Abstract
The objective of the present study was to investigate the effects of processing variables and formulation factors on the characteristics of hot-melt extrudates containing a copolymer (Kollidon® VA 64). Nifedipine was used as a model drug in all of the extrudates. Differential scanning calorimetry (DSC) was utilized on the physical mixtures and melts of varying drug–polymer concentrations to study their miscibility. The drug–polymer binary mixtures were studied for powder flow, drug release, and physical and chemical stabilities. The effects of moisture absorption on the content uniformity of the extrudates were also studied. Processing the materials at lower barrel temperatures (115–135°C) and higher screw speeds (50–100 rpm) exhibited higher post-processing drug content (~99–100%). DSC and X-ray diffraction studies confirmed that melt extrusion of drug–polymer mixtures led to the formation of solid dispersions. Interestingly, the extrusion process also enhanced the powder flow characteristics, which occurred irrespective of the drug load (up to 40% w/w). Moreover, the content uniformity of the extrudates, unlike the physical mixtures, was not sensitive to the amount of moisture absorbed. The extrusion conditions did not influence drug release from the extrudates; however, release was greatly affected by the drug loading. Additionally, the drug release from the physical mixture of nifedipine–Kollidon® VA 64 was significantly different when compared to the corresponding extrudates (f 2 = 36.70). The extrudates exhibited both physical and chemical stabilities throughout the period of study. Overall, hot-melt extrusion technology in combination with Kollidon® VA 64 produced extrudates capable of higher drug loading, with enhanced flow characteristics, and excellent stability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
It has been estimated that more than 40% of all new molecular entities suffer from solubility-limited bioavailability due to the advent of high throughput screening and combinatorial chemistry [1]. Oral delivery of such compounds presents one of the most frequent and formidable challenges to formulation scientists. A myriad of techniques such as spray drying [2], solvent evaporation [3], nanocrystal formation [4], complexation [5], and micronization of active pharmaceutical ingredient (API) [6] have been widely utilized in the industry to improve the solubility and/or dissolution rate of such BCS class II drugs, thereby enhancing their bioavailability. During the past couple of decades, hot-melt extrusion (HME) technology has gained enormous interest among researchers for improving the bioavailability of drug substances, especially those having low water solubility, by the formation of solid dispersions [7–9]. Compared to other techniques, HME offers several advantages such as being solvent-free, a potentially continuous process, and involving fewer processing steps in general.
HME is the process of pumping raw materials with rotating screws under elevated temperatures through a die into a product of uniform character and shape. These final extrudates may be in the form of films or rods that can be cut as tablets and pellets or milled into powder as desired. To date, many research papers have been published by scientists that focused on the preparation of solid dispersions/solutions of numerous model drugs with low solubility such as clotrimazole, indomethacin, nifedipine etc., by utilizing HME technology. Shibata et al. produced solid solutions of indomethacin and crospovidone using a twin screw extruder. It was demonstrated that parameters such as residence time, screw speed, and heating temperatures play a significant role in the extrusion process [10]. On the other hand, in a study by Verhoeven et al., the effect of process parameters (screw design, feed rate, and screw speed) on metoprolol tartarate and ethyl cellulose mini matrices were studied, and revealed that the process parameters had no significant influence on drug release, indicating the robust nature of HME [11]. The role of the kneading elements and operating conditions of a twin screw extruder on nifedipine and HPMCP extrudates were studied by Nakamichi et al. [12], and the authors concluded that all of these factors played an important role in obtaining an ideal solid dispersion. It should be noted that most of the previous studies revealed that the influence of the processing parameters is dependent on both the physical and chemical properties of the API and the carrier matrix.
In this current study, nifedipine (a dihydropyridine calcium channel antagonist) was employed as a model drug, and Kollidon® VA 64 was utilized as the polymeric carrier. Nifedipine is a photosensitive yellow powder which is practically insoluble in water and has a melting point of 172–174°C. It is a calcium channel blocker used in the treatment of hypertension and angina. Nifedipine is commercially available as both immediate release (10–30 mg three times daily) and extended release (30–60 mg once daily) tablet dosage forms. Kollidon® VA 64 is a vinyl pyrrolidone/vinyl acetate copolymer at a ratio of 6:4. It is a hygroscopic white powder, soluble both in water and alcohols and has a glass transition temperature of approximately 106°C. It has several pharmaceutical applications such as a tablet binder, a granulating agent, and a film former. Nifedipine’s poor oral bioavailability (approximately 45–56%), primarily due to solubility limitations, and the soluble nature of the polymer, as well as its low glass transition temperature, makes them an appropriate choice as the drug and the carrier for HME processing at relatively lower temperatures.
The nature of Kollidon® VA 64 as a matrix forming polymer in HME has been previously studied by researchers to some extent. For example, rapidly disintegrating tablets containing indomethacin and Kollidon® VA 64 extrudates were prepared by Dinunzio et al. [13]. In addition, Jijun et al. prepared solid dispersion tablets of nimodipine with a mixture of Eudragit® EPO and Kollidon® VA 64 by combining HME with subsequent direct compression [14]. However, a further in-depth characterization of Kollidon® VA 64 hot-melt extrudates focusing on the processing and formulation parameters is warranted for a thorough understanding of the dispersed systems formed utilizing this polymer. Therefore, in this current study, we have extensively studied the influence of various process parameters (i.e., screw speed and extrusion temperature) in addition to formulation factors (i.e., drug load, plasticizer type, and concentration), on drug release and physical and chemical stabilities. Additionally, the effect of moisture on content uniformity and dissolution, as well as the effect of melt extrusion processing on powder flow characteristics, have also been investigated.
MATERIALS AND METHODS
Materials
Nifedipine USP was purchased from LETCO Medical (Decatur, AL, USA); polyethylene glycol 3350, triethyl citrate, stearic acid, vitamin E succinate, propyl paraben, sodium lauryl sulfate (SLS), potassium phosphate monobasic, and sodium hydroxide were obtained from Fisher Scientific (Fair Lawn, NJ, USA). Kollidon® VA 64 was kindly gifted by BASF Corporation. High-performance liquid chromatography (HPLC) grade water was freshly prepared in the laboratory by Nanopure systems (Barnstead, Dubuque, IA, USA). All solvents utilized in the study were of analytical grade and obtained from Fisher Scientific (Fair Lawn, NJ, USA).
Analytical Method
An in-house reverse phase high-performance liquid chromatography (HPLC) analytical method was developed for the quantification of nifedipine. This method was validated according to ICH and FDA guidelines for chromatographic methods. An HPLC equipped with UV detector, Waters Symmetry shield 5 μ C18 column (250 × 4.6 mm), and an isocratic mode of elution with mobile phase containing acetonitrile and water (55:45) at a flow rate of 1.0 mL/min were employed to quantify the drug at a wavelength (λmax) of 235 nm. The acquired data was processed using Empower 2 build 2154 software (Waters Inc., Mount Holly, NJ, USA).
Solubility Parameter Calculations
Hansen solubility parameters were obtained from the literature. Molecular Modeling Pro, v6.2.8 (ChemSW, Fairfield, CA, USA) was used to calculate the solubility parameters for all materials not referenced in the literature by using their respective molecular structures and melting points.
Thermal Gravimetric Analysis
The thermal stability of the nifedipine, Kollidon® VA 64, their physical mixture, and the melt-extruded samples were determined as a function of weight loss. The analysis was performed on the samples (approximately 4–5 mg) using a PerkinElmer Pyris 1 thermogravimetric analyzer (Norwalk, CT, USA) operated a ramp rate of 20°C/min from a temperature of 50 to 250°C. The percent weight loss for all of the samples tested was recorded using the Pyris 1 thermal gravimetric analysis (TGA) software. All of the TGA runs were performed in an open pan under an ultra-high purity nitrogen purge at a flow rate of 40 mL/min.
Differential Scanning Calorimetry Analysis
Differential scanning calorimetry (DSC) was used to characterize the miscibility of nifedipine in Kollidon® VA 64, utilizing a PerkinElmer Diamond DSC instrument (Shelton, CT, USA). A 2–3 mg sample of the pure drug and the polymer and their physical mixtures with varying drug concentrations (5, 10, 20, 40, 60, and 80%) were weighed using a Mettler Toledo™ Excellence Plus (model no. XP 204) analytical balance, sealed in aluminum crimped pans (Kit 0219–0062, PerkinElmer Instruments, Shelton, CT, USA), and heated from 40 to 245°C at a ramp rate of 20°C/min under nitrogen purge at a flow rate of 20 mL/min. The hot-melt extrudates were prepared by varying the drug concentrations as mentioned above and subjected to an initial heat–cool cycle (by heating to 140°C at the rate of 20°C/min and held for 5 min followed by cooling) to remove the thermal history of the samples. A second heat cycle was initiated wherein the samples were heated from 40 to 230°C at a ramp rate of 20°C/min under nitrogen purge at a flow rate of 20 mL/min.
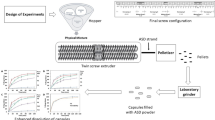
Hot-Melt Extrusion
Nifedipine and Kollidon® VA 64 were used as a model drug and carrier matrix, respectively. Prior to processing, the raw materials had been passed through a US no. 40 mesh sieve and accurately weighed. The materials were mixed in a twin shell V-blender (The Patterson-Kelly Co., Inc., East Stroudsburg, PA, USA) at a speed of 25 rpm for 10 min to obtain a homogenous physical mixture. During the extrusion process, the drug–polymer mixture was fed into the hopper and its opening was sealed immediately with an aluminum foil. For the most part, the material being extruded was in an enclosed condition and was very minimally exposed to the light, if any. Each of these resultant drug–polymer binary mixtures containing 25% of the drug were extruded at three different temperature profiles (115, 135, and 155°C) utilizing a bench top corotating twin screw extruder (Prism 16 mm EuroLab, Thermo Electron Corporation, Newington, NH, USA) equipped with an 8-mm round opening die. The effects of three different screw speeds (25, 50, and 100 rpm) were also investigated for each of the temperature profiles. A total of nine batches of extrudates of each of the formulations were obtained. In addition, the effects of processing aids on the extrudates containing 10/90% w/w and 40/60% w/w drug/polymer mixtures were evaluated. All of the extrudates were stored in foil-lined polyethylene bags and stored in under refrigeration until further analysis.
Post-Processing Drug Content
A randomly selected portion of each of the extruded formulations was crushed into a fine powder using a mortar and pestle and stored in amber-colored glass bottles. These powdered extrudates were analyzed for post-extrusion drug content immediately after processing. A known amount of the extrudates and physical mixture were dissolved in 3:2 methanol:water, diluted and filtered using 0.2 μm, 13 mm PTFE membrane filters (Whatman, Piscataway, NJ, USA), and analyzed utilizing a HPLC at a wavelength of 235 nm.
X-Ray Diffraction
X-ray diffraction (XRD) measurements were conducted using a Philips Model 1710 X-ray diffractometer (Philips Electronic Instruments Inc., Mahwah, NJ, USA) to assess crystallinity. Pure nifedipine, the polymer, the physical mixtures, and the powdered extrudates were passed through a US no. 40 mesh screen prior to testing. The physical mixtures were prepared by premixing nifedipine and Kollidon® VA 64 in the appropriate ratio (25% drug/75% polymer). The diffractometer was operated at an accelerating voltage of 40 kV and 30 mA. The samples were placed into channeled stages and the diffraction profile was measured from 3.0° to 50.0° using a 2θ step size of 0.02° and dwell time of 3 s.
Moisture Absorption
Moisture absorption by the drug, polymer, physical mixture (1:3; drug:polymer), and the extrudates was investigated by placing each of the samples in opened amber-colored borosilicate glass bottles. The opened bottles were stored in a 40°C/75% RH environment for 7 days. In addition to moisture absorption, the samples were also examined for content uniformity both before and after the absorption study.
The moisture absorbed by the samples is reported in terms of their percent weight gain (G) and was calculated using Eq. 1:
where S is the weight of the sample before moisture absorption, wi is the initial weight of a glass bottle with sample (before moisture absorption), and wf is the final weight of a glass bottle with sample (after moisture absorption). All measurements were performed in triplicate and were compared with the initial data.
Flow Properties
The pure polymer, drug–polymer physical mixtures with varying drug loads (10, 25, and 40% w/w) and their corresponding extrudates have been assessed for their flow characteristics using the traditional “angle of repose” measurement. In this experiment, a funnel was set to a fixed height using a clamp such that the distance between the lower tip of the funnel and the surface of a graph paper placed below is constant. The powder was slowly poured through the funnel on to the paper until the bulk material formed a conical pile with its tip touching the base of the funnel. The angle of repose (θ) for each of these powder samples was then measured by Eq. 2:
In Eq. 2, h is the height of the pile of powder and d is the diameter of the surface covered by the powder.
In Vitro Dissolution
Dissolution testing (USP XXXI, Apparatus II; 50 rpm) was performed utilizing a Hanson SR8-plus™ dissolution test station (Hanson Research Corporation, Chatsworth, CA, USA). Drug release from the various formulations was evaluated. Additionally, the effect of moisture absorption on the drug-loaded samples (10, 25, and 40% w/w) was evaluated. An amount equivalent to 25.0 mg nifedipine (theoretical drug loading) from the physical mixture and the previously ground extrudates was accurately weighed and filled in gelatin capsule no, 3. These capsules were added to the dissolution vessel (preheated to 37 ± 0.5°C) containing 900 mL of pH 6.8 phosphate buffer with 1% w/v sodium lauryl sulfate (SLS). During testing, 1.5 mL samples were removed from the dissolution vessels at predetermined time intervals and replaced with an equal volume of fresh dissolution medium. Samples were immediately filtered using 13-mm PTFE membrane filters (Whatman, Piscataway, NJ, USA) with a pore size of 0.2 μm and analyzed using HPLC at a λ max of 235 nm. Drug concentration was calculated from a standard calibration plot and expressed as cumulative percent of drug dissolved. The release studies were performed in triplicate, and the mean values were compared.
Stability Studies
A portion of the nifedipine–Kollidon® VA 64 extrudates were stored in amber-colored borosilicate glass bottles at three different temperatures (4, 25, and 40°C) and analyzed at predetermined time intervals for the amount of nifedipine present using HPLC. The stability of these extrudates was then compared to the physical mixture. The results of the stability studies are expressed as a percentage of nifedipine degraded at predetermined time intervals.
Statistical Analysis
To compare between different formulations, statistical analysis was performed utilizing one-way analysis of variance (ANOVA). A statistically significant difference was considered to be when P < 0.05.
RESULTS AND DISCUSSION
Analytical Method
Validation was carried out as per the ICH and FDA guidelines for chromatographic methods. The linear calibration range for the detection of nifedipine was found to be 5–200 μg/mL, with the coefficient of determination (R 2) of 0.999. The limit of detection and quantitation for the drug were 0.2 μg/mL and 1.0 μg/mL, respectively, and the retention time for nifedipine was ~7.8 min. The percent relative standard deviation (RSD) of the replicates was less than 2%, demonstrating the reproducibility of this method. Precision was tested by injecting a single drug concentration of 20 μg/mL, and the peak area (n = 10) was recorded. An excellent precision was observed with less than 0.5% RSD.
Solubility Parameter Calculations
A three-dimensional Hansen solubility parameter obtained from either literature or Molecular Modeling ProTM software represents dispersive, polar, and hydrogen bonding interactions of a molecule, and it could be used to estimate the interactions among the components in a formulation [15]. From a pharmaceutical applications standpoint, this parameter is a very useful tool in predicting the API morphology, drug–polymer miscibility in the solid dispersions, and the drug–excipient interactions in the development of solid oral dosage forms. The effective solubility parameter obtained from Hansen method for nifedipine and Kollidon® VA 64 were 17.9 and 21.1 MPa1/2, respectively. Theoretically, in order to observe at least partial miscibility between the drug and the polymer, the solubility parameter difference (Δδ) between them is required to be less than 7.0 MPa1/2 [16, 17]. In the present scenario, Δδ is 3.2, which is significantly less than 7.0 MPa1/2, indicating miscibility of nifedipine in Kollidon® VA 64.
Thermal Gravimetric Analysis
It is crucial to study the thermal profiles of the API as well as the individual excipients employed in a formulation prior to melt extrusion. To evaluate the active and excipient processability and stability during extrusion, the materials were subjected to heat ramp and identified that both the pure components and their physical mixtures exhibited less than 2% weight loss throughout the temperature range studied (Fig. 1). It was, therefore, concluded that the processing temperatures studied were suitable to employ during the extrusion process.
Differential Scanning Calorimetry Analysis
An important prerequisite to attain a solid dispersion is an understanding of the miscibility or interaction between the API and polymeric carrier. In the present study, the miscibility of nifedipine at various concentrations in Kollidon® VA 64 was investigated using a PerkinElmer diamond DSC. In the thermograms shown in Fig. 2, the temperature is plotted on the X-axis and heat flow (endotherm up) on the Y-axis. The DSC thermogram of the pure drug revealed a characteristic melting endotherm of nifedipine at 172–174°C [18], while the physical mixtures exhibited a slight melting point depression of the drug in presence of the polymer. This is indicative of the API’s miscibility in the carrier. Nifedipine was completely miscible only at a concentration of 5% w/w in the physical mixtures, whereas the hot-melt extrudates demonstrated good miscibility of the drug (up to 40% w/w) in the polymeric matrix. The peak corresponding to the melting of nifedipine was clearly evident in the DSC thermogram of the physical mixtures at 40% w/w of the active, whereas it disappeared in the melt extrudates (loss of crystallinity) at same drug load. This could be attributed to the formation of an amorphous solid dispersion of nifedipine in the Kollidon® VA 64 matrix. However, nifedipine at a concentration of 60 and 80% w/w in the melt extrudates does not seem to be completely miscible.
Hot-Melt Extrusion
Effect of Processing Parameters (Temperature and Screw Speed)
The process parameters employed in this study are provided in Table I. All of the formulations exhibited a maximum die pressure of 6–7 bars with a consistent material feed rate. The extrudates processed at lower barrel temperatures (115 and 135°C), irrespective of screw speeds, exhibited higher post-processing drug content (97–100%). However, for the formulations processed at a higher extrusion temperature of 155°C, the post-processing drug content decreased with decrease in screw speed from 100 to 25 rpm (Fig. 3). In particular, the formulation produced at a higher temperature (155°C) and lower screw speed (25 rpm) exhibited only 45% drug remaining post-processing. The lower screw speed of 25 rpm displayed relatively longer material residence time in the extruder barrel (approximately 6–7 min) in comparison to the higher screw speeds, and the exposure of the active at 155°C for such a relatively long period of time might have caused drug degradation. The results were in accord with the findings of Caira et al., where the authors identified the existence of a solvated crystal form of nifedipine using single-crystal X-ray technique. This solvated crystal form undergoes desolvation leading to a complete disruption of crystalline structure and conversion into a monoclinic polymorph that degrades at relatively high temperatures of 150–153°C [18]. Both of the processing parameters, temperature and screw speed, were found to have a significant impact on the post-processing drug content of the final extrudates.
Effect of Formulation Factors: Drug Load and Process Aids
It was identified from the process evaluation that use of lower temperatures was suitable to produce extrudates with minimal drug degradation. In addition, when the preliminary extrusion runs were conducted at a screw speed of 100 rpm, although the run demonstrated good post-processing drug content, it was noticed that the residence time of the material inside the extruder barrel was only 1–2 min. On the other hand, the run with 50-rpm screw speed has not only exhibited excellent post-processing drug content, but also provided relatively longer residence time (about 3–4 min) inside the barrel. This extra residence time obtained at 50-rpm screw speed could potentially provide a better mixing opportunity for the molten materials leading to uniform drug distribution throughout the extrudate matrix, without affecting the drug content. Consequently, 10, 25, and 40% w/w nifedipine formulations were extruded at 135°C and 50-rpm screw speed, which resulted in formulations with excellent post-processing drug content (99–100%). The drug concentrations in the formulations were chosen based on the results obtained from preliminary DSC studies that supported the formation of a solid dispersion in mixtures containing up to 40% w/w drug.
Furthermore, to produce a flexible formulation with greater ease, and at lower temperatures, processing aids are very useful. Kollidon® VA 64, as mentioned earlier, softens above 100°C and may necessitate the use of a processing aid in order to produce a uniform melt at relatively low processing temperatures. For this purpose, several hydrophilic and hydrophobic additives such as polyethylene glycol 3350, triethyl citrate, stearic acid, vitamin E succinate, and propyl paraben, each at two different concentrations (2.5 and 5% w/w), were investigated as processing aids to evaluate their miscibility in drug-loaded Kollidon® VA 64 blends. Each of these physical mixtures in the preliminary set of experiments, containing 25% w/w drug and 70–72.5% w/w of the polymer as well as the processing aids, was prepared into polymeric patches. This was accomplished using the melt casting method on a brass plate heated to 130°C and using a punch and an 8-mm round die. The physical properties of the patches were evaluated visually for flexibility and texture. None of the chemical processing aids utilized in the formulation exhibited flexibility in the melt casted films. Moreover, they produced either glassy or sticky products at the concentrations employed. Nevertheless, Kollidon® VA 64 was extrudable at 90% w/w, with only a model drug in the formulation.
X-Ray Diffraction
DSC studies indicated drug–polymer miscibility in the hot extrudates (data not shown), which has been further confirmed by the XRD analysis. The diffraction pattern of pure nifedipine exhibited sharp and highly intense peaks at 2θ degrees of 8.0, 11.9, 19.5, and 24.0, indicating the crystalline nature of the drug [19]. However, the physical mixtures containing drug and polymer at 1:3 ratios, when subjected to X-ray diffraction, exhibited relatively less intense and more diffused peaks. The 2θ angles of the peaks remained unaffected (Fig. 4). These changes in peak intensity could be attributed to the dilution of the active in the Kollidon® VA 64 matrix, and the unchanged 2θ angles of the peaks indicate existence of original crystalline form of the drug in the physical mixture. Conversely, the XRD pattern of the melt-extruded formulations produced using different process parameters did not demonstrate any sign of crystallinity or characteristic peaks at specific 2θ angles. This indicated the formation of an amorphous solid dispersion in those formulations. This data further supports the DSC data discussed earlier where the active was completely miscible in the melt extrudates up to 40% w/w in which there was no sign of the characteristic melting endotherm of nifedipine.
Moisture Absorption
It was hypothesized that variability in the intimacy of mixing in different powder samples would be explained by investigating the extent of moisture absorption, which is directly proportional to the amount of hygroscopic surface area of the material being tested [20]. Thus, the extent of moisture absorption could be indicative of the intimate mixing of powders. High moisture content in the powder mixtures could lead to an inconsistency in the drug content and drug dissolution. The extent of moisture absorption was estimated by percentage weight gained by the powder mixtures when subjected to high relative humidity conditions. Although the mixtures were exposed to high humidity conditions for 7 days, all of the samples under test reached equilibrium within 24 h. The extrudates (at 25% w/w drug loading) exhibited 15–20% decrease in weight gain compared to their corresponding physical mixture, and about 30% decrease in weight gain in comparison to the pure polymer when subjected to the same conditions. Among the extrudates, the formulations produced utilizing different processing temperatures and screw speeds did not demonstrate any significant difference in the percent of moisture absorbed (data not presented), which was indicated by unchanged percentage weight gain of the mixtures. However, as the drug concentration increased from 10 to 40% w/w in the extrudates, the decrease in weight gain in the milled extrudates changed from 25 to 45%, when compared to the pure polymer (Fig. 5). This change in the percent weight gain, or reduced moisture uptake, could be attributed to the presence of low concentrations of hygroscopic polymer in the formulations with higher drug loading.
Additionally, the content uniformity of nifedipine in the milled extrudates containing 10, 25, and 40% w/w drug loads and in 25% drug to 75% polymer physical mixture, prior to and after exposure to high humidity conditions for a period of 7 days, was evaluated. From the data presented in Fig. 6, it is obvious that the milled extrudate samples did not show any great change in the drug content uniformity before and after being exposed to a high humidity environment. This finding was irrespective of their drug concentrations. Although the percent weight gain varied among the milled extrudates with different drug loading, the drug distribution within the polymeric mixtures was not affected by the presence of moisture. This could be due to the drug being uniformly distributed at a molecular level in the polymeric matrix during the melt extrusion process, which provides intimate contact between both of the components in a formulation. Further, nifedipine content uniformity in the physical mixtures decreased from 96 to 87% after exposure to high humidity levels. This variability in the content could be attributed to high moisture absorbing tendency of the physical mixtures, where the drug might form discrete agglomerates in the moisture-rich polymer phase during physical mixing, and ultimately result in a nonuniform distribution of active in the polymeric carrier.
Flow Properties
The manufacturing of dosage forms such as tablets and capsules is a very simple and cost-effective approach that has been in practice over many decades. In order to produce such formulations with good content uniformity, it is essential to consider the powder characteristics such as particle shape, density, and coefficient of friction of the material. The angle of repose, utilized to measure the flow properties, is also related to the attributes mentioned above. Smaller angles correspond to wide spread of the bulk material, which indicates acceptable flow properties.
From the results of the study presented in Table II, Kollidon® VA 64 demonstrated excellent flow properties by itself. However, an increase in the drug load from 10 to 40% w/w in the physical mixtures resulted in larger angles of repose, which indicate poor flow characteristics. This behavior could be attributed to the difference in particle sizes between the API and the polymer and to the cohesive nature of API diluting the free flowing polymer. On the other hand, similar binary physical mixtures, when processed utilizing hot-melt extrusion technology, followed by milling into fine powder, demonstrated superior powder flow characteristics independent of drug loading. A possible explanation for this observation could be the formation of a solid dispersion, post-extrusion. As previously confirmed by DSC and XRD studies, where the drug dissolves at a molecular level in the hydrophilic polymer matrix, there is a resulting change in the API physical properties. Based on these results, it is obvious that hot-melt extrusion technology could be utilized to enhance the flow characteristics of the bulk material that could potentially minimize the content uniformity issues in conventional dosage forms.
In Vitro Dissolution
Dissolution testing of the melt extrudates in vitro is another important aspect that provides an implication of how a formulation behaves in vivo in terms of drug release. All of the extruded formulations, produced at different screw speeds and temperatures, showed similar release profiles; in addition, more than 90% of the drug was released within 1 h from all of the formulations tested (Fig. 7a, b). The similarity factor f 2 was utilized to compare the dissolution profiles among different formulations [21] and was calculated using Eq. 3. Here, n is the total number of sampling intervals and R t and T t are cumulative percent of drug released from reference and test formulations at any time interval t.
All of the obtained f 2 values, when compared among the dissolution profiles, were between 50 and 100 which indicated that the drug release was similar for all of the formulations under investigation.
Similarly, extrudates with varying drug loads of 10, 25, and 40% w/w were analyzed for drug release, and their release patterns are presented in Fig. 8. The formulation containing 10% drug loading demonstrated a higher release rate compared to 25%, followed by 40% w/w drug load. The extrudates with lower drug concentration (10% w/w) behaved as an immediate release dosage forms with more than 90% of the drug being released within the first 20 min while those containing 25% w/w drug loading demonstrated similar release profiles within 60 min. From this data, it is apparent that the drug load has a great impact on the release characteristics of the API. This may be attributed to the presence of more amount of hydrophilic matrix, in the solid dispersion formulations containing 10% w/w nifedipine. The extrudates containing 40% w/w nifedipine demonstrated slower drug dissolution when compared to the extrudates with lower drug loading. This could be attributed to the low solubility of the drug in the chosen dissolution media, and poor wettability of the active.
Drug release from the extrudates with varying drug concentrations was studied before and after subjecting samples to the moisture absorption study. There was no significant difference observed in the release profiles (data not shown) before and after the moisture absorption studies. Moreover, the release of nifedipine from the 25% w/w extrudates was found to be significantly enhanced (Fig. 8) compared to its corresponding 25% w/w physical mixture (f 2 = 36.70). This is indicative of the formation of solid dispersion (previously evidenced by the DSC and XRD data) utilizing hot-melt extrusion technology.
Stability Studies
Achieving a stable formulation with minimal or no API degradation throughout the product’s shelf-life is of paramount importance for any given dosage form. Conventional dosage forms have numerous excipients incorporated in their formulations that lead to an increased possibility of drug–excipient incompatibilities which can result in drug degradation. In this study, the extrudates have the additional advantage being comprised of a binary mixture of the drug and the polymeric carrier, thus minimizing the risk of subsequent drug instability. Additionally, it is also well known that temperature is a key factor that influences chemical reaction rates, and consequently plays an important role in drug degradation [22]. As per the Arrhenius equation, a pharmaceutical system under investigation, when subjected to excessive thermal exposure, undergoes accelerated degradation. However, the assumption inherent in the Arrhenius equation may render it somewhat inaccurate for various systems due to the unexpected phase changes or adsorbed moisture present. It is, therefore, advisable to use the lowest temperatures for the study that produces measurable failure. The chemical stability of the extruded formulations was studied by storing the samples at three different temperatures (4, 25, and 40°C) and analyzing them for percent active remaining in the extrudates, which represents the scale on Y-axis (Fig. 9). The API degradation in all of the formulations under study was found to be less than 5% for up to 3 months. These results indicate that the extrudates were chemically stable, and that the processing conditions did not significantly impact the stability of nifedipine in the extrudates.
This data was further corroborated with the physical stability results. As previously mentioned, elevated temperatures tend to promote chemical reactions while also accelerating the kinetics. The typical elevated storage temperature for accelerated degradation of a pharmaceutical system is 40°C. Therefore, the physical stability of the extrudates stored at this temperature for 3 months was assessed for change in the crystallinity or amorphous nature of the formed solid dispersions. XRD patterns (Fig. 10) of the extrudates under investigation did not demonstrate any sign of crystallization after exposing to the elevated temperature for such a long period, demonstrating their physical stability. In addition, all of the extruded formulations with 10, 25, and 40% drug loading were also found to be physically and chemically stable up to 3 months (data not presented) under the aforementioned conditions.
CONCLUSIONS
In this study, melt extrusion technology in combination with Kollidon® VA 64 produced chemically and physically stable extrudates with higher drug loading and enhanced drug release. Nifedipine was found to be miscible in Kollidon® VA 64 up to 40% w/w drug loading without demonstrating the need for any processing aids. For example, Kollidon® VA 64 was extrudable at 60–90% w/w with only nifedipine in the formulation. The processing parameters had a significant impact on the post-extrusion drug content, and the influence of drug load on release from extrudates was quite remarkable. These results demonstrate the importance of selecting a suitable carrier matrix for HME processes as well as appropriate processing conditions based on the physicochemical properties of the active, which will greatly influence the properties of the extruded formulations.
REFERENCES
Crowley MM, Zhang F, Repka MA, Thumma S, Upadhye SB, Battu SK, et al. Pharmaceutical applications of hot-melt extrusion: part I. Drug Dev Ind Pharm. 2007;33(9):909–26.
Paradkar A, Ambike AA, Jadhav BK, Mahadik KR. Characterization of curcumin–PVP solid dispersion obtained by spray drying. Int J Pharm. 2004;271(1–2):281–6.
Arora SC, Sharma PK, Irchhaiya R, Khatkar A, Singh N, Gagoria J. Development, characterization and solubility study of solid dispersions of azithromycin dihydrate by solvent evaporation method. J Adv Pharm Technol Res Apr;1(2):221–8
Hecq J, Deleers M, Fanara D, Vranckx H, Amighi K. Preparation and characterization of nanocrystals for solubility and dissolution rate enhancement of nifedipine. Int J Pharm. 2005;299(1–2):167–77.
Battu SK, Repka MA, Maddineni S, Chittiboyina AG, Avery MA, Majumdar S. Physicochemical characterization of berberine chloride: a perspective in the development of a solution dosage form for oral delivery. AAPS PharmSciTech Sep;11(3):1466–75
Vogt M, Kunath K, Dressman JB. Dissolution enhancement of fenofibrate by micronization, cogrinding and spray-drying: comparison with commercial preparations. Eur J Pharm Biopharm. 2008;68(2):283–8.
Repka MA, Battu SK, Upadhye SB, Thumma S, Crowley MM, Zhang F, et al. Pharmaceutical applications of hot-melt extrusion: part II. Drug Dev Ind Pharm. 2007;33(10):1043–57.
Repka MA, Majumdar S, Kumar Battu S, Srirangam R, Upadhye SB. Applications of hot-melt extrusion for drug delivery. Expert Opin Drug Deliv. 2008;5(12):1357–76.
Repka MA, Shah S, Lu J, Maddineni S, Morott J, Patwardhan K, et al. Melt extrusion: process to product. Expert Opin Drug Deliv Jan;9(1):105–25
Shibata Y, Fujii M, Sugamura Y, Yoshikawa R, Fujimoto S, Nakanishi S, et al. The preparation of a solid dispersion powder of indomethacin with crospovidone using a twin-screw extruder or kneader. Int J Pharm. 2009;365(1–2):53–60.
Verhoeven E, De Beer TR, Van den Mooter G, Remon JP, Vervaet C. Influence of formulation and process parameters on the release characteristics of ethylcellulose sustained-release mini-matrices produced by hot-melt extrusion. Eur J Pharm Biopharm. 2008;69(1):312–9.
Nakamichi K, Nakano T, Yasuura H, Izumi S, Kawashima Y. The role of the kneading paddle and the effects of screw revolution speed and water content on the preparation of solid dispersions using a twin-screw extruder. Int J Pharm. 2002;241(2):203–11.
Dinunzio JC, Schilling SU, Coney AW, Hughey JR, Kaneko N, McGinity JW. Use of highly compressible Ceolus™ microcrystalline cellulose for improved dosage form properties containing a hydrophilic solid dispersion. Drug Dev Ind Pharm Feb;38(2):180–9.
Jijun F, Lishuang X, Xiaoli W, Shu Z, Xiaoguang T, Xingna Z, et al. Nimodipine (NM) tablets with high dissolution containing NM solid dispersions prepared by hot-melt extrusion. Drug Dev Ind Pharm Aug;37(8):934–44.
Hancock BC, York P, Rowe RC. The use of solubility parameters in pharmaceutical dosage form design. Int J Pharm. 1997;148(1):1–21.
Forster A, Hempenstall J, Tucker I, Rades T. Selection of excipients for melt extrusion with two poorly water-soluble drugs by solubility parameter calculation and thermal analysis. Int J Pharm. 2001;226(1–2):147–61.
Greenhalgh DJ, Williams AC, Timmins P, York P. Solubility parameters as predictors of miscibility in solid dispersions. J Pharm Sci. 1999;88(11):1182–90.
Caira MR, Robbertse Y, Bergh JJ, Song M, De Villiers MM. Structural characterization, physicochemical properties, and thermal stability of three crystal forms of nifedipine. J Pharm Sci. 2003;92(12):2519–33.
Chutimaworapan S, Ritthidej GC, Yonemochi E, Oguchi T, Yamamoto K. Effect of water-soluble carriers on dissolution characteristics of nifedipine solid dispersions. Drug Dev Ind Pharm. 2000;26(11):1141–50.
Fukuda M, Miller DA, Peppas NA, McGinity JW. Influence of sulfobutyl ether beta-cyclodextrin (Captisol) on the dissolution properties of a poorly soluble drug from extrudates prepared by hot-melt extrusion. Int J Pharm. 2008;350(1–2):188–96.
Battu SK, Repka MA, Majumdar S, Madhusudan RY. Formulation and evaluation of rapidly disintegrating fenoverine tablets: effect of superdisintegrants. Drug Dev Ind Pharm. 2007;33(11):1225–32.
Zolnik BS, Leary PE, Burgess DJ. Elevated temperature accelerated release testing of PLGA microspheres. J Control Release. 2006;112(3):293–300.
ACKNOWLEDGMENTS
This project was supported by Grant No. P20RR021929 from the National Center for Research Resources (NIH/NCRR). The authors would also like to thank BASF Corporation for its generous supply of Kollidon®VA 64, as well as Dr. James W. McGinity for his assistance in conducting XRD studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maddineni, S., Battu, S.K., Morott, J. et al. Influence of Process and Formulation Parameters on Dissolution and Stability Characteristics of Kollidon® VA 64 Hot-Melt Extrudates. AAPS PharmSciTech 16, 444–454 (2015). https://doi.org/10.1208/s12249-014-0226-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-014-0226-4