Abstract
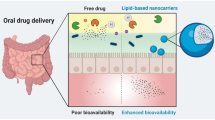
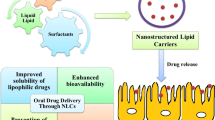
Oral drug delivery route is one of the most convenient and extensively utilised routes for drug administration. But there exists class of drugs which exhibit poor bioavailability on oral drug administration. Designing of drug–lipid conjugates (DLCs) is one of the rationale strategy utilised in overcoming this challenge. This review extensively covers the various dimensions of drug modification using lipids to attain improved oral drug delivery. DLCs help in improving oral delivery by providing benefits like improved permeability, stability in gastric environment, higher drug loading in carriers, formation of self-assembled nanostructures, etc. The clinical effectiveness of DLCs is highlighted from available marketed drug products along with many DLCs in phase of clinical trials. Conclusively, this drug modification strategy can potentially help in augmenting oral drug delivery in future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Effective delivery of drug to desired site is an absolute necessity to achieve pharmacological benefits. Drug delivery systems play major role in safe and effective dispatch of active drug to desired site of action. These delivery systems can be administered through various routes like oral, intravenous, transdermal, topical, nasal, etc. Amongst all, oral route is most preferred and extensively utilised for drug administration. It provides lucrative benefits like ease of delivery, possibility of self-administration, high-dose drug administration, cost-effective manufacturing, less stringent storage conditions, etc. Therefore, it is not surprising that roughly 80% of drugs are preferentially administered through oral route (1). But to achieve all the benefits using oral delivery, the given drug candidate must show good absorption from gastrointestinal tract (GIT). Absorption of drugs from GIT takes place through any one or combination of mechanisms like passive, transcellular, passive paracellular, carrier-mediated, and receptor-mediated endocytosis (2). Basic physicochemical properties of drug like aqueous solubility at gastrointestinal pH, lipophilicity, stability in gastric environment and ionisation coefficient determines the extent of drug absorption from GIT which ultimately affects oral bioavailability. Therefore, although there is plethora of drugs with good oral bioavailability profile, there also exists class of drugs which show very poor bioavailability after oral administration. Possible reason for this bioavailability failure could be drug instability at GI pH, enzymatic degradation, insufficient drug lipophilicity to cross GI membranes, excessive ionisation of drug, poor solubility and extensive metabolism in liver due to first-pass effect (3).
To circumvent the challenge of poor oral bioavailability, alternative routes like intravenous, transdermal and pulmonary could be utilised. But this strategy does not decipher the root cause of problem. Therefore, tailoring of drug properties as per the basic needs of oral route is the only way to resolve the problem. The strategy of chemical modification of drug is a double-edged sword which can help in altering drug properties, but at the same time, it also affects the safety, intrinsic activity and pharmacological efficacy of parent drug. The emergence of the science for prodrug designing provides the best-fit solution to accost this challenge. This approach helps in effectively altering drug properties as per the requirement of therapy by keeping similar safety and efficacy profile of the parent drug (4). Prodrugs are molecules which are devoid of intrinsic biological activity but on metabolism generates biologically active parent drug. Prodrug strategy is usually used to circumvent the challenges associated with formulation development, solubility, absorption, instability, toxicity, etc. Prodrugs majorly fall into two categories, i.e. carrier-linked prodrugs and bioprecursor prodrugs (Fig. 1). In carrier-linked prodrugs, active drug is covalently conjugated to carrier group which gets cleaved in vivo by enzymatic action, while bioprecursor prodrugs are generated by modifying parent drug in such a way that it will act as substrate for metabolic enzymes to generate active drug. For example, if parent drug contains carboxylate group, the bioprecursor could be an alcohol derivative, which on oxidative metabolism converts back into aldehyde followed by carboxylate drug (5,6,7).
Effectiveness of prodrugs in improving oral drug delivery can be easily explained using Fick’s first law which gives estimate of steady-state flux of drug across gastrointestinal walls.
where Jwall is drug flux across intestinal wall, Pwall is the permeability coefficient for intestinal wall, and Cwall is the concentration of drug at the membrane surface (which is indicated by drug solubility under sink conditions). From the equation, it is very clear that the absorption from GI walls depends on solubility of drug in GI environment and its partition coefficient. The drugs with good solubility profile may also fail to get effectively absorbed, if they lack sufficient partition coefficient. Therefore, optimisation of solubility and lipophilicity is required for effective oral bioavailability. Although the relationship between chemical structure of drug and its permeability is quite complex, the general trend proves that the drugs with sufficient hydrophobic functional groups usually have good GI permeability. Therefore, chemical modification of drug structure using hydrophobising moieties like lipids is a practically effective strategy to improve its diffusivity, permeability through GI walls and stability in GI environment (3) (Fig. 2).
DLCs—THE CONCEPT
Drug–lipid conjugates (DLCs) are the class of prodrugs in which active drug is conjugated with the lipidic moiety through covalent or noncovalent interactions (8). Basically, the prodrug designing involves the simple chemistry, where the active functional group of the drug is conjugated with the corresponding functional group of lipids. These prodrugs after undergoing metabolism releases parent drug along with lipid derivatives. The conjugated lipid can belong to diverse group of lipid derivatives like fatty acids, phospholipids, glyceride sterols, etc. The lipids are used because of their typical properties such as biocompatibility, safety, additional functional roles in drug targeting or self-assembly and versatility of chemical modification.
As the prodrugs are inactive themselves, they need to get converted back into the active form to show their pharmacological action. The conversion could be triggered by any in-vivo biological stimulation, for example by pH or enzymatic transformation. However, majority of biotransformations are driven by physiological enzymes. The speed and degree of the biotransformation depends mainly on the enzyme expression levels (which in turn depends on pharmacogenomics of patients). Therefore, while designing prodrugs, it is crucial to take into consideration the genetic polymorphism and race-specific enzyme expression levels. In general, DLCs are designed in such a manner that they will get metabolised by enzymes having broad substrate specificity, e.g. esterase, amidases and hydrolases. Chemical bonds which get cleaved by highly substrate specific enzymes are usually avoided due to the risk of enzyme saturation and patient response variability. The enzymes responsible for prodrug conversion into active drug could be present at various sites like blood, liver, GIT and organ-specific tissues. Esterase, hydrolases, amidases, paraoxonases and cytochrome P450-based enzymes are major classes of enzymes responsible for bioconversion of prodrugs (9,10,11,12).
MECHANISM OF DRUG RELEASE FROM DLC-BASED FORMULATIONS
In DLC-based formulations, majorly prodrug is loaded into inert carrier system like tablet, capsule and/or nanocarrier. Therefore, the drug release from DLC-loaded formulations follows two critical steps: first, the release of DLC from drug carrier system via diffusion and/or erosion, followed by bioconversion of DLC into parent drug by gastrointestinal enzymes or other physiological enzymes. Second stage directly impacts the bioavailability and efficacy of DLC. Therefore, while designing DLC-based prodrugs, the enzyme specificity and relative abundance of enzyme at particular physiological site must be critically analysed to avoid failures in further drug development stages. The lipids which get released after cleavage of DLC are easily metabolised and eliminated from the body. Low water solubility of DLCs prevents initial burst release from drug carriers. Two rate kinetics play part in drug release process from DLC-based formulations (13). DLC-loaded carrier systems are considered as a heterogeneous system. Rate of drug release from such systems is influenced by hydrolysis of DLC as well as the rate of drug diffusion from bioerosion of the associated delivery carrier. The release of 5-fluorouracil from a 1-(2-methacryloyloxy)ethylcarbamoyl-5-fluorouracil matrix showed that both hydrolysis rate constant and diffusion rate constant play major role in modulating drug release kinetics. Although this study considers polymer-based prodrug, the findings still can be easily extrapolated to the DLCs due to the involvement of similar factors in drug release (13,14,15,16).
Release of DLC from carrier system occurs through diffusion and/or erosion which could be analysed by models like Higuchi or Korsmeyer–Peppas or Hixson–Crowell equations (17,18,19), while release of drug from DLC (which is dependent on enzymes) follows zero- or first-order release kinetics. Hydrolysis rate constant for DLC depends on chemical nature of linkage, lipid structure and stearic hindrance. The rate of hydrolysis of ester-based linkages depends on extent of hydration; therefore, hydrophilic neighbouring groups can assist in faster hydrolysis. On the other hand, hydrophobic groups retard hydrolysis rate. This phenomenon can be used in manipulating the rate of drug release by changing the ratio of hydrophilic and hydrophobic groups on lipid structure (15,20). The rate by which DLC gets converted into parent drug also depends on formulation factors like hydrophobicity of drug, composition of release media, in vivo environment like pH and presence of enzymes (21,22,23). Composition of delivery carrier can also affect the release kinetics of drug. For example, lipid conjugate of mitomysin C showed faster release after incorporation into hydrogenated soybean phosphatidylcholine-based liposomes than in cholesterol-based liposomes. Better membrane stability of cholesterol-based liposomes could possibly be responsible for this behaviour. In summary, the successful development of effective DLC requires in-depth understanding of mechanism of release which ultimately helps in the tailoring of drug release rate for intended therapeutic application (24).
SELECTION PARAMETERS FOR DLC DESIGNING
Careful selection of the lipid and the drug based on their structure compatibility with each other is necessary in DLC designing. The selection of the drug and the lipid is done by considering multiple factors as follows:
Selection of the Lipids
Lipids are selected by considering points like functional role of lipids, their melting point, drug structure, its stability in GIT, solubility of lipid and solubility of resulting DLC in GIT. The lipids should possess sufficient number of functional groups for easy conjugation with drug. Four different classes of the lipids could be selected, such as fatty acids, steroids, glycerides and phospholipids (Fig. 3). Functional role of lipid is key determinant in the selection process of lipids for DLC development. For example, glyceride-based prodrugs are majorly taken up into systemic circulation through lymphatic route. Therefore, this strategy could be used in designing targeted drug delivery to lymphatics which is useful in treating lymphatic cancer or infections. Also, uptake through lymphatic route helps to bypass first-pass metabolism. Therefore, glyceride-based prodrugs might prove useful to design oral delivery system for drugs which undergo extensive first pass effect, e.g. alpha-blockers, L-dopa. Similarly, phospholipid conjugates of nonsteroidal anti-inflammatory drugs (NSAIDs) can help to reduce gastric ulceration by providing protective hydrophobic barrier on GI mucosal surface to prevent GI tissue from damage. The length of fatty acid chain also plays major role in determining the fate of drug uptake from GIT. In general, medium-chain fatty acid derivatives are absorbed through lymphatic route while fatty acids with carbon chain length less than 12 are mainly absorbed by portal blood circulation route (14,25).
Selection of the Drugs
Drug candidates with poor oral absorption, instability in GIT and low drug encapsulation efficiency are usually good candidate for DLC development. Since conjugation of lipids with drugs help in improving their GI permeability by modulating lipophilicity of drug, drug candidates with biopharmaceutical classification system (BCS) class III and IV are better suited for DLC development. Although this generalisation cannot be used as rule of thumb and careful assessment of physicochemical and biopharmaceutical properties of individual drug candidate is essential before development of DLC. Drugs with the amine or alcohol functionality can be covalently conjugated with the lipid to form ester- or amide-based prodrugs which have higher lipophilicity. Drugs with carboxylate functional groups are usually coupled with diglycerides via ester linkage. The drugs resembling to nucleoside analogue structure (e.g. acyclovir, decitabine and rifampicin) can be coupled to the phosphate monophosphate diacylglycerol, diphosphate diacylglycerol and triphosphate diacylglycerol (14,26).
VARIOUS LIPID MOIETIES USED IN DLC DESIGNING
Fatty Acids
Fatty acids are mostly used candidate for conjugation of drugs to confer lipophilicity to the conjugates. Fatty acids commonly have a long acyl chain and a reactive carboxyl group which readily interacts with an amide or hydroxyl group in the parent drug molecule. Unsaturated fatty acids (UFAs) have demonstrated a higher affinity to tumour cells and are also biocompatible. Hence, they have been widely studied as conjugating moiety for anticancer drugs. UFAs also play nutritional role and improve cardiovascular health by reducing cholesterol and triglycerides (27,28,29,30,31,32,33,34). Fatty acids could be conjugated to the drug by two possible ways. Firstly, the drug can be directly attached to free carboxyl group of fatty acids to make stable ester or amide bond. In another case, drug can be conjugated to the modified ω-atom by keeping carboxyl group free. These types of prodrugs utilise the endogenous metabolic pathways for fatty acids, because the presence of free carboxyl group is essential for the recognition of fatty acids by cell membrane transporters and also for binding of fatty acids to serum albumin. Effective utilisation of endogenous metabolic pathways is not possible in DLCs which devoid free carboxyl functionality (35,36). The carbon chain length of fatty acids also plays major role in determining the fate of drug delivery. In general, short- and medium-chain fatty acids got absorbed through portal network, while long-chain fatty acids follow lymphatic uptake. Unsaturated fatty acids (UFAs) additionally possess intrinsic tumour-targeting properties which have been intelligently utilised by conjugating UFA with anticancer drugs. Examples like gemcitabine-eladic acid, paclitaxel-docosahexaenoic acid and cytarabine-elaidic acid have shown promising benefits in clinical trials. Although these conjugates are intended for intravenous application, their clinical results highlight towards promising role of drug–fatty acid conjugates for improving drug delivery 37,38,39.
Effective improvement of oral drug delivery is possible with fatty acid conjugation. For example, anticancer drug, 7-ethyl-10-hydroxycamptothecin, belongs to BCS class IV and exhibits poor stability in GI environment. Fatty acid–based prodrug has been developed for this drug by conjugating undecanoic acid at C20 position of 7-ethyl-10-hydroxycamptothecin which substantially improved intestinal permeability and stability of parent drug. Oral delivery of this prodrug exhibited 100 times higher area under the curve (AUC) of parent drug in comparison with oral solution of unmodified drug (40). Similarly, when cytarabine (with ~ 20% oral bioavailability) conjugated lauric acid, the conjugate exhibited higher lipophilicity, permeability and improved the gastric stability by protecting the deamination of amino group in cytarabine. The effectiveness of this prodrug can be easily observed from the pharmacokinetic parameters in which prodrug showed 6.6-, 5- and 32-fold improvement in elimination half-life, peak plasma concentration and bioavailability respectively when compared with unmodified free drug (41). Controlling the drug release at specific site of GIT is also possible by this strategy. The drug, 5-aminosalicylic acid (5-ASA) (used in treatment of Crohn’s disease and ulcerative colitis), has been conjugated with functional fatty acids (having intrinsic anti-inflammatory activity) caprylic acid and eicosapentaenoic acid. This ester-based prodrug showed controlled release of 5-ASA in ileum and colon region. The prodrug was stable in gastric region and gets hydrolysed in intestinal environment selectively. This strategy helped in decreasing systemic exposure of 5-ASA, hence improving the safety of therapy (42).
Glycerides
Another interesting option for conjugation is triglycerides (TGs). TGs have a unique pathway for metabolism involving deacylation–reacylation. The TG is first broken down into 2-monoglycerides and free fatty acids in the GIT, following which the monoglyceride is reacylated in the enterocytes to TG again. This TG is then taken up by the lipoproteins and swept into the lymphatic system. Hence, this approach to conjugation aims at improving drug absorption and lymphatic targeting of drugs. TGs are formed by coupling glycerol to three fatty acids through an ester bond. Commonly, the second fatty acid chain is replaced by the drug molecule. Mycophenolic acid has been combined with TG at the position 2, with two palmitoyl groups at positions 1 and 3. It has also shown to undergo the above predicted pathway. The drug mycophenolic acid was modified with triglyceride conjugation to form 1,3-dipalmitoyl-2-mycophenoloyl glycerol. This prodrug utilised triglyceride deacylation-reacylation pathway to improve lymphatic uptake after administration through oral route (43,44). Similarly, GI stability of testosterone has been improved by conjugation with triglycerides. The modified prodrug showed almost 90-fold improvement in oral bioavailability of testosterone as compared to free testosterone (45).
Steroids
All steroids unambiguously have a four-ringed framework, usually with flanking hydroxyl group(s). These hydroxyl groups are useful in conjugating drug molecules with these steroidal moieties. The most prominent member of this group is cholesterol. It has tumour-targeting properties and is taken up by the overexpressed LDL receptors which are present on the tumour cells. Thus, conjugation with cholesterol is beneficial as it assists drug loading into lipoproteins which consequently enhances targeted delivery, lessens side effects and hence increases cellular uptake. For example, 5-fluorouracil had an upgraded anticancer effect when coupled with the singular hydroxyl group of cholesterol (21,46,47,48). Cholic acid derivatives have also been utilised for conjugation in the form of ursodeoxycholic acid (UDCA) and lithocholic acid (LCA). Unlike cholesterol, UDCA has three hydroxyl groups out of which the one farthest from the steroidal framework has been used for conjugation. Bile acid has also shown prospective in conjugation of drugs (21).
Phospholipids
Phospholipids can also be used as an effective conjugating moiety. Either the phosphate group or the position 2 of the glycerol backbone in the phospholipid could be exploited to form the desired drug conjugates. Conjugates hence prepared have a higher tendency to be incorporated into liposomes or any delivery system based on phospholipids. In case of unsaturated phospholipids (e.g. egg phosphatidylcholine), it has been observed that efficient drug incorporation necessitated the inclusion of cholesterol into the liposome, whereas this has not been observed in case of saturated phospholipids due to the structural similarity between the linear acyl chains (21). Valproic acid was conjugated with phospholipids at sn-2 position. This conjugate showed ~ 60% of drug absorption through lymphatic uptake due to resemblance with natural phospholipids. The conjugate showed improved intestinal permeation followed by entry into systemic circulation via lymphatic uptake (49,50,51,52,53). Similarly, phospholipid conjugates of indomethacin and diclofenac were developed which showed improved drug delivery and bioavailability (54,55).
CHARACTERISATION OF DLCS
Characterisation of newly developed DLCs is very important to assure the therapeutic efficacy and biological safety of conjugates. Assessment of chemical structure can be done using techniques like FTIR, NMR and mass spectrometry, while physicochemical properties can be determined by techniques like log P estimation, DSC, X-ray diffraction analysis, solubility and stability studies. Evaluation of therapeutic efficacy of developed conjugates is carried out using various bioassays and in vivo studies. Metabolism studies are also carried out to ensure the effective in vivo bioconversion of prodrug into parent molecule, e.g. analysis of drug conversion in liver homogenate, in vitro analysis of drug conversion in presence of specific metabolising enzyme and in vivo analysis of drug bioconversion. Furthermore, characterisation of DLC-loaded formulations is essential to ensure dosage uniformity (Table I). DLC-loaded formulations like tablets, capsules, emulsions, suspensions or nanocarriers are usually characterised for conventional parameters like amount of drug present per unit volume of formulation, impurity profiling, stability analysis, content uniformity, in vitro drug release kinetics, assessment of pharmacokinetic and pharmacodynamics parameters using in vivo studies, and toxicity analysis (56,57,58,59).
ADVANTAGES OF DLCS
Large number of drugs face critical hurdles in the formulation stage and clinical setup due to the undesirable pharmaceutical properties like poor solubility, permeability, drug loading in delivery carrier and gastric instability. Conjugation offers an edge over other strategies especially from the standpoint of oral drug delivery. The chemical attributes of the DLCs can be fine-tuned to encompass variety of desirable characteristics to the formulation (Fig. 4). Some of the benefits are explained in the following:
Increased Absorption and Permeability
Lipophilicity of drug is major determinant of oral bioavailability. Hydrophilic drugs usually show poor absorption from GIT due to insufficient permeation from GIT. Polar functional groups present in drug structure majorly determine relative hydrophilicity of drug. Therefore, in prodrug designing, polar functional groups like carboxylates are converted into less polar groups which can effectively cross GI permeation barriers. For example, carboxylate groups on conversion into less polar ester by fatty acid conjugation can significantly improve GI permeation, followed by hydrolysis of esters in bloodstream to the parent drug. Similar strategies can be used for functional groups like phosphate, alcohols and phenols (4,21,60).
Various drugs display limited absorption, and hence little efficacy, owing to their degradation by metabolic enzymes and the notorious first-pass effect. In order to evade these metabolic obstructions, lipidic moieties have been utilised to conjugate such drug molecules to affect their absorption in a befitting manner. Lipid conjugation provides the molecule an enhanced lipophilic nature and makes it a favourable candidate for the lymphatic uptake. Hence, the conjugates successfully assist in avoiding the impetuous degradation of the drug in its early stages and augment the cellular interactions. Cytarabine ocfosfate showed higher lipophilicity than cystarabine 5′-monophosphate and could be given orally and was resistant to deamination too. Apomorphine has shown a monumental improvement in the log P values when conjugated with dipalmitoyl or dilauryl groups in a SEDDS formulation (the free drug having logP of 2.0 whereas the conjugated systems having values of 17.1 and 12.9, respectively). An antineoplastic agent, SN-38, was linked to undecanoic acid (C20). The liphophilic conjugate enhanced the entry into the enterocytes leading to more than double the drug permeation with respect to the free drug in murine intestinal epithelium (21,61,62,63,64).
Increase in Stability
Vitamins such as A and E have an intrinsic chemical stability. They are more frequently utilised prodrugs which may also benefit them from the perspective of absorption and the successive lymphatic journey. Vitamin E is often linked to an alkyl ether group whereas vitamin A is employed as a fatty acid derivative. The lipophilic groups are hydrolysed in the intestine, and the unconjugated drug is then absorbed. Lipid conjugation can also prevent the metabolic instability of chemotherapeutic agents. Conjugation of unsaturated fatty acids to demethyldeoxypodophyllotoxin has improved the stability of the drug and has also boosted the chemotherapeutic efficacy. Incorporating the pivaloyl oxymethyl (POM) moiety to 9-(2-phosphonylmethoxyethyl) adenine (PMEA) exhibited enhanced activity against HSV. Bis(POM)-PMEA portrayed stability at pH 2.0, indicating an opportunity to use it orally, showing 100-fold increase in metabolic stability and a 5-fold increase in the oral bioavailability. A series curcuminoid prodrugs comprising of succinyl ester moieties were prepared which showed better stability and anticancer activity (4,26,65,66,67,68).
Altering Drug Uptake Pathway Through Intestinal Lymphatics
The intestinal lymphatic system serves as specific absorption and transport pathway for the lipidic moieties. The lipidic moiety whenever reaches intestinal lymphatics (by the aid of the lipoprotiens) follows a series of pathway before reaching to systemic circulation. This journey can be shortly described as from intestinal lymphatics, lipophilic drug reaches to the mesenteric lymphatics, then into the cysterna chili. The cysterna chili then transports drug into thoracic lymph duct, which ultimately delivers lipidic moiety into the systemic circulation via left subclavian and left internal jugular veins. This physiological pathway can be utilised as strategy for drug delivery to offer several advantages, such as the bypassing of the first-pass metabolism, improved mesenteric targeting, and reduced toxicity, altering the overall drug distribution profile to provide sustained drug action. Conjugation of lipids to drug helps in improving their lipophilicity which is essential for preferential lymphatic uptake. Therefore, DLC-based prodrug design can be used to alter the drug uptake pathway through intestinal lymphatic system. Examples of drug derivatives which are transported by the intestinal lymphatics after oral administration are as follows: lipophilic vitamins, halofantrine, naftifine, mepitiostane, probucol and cyclosporin. Mere high lipophilicity of prodrug does not guarantee the lymphatic uptake. Sometimes, drug analogues with very high lipophilicity can also have very low degree of lymphatic transport. This indicates that factors other than lipophilicity, such as conversion of the prodrugs before absorption, or the metabolism of the prodrug by the enterocytes also govern the transport. Therefore, for understanding the effective transport of the drug to the lymphatics, in-depth understanding of the lipid metabolism process is necessary (69).
Reduced Side Effects
Numerous drugs have multiple sites of action due to which they exhibit undesirable effects that often diminishes the patient compliance and may affect the patient adversely. Certain molecules that are orally administered cause gastric irritation by disrupting the gastric mucous layer. Thus, various strategies have been adopted to reduce or eliminate these effects and consequently build a more efficacious molecule. Conjugation with different moieties to produce prodrugs with suitable properties has proven to be constructive (69). Glyceride prodrugs of NSAIDs like naproxen, aspirin and indomethacin has showed lower incidences of gastric irritation. Voglibose is used to decrease postprandial drug sugar levels by inhibiting α-glycosidase. Its high solubility is responsible for flatulence, diarrhoea and constipation. Linking the drug molecule to lipidic moiety decreased the solubility and hence decreased its gastrointestinal side effects (69,70,71,72,73).
A 5-aminosalicylic acid (5-ASA) derivative, balsalazide, contains a 4-aminobenzoyl β-alanine connected through an azo bond to 5-ASA. Balsalazide averts the proximal absorption and is transformed only by the azo-reducing bacteria present in the colon to 5-ASA. Thus, it does not have the side effects occurring due to the sulphapyridine group (with which 5-ASA was originally formulated), and have auxillary dose-related advantages. Our group also effectively designed lipid conjugate of amphotericin B using fatty acid (oleic acid). The amphotericin DLC was found to help in remarkably reducing aggregation-induced toxicities of amphotericin B and also simultaneously improved its GI stability (56).In another instance, doxorubicin was coupled with a tetrapeptide via a functionalized linker. Doxorubicin is associated with a large number of side effects such as myelosuppression and cardiotoxicity due to its nonspecific nature. The coupling of the peptide proved advantageous as it was hydrolysed by caspase-3, which is particularly overexpressed in the apoptotic cells. Thus, this could be utilised by exogenously radiating the specific area to form apoptotic cells that will activate caspase-3, and hence prevent the off-target effects (4,74,75).
Self-Assembly
Self-assembly is induced by the formation of noncovalent bonds such as hydrogen bonding, hydrophobic interactions and Van der Waals forces of lipidic molecules. This property can be utilised by forming drug conjugates with biocompatible lipids which demonstrate self-assembly into nanoparticles in the presence of water. This feature forms a ground for enhanced drug delivery, particularly anticancer drugs, from the standpoint of improved intracellular penetration, higher drug concentrations and diminished side effects. Self-assembly encompasses the spontaneous arrangement of the building blocks into an ordered structure as a result of local hydrophobic interactions. One the basic requirement for self-assembly is the amphiphilic nature of the constituents involved in self-assembly. Squalenoylation is one of the most extensively used strategies to design self-assembled DLCs. In this, drug is covalently conjugated to the terpene lipid, squalene, which can self-assemble in aqueous solution to form nanoparticles. Squalenoylation of gemcitabine assisted the formation of self-assembled structures that lead a lower clearance rate and a slower absorption rate as compared to gemcitabine while maintaining the same distribution profile as gemcitabine which proved beneficial for the oral treatment of gastrointestinal cancers (76,77).
Improved Drug Loading into Delivery Carriers
Hydrophilic drugs usually show poor drug loading into delivery carriers due to drug leakage. Conjugation of lipid to hydrophilic drug increases lipophilicity of parent drug and help in significantly improving the drug loading. The conjugation can also enhance affinity between drug and lipidic components of carrier which leads to reduction in drug leakage. Conjugation of paclitaxel with behenic acid leads to increase in drug loading from 10 to 47% inside nanoparticles. Similar trend was noted in case of 4-(N)-stearoyl gemcitabine conjugate (78,79).
Achieve Extended Drug Release
DLCs show low aqueous solubility and therefore help in providing extended drug release on administration. The properties of synthesised conjugate like aqueous solubility, partition coefficient and melting point can be effectively controlled by careful selection of lipids which helps tailoring the drug release profile. There are several FDA-approved drug formulations which are based on this strategy like paliperidione palmitate, which is palmitic acid conjugate of antipsychotic drug paliperidone, along with drugs like haloperidol decanoate, aripiprazole lauroxil and fluphenazine enanthate (21).
Improvement in Pharmacological Activity
Lipid conjugation of drug may also serve to improve the therapeutic efficacy of parent drug. The reports on curcumin-phospholipid complex showed that the lipid-conjugated derivatives have better hepatoprotective activity than free drug. Also, conjugation of functional lipidic moieties may help to provide additional therapeutic activities, e.g. drug conjugates with tocopherol groups can provide additional antioxidant action along with providing hydrophobicity (80).
GLIMPSES OF DLC-BASED DRUG DEVELOPMENT PIPELINE
DLC strategy is one of the most successful approaches for prodrug designing for improving biopharmaceutical performance of the drugs. There are significant number of regulatory approved products in the market and many more in clinical trials showing promising improvement in performance of parent drug. The following section describes few examples of marketed/under clinical trial DLC-based prodrugs which gives the brief idea about benefits of lipid conjugation strategy.
Paliperidone Palmitate
This drug is used in treatment of psychic disorders. The paliperidone is a parent drug which has been converted into palmitate ester form to attain sustained release effect. The drug gets bioconverted into active form (paliperidone) by the action of systemic esterase enzymes. This drug is currently available in the market under the brand name of Invega Sustenna (1-month sustain-release injection) and Invega Trinza (3-month sustain-release injection) (81).
Aripiprazole Lauroxil
Aripiprazole lauroxil has been approved by USFDA in 2015 for the treatment of schizophrenia. It is N-acyloxymethyl derivative of aripiprazole, providing extremely lipophilic prodrug. As aripiprazole lacks hydroxyl group in its structure, this prodrug has been developed by firstly forming N-hydroxymethyl group by reaction of formaldehyde with amide in aripiprazole, which is further conjugated with lauric acid to give DLC. This prodrug is administered intramuscularly and provides drug release for 6 weeks. The strategy of using higher alkyl chain fatty acid esters is usually used to design prodrugs for extended release profile. Fatty acids like decanoic acid, palmitic acid and valerates are majorly used for this purpose. Similar strategy has been utilised to develop several marketed prodrugs of oestrogen, testosterone, fluphanazine, flupentixol, haloperidol, pipotiazine, etc. (82,83,84).
Sapacitabine
This is amide-based fatty acid conjugate of the nucleoside analogue 2′-C-cyano-2′-deoxy-1-β-D-arabino-pentofuranosylcytosine (CNDAC). In this, palmitoyl group is conjugated to N4- group of the cytosine which makes the conjugate permeable from GIT stable in gastric environment and also reduces inactivation by enzymatic deamination. After absorption through intestine, sapacitabine gets bioconverted by amidases in liver and plasma to active form CNDAC, which shows its cytotoxic action by DNA strand break. This prodrug is currently in phase 2 clinical trial for relapsed chronic lymphocytic leukaemia and small lymphocytic lymphoma (84,85).
CMX157
This is an orally active prodrug of tenofovir with increased bioavailability, reduced toxicity and liver targeting potential as compared to tenofovir. Chemically, it is an alkoxyalkyl-based prodrug in which hydroxyl groups of tenofovir are conjugated to the hexadecyloxypropyl moiety. This drug gets bioconverted to active form, i.e. tenofovir by enzyme phospholipase C. This enzyme is absent in plasma and GI environment, making the conjugate stable in blood and GIT. The enzyme specificity helps in ensuring the prodrug stability during oral absorption and transport in systemic circulation to the tissues. Currently, this prodrug is under phase 2 clinical trials for HBV infections (NCT02710604) (84,86).
CONCLUSION
Lipid–drug conjugation is an effective prodrug strategy to improve the oral bioavailability of drugs. The bioavailability benefit offered by DLCs is due to improved drug lipophilicity, better GI stability, improved drug loading in carriers, reduced toxicities or selective uptake mechanism. This drug designing strategy can substantially help to improve drug loading in delivery carriers and can also provide carrier-free drug delivery through self-assembled nanostructures. Careful selection of lipids and drug, detailed understanding of lipid digestion and absorption mechanisms is necessary while designing the DLC-based prodrug to achieve maximum benefits and minimise toxicities. There are many successfully marketed products based on DLC approach and many more under clinical exploration which are providing significant clinical benefits over the conventionally used parent drugs. In upcoming future, this drug modification strategy can provide ray of hope to successfully deliver drugs with poor oral bioavailability. Rising research exploration in the field of nanocarrier-based delivery platforms can further help in future to enhance therapeutic potential of DLCs. In brief, DLC strategy has potential to provide satisfactory solutions to the challenges in oral drug delivery in coming future.
References
Sastry SV, Nyshadham JR, Fix JA. Recent technological advances in oral drug delivery—a review. Pharm Sci Technolol Today. 2000;3:138–45.
Charman WN. Lipids, lipophilic drugs, and oral drug delivery—some emerging concepts. J Pharm Sci. 2000;89:967–78.
Taylor MD. Improved passive oral drug delivery via prodrugs. Adv Drug Deliv Rev. 1996;19:131–48.
Abet V, Filace F, Recio J, Alvarez-Builla J, Burgos C. Prodrug approach: an overview of recent cases. Eur J Med Chem. Elsevier Masson. 2017;127:810–27.
Wermuth CG. Designing prodrugs and bioprecursors. In: Pract Med Chem. 3rd ed. Cambridge: Academic Press; 2008. p. 721–46.
Kokil GR, Rewatkar PV. Bioprecursor prodrugs: molecular modification of the active principle. Mini-Reviews Med Chem. 2010;10:1316–30.
Silverman RB, Holladay MW. The organic chemistry of drug design and drug action. 3rd ed. Drug Dev. Res. The Academic Press; 2014.
Lambert DM. Rationale and applications of lipids as prodrug carriers. Eur J Pharm Sci. Elsevier. 2000;11:S15–27.
Liederer BM, Borchardt RT. Enzymes involved in the bioconversion of ester-based prodrugs. J Pharm Sci Elsevier. 2006;95:1177–95.
Huttunen KM, Raunio H, Rautio J. Prodrugs—from serendipity to rational design. Pharmacol Rev. 2011;63:750–71.
Clas S-D, Sanchez RI, Nofsinger R. Chemistry-enabled drug delivery (prodrugs): recent progress and challenges. Drug Discov Today. Elsevier Current Trends. 2014;19:79–87.
Lesniewska-Kowiel MA, Muszalska I. Strategies in the designing of prodrugs, taking into account the antiviral and anticancer compounds. Eur J Med Chem Elsevier Masson. 2017;129:53–71.
Müller RH, Olbrich C. Lipid matrix-drug conjugates particle for controlled release of active ingredient [Internet]. 2000 [cited 2018 Nov 22]. Available from: patents.google.com/patent/US6770299B1/en. Accessed 19 Sept 2018.
Adhikari P, Pal P, Das AK, Ray S, Bhattacharjee A, Mazumder B. Nano lipid-drug conjugate: an integrated review. Int J Pharm. 2017;529:629–41.
Kondo S, Hosaka S, Hatakeyama I, Kuzuya M. Mechanochemical solid-state polymerization. IX. Theoretical analysis of rate of drug release from powdered polymeric prodrugs in a heterogeneous system. Chem Pharm Bull (Tokyo). The Pharmaceutical Society of Japan. 1998;46:1918–23.
D’Souza AJM, Topp EM. Release from polymeric prodrugs: linkages and their degradation. J Pharm Sci. 2004;93:1962–79.
Dash S, Murthy PN, Nath L, Chowdhury P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol Pharm. 67:217–23.
Siepmann J. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv Drug Deliv Rev Elsevier. 2012;64:163–74.
Siepmann J, Kranz H, Bodmeier R, Peppas NA. HPMC-matrices for controlled drug delivery: a new model combining diffusion, swelling, and dissolution mechanisms and predicting the release kinetics. Pharm Res. Kluwer Academic Publishers-Plenum Publishers. 1999;16:1748–56.
Pitt GG, Cha Y, Shah SS, Zhu KJ. Blends of PVA and PGLA: control of the permeability and degradability of hydrogels by blending. J Control Release. Elsevier. 1992;19:189–99.
Irby D, Du C, Li F. Lipid–drug conjugate for enhancing drug delivery. Mol Pharm. American Chemical Society. 2017;14:1325–38.
Teshima M, Fumoto S, Nishida K, Nakamura J, Ohyama K, Nakamura T, et al. Prolonged blood concentration of prednisolone after intravenous injection of liposomal palmitoyl prednisolone. J Control Release. Elsevier. 2006;112:320–8.
Signorell RD, Luciani P, Brambilla D, Leroux J-C. Pharmacokinetics of lipid-drug conjugates loaded into liposomes. Eur J Pharm Biopharm. Elsevier. 2018;128:188–99.
Zalipsky S, Gabizon AA. Conjugate having a cleavable linkage for use in a liposome [Internet]. 2000 [cited 2018 Nov 22]. Available from: https://patents.google.com/patent/US6365179B1/en. Accessed 19 Sept 2018.
McDonald GB, Weidman M. Partitioning of polar fatty acids into lymph and portal vein after intestinal absorption in the rat. Q J Exp Physiol. Wiley/Blackwell (10.1111). 1987;72:153–9.
Alexander P, Kucera G, Pardee TS. Improving nucleoside analogs via lipid conjugation: is fatter any better? Crit Rev Oncol Hematol. Elsevier. 2016;100:46–56.
Wang Y, Li L, Jiang W, Larrick JW. Synthesis and evaluation of a DHA and 10-hydroxycamptothecin conjugate. Bioorg Med Chem Pergamon. 2005;13:5592–9.
Dichwalkar T, Patel S, Bapat S, Pancholi P, Jasani N, Desai B, et al. Omega-3 fatty acid grafted PAMAM-paclitaxel conjugate exhibits enhanced anticancer activity in upper gastrointestinal cancer cells. Macromol Biosci. Wiley-Blackwell. 2017;17:1600457.
Bedikian AY, DeConti RC, Conry R, Agarwala S, Papadopoulos N, Kim KB, et al. Phase 3 study of docosahexaenoic acid-paclitaxel versus dacarbazine in patients with metastatic malignant melanoma. Ann Oncol Oxford University Press. 2011;22:787–93.
Venugopal B, Awada A, Evans TRJ, Dueland S, Hendlisz A, Rasch W, et al. A first-in-human phase I and pharmacokinetic study of CP-4126 (CO-101), a nucleoside analogue, in patients with advanced solid tumours. Cancer Chemother Pharmacol. 2015;76:785–92.
Pardini RS. Nutritional intervention with omega-3 fatty acids enhances tumor response to anti-neoplastic agents. Chem Biol Interact Elsevier. 2006;162:89–105.
Effenberger K, Breyer S, Schobert R. Modulation of doxorubicin activity in cancer cells by conjugation with fatty acyl and terpenyl hydrazones. Eur J Med Chem. Elsevier Masson. 2010;45:1947–54.
Igarashi M, Miyazawa T. Newly recognized cytotoxic effect of conjugated trienoic fatty acids on cultured human tumor cells. Cancer Lett Elsevier. 2000;148:173–9.
Sun B, Luo C, Cui W, Sun J, He Z. Chemotherapy agent-unsaturated fatty acid prodrugs and prodrug-nanoplatforms for cancer chemotherapy. J Control Release. Elsevier. 2017;264:145–59.
Bontemps L, Demaison L, Keriel C, Pernin C, Mathieu JP, Marti-Batlle D, et al. Kinetics of (16 123I) Iodohexadecenoic acid metabolism in the rat myocardium, influence of glucose concentration in the perfusate and comparison with (1 14C) palmitate. Eur Heart J Oxford University Press. 1985;6:91–6.
Charbon V, Latour I, Lambert DM, Buc-Calderon P, Neuvens L, De Keyser J, et al. Targeting of drug to the hepatocytes by fatty acids. Influence of the carrier (albumin or galactosylated albumin) on the fate of the fatty acids and their analogs. Pharm Res. Kluwer Academic Publishers-Plenum Publishers. 1996;13:27–31.
Sparreboom A, Verweij J, van der Burg ME, Loos WJ, Brouwer E, Viganò L, et al. Disposition of Cremophor EL in humans limits the potential for modulation of the multidrug resistance phenotype in vivo. Clin Cancer Res American Association for Cancer Research. 1998;4:1937–42.
Stuurman FE, Voest EE, Awada A, Witteveen PO, Bergeland T, Hals P-A, et al. Phase I study of oral CP-4126, a gemcitabine derivative, in patients with advanced solid tumors. Invest New Drugs Springer US. 2013;31:959–66.
Pignata S, Amant F, Scambia G, Sorio R, Breda E, Rasch W, et al. A phase I-II study of elacytarabine (CP-4055) in the treatment of patients with ovarian cancer resistant or refractory to platinum therapy. Cancer Chemother Pharmacol Springer-Verlag. 2011;68:1347–53.
Bala V, Rao S, Bateman E, Keefe D, Wang S, Prestidge CA. Enabling oral SN38-based chemotherapy with a combined lipophilic prodrug and self-microemulsifying drug delivery system. Mol Pharm American Chemical Society. 2016;13:3518–25.
Liu J, Liu J, Zhao D, Ma N, Luan Y. Highly enhanced leukemia therapy and oral bioavailability from a novel amphiphilic prodrug of cytarabine. RSC Adv. The Royal Society of Chemistry. 2016;6:35991–9.
Kandula M, Sunil Kumar K, Palanichamy S, Rampal A. Discovery and preclinical development of a novel prodrug conjugate of mesalamine with eicosapentaenoic acid and caprylic acid for the treatment of inflammatory bowel diseases. Int Immunopharmacol Elsevier. 2016;40:443–51.
Han S, Hu L, Quach T, Simpson JS, Trevaskis NL, Porter CJH. Profiling the role of deacylation-reacylation in the lymphatic transport of a triglyceride-mimetic prodrug. Pharm Res Springer US. 2015;32:1830–44.
Han S, Hu L, Gracia, Quach T, Simpson JS, Edwards GA, et al. Lymphatic transport and lymphocyte targeting of a triglyceride mimetic prodrug is enhanced in a large animal model: studies in greyhound dogs. Mol pharm. Am Chem Soc. 2016;13:3351–61.
Hu L, Quach T, Han S, Lim SF, Yadav P, Senyschyn D, et al. Glyceride-mimetic prodrugs incorporating self-immolative spacers promote lymphatic transport, avoid first-pass metabolism, and enhance Oral bioavailability. Angew Chemie. Wiley-Blackwell. 2016;128:13904–9.
Radwan AA, Alanazi FK. Targeting cancer using cholesterol conjugates. Saudi Pharm J Elsevier. 2014;22:3–16.
Radwan A, Alanazi F, Radwan AA, Alanazi FK. Design and synthesis of new cholesterol-conjugated 5-fluorouracil: a novel potential delivery system for cancer treatment. Molecules Multidisciplinary Digital Publishing Institute. 2014;19:13177–87.
Wolfrum C, Shi S, Jayaprakash KN, Jayaraman M, Wang G, Pandey RK, et al. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat Biotechnol Nature Publishing Group. 2007;25:1149–57.
Dahan A, Duvdevani R, Shapiro I, Elmann A, Finkelstein E, Hoffman A. The oral absorption of phospholipid prodrugs: in vivo and in vitro mechanistic investigation of trafficking of a lecithin-valproic acid conjugate following oral administration. J Control Release Elsevier. 2008;126:1–9.
Labiner DM. DP-VPA D-Pharm. Curr Opin Investig Drugs. 2002;3:921–3.
Isoherranen N, Yagen B, Bialer M. New CNS-active drugs which are second-generation valproic acid: can they lead to the development of a magic bullet? Curr Opin Neurol. 2003;16:203–11.
Bialer M, Johannessen S, Kupferberg H, Levy R, Loiseau P, Perucca E. Progress report on new antiepileptic drugs: a summary of the Sixth EILAT Conference (EILAT VI). Epilepsy Res. Elsevier. 2002;51:31–71.
Bialer M, Johannessen S, Kupferberg H, Levy R, Loiseau P, Perucca E. Progress report on new antiepileptic drugs: a summary of the Fifth EILAT Conference (EILAT V). Epilepsy Res Elsevier. 2001;43:11–58.
Dahan A, Duvdevani R, Dvir E, Elmann A, Hoffman A. A novel mechanism for oral controlled release of drugs by continuous degradation of a phospholipid prodrug along the intestine: in-vivo and in-vitro evaluation of an indomethacin–lecithin conjugate. J Control Release. Elsevier. 2007;119:86–93.
Dahan A, Markovic M, Epstein S, Cohen N, Zimmermann EM, Aponick A, et al. Phospholipid-drug conjugates as a novel oral drug targeting approach for the treatment of inflammatory bowel disease. Eur J Pharm Sci Elsevier. 2017;108:78–85.
Thanki K, Prajapati R, Sangamwar AT, Jain S. Long chain fatty acid conjugation remarkably decreases the aggregation induced toxicity of amphotericin. B. Int J Pharm. Elsevier. 2018;544:1–13 Available from: https://www.sciencedirect.com/science/article/pii/S0378517318302205. Accessed 19 Sept 2018.
Kushwah V, Katiyar SS, Agrawal AK, Gupta RC, Jain S. Co-delivery of docetaxel and gemcitabine using PEGylated self-assembled stealth nanoparticles for improved breast cancer therapy. Nanomed Nanotechnol Biol Med. Elsevier. 2018;14:1629–41 Available from: https://www.sciencedirect.com/science/article/pii/S1549963418300819. Accessed 19 Sept 2018.
Olbrich C, Gessner A, Kayser O, Müller RH. Lipid-drug-conjugate (ldc) nanoparticles as novel carrier system for the hydrophilic antitrypanosomal drug diminazenediaceturate. J Drug Target. 2002;10:387–96 Available from: http://www.tandfonline.com/doi/full/10.1080/1061186021000001832. Accessed 19 Sept 2018.
Wissing S, Kayser O, Müller R. Solid lipid nanoparticles for parenteral drug delivery. Adv Drug Deliv Rev. Elsevier. 2004;56:1257–72 Available from: https://www.sciencedirect.com/science/article/pii/S0169409X04000456. Accessed 19 Sept 2018.
Trevaskis NL, Kaminskas LM, Porter CJH. From sewer to saviour—targeting the lymphatic system to promote drug exposure and activity. Nat Rev Drug Discov Nature Publishing Group. 2015;14:781–803.
Braess J, Freund M, Hanauske A, Heil G, Kaufmann C, Kern W, et al. Oral cytarabine ocfosfate in acute myeloid leukemia and non-Hodgkin’s lymphoma—phase I/II studies and pharmacokinetics. Leukemia Nature Publishing Group. 1998;12:1618–26.
Saneyoshi M, Morozumi M, Kodama K, Machida H, Kuninaka A, Yoshino H. Synthetic nucleosides and nucleotides. XVI. Synthesis and biological evaluations of a series of 1-.BETA.-D-arabinofuranosylcytosine 5′-alkyl or arylphosphates. Chem Pharm Bull (Tokyo). The Pharmaceutical Society of Japan. 1980;28:2915–23.
Borkar N, Li B, Holm R, Håkansson AE, Müllertz A, Yang M, et al. Lipophilic prodrugs of apomorphine I: preparation, characterisation, and in vitro enzymatic hydrolysis in biorelevant media. Eur J Pharm Biopharm Elsevier. 2015;89:216–23.
Bala V, Rao S, Li P, Wang S, Prestidge CA. Lipophilic prodrugs of SN38: synthesis and in vitro characterization toward oral chemotherapy. Mol Pharm. American Chemical Society. 2016;13:287–94.
Fleisher D, Bong R, Stewart BH. Improved oral drug delivery: solubility limitations overcome by the use of prodrugs. Adv Drug Deliv Rev. Elsevier. 1996;19:115–30.
You Y-J, Kim Y, Nam N-H, Ahn B-Z. Antitumor activity of unsaturated fatty acid esters of 4′-demethyldeoxypodophyllotoxin. Bioorg Med Chem Lett Pergamon. 2003;13:2629–32.
Naesens L, Neyts J, Balzarini J, Bischofberger N, De Clercq E. In vivo antiretroviral efficacy of oral bis(POM)-PMEA, the bis(pivaloyloxymethyl)prodrug of 9-(2-phosphonylmethoxyethyl) adenine (PMEA). Nucleosides Nucleotides Nucleic Acids. 1995;14:767–70.
Wichitnithad W, Nimmannit U, Wacharasindhu S, Rojsitthisak P, Wichitnithad W, Nimmannit U, et al. Synthesis, characterization and biological evaluation of succinate prodrugs of curcuminoids for colon cancer treatment. Molecules Molecular Diversity Preservation International. 2011;16:1888–900.
Charman WN, Porter CJH. Lipophilic prodrugs designed for intestinal lymphatic transport. Adv Drug Deliv Rev. 1996;19:149–69.
Sugihara J, Furuuchi S, Ando H, Takashima K, Harigaya S. Studies on intestinal lymphatic absorption of drugs. II. Glyceride prodrugs for improving lymphatic absorption of naproxen and nicotinic acid. J Pharmacobiodyn The Pharmaceutical Society of Japan. 1988;11:555–62.
Kumar R, Billimoria JD. Gastric ulceration and the concentration of salicylate in plasma in rats after administration of 14C-labelled aspirin and its synthetic triglyceride, 1,3-dipalmitoyl-2(2′-acetoxy-[14C]carboxylbenzoyl) glycerol. J Pharm Pharmacol. Wiley/Blackwell (10.1111). 1978;30:754–8.
Paris GY, Garmaise DL, Cimon DG, Swett L, Carter GW, Young P. Glycerides as prodrugs. 3. Synthesis and antiinflammatory activity of [1-(p-chlorobenzoyl)-5-methoxy-2-methylindole-3-acetyl]glycerides (indomethacin glycerides). J Med Chem Am Chem Soc. 1980;23:9–13.
Paris GY, Garmaise DL, Cimon DG, Swett L, Carter GW, Young P. Glycerides as prodrugs. 2. 1,3-Dialkanoyl-2-(2-methyl-4-oxo-1,3-benzodioxan-2-yl)glycerides (cyclic aspirin triglycerides) as antiinflammatory agents. J Med Chem Am Chem Soc. 1980;23:79–82.
Hanauer SB. Review article: high-dose aminosalicylates to induce and maintain remissions in ulcerative colitis. Aliment Pharmacol Ther. Wiley/Blackwell (10.1111). 2006;24:37–40.
Keum N, Greenwood DC, Lee DH, Kim R, Aune D, Ju W, Hu FB, Giovannucci EL. Adult weight gain and adiposity-related cancers: a dose-response meta-analysis of prospective observational studies. JNCI: Journal of the National Cancer Institute. 2015;107(2).
Fumagalli G, Marucci C, Christodoulou MS, Stella B, Dosio F, Passarella D. Self-assembly drug conjugates for anticancer treatment. Drug Discov Today. Elsevier Current Trends. 2016;21:1321–9.
Reddy LH, Marque P-E, Dubernet C, Mouelhi S-L, Desmaële D, Couvreur P. Preclinical toxicology (subacute and acute) and efficacy of a new squalenoyl gemcitabine anticancer nanomedicine. J Pharmacol Exp Ther American Society for Pharmacology and Experimental Therapeutics. 2008;325:484–90.
Kawabata K, Takakura Y, Hashida M. The fate of plasmid dna after intravenous injection in mice: involvment of scavenger receptors in its hepatic uptake. Pharm Res. 1995;12:825–30 Available from: https://springerlink.bibliotecabuap.elogim.com/article/10.1023/A:1016248701505. Accessed 19 Sept 2018.
Gupta A, Asthana S, Konwar R, Chourasia MK. An insight into potential of nanoparticles-assisted chemotherapy of cancer using gemcitabine and its fatty acid prodrug: a comparative study. J Biomed Nanotechnol. 2013;9:915–25.
Maiti K, Mukherjee K, Gantait A, Saha BP, Mukherjee PK. Curcumin–phospholipid complex: preparation, therapeutic evaluation and pharmacokinetic study in rats. Int J Pharm. 2007;330:155–63.
Chue P, Chue J. A review of paliperidone palmitate. Expert Rev Neurother. 2012;12:1383–97.
ARISTADA® (aripiprazole lauroxil) | Every 2 Months (1064 mg) [Internet]. [cited 2018 Sep 15]. Available from: https://www.aristada.com/. Accessed 19 Sept 2018.
Meltzer HY, Risinger R, Nasrallah HA, Du Y, Zummo J, Corey L, et al. A randomized, double-blind, placebo-controlled trial of aripiprazole lauroxil in acute exacerbation of schizophrenia. J Clin Psychiatry. 2015;76:1085–90.
Rautio J, Kärkkäinen J, Sloan KB. Prodrugs—recent approvals and a glimpse of the pipeline. Eur J Pharm Sci. Elsevier B.V. 2017;109:146–61.
Hanaoka K, Suzuki M, Kobayashi T, Tanzawa F, Tanaka K, Shibayama T, et al. Antitumor activity and novel DNA-self-strand-breaking mechanism of CNDAC (1-(2-C-cyano-2-deoxy-?-d-ARABINO-Pentofuranosyl) cytosine) and itsN4-palmitoyl derivative (CS-682). Int J Cancer Wiley-Blackwell. 1999;82:226–36.
Painter GR, Almond MR, Trost LC, Lampert BM, Neyts J, De Clercq E, et al. Evaluation of hexadecyloxypropyl-9-R-[2-(Phosphonomethoxy)propyl]- adenine, CMX157, as a potential treatment for human immunodeficiency virus type 1 and hepatitis B virus infections. Antimicrob Agents Chemother. American Society for Microbiology Journals. 2007;51:3505–9.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editor: Sanyog Jain
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Date, T., Paul, K., Singh, N. et al. Drug–Lipid Conjugates for Enhanced Oral Drug Delivery. AAPS PharmSciTech 20, 41 (2019). https://doi.org/10.1208/s12249-018-1272-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-018-1272-0