time. As such, determining the extent and rate of drug delivery from TDS is critical for ensuring the safety of the patients wearing the systems. Strength of transdermal systems is expressed as a rate of drug delivery, and frequently, manufacturers derive this rate by human pharmacokinetic studies or determining the residual drug in a TDS after it has been worn (1). Exploring and understanding the differences between the two methods becomes vitally important when defining strength and subsequently in developing labeling across the dosage form. The need for identifying potential complications in developers utilizing two differing methods for strength determination, as well as the need for consistency and clinically meaningful labeling, is further underscored by the diversity of system designs, clinical indications, and doses of TDS being developed.
As an initial model drug, the Transderm Scōp® (scopolamine) TDS is a circular, 0.2 mm thick, 2.5 cm2 film containing 1.5 mg of scopolamine, indicated for prevention of motion sickness and nausea with a labeled strength of 1 mg/72 h (2). This study determined serum drug concentrations in healthy adults after wearing a scopolamine TDS and quantified residual drug post-wear.
The two primary approaches for determining strength for transdermal systems were compared and contrasted with the broader goal of facilitating the future development of a single, robust, and clinically meaningful method by which strength can be characterized across the dosage form.
MATERIALS AND METHODS
Materials
Scopolamine TDS, 1 mg/72 h (Transderm Scōp®, ALZA Corporation, Vacaville, CA) were purchased through the Investigational Drug Services Pharmacy at the University of Iowa. Scopolamine hydrobromide injection, USP, 0.4 mg/mL, was purchased from Omega Laboratories, LTD (Montréal, Québec Canada). Webcol™ alcohol swabs (Covidien, Mansfield, MA) were used in all studies. Chloroform, sulfuric acid, and glacial acetic acid were purchased from EMD Millipore (Burlington, MA, USA); methanol and ammonium hydroxide were purchased from Fisher Chemical (Pittsburgh, PA, USA). Ammonium acetate was purchased from Sigma Aldrich (St. Louis, MO, USA). The Milli-Q Integral water purification system was used for all TDS extraction studies.
Clinical Procedures
This was a two-arm, open-label, crossover pharmacokinetic study in 26 healthy adult volunteers. Procedures were approved by the University of Iowa Institutional Review Board and the Research Involving Human Subjects Committee (RIHSC) of the FDA and fulfilled the principles set forth in the Declaration of Helsinki. Written informed consent was provided by all subjects prior to enrollment. Study procedures were performed in the Clinical Research Unit at the University of Iowa Hospitals and Clinics. Healthy, non-smoking individuals between 18 and 65 years of age were enrolled. Suitability for participation was determined through baseline blood samples (complete blood count, basic metabolic panel, liver function tests, serum pregnancy test if applicable), urine samples (basic urinalysis and drug of abuse screen), and a complete drug/medical history and physical exam by the study physician. Subjects were excluded for the following criteria: < 18 or > 65 years of age, body mass index > 29.9, diagnosis of any chronic medical conditions, dermatologic conditions/cancers (exception of superficial basal cell carcinomas not involving the investigative site), and pregnant or lactating women. Subjects did not take any prescription medications within 30 days of study enrollment (oral contraceptives and vitamins were allowed) or any over-the-counter drugs within 3 days prior to and during the study.
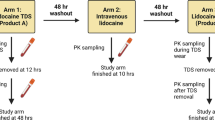
There were two study arms: (1) transdermal system application (Transderm Scōp®), worn for the prescribed wear time of 72 h; and (2) IV administration of scopolamine hydrobromide (0.4 mg dose for all subjects). Subjects in Group 1 completed the transdermal study arm first, followed by the IV study arm; those in Group 2 completed the study arms in the opposite order. There was a minimum washout period of 7 days between study arms, regardless of study group. Vital signs (blood pressure, heart rate, respiratory rate, and temperature) were measured and blood samples were collected throughout the study at predetermined time points for both study arms. The study schedule can be seen in Fig. 1.
TDS Application and Removal
Prior to TDS application, the postauricular area (behind the ear) was visually examined to ensure that the skin was healthy, clean, and relatively free of hair (excessive hair was clipped but not shaved). The area was gently cleansed with water and allowed to dry. A primary skin irritation assessment was performed prior to TDS application and graded from 0 to 4 using the scale described in Table I (3). System adhesion during wear was measured on a scale of 0 to 4, where 0 indicated ≥ 90% adhesion or no issues with adhesion and 4 indicated complete detachment of the TDS (Table II) (4).
A study team member removed the release liner from the TDS and applied the drug product to the postauricular area by applying firm pressure for ~ 30 s. The release liner was retained until system removal. To collect any residual drug that may have transferred to the drug pouch during storage, or to the investigator’s glove during application, an alcohol swab was used to wipe the inside of the pouch and any portion of the gloves that came in contact with the system. The alcohol swab was placed back into its original package and, along with the system’s coverslip, was retained for analysis (the coverslip is an additional clear liner included in the packaging of Transderm Scōp®). During storage, this liner rests on top of the TDS and is discarded during use. For study purposes, the coverslip was retained for analysis. Upon removal at 72 h, the system was placed back onto the release liner. The skin at the wear site and the gloves used during TDS removal were wiped with an alcohol swab that was retained for analysis. All TDS materials and alcohol swabs were shipped overnight at ambient conditions for residual drug content analysis.
Administration of Scopolamine Hydrobromide Injection
An IV catheter was placed in the antecubital vein to facilitate drug administration; this was placed in the opposite arm from the catheter used for serial blood sampling. Scopolamine hydrobromide, 0.4 mg dose, was injected slowly over 3 min. After completion of the injection, the IV catheter used for drug administration was removed.
Pharmacokinetic Sampling
During each study arm, an IV catheter was inserted into the subject’s antecubital vein on the first day to facilitate multiple blood draws (the catheter was removed before the subject left the clinic that evening). Serial blood draws were collected into vacutainer tubes. A baseline sample prior to drug administration was collected for both study arms. The TDS was applied, and then blood samples were collected at the following time points while the subject was wearing the TDS: 1, 2, 3, 4, 5, 6, 8, 10, 12, 24, 36, 48, 60, and 72 h. The TDS was removed at 72 h and additional samples were taken at 73, 74, 78, 84, 96, 108, and 120 h (these times correspond to hours after initial application of the TDS). Following IV administration of scopolamine hydrobromide, blood samples were collected within the first hour at the following times: 2.5, 5, 10, 20, 30, and 45 min. Additional samples were then collected at 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 24, 36, and 48 h (after the IV administration). After collection, each sample was set aside to rest at room temperature for ~ 1 h until the blood clotted, followed by centrifugation at 1300×g at 4°C for 20 min. Scopolamine is stable in human serum and there were no concerns for drug degradation during this time (5). The serum was immediately pipetted off and stored at − 80°C until analysis.
Extraction of Scopolamine from TDS and Alcohol Swabs
The TDS and resulting components from the procedure were placed in individual centrifuge tubes. For analysis of worn TDS samples, Tube 1 contained alcohol swabs used to wipe glove fingers, pouch, and coverslip; Tube 2 contained alcohol swabs used to clean adhesive and drug residue off the skin following TDS removal; Tube 3 contained the worn transdermal system, release liner, and alcohol swabs used to wipe the gloves. Unworn TDS from the same lot were analyzed as controls. For each control sample, Control Tube 1 contained one alcohol swab used to wipe both sides of the coverslip and the inside of the pouch; Control Tube 2 contained one unused TDS and its attached release liner. Ten milliliters of chloroform was added to each of the tubes. The samples were heated in a water bath (Fisher Scientific Isotemp GRD 05 Water Bath, Pittsburgh, PA, USA) at 60°C for 30 min and intermittently vortexed. The tubes were removed from the water bath and vortexed vigorously for 1 min and then allowed to cool to room temperature. Ten milliliters of 0.01 N sulfuric acid was added to each tube, which were then vortexed vigorously for 2 min. The tubes were centrifuged at 2000 rpm for 10 min (Thermo Jouan GR 422 refrigerated floor centrifuge, Pittsburgh, PA, USA) and an aliquot of the acid (upper) layer was transferred to a high-pressure liquid chromatography (HPLC) vial for analysis.
High-Pressure Liquid Chromatography Assay
Scopolamine concentrations in TDS and alcohol swabs were quantified using high-pressure liquid chromatography (HPLC) with UV detection. The HPLC system consisted of an Agilent-1260 HPLC system with a 1260 DAD G4212B detector, 1260 G1329B injector, and 1260 Bin Pump G1312B. The mobile phase was 60:40 water:methanol 0.075 M ammonium acetate, adjusted to pH 7.2. A USP L1 Axxiom ODS column (3 μm, 50 × 4.6 mm) was used. The UV wavelength was 220 nm, 1.0 mL/min flow rate, and 10 μL injection volume. Scopolamine hydrobromide standards were prepared in 0.01 N sulfuric acid over in the range of 5–200 μg/mL. All standards displayed excellent linearity over the entire concentration range (R2 = 0.9999).
Serum Drug Extraction Assay
Scopolamine was extracted from 0.5 mL of serum using a solid-phase extraction assay that was developed in-house and previously published (6). Extracted drug was dissolved in water:acetonitrile (90:10) containing 0.1% formic acid, transferred to glass vials and centrifuged at 10,000 rpm for 5 min, then analyzed by LC-MS/MS. The chromatographic separation was performed using a Zorbax XDB-C18 column (4.6 × 50 mm, 1.8 μm, and 600 bar) on an Agilent 1200 series HPLC; a gradient elution with water and acetonitrile containing 0.1% v/v formic acid was the mobile phase; flow rate was 0.5 mL/min. The precursor to product ion transition for scopolamine was 304.10 → 138.10. Samples were quantified in positive ion mode using a TSQ Quantum Classic mass spectrometer (Thermo Fisher) housed at the Clinical Pharmacology Analytical Service lab (CPAS) of the College of Pharmacy, University of Minnesota. Extraction and detection procedures were validated for accuracy (96%), precision (6.3%), and intra-day and inter-day variability. The lower limit of quantification of the assay was 5 pg/mL.
Pharmacokinetic Analyses
Analyses of Scopolamine Administered as an IV Bolus Injection
Phoenix Winonlin 7.0 (Certara) software was used for pharmacokinetic modeling of scopolamine concentration-time profiles. Area under the serum drug concentration-time curve (AUC0-t) was determined from 0 to 48 h. In addition to non-compartmental analysis (NCA), compartmental analysis was conducted by fitting a 1-compartment or a 2-compartment model to the data. The best model was selected based on the visual assessment of the best-fit curve to the data and the residual plots as well as by comparing the diagnostic parameters: Weighted Sum Square Residuals (WSSR), Akaike Information Criteria (AIC), and Schwarz Bayesian Criteria (SBC). Iterative reweighting (1/y2) was employed to improve the model fit. Further, F-ratio test (Eq. 1) was used to assess the significance of fitting the data to a 2-compartment model vs a 1-compartment model (7,8,9).
where df(1-comp) = 14 df (2-comp) = 12; F(2,12) was calculated using Eq. 1 and the calculated values were compared with the table F(2,12) values at p = 0.05.
Analyses of Scopolamine After TDS Administration
Pharmacokinetic parameters were obtained by performing NCA using Phoenix WinNonlin 7.0 (Certara) and graphical analysis. Maximum serum concentration during TDS wear (Cmax), time of maximum serum concentration (Tmax), elimination rate constant (Kel), serum elimination half-life (T1/2), and clearance (CL) were estimated for both study arms (Fig. 2). The AUC was calculated for 2 periods: 0–72 h (time of wear) and 0–120 h (total sampling time).
For the TDS arm, the concentration at steady state (Css) after application of TDS was determined in two ways: (a) according to Eq. 2 where AUC0–72 was obtained by NCA, indicated as Css (Average), and (b) by graphical estimation using Eq. 3Css (graph).
Equation 2 above represents average concentration over a specific period of time and is not a universal formula to estimate Css for a transdermal system. In this analysis, scopolamine approached steady state rapidly within 12 h after TDS application (Fig. 2). Hence, Css(Average) over 72 h is expected to provide an approximation of steady-state concentration.
From the concentration-time plot, the average concentration between 24 and 72 h after TDS administration was estimated using 5 time points at 12-h intervals C24, C36, C48, C60, and C72 (Fig. 2). These data points were chosen based on visual assessment of the concentration-time plot obtained after TDS application.
The Kel was estimated using serum concentrations obtained after TDS removal at 72 h through the end of the sampling period (i.e., from 72 to 120 h). Lag time (Tlag), which is the time required to reach detectable serum drug concentrations at the limit of quantification (5 pg/mL), was determined using a model-independent method as described previously (10). Briefly, a quadratic equation was fitted to at least the first three serum concentrations above the limit of quantification. Tlag was then estimated by extrapolation of the curve to the time axis.
Rate and extent of drug released from TDS to subject: Total drug released from the TDS to the subject, referred to as strength, was estimated according to Eq. 4:
where 1.61 mg is the average amount of scopolamine base present in the TDS, determined from assaying unused TDS (n = 3; SD = 0.03; 1.9% RSD), A is scopolamine recovered from the packaging pouch (mg), B is the scopolamine recovered from cotton swabs that were used to wipe the skin surface that was in contact with the TDS (mg), and C is drug recovered from worn TDS (mg). Drug strength for this study is described per wear period (72 h), and the rate per hour can be estimated according to Eq. 5:
Pharmacokinetic determination of rate and extent of drug release: Rate of scopolamine release from TDS was determined by (1) numeric deconvolution and (2) using serum Css estimates.
Determination of Absorption Rate Using the Deconvolution Method
A numerical deconvolution method, which is a model-independent method that does not assume first order absorption, was employed to evaluate drug release and absorption from scopolamine TDS (11,12). Several assumptions were made when applying the numeric deconvolution model to estimate input rate. First, each and every scopolamine molecule entering the body via skin has the same probability of reaching the drug sampling site (blood). Second, scopolamine transport from skin to blood is a random and an independent event and any potential intermolecular scopolamine interactions do not affect this transit. Third, processes influencing scopolamine transit from point skin to blood are time-invariant. Fourth, scopolamine concentration in blood and serum is the same. Last, no assumptions regarding kinetics of scopolamine absorption are made. However, distribution and elimination are assumed to follow linear kinetics. Under these conditions, the scopolamine input rate can be estimated using Eq. 6:
where C(t) is scopolamine concentration in serum at time t after TDS application. The f(t) denotes scopolamine input rate from TDS; * is a convolution operator; Cδ(t) denotes unit impulse response (UIR). This was obtained by initially fitting the serum concentration-time profile of each subject obtained after IV bolus administration to a 2-compartment PK model. As the UIR denotes response from one drug unit, the resulting parameters A and B were divided by the stripping dose (0.4 mg used in IV studies) to obtain A1 and A2. The micro rate constants Alpha and Beta served as α1 and α2 and then the UIR was estimated using Eq. 7, where N denotes the number of exponential terms.
Since the UIR was obtained from a 2-compartment fit, N = 2. The drug input rate f(t) was obtained via an iterative procedure known as the deconvolution through convolution (DTC) method and then the extent of scopolamine released was calculated as the cumulative drug input (Eq. 8):
Estimation using Css
As an alternative method, the Css of scopolamine was estimated from Eqs. 2 and 3 and the input rate was calculated using Eq. 9:
The CL was obtained from 2-compartment fit of plasma concentration vs. time data following IV bolus administration for the same individual. The cumulative amount of drug that reached serum over 72 h was estimated by Eq. 10:
RESULTS
Twenty-six subjects enrolled in the study, and 23 of them completed both arms. One subject withdrew consent for undisclosed reasons after the TDS arm. One subject was withdrawn by the study team after the IV arm for no longer meeting inclusion/exclusion criteria, and the other withdrew after the IV study arm. The Mean ± SD age for the 26 subjects was 24.3 ± 6.3 years, and there were 10 males and 16 females. Demographics of the subjects are provided in Table S1.
Adverse Events and TDS Adhesion
No unexpected, serious, or life-threatening adverse events occurred. The most common events during the TDS arm were dry mouth/increased thirst, decreased heart rate, and vision disturbances. The most common events during the IV arm were dizziness/lightheadedness, decreased heart rate, and fatigue (Table III). There was minimal to no skin irritation from TDS wear; all primary skin irritation assessment scores were 0 or 1 in all subjects after TDS removal. TDS adhesion scores were “0” for all subjects through the 72 h TDS wear time (indicating full adhesion to the skin), with only one subject with a score of “1” recorded immediately before the TDS was removed.
Pharmacokinetic Serum Analysis
Concentration-time profiles of scopolamine after IV bolus administration were analyzed by noncompartmental analysis (NCA) and compartmental modeling. Data fitted to a 2-compartment model had the lowest AIC, WSSR, and SBC (Table S2) and calculated F-ratio values were high for most subjects compared with the tabled F(2, 12) value at p = 0.05 of 19.41. Calculated F values were lower than tabled F values for Subjects 7 and 13. However, the 2-compartment fit was still applied to these subjects taking into consideration the other diagnostic parameters (AIC and SBC). Pharmacokinetic parameters obtained by 2-compartmental modeling and NCA are presented in Tables S3 and S4, respectively.
Of the 23 subjects that completed both arms of the study, IV data for two subjects exhibited unusual serum concentration vs time profiles. Subject 2 exhibited unusually low serum concentrations and Subject 8 exhibited Cmax and Tmax at 0.5 h. Hence, these subjects were not considered for final parameter estimates. Pharmacokinetic analyses of data from the 21 out of 23 subjects who completed both IV and TDS arms (Table IV) were used in the final estimation of rate and extent of scopolamine delivery from the TDS.
Residual Drug in Worn TDS and Calculated Drug Release
Mean (± SD) drug loading of three unworn control TDS samples was 1.61 ± 0.03 mg (n = 3; 1.9% RSD). No scopolamine was detected in any alcohol swabs used to wipe the inside of the drug pouch or application gloves (A in Eq. 4). Conversely, drug was detected in all swabs used to clean drug residue and adhesive from the skin following TDS removal (B in Eq. 4). Mean drug recovery of 24 worn TDS was 0.47 mg ± 0.11 (23% RSD). Using residual drug analysis and Eq. 4, the mean drug released to the subject (i.e., delivered as calculated by this method) in 72 h was 1.09 mg ± 0.11 (10% RSD) (Table V).
Pharmacokinetic Determination of Rate and Extent of Release
In pharmacokinetic analyses, scopolamine absorption rate (mean ratein ± SD) and cumulative amount released was estimated by numerical deconvolution as well as using Css estimated from Eqs. 2 and 3. The absorption rates obtained by these methods are presented in Table VI. The absorption rate estimated using the deconvolution method was 0.016 ± 0.006 mg/h (35% RSD). From the Css (graph) value obtained by graphical analysis and CL estimated from 2-compartment fit of the IV bolus data (Fig. 2), the absorption rate was determined to be 0.015 ± 0.005 mg/h (34% RSD). Using Css (average), the calculated absorption rate was 0.015 ± 0.007 mg/h (44% RSD). Total drug released over 72 h as estimated by these approaches was 1.12 ± 0.4 mg (35% RSD), 1.07 ± 0.35 mg (33% RSD), and 1.07 ± 0.45 mg (42% RSD), respectively.
DISCUSSION
The typical convention for describing drug delivery rate from a TDS is mass of drug delivered per dosage form per unit of time, delivered throughout the prescribed wear time. This rate also quantifies TDS strength. Because of diffusion principles governing passive drug delivery, a significant drug amount remains in TDS after being worn. This residual drug, and pharmacokinetic data from humans, provides complementary means for assessing product strength. Based on assumptions of each method when calculating strength, there are limitations to both approaches and thus the methods require further scrutiny and direct comparison.
Scopolamine is a small molecule with a molecular weight of 303 Da and logP of 0.98 (13). These are within typical parameters of drugs suitable for transdermal delivery (14). Transderm Scōp® contains 1.5 mg of scopolamine base. In control samples, we estimated scopolamine amount to be 1.61 mg. According to prescribing information, the TDS is formulated to deliver ~ 1 mg of scopolamine over 3 days. Our calculation of total (mean ± SD) scopolamine in serum over the 72-h period was 1.09 ± 0.11 mg, which agrees with the prescribing information.
The main objective of this work was to compare two approaches for estimating rate and extent of delivery from a transdermal system to calculate strength. Here, we explored two methods of analysis for quantifying drug delivery from TDS in healthy individuals wearing a scopolamine TDS: analysis of serum drug concentrations and determination of drug remaining in TDS after prescribed wear (72 h). The estimates obtained by both methods were similar. Using residual drug analysis approach, the rate of scopolamine delivery was 1.09 ± 0.11 mg/72 h (0.015 ± 0.002 mg/h). Pharmacokinetic analyses were conducted using three different methods. All of them estimated the extent of delivery (i.e., strength) in 72 h as 1.07–1.12 mg with standard deviations ranging between 0.35–0.45 mg, whereas the rates of delivery were between 0.015 and 0.016 mg/h with standard deviations ranging between 0.005–0.007 mg/h. Thus, the estimates obtained by both approaches were very similar, though there was considerably greater variability associated with the estimated rate using the pharmacokinetic approach. Large inter-subject variabilities in pharmacokinetic parameters have been previously reported following scopolamine administration by IV (15,16), transdermal (17), and other routes (18,19). The %RSD in these previous studies was as large as 50%. For example, in IV studies performed by Putcha et al., the reported pharmacokinetic estimates translated to 90%, 50%, and 19% RSD, for T1/2, volume of distribution, and CL, respectively (15). In IV studies reported by Ebert et al., %RSD for CL and volume of distribution were 56% and 37%, respectively (16).
Here, we observed up to 44% RSD for scopolamine delivery rate and extent when determined via the pharmacokinetic method. This likely arises from the variability in associated pharmacokinetic parameters that were used in the calculations (e.g., RSD for AUC0–72 and CL were 38.4% and 37.5% respectively). For transdermal systems, AUC is dependent on percutaneous absorption rate, systemic uptake, and CL. Factors such as body and skin temperature, hydration, and ethnicity-based skin variations can affect absorption through the skin (20). Subjects of different races and ethnicities participated in our study, and while this makes the data more generalizable overall, inter- and intra-subject differences could contribute to variation in the pharmacokinetic parameters.
CONCLUSIONS
This study demonstrates that analyses of residual drug in worn TDS and the corresponding pharmacokinetic parameters result in similar nominal delivery rates, though the residual drug analysis method had less variability overall. The residual drug method had the advantage that it did not necessitate the administration of an IV, blood sampling, or plasma drug extraction. Thus, these analyses could be less time intensive and potentially less burdensome for drug developers and study subjects. Additional studies with other active pharmaceutical ingredients, intended delivery rates, and transdermal system designs are necessary to work towards the long-term goal of developing a single method by which strength can be characterized across the dosage form.
Abbreviations
- TDS:
-
Transdermal delivery system
References
Strasinger C, Raney SG, Tran DC, Ghosh P, Newman B, Bashaw ED, et al. Navigating sticky areas in transdermal product development. J Control Release. 2016;233:1–9.
Transderm Scōp® package insert. In: Alza C, editor. Vacaville, CA.2017.
Farage MA, Maibach HI, Andersen KE, Lachapelle JM, Kern P, Ryan C, et al. Historical perspective on the use of visual grading scales in evaluating skin irritation and sensitization. Contact Dermatitis. 2011;65(2):65–75.
FDA. Transdermal and Topical Delivery Systems – Product development and quality considerations draft guidance for industry. 2019. Available from: https://www.fda.gov/media/132674/download Last accessed March 6, 2020.
John H, Binder T, Hochstetter H, Thiermann H. LC-ESI MS/MS quantification of atropine and six other antimuscarinic tropane alkaloids in plasma. Anal Bioanal Chem. 2010;396(2):751–63.
Swaminathan SK, Fisher J, Brogden NK, Kandimalla KK. Development and validation of a sensitive LC-MS/MS method for the estimation of scopolamine in human serum. J Pharm Biomed Anal. 2018;164:41–6.
Schwarz G. Quantitative analysis of activation and inactivation of asymmetry currents in biological membranes, based on a conformational transition model. J Membr Biol. 1978;43(2–3):149–67.
Boxenbaum HG, Riegelman S, Elashoff RM. Statistical estimations in pharmacokinetics. J Pharmacokinet Biopharm. 1974;2(2):123–48.
Bourne, DWA. Mathematical modeling of pharmacokinetic data. (Technomic Publishing Co., Inc: Lancaster, PA, 1995).
Csizmadia F, Endrenyi L. Model-independent estimation of lag times with first-order absorption and disposition. J Pharm Sci. 1998;87(5):608–12.
Gibaldi M, Perrier D. Pharmacokinetics. 2nd edn. CRC Press (Taylor and Francis Group, Boca Raton, FL, 1982).
Kim YC, Song JM, Lipatov AS, Choi SO, Lee JW, Donis RO, et al. Increased immunogenicity of avian influenza DNA vaccine delivered to the skin using a microneedle patch. Eur J Pharm Biopharm eV. 2012;81(2):239–47.
Pubchem. [Available from: https://pubchem.ncbi.nlm.nih.gov/compound/5184.] Last accessed April 8, 2018.
Chandrashekar NS, Shobha Rani RH. Physicochemical and pharmacokinetic parameters in drug selection and loading for transdermal drug delivery. Indian J Pharm Sci. 2008;70(1):94–6.
Putcha L, Cintron NM, Tsui J, Vanderploeg JM, Kramer WG. Pharmacokinetics and oral bioavailability of scopolamine in normal subjects. Pharm Res. 1989;6(6):481–5.
Ebert U, Grossmann M, Oertel R, Gramatte T, Kirch W. Pharmacokinetic-pharmacodynamic modeling of the electroencephalogram effects of scopolamine in healthy volunteers. J Clin Pharmacol. 2001;41(1):51–60.
Nachum Z, Shupak A, Gordon CR. Transdermal scopolamine for prevention of motion sickness : clinical pharmacokinetics and therapeutic applications. Clin Pharmacokinet. 2006;45(6):543–66.
Ebert U, Siepmann M, Oertel R, Wesnes KA, Kirch W. Pharmacokinetics and pharmacodynamics of scopolamine after subcutaneous administration. J Clin Pharmacol. 1998;38(8):720–6.
Kanto J, Kentala E, Kaila T, Pihlajamaki K. Pharmacokinetics of scopolamine during caesarean section: relationship between serum concentration and effect. Acta Anaesthesiol Scand. 1989;33(6):482–6.
Singh I, Morris AP. Performance of transdermal therapeutic systems: effects of biological factors. Int J Pharm Investig. 2011;1(1):4–9.
Funding
This project was funded by the following grants: National Institute for Pharmaceutical Technology and Education U01 Critical Patch Manufacturing Sector Research Initiative (5U01FD004275), and NIH/NCATS CTSA U54TR001356.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Disclaimer
Views expressed in this manuscript do not necessarily reflect official policies of the United States Food and Drug Administration; nor does any mention of trade names, commercial practices, or organization imply endorsement by the United States Government.
Additional information
Guest Editors: Ajaz S. Hussain, Kenneth Morris, and Vadim J. Gurvich
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 31 kb)
Rights and permissions
About this article
Cite this article
Swaminathan, S.K., Strasinger, C., Kelchen, M. et al. Determination of Rate and Extent of Scopolamine Release from Transderm Scōp® Transdermal Drug Delivery Systems in Healthy Human Adults. AAPS PharmSciTech 21, 117 (2020). https://doi.org/10.1208/s12249-020-01658-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-020-01658-4