Abstract
Application of heat (hyperthermic conditions) on skin is known to enhance drug transfer and facilitate skin penetration of molecules. The aim of this work was to study the effect of hyperthermia on the drug release and skin permeation from nicotine transdermal patches. The drug release and skin permeation were characterized by in vitro release test and in vitro permeation test. The temperature was maintained at 32 °C as control (simulating normal physiological skin temperature) and 42 °C as hyperthermia condition. The in vitro release test was carried out using USP apparatus 5-Paddle over disk method for a transdermal patch. Skin permeation study was carried out across porcine skin using the flow through cells (PermeGear, Inc.) with an active diffusion area of 0.94 cm2. Mechanistic studies (parameters such as partition coefficient, TEWL and electrical resistivity) were also performed to understand the mechanisms involved in determining the influence of hyperthermia on drug delivery from transdermal patches of nicotine. The rate and extent of drug release from nicotine patch was not significantly different at two temperatures (Cumulative release after 12 h was 43.99 ± 3.29% at 32 °C and 53.70 ± 5.14% at 42 °C). Whereas, in case of in vitro permeation studies, the nicotine transdermal permeation flux for patch was threefold higher at 42 °C (100.1 ± 14.83 μg/cm2/h) than at 32 °C (33.3 ± 14.83 μg/cm2/h). The mechanistic studies revealed that the predominant mechanism of enhancement of drug permeation by hyperthermia condition is by the way of increasing the skin permeability. There is a potential concern of dumping of higher dose of nicotine via transdermal route.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Transdermal route of delivery of drugs has been considered as an alternative to oral dosage forms, owing to its ability to overcome the limitations associated with oral route or delivery, such as first pass metabolism, poor bioavailability, and patient noncompliance (1). Although stratum corneum is a formidable barrier to the transdermal administration of therapeutic agents due to its unique structure and composition, it is significantly permeable to moderately lipophilic smaller molecular weight substances. Studies have reported that higher temperatures could modulate skin permeability thus leading to enhanced bioavailability of drugs (2,3). Most of the earlier studies investigated the influence of temperature on skin permeability using in vitro models at temperatures higher than physiologically relevant. Several reports are available in literature as examples where the exposure of transdermal patches in patients to elevated temperatures, mainly from heating pads, climate, exercise, saunas, and fever has led to enhanced performance of transdermal patches (4,5).
Cases have been reported on elevated absorption of nitroglycerine from the patches, on exposure to heat (3,6,7). Similar cases have also been reported with nicotine patch, which showed an elevated drug uptake on exposure to heat (8,9). Though, the effect of heat has been studied extensively both in vitro and in vivo, various parameters such as the amount of drug permeated, the change in flux values and the mechanism by which an enhanced release is obtained is still unknown. Some studies reported that, heat could lead to increased kinetic energy/diffusion coefficient of drug in the patch system, which in turn could lead to increased drug release rate and enhanced systemic drug absorption (4,5). In one of the studies, Petersen and coworkers investigated transdermal uptake from nicotine patch on human volunteers at locally applied controlled heating at 43 °C and they concluded that increased uptake of nicotine was due to increased regional blood flow caused due to increased body temperature (10,11,12). Shoemaker and others examined the effects of locally applied heat on the systemic delivery of fentanyl through different transdermal delivery system (13,14,15). The relative contribution of mechanisms appears to vary with the kind of transdermal systems and also is apparently driven by the physicochemical properties of the active pharmaceutical ingredient.
Hyperthermia is a condition in which the body temperature elevates by a few degrees Celsius. The reason for hyperthermia could be due to exposure, contact of physiological abnormality. The influence of hyperthermia condition on the bioavailability of drugs from transdermal patches is of interest from the perspective of understanding the potential for drug-induced toxicity and side effects. The aim of the present study was to investigate the mechanism involved and the impact of hyperthermia (temperature higher than body temperature) condition on the drug delivery of nicotine from transdermal patches. Nicotine is a colorless liquid with a fishy odor. It has a melting point of − 80 °C. It is miscible in water and highly soluble in alcohol. Nicotine is a model lipophilic molecule and represents many such moderately lipophilic molecules available in the form of transdermal patches.
MATERIALS AND METHODS
Materials
Nicotine transdermal system patches (Equate, Walmart Stores, Inc., Bentonville, AR 72716), (dose, 21 mg over 24 h) was purchased from the local pharmacy. Nicotine was purchased from Alfa Aesar, USA. HPLC grade solvents like methanol and ethanol were purchased from Fisher Chemicals, USA. The porcine skin was obtained from Pontotoc slaughtering house, MS, USA.
Methods
Analytical Method
Nicotine was quantified by a validated method using HPLC. The HPLC system consisted of a variable wavelength UV detector and an analytical C18 column (4.6 × 250 mm). The solution of nicotine in water system was prepared at 0.5–8000 ng/ml for developing a calibration curve by HPLC method. Calibration curve was plotted for nicotine in standard and fitted by linear regression. The HPLC conditions was developed and standardized (flow rate = 1 mL/min, with isocratic mobile phase of 50:50 v/v, methanol and 10 mM phosphate buffer, λmax = 260 nm).
Evaluation of Transdermal Patches
The transdermal patch system was obtained and subjected to in vitro release testing and in vitro permeation testing at physiological (32 ± 1 °C) and hyperthermia condition 42 ± 1 °C.
In vitro Release Test
The in vitro release studies were carried out as per the United States Pharmacopeia using apparatus 5, paddle over disk method for transdermal patches. The release media consisted of 150 ml of phosphate buffer solution (PBS) at pH 6.6. The study was conducted at a temperature of 32 °C and 42 °C (hyperthermia temperature) with a paddle speed of 50 rpm (16). At predetermined time intervals of 0, 2, 4, 6, 8, 10, and 12 h, 1 ml of the sample was withdrawn (and filtered using a 10-μm filter) and the receiver media was replaced with fresh PBS of the same volume. The samples withdrawn were then analyzed by HPLC.
In vitro Permeation Test
The freshly excised porcine skin samples were washed carefully with saline. Skin permeation study was carried out in Flow through cell apparatus (ILC07 automated system, PermeGear, Inc., USA) having a diffusion area of 0.94 cm2. Full thickness porcine skin was sandwiched between the donor and the receiver chamber of the flow through cells. The rate of flow of receiver fluid was set to 66 μL/min. Temperature was maintained at 32 °C and 42 °C as hyperthermia temperature. Sampling was carried out at predetermined time intervals of 0, 2, 4, 6, 8, 10, and 12 h. The samples were analyzed for nicotine using HPLC.
Mechanistic Studies
Partition Coefficient
Partition coefficient for nicotine at two temperatures (32 °C and 42 °C) was determined using octanol-water solvent system (17). A known amount of nicotine was dissolved in octanol saturated with water in 1:1 v/v ratio. The entire setup was stirred at 32 °C and 42 °C for 24 h. The phases were separated and analyzed with HPLC. The partition coefficient was calculated as the logarithm of the ratio of solute concentration between the two solvents.
In vitro Trans Epidermal Water Loss Measurements
Full thickness porcine skin was mounted between the donor and receiver compartments of vertical Franz diffusion cells. The receiver compartment was filled with 5 ml of PBS (pH = 7.2) and allowed to equilibrate for 2 h at 32 ± 1 °C (control) and 42 ± 1 °C (hyperthermia condition). Trans epidermal water loss (TEWL) was measured using a Vapometer® (Delfin technologies INC., Stamford, CT). The Franz cell adapter of the Vapometer® was placed on the donor compartment and the TEWL measurements were recorded at regular time intervals (18).
Electrical Resistance
Full thickness porcine skin was sandwiched between donor and receiver compartments of the Franz diffusion cell. Both the compartments were filled with 400 μl (donor) and 5 ml (receiver) of PBS (pH = 7.2). The electrical resistance across the skin was measured at 32 ± 1 °C and 42 ± 1 °C by placing a load resistor RL (100 kΩ) in series with skin. The voltage drop across the whole circuit (VO) and skin (Vep) was approximated with the aid of a waveform generator and a digital multimeter. A voltage of 100 mV was applied at 10 Hz and the skin resistance in kΩ was approximated. After the inherent electrical resistivity of the skin was recorded, the resistance drop across the skin was investigated (18,19). The skin resistance was approximated using the formula:
where Rep is skin resistance and RL is the load resistance in kΩ.
Statistical Analysis
All the data were analyzed with Graph Pad Prism 7 using Student’s t test for single unpaired comparison between two groups. The difference was considered significant when p value < 0.05. Each data point represents a mean of three in all experiments except in vitro permeation test, in which each data point was a mean of five trials ± standard deviation (SD).
RESULTS AND DISCUSSION
In vitro Release Test
The percentage drug release from the nicotine patch after 12 h was 53.70 ± 5.14% at 42 °C and 43.99 ± 3.29% at 32 °C. The in vitro release profile is shown in Fig. 1. Based on the R2 values obtained from various kinetic models, the mechanism of release profile was best explained by using Higuchi kinetics as shown in Fig. 2. The release rate of drug is apparently showed an increasing trend at hyperthermia condition. However, statistical calculations did not demonstrate a significant difference between the flux values obtained at two temperatures.
In vitro Permeation Test
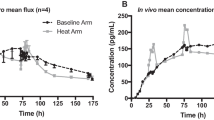
The in vitro permeation profile across the skin model is a great tool to understand the difference in performance of transdermal systems subjected to different experimental variables. The results of permeation profile of nicotine at temperature 32 °C and at 42 °C are shown in Fig. 3. The cumulative amount of nicotine that permeated in 12 h across the skin at 32 °C and 42 °C was 413.8 ± 83. 70 μg/cm2 and 1279.5 ± 289.58 μg/cm2 respectively (p value < 0.05). This was due to a threefold difference transdermal flux of nicotine (Fig. 4) at 42 °C (100.1 ± 14.83 μg/cm2/h) compared to that at 32 °C (33.3 ± 14.83 μg/cm2/h). There could be two possible mechanisms responsible for an enhanced permeation of nicotine at hyperthermia condition. First is due to interaction of heat with the patch leading to increased drug release. Second is due to interaction of heat with the skin thus leading to modulation of skin permeability to nicotine. Therefore, to better understand the relative contribution of these two mechanisms, further mechanistic studies were undertaken.
Mechanistic Studies
Partition Coefficient
The partition coefficient (Koctanol/Water) for nicotine at 32 °C and 42 °C was 11.058 ± 0.021 and 8.937 ± 0.024 respectively (p < 0.05). The small decrease in Koct/water value could be due to an increased solubility of nicotine at higher temperatures, which is similar to previously reported cases (Table I) (19,20). Bahadur et al. studied the effect of temperature (4 to 45 °C) for the partition coefficient of chlorobenzene in an octanol-water system where a decrease in partition coefficient was seen with increasing temperature range. Temperature dependence on partition coefficient is an important variable but is usually not considered. Partition coefficient has been correlated with skin permeability. Significant change in this property influenced by the temperature could affect the transdermal absorption of drugs. In the present study, it is evident that elevated temperature did not lead to notable change in the log p value.
In vitro Transepidermal Water Loss Measurements
Water evaporates at a constant rate from the interior of the skin to the surface of skin due to the water gradient across the skin layers. TEWL is an important characteristic of skin that indicates the skin integrity. Any damage to the stratum corneum would lead to changes in the TEWL. It has been reported that body temperature influences the TEWL (21,22). The TEWL can be used as an indirect measure of skin permeability and barrier function in the absence of sweating (23,24). It was found that the TEWL for skin at 42 °C was threefold higher than for the porcine skin at 32 °C. Transepidermal water loss for skin at 42 °C was 42.07 ± 1.96 (g/m2/h) and 18.43 ± 3.16 (g/m2/h) at 32 °C (p value < 0.05).
Electrical Resistance
Transdermal electrical resistance is one of the tools used for evaluation of skin barrier integrity (25,26). A drop in the resistance indicates a compromise in the stratum corneum barrier integrity. In this study, the effect of temperature was on the integrity of porcine skin was investigated. Which could be a parameter affecting enhanced permeation of nicotine (18,27). It was found that there was a sudden drop (38%) in electrical resistance at 42 °C (43 ± 2.30 Ω) within 10 min, which remained constant over the period of experiment. The electrical resistivity at 32 °C did not change significantly over the course of the experiment (Fig. 5). Whereas for the skin at 42 °C, a drop in electrical resistivity was seen after 10 min which after 20 min dropped to significance level as compared to drop in electrical resistivity observed at 32 °C. This drop in electrical resistance along with increased TEWL supports the fact the mild hyperthermia conditions increase the skin permeability (28).
Overall results indicate that elevated temperature has led to an increase in the skin permeability which in turn would reflect the increased performance of nicotine patch at elevated temperature. However, the role of enhanced regional blood circulation at hyperthermia could not be delineated in this work due to limitation of the in vitro model used in the experiment.
CONCLUSION
Hyperthermia condition was found to increase the release of nicotine from the transdermal patches at levels that were not statistically significant. However, the permeability of skin was enhanced significantly at hyperthermia condition as indicated by enhanced drug flux, drop in electrical resistance of the skin and increased TEWL values. Hyperthermia condition could lead to enhanced bioavailability of drugs. Often it could lead to surge in the plasma drug levels causing potential side effects.
Abbreviations
- HPLC:
-
High performance liquid chromatography
- UV:
-
Ultraviolet
- PBS:
-
Phosphate buffer saline
- TEWL:
-
Trans epidermal water loss
- RL :
-
Load resistor
- V O :
-
Voltage drop across entire circuit
- V ep :
-
Voltage drop across skin
- R ep :
-
Skin resistance
- USP:
-
United States Pharmacopeia
References
Petersen KK, Rousing ML, Jensen C, Arendt-Nielsen L, Gazerani P. Effect of local controlled heat on transdermal delivery of nicotine. Int J Physiol Pathophysiol Pharmacol. 2011;3(3):236–42.
Vanakoski J, Seppälä T, Sievi E, Lunell E. Exposure to high ambient temperature increases absorption and plasma concentrations of transdermal nicotine. Clin Pharmacol Ther. 1996 Sep 1;60(3):308–15.
Rose PG, Macfee MS, Boswell MV. Fentanyl transdermal system overdose secondary to cutaneous hyperthermia. Anesth Analg. 1993;77:390–1.
Vanakoski J, Seppala T. Heat exposure and drugs. A review of the effects of hyperthermia on pharmacokinetics. Clin Pharmacokinet. 1998;34:311–22.
U.S. Food and Drug Administration. FDA issues second safety warning on fentanyl skin patch. Deaths and serious injuries from improper use; 2007; Available from: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2007/ucm109046.htm.
Newshan G. Heat-related toxicity with the fentanyl transdermal patch. J Pain Symptom Manag. 1998;16:277–8.
Barkve TF, Langseth-Manrique K, Bredesen JEandGjesdal K. Increased uptake of transdermal glyceryl trinitrate during physical exerciseand during high ambient temperature. Am Heart J. 1986;112:537–41.
Sindali K, Sherry K, Sen S, Dheansa B. Life-threatening coma and full-thickness sunburn in a patient treated with transdermal fentanyl patches: a case report. J Med Case Rep. 2012;6:220.
Shin SH, Ghosh P, Newman B, Hammell DC, Raney SG, Hassan HE, et al. On the road to development of an in vitro permeation test (IVPT) model to compare heat effects on transdermal delivery systems: exploratory studies with nicotine and fentanyl. Pharm Res. 2017;34(9):1817–30. https://doi.org/10.1007/s11095-017-2189-0.
Moore KT, Sathyan G, Richarz U, Natarajan J, Vandenbossche J. Randomized 5-treatment crossover study to assess the effects of external heat on serum fentanyl concentrations during treatment with transdermal fentanyl systems. J Clin Pharmacol. 2012;52:1174–85.
Forster M, Bolzinger MA, Fessi H, Briancon S. Topical delivery of cosmetics and drugs. Molecular aspects of percutaneous absorption and delivery. Eur J Dermatol. 2009;19:309–23.
Paudel KS, Milewski M, Swadley CL, Brogden NK, Ghosh P, Stinchcomb AL. Challenges and opportunities in dermal/transdermal delivery. Ther Deliv. 2010;1:109–31.
Wiedersberg S, Guy RH. Transdermal drug delivery: 30+ years of war and still fighting! J Control Release. 2014;190:150–6.
Park J-H, Lee J-W, Kim Y-C, Prausnitz MR. The effect of heat on skin permeability. Int J Pharm. 2008;359:94–103.
Shomaker TS, Zhang J, Ashburn MA. Assessing the impact of heat on the systemic delivery of fentanyl through the transdermal fentanyl delivery system. Pain Med. 2000;1:225–30.
Thong HY, Zhai H, Maibach HI. Percutaneous penetration enhancers: an overview. Skin Pharmacol Physiol. 2007;20:272–82.
Hafeez F, Chiang A, Hui X, Maibach H. Role of partition coefficients in determining the percutaneous penetration of salicylic acid and formaldehyde under varying occlusion durations. Drug Dev Ind Pharm. 2014;40(10):1395–401. https://doi.org/10.3109/03639045.2013.828218.
Manda P, Angamuthu M, Hiremath SR, Raman V, Murthy SN. Iontophoretic drug delivery for the treatment of scars. J Pharm Sci. 2014;103(6):1638–42. https://doi.org/10.1002/jps.23946.
Noubigh A, Mgaidi A, Abderrabba M. Temperature effect on the distribution of some phenolic compounds: an experimental measurement of 1-octanol/water partition coefficients. J Chem Eng Data. 2010;55(1):488–91. https://doi.org/10.1021/je900271h.
Noubigh A, Abderrabba M, Provost E. Temperature and salt addition effects on the solubility behaviour of some phenolic compounds in water. J Chem Thermodyn. 2007;39:297–303.
Plessis J d, Stefaniak A, Eloff F, John S, Agner T, Chou T, et al. International guidelines for the in vivo assessment of skin properties in non-clinical settings: part 2. Transepidermal water loss and skin hydration. Skin Res Technol. 2013;19(3):265–78. https://doi.org/10.1111/srt.12037.
Berardesca E, Maibach HI. Racial differences in sodium lauryl sulphate induced cutaneous irritation: black and white. Contact Dermatitis. 1988a;18:65–70.
Mathias CGT, Wilson D, Maibach HI. Transepidermal water loss as a function of skin temperature. J Invest Dermatol. 1981;77:219–20.
Rogiers V, EEMCO Group. EEMCO guidance for the assessment of transepidermal water loss in cosmetic sciences. Skin Pharmacol Appl Ski Physiol. 2001;14(2):117–28. https://doi.org/10.1159/000056341.
Berardesca E, Maibach HI. Sodiumlauryl- sulphate-induced cutaneous irritation: comparison of white and Hispanic subjects. Contact Dermatitis. 1988b;19:136–40.
Berardesca E, Maibach H. Ethnic skin: overview of structure and function. J Am Acad Dermatol. 2003;48:S139–42.
Narasimha Murthy S, Sen A, Zhao Y, Hui SW. Temperature influences the postelectroporation permeability state of the skin. J Pharm Sci. 2004;93(4):908–15. https://doi.org/10.1002/jps.20016.
Prausnitz MR. The effects of electric current applied to skin: a review for transdermal drug delivery. Adv Drug Deliv Rev. 1996;18(3):395–425. https://doi.org/10.1016/0169-409X(95)00081-H.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Panda, A., Sharma, P.K. & Narasimha Murthy, S. Effect of Mild Hyperthermia on Transdermal Absorption of Nicotine from Patches. AAPS PharmSciTech 20, 77 (2019). https://doi.org/10.1208/s12249-019-1299-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1299-x