Abstract
Background
People all around the world are affected by primary liver cancers like hepatocellular carcinoma (HCC), which is usually associated with cirrhosis. Early HCC detection is crucial for better prognosis, but effective biomarkers are still needed. Hepcidin, a hormone-regulating iron homeostasis, has been implicated in liver diseases. In this study, blood hepcidin levels were evaluated in cirrhotic individuals as a possible biomarker for HCC.
Methods
There were three groups involved in this case-control study: cirrhotic patients with no HCC (group I), cirrhotic patients diagnosed with HCC (group II), and healthy controls (group III). Clinical and laboratory data, such as those from tests indicating the liver function, hepcidin levels, and imaging, were all analyzed using a number of statistical tests.
Results
When compared to those with cirrhosis, serum hepcidin levels were significantly lower in HCC patients, but there was no significant difference statistically between the two studies involved: cirrhotic groups and the controls. Serum alpha-fetoprotein (AFP) was also significantly greater in HCC patients.
Conclusions
The start and progression of liver diseases, such as HCC in cirrhotic people, appear to be influenced by hepcidin. It can be utilized as a potential HCC biomarker when cirrhotic liver is present, despite the fact that it cannot be used to diagnose cirrhosis by itself.
Similar content being viewed by others

Background
HCC, which is the fourth most frequent cancer in Egypt and also the sixth major reason behind cancer-related deaths woldwide, has a huge impact on global health [1]. Cirrhosis in particular, a known risk factor for the HCC development, is closely related to chronic liver diseases [2]. The HCC prognosis is greatly influenced by early detection, as timely intervention can improve treatment outcomes and patient survival rates. Therefore, the search for reliable biomarkers to aid in early HCC diagnosis in cirrhotic patients has become a crucial focus of research [3]. AFP has been employed traditionally as HCC biomarker; however, it has limits regarding sensitivity and specificity, particularly among those individuals with cirrhosis and other liver conditions [4]. As a result, there is a growing interest in exploring alternative biomarkers that can more accurately detect HCC in the presence of cirrhosis.
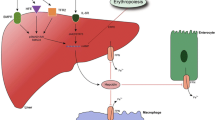
One such choice is hepcidin, a 25-amino acid hormone that is mostly produced by the liver and crucial for maintaining the body’s iron homeostasis [5].
Numerous liver conditions, such as cirrhosis and HCC, have been linked to iron metabolism and hepcidin dysregulation [6]. Hepatic fibrosis, which is a precursor to cirrhosis, has been connected to hepcidin deficit, and cirrhosis’ altered expression of hepcidin may contribute to the HCC onset and progression [7]. Accordingly, blood hepcidin levels may be used as a possible biomarker to identify cirrhotic individuals’ risk for developing and progressing HCC.
Methods
The study was conducted during 2021 and 2022 at the Alexandria University at Faculty of Medicine’s Tropical Medicine Department, Egypt. The study enrolled three groups of participants: Group I consisted of 30 established cirrhotic patients (HCV-induced liver cirrhosis) without HCC, group II of 30 cirrhotic patients (HCV-induced liver cirrhosis) with HCC, and group III of 30 healthy controls with same age and sex. Before being enrolled in the study, every subject gave their informed consent. Each participant’s demographic information, clinical complaints, and results of laboratory tests were gathered. All individuals had thorough clinical evaluations and in-depth history collection. Complete blood count, tests of liver function (AST, ALT [total and direct serum bilirubin] level, prothrombin time [PT], international normalized ratio [INR], serum albumin, alkaline phosphatase), renal function tests (serum urea and serum creatinine), fasting blood glucose, serum iron, and total iron-binding capacity (TIBC) were all performed as part of routine laboratory investigations. Enzyme-linked immunosorbent assay (ELISA) methods were also used to measure the AFP and serum hepcidin levels. All participants performed radiological examinations, including an abdominal ultrasound and a triphasic CT abdomen for those who had ultrasound-proven hepatic focal lesions. Exclusion criteria included those with HCV unrelated hepatic tumors, hemolytic-related diseases, concurrent hepatitis B virus infection, hemochromatosis family history, and patients with recent bleeding or blood transfusions histories of and also those diagnosed with autoimmune liver disease.
Statistical analysis
Using the IBM SPSS software program, version 20.0 (Armonk, NY, USA, IBM Corp.), the data were statistically analyzed. Quantitative information was displayed as percentages and figures. For quantitative data, the Shapiro-Wilk test was employed to verify the normality of the distribution. Several descriptive statistics, such as range (minimum and maximum), mean, standard deviation, median, and interquartile range (IQR), were available for quantitative data. The 5% level was used to establish the statistical significance results.
The subsequent statistical tests were applied to compare various groups:
-
1.
Using the chi-square test, for comparing the categorical variables between different groups
-
2.
Fisher’s test exact correction: Used to correct the chi-square statistic when 20% or more of the cells have a count of less than 5
-
3.
The one-way ANOVA test is used when comparing data that are normally distributed between more than two groups, and for pairwise comparisons, a post hoc test (Tukey) is included.
-
4.
Kruskal-Wallis test: It is used to compare numerical data across groups of more than two when it is not normally distributed, with a test for pairwise comparisons called post hoc (Dunn’s multiple test).
Power calculation
A power calculation was performed to calculate the required sample size for the investigation, based on the expected effect size and significance level. This ensured that the study had the ability to detect any found significant differences statistically among the groups if they existed.
Ethical considerations
The study adhered to ethical principles and guidelines, ensuring the privacy and confidentiality of participants’ data. Before including any patients or controls in the study, informed permission was acquired from each of them. Data handling and analysis followed standard ethical practices.
Results
Table 1 compares the three study groups in terms of demographic information. Group I had 16 males (53.3%), while there were 14 females (46.7%). The ages of the patients varied from 50 to 76 years old, with a mean ± SD of 59.23 and 7.59. Males made up 63.3% of group II, while females made up 36.7%. The ages of the patient ranged within the range of 50 to 78 years old having a mean ± SD of 60.90 ± 7.84 years old. Thirteen patients (43.3%) were female, and 17 (56.7%) were male in group III. The patients’ age ranged between 23 and 69 years old (mean ± SD of 56.53 ± 10.27 years old). Regarding age and sex, there was difference but not statistically significant between the three study involved groups (p = 0.727 and 0.149, respectively).
A 43.3% of Child-Pugh score of C was achieved by the patients in groups I and II as a whole. When comparing the Child-Pugh scores of the two groups, there was no discernible difference (p = 0.935) (Table 2).
Patients with cirrhosis and HCC had a greater chance of developing multiple tumors (11/26; 36.7%). Tumors often have the maximum diameter between 1.5 and 18 cm, with a mean SD of 7.49 and 5.08 cm (Table 3). The portal vein involved thrombosis diagnosis which was made in 17 patients (56.7%), and infiltration was seen in 6 individuals (20%). Seven patients (23.3%) experienced lymph node metastases, while three patients (10%) reported distant metastases. (Table 3). The majority of the patients had BCLC type D (43.3%) (Table 3).
Overall, group II exhibited a substantial increase in serum AFP (ng/ml) compared to groups I, II, and III (p 0.001). Group I also had greater AFP (ng/ml) than group III did (p = 0.034). The three groups differed statistically significantly in terms of AFP serum levels (ng/ml) (Table 4).
Hepcidin (ng/ml) was statistically greater in group I compared to group II (p1 = 0.001*), but neither group I nor group II showed a statistically significant difference with group III. respectively (p = 0.122 and 0.080) Table 5.
When comparing serum iron levels (μg/dL) in micrograms per deciliter, there was no significance statistically between the three involved study groups (p = 0.284) (Table 6).
No statistically significant correlation between hepcidin and serum AFP, APRI score, and size of tumor was detected among cirrhotic patients diagnosed with HCC (p = 0.759, 0.932, and 0.714) respectively, as illustrated in Table 7.
Furthermore, there was no significant association statistically between Hepcidin and the BCLC score, tumour number, or distant metastasis in Cirrhotic patients with HCC (p = 0.990, 0.182 and 0.283) respectively as illustrated in Table 8.
The diagnostic performance of hepcidin and serum AFP to distinguish cirrhotic individuals with HCC from cirrhotic patients without HCC was evaluated using ROC curve analysis (Fig. 1).
Hepcidin demonstrated sensitivity of 73.33% and specificity of 76.67%, 75.9% (PPV), and 74.2% (NPP) to differentiate between the two groups at a cut-off value of 1.1164 (ng/ml) (p 0.001) (Table 9).
Furthermore for discrimination between both groups, serum AFP at a threshold of more than 12 ng/ml displayed a 73.33% sensitivity, 80% specificity, PPV of 78.6%, and NPP of 75% (p < 0.001) (Table 9), while a combination of both hepcidin and serum AFP to discriminate between both groups exhibited 73.33% sensitivity, 100.0% specificity, 100.0% PPV, and 78.95% NPP (p < 0.001) (Table 9).
Discussion
The fourth most prevalent malignancy in Egypt and the sixth most common malignancy in the globe are primary liver cancer. With between 70 and 85% of all cases being HCC, it is the most common kind of 1ry hepatic malignancy and is linked to high morbidity and death [8, 9].
Regardless of the cause, cirrhosis is a substantial risk factor for HCC. With an annual incidence of 1–6%, HCC is the primary killer of cirrhotic individuals [10]. It is crucial to understand that HCC has a better prognosis the sooner it is diagnosed [11]. It is strongly advised to employ various blood indicators to identify the early diagnosis of HCC. Numerous studies are being conducted to find a more accurate and focused marker for the diagnosis of HCC [12].
The 25-AA hormone hepcidin is released by the liver and keeps the body’s systemic iron homeostasis stable. Hepcidin and its modulators must also be dysregulated as Fe sensing is dysregulated in HCC. Hepcidin deficiency enhances the development of hepatic fibrosis, a risk factor for HCC, in animal studies. Additionally, cirrhosis has decreased hepatic hepcidin expression [13]. This also raises the chance of developing HCC. All of this points to the significant role that hepcidin plays in the development, progression, and metastasis of cancer as well as the formation and advancement of liver disease [14]. The goal of the current study was to assess the serum hepcidin level as a potential biomarker for HCC in cirrhotic people.
The individuals in the current study were similar in age and gender in all groups that were analyzed. This was consistent with Mohamed et al. [15]. Most of the participants in this research were assigned a Child-Pugh C (43.3%, for both groups). Regarding the Child-Pugh score, the two research groups did not vary significantly statistically. Seemingly matching our results, Sheta et al. [16] observed no discernible difference in Child-Pugh scores between HCC patients and cirrhotic patients; however, contrary to our results, the majority of HCC cases (68.8%) and cirrhotic cases (64.6%) were assigned to Child-Pugh class B. On the other hand, in Li et al. [17] cohort analysis, almost all HCC and patients diagnosed with cirrhosis were assigned to class A of Child-Pugh.
The patient’s majority (36.7%) in the present research who had HCC had more than three tumors. The biggest dimension of the mean tumor size was7.49 ± 5.08 cm. In 56.7%, 20%, 23.3%, and 10% of cases of group II, respectively, there was portal vein thrombosis, infiltrates, lymph node metastases, and distant metastases. This agreed with research done by Salem et al. [17]. They discovered that the majority of the HCC patients in their research had multifocal lesions (61.4%), those that were larger than 5 cm (86.5%), and those that had lymph node, portal vein thrombosis, and distant metastases.
In comparison, using CT, Mohamed et al. [15] analyzed 20 individuals with HCC. They discovered that the majority of patients had numerous hepatic tumors that were less than 5 cm in diameter and lacked lymph node metastases and distant metastases. While in Ismail et al. [12] study, according to the findings of the radiological evaluation of the HCC patients, 63.33% of them had a single focal lesion without abdominal lymph node metastases or portal vein invasion.
According to Ibrahim et al. research [16], which found that half of HCC patients were categorized as BCLC D, the patient’s majority in this investigation (43.3%) were BCLC D. However, this outcome was distinct from Matboli et al. [17] who found that the majority of HCC were categorized as BCLC A (88.2%).
AFP serum levels (ng/ml) in the HCC-diagnosed patients in the current research were significantly higher than those in the control individuals and cirrhotic patients diagnosed with no HCC (P 0.001). Furthermore, levels of AFP were higher in patients diagnosed with cirrhosis and no HCC than those involved in the controls (P = 0.034). This was in line with other earlier investigations [16, 18, 19]. In contrast, Matboli et al. [17] observed no discernible variation in AFP levels between HCC patients, cirrhotic patients, and controls.
Various studies indicated low AFP sensitivity [12, 20]. AFP is often used for screening in individuals with a high risk of developing HCC; however, when chronic liver disease and acute hepatitis are both exacerbating, serum AFP temporarily rises, making diagnosis challenging. It could also be typical in certain HCC patients [21]. This may explain the difference between the results of studies regarding AFP.
Despite the fact that many iron metabolism indicators, such as ferritin and hepatic iron, are frequently used tools for diagnosis in identifying the load of iron as a liver fibrosis risk factor, hepcidin has gained attention as a recently discovered substance that primarily functions as an iron efflux controller from cells [7].
In the present investigation, HCC patients’ serum hepcidin levels (ng/ml) significantly decreased as compared to those with cirrhosis (P = 0.001). However, the hepcidin level differences between cirrhotic patients and controls, as well as between HCC patients and controls, were not statistically significant, showing that serum hepcidin can be a crucial biochemical parameter in the development of cirrhosis into HCC but is not a diagnostic marker for either cirrhosis or HCC.
On the contrary to our results, Tsochatzis et al. [22] found that in comparison to healthy control individuals, serum hepcidin levels were lower considerably among HCC patients, and Mohamed et al. [15] stated that serum hepcidin level was significantly lower among cirrhotic ones than those in the investigation control group, but similar to our outcome, level of hepcidin significantly decreased among cases with HCC than those with liver cirrhosis.
Wang et al. [23] found that levels of hepcidin in HBV-cirrhotic patients are lower than those in HBV patients with non-cirrhosis. In addition, they said that when compared to healthy controls, hepcidin levels do not alter, which was consistent with our findings. Contrary to our findings, Wang et al. [23] hypothesized that individuals with cirrhosis caused by HBV had lower mean hepcidin levels than those with HCC caused by HBV or those with HBV without cirrhosis.
This discrepancy may be clarified by the hypothesis that serum hepcidin levels are influenced by the cirrhosis etiology but not by its severity [22].
No significant correlation statistically between level of hepcidin and serum AFP, APRI score and size of tumor was detected among cirrhotic patients with HCC (P = 0.759, 0.932, and 0.714) respectively which was contradictory with Mohamed Amal et al. [15] who mentioned revealed hepcidin level, and each of AFP, iron, AST, and ALT showed a highly significant inverse association, while there was no such link with albumin. But this was in agreement with Detivaud et al. [24] who discovered a correlation with no statistical significance between serum hepcidin and both fibrosis degrees.
We observed no statistically significance difference in serum iron (μg/dL) in both HCC and cirrhotic patients compared to controls, but Marzouk et al. [25] discovered that CLD patients’ serum iron levels were lower than those of the control group. These findings aligned with those discovered that the moderate anemic condition of CHC patients was a problem. The substantially decreased hepatic hepcidin synthesis in these patients may potentially be influenced by this anemia. In contrast, Mohamed et al. [15] concluded that serum iron was significantly higher in CHC patients compared to the control group. Also, Wang et al. [23] observed that the HCC patients had blood iron levels that were considerably greater than those of the control group, showing that iron is required for the growth of cancerous cells. The discrepancy between our finding and others may be due to most of our patient was in Child-Pugh C. Given that ferritin is a protein in the acute phase, and that there is not enough information in the literature to specify the precise changed amount, our study did not examine ferritin levels, which led to a misunderstanding about the study’s conclusion [26].
Using ROC curve analysis, the ability of hepcidin and AFP level in serum to distinguish those with cirrhosis and HCC from other patients with cirrhosis and no HCC was assessed.
Hepcidin demonstrated sensitivity of 73.33%, specificity of 76.67%, 75.9% (PPV), and 74.2% (NPP) to differentiate between the two groups at a cut-off value of 1.1164 (ng/ml) (p 0.001), which was agreeing with Mohamed Safia et al.’s report [27], which stated that the point of cutoff for serum hepcidin levels in HCC identification from the control group was 2.1 (ng/ml), with an AUC = 1, 100% sensitivity, specificity, positive, and NPV.
Moreover, Mohamed et al. [15] determined that the optimum cutoff value of serum hepcidin for distinguishing HCC patients from those with other liver disorders was 42.7 (ng/ml), AUC = 0.9, with sensitivity of 92%and specificity of 90%. The difference in the number of HCC patients included in each study may be the cause of this discrepancy.
Also, to discriminate between both groups, serum AFP at a cut-off value higher than 12 ng/ml displayed a sensitivity of 73.33%, 80% specificity, PPV of 78.6%, and NPP of 75% (p < 0.001), agreeing with Mohamed Amal et al. [15] who claimed that for the diagnosis of HCC patients, AFP had a cut-off value of 21 ng/ml and had a 66.7% sensitivity and 72% specificity (p < 0.001). Similar results were found by Toyoda et al. [28], who concluded that the use of AFP alone for the monitoring of hepatocellular carcinoma is not recommended since it has a limited role in the detection and diagnosis of HCC.
So, using serum AFP alone for diagnosis of HCC in cirrhotics is better than using serum hepcidin alone as it showed more specificity (80%), PPV of 78.6%, and NPP of 75% (p < 0.001).
A combination of both hepcidin and serum AFP to discriminate between both groups showed a 73.33% sensitivity, 100.0% specificity, 100.0% PPV, and 78.95% NPP. (p<0.001) which is better than using either serum AFP or serum hepcidin alone.
This research has several restrictions. First off, because this research only included a limited sample size from one hospital, it is important to be cautious when interpreting the results. Second, this investigation produced no information about the timing and manner of the change in serum levels in these individuals without serial assessment of the circulating hepcidin levels. Hepcidin tests were taken after the HCC; therefore, they may not correctly represent pre-HCC exposure.
Conclusions
We concluded that although hepcidin levels cannot be utilized as a diagnostic marker for cirrhosis or HCC, they may be employed as an essential biochemical parameter in progress of cirrhosis up to HCC. Using ROC curve analysis, the ability of combined use of hepcidin and AFP level in serum to distinguish those with cirrhosis and HCC from other patients with cirrhosis and no HCC is better than AFP alone or hepcidin alone.
Availability of data and materials
The article contains the information utilized to support the conclusions of this study.
Abbreviations
- HCV:
-
Hepatitis C virus
- HCC:
-
Hepatocellular cancer
- CBC:
-
Complete blood count
- ESR:
-
Erythrocyte sedimentation rate
- CRP:
-
C-reactive protein
- FBG:
-
Fasting blood sugar
- AST:
-
Aspartate transferase
- ALT:
-
Alanine transferase
- ALP:
-
Alkaline phosphatase
- PA:
-
Prothrombin activity
- INR:
-
International normalized ratio
- AFP:
-
Alfa-fetoprotein
- APRI:
-
AST-to-platelet ratio index
- ELISA:
-
Enzyme-linked immunosorbent assays
- PVT:
-
Portal vein thrombosis
- ULN:
-
Upper limit of normal
- μg/dL:
-
Micrograms per deciliter
References
Hanif H, Ali MJ, Susheela AT, Khan IW, Luna-Cuadros MA, Khan MM et al (2022) Update on the applications and limitations of alpha-fetoprotein for hepatocellular carcinoma. World J Gastroenterol 28(2):216–29
Yang JD (2019) Detect or not to detect very early stage hepatocellular carcinoma? The Western perspective. Clin Mol Hepatol 25(4):335–43
Paganoni R, Lechel A, Vujic Spasic M. Iron at the interface of hepatocellular carcinoma. Int J Mol Sci. 2021;22(8).
Agarwal AK, Yee J (2019) Hepcidin. Adv Chronic Kidney Dis 26(4):298–305
Tarao K, Nozaki A, Ikeda T, Sato A, Komatsu H, Komatsu T et al (2019) Real impact of liver cirrhosis on the development of hepatocellular carcinoma in various liver diseases-meta-analytic assessment. Cancer Med 8(3):1054–65
Chidambaranathan-Reghupaty S, Fisher PB, Sarkar D (2021) Hepatocellular carcinoma (HCC): epidemiology, etiology and molecular classification. Adv Cancer Res 149:1–61
Vela D (2018) Low hepcidin in liver fibrosis and cirrhosis; a tale of progressive disorder and a case for a new biochemical marker. Mol Med (Cambridge, Mass) 24(1):5
Tampaki M, Papatheodoridis GV, Cholongitas E. Management of hepatocellular carcinoma in decompensated cirrhotic patients: a comprehensive overview. Cancers (Basel). 2023;15(4).
Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR (2019) A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterolhepatol 16(10):589–604
Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S et al (2021) Hepatocellular carcinoma. NatRev Dis Primers 7(1):6
Vogel A, Martinelli E (2021) Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann Oncol 32(6):801–5
Ismail SA, El Saadany S, Ziada DH, Zakaria SS, Mayah WW, Elashry H et al (2017) Cytokeratin-18 in diagnosis of HCC in patients with liver cirrhosis. Asian Pac J Cancer Prev 18(4):1105–11
Kessler SM, Barghash A, Laggai S, Helms V, Kiemer AK (2015) Hepatic hepcidin expression is decreased in cirrhosis and HCC. J Hepatol 62(4):977–9
Joachim JH, Mehta KJ (2022) Hepcidin in hepatocellular carcinoma. Br J Cancer 5(1):123–30
Mohamed A, Badawy I, El Amir N, Shalaby H, Sief S, Maklad S et al (2014) Evaluation of serum hepcidin and iron levels in some liver diseases. MEJAS. 4(2):166–74
Ibrahim MH, El-Raey FM, Fathy N, El-Bakrey K (2020) Transthyretin as a novel biomarker for diagnosis of hepatocellular carcinoma in cirrhotic patients. Int J Med Arts 2(2):412–9
Matboli M, Shafei AE, Ali MA, Ashry AM, Kamal KM, Agag MA et al (2019) circRNAs are novel potential biomarkers in hepatocellular carcinoma. J Cell Biochem 120(5):7711–24
Takaya H, Namisaki T, Kitade M, Kaji K, Nakanishi K, Tsuji Y et al (2019) VWF/ADAMTS13 ratio as a potential biomarker for early detection of hepatocellular carcinoma. BMC Gastroenterol 19(1):167
Sheta T, Selim M, Sabry M, Saed A (2021) Serum thioredoxin as a diagnostic marker for hepatocellular carcinoma in cirrhotic hepatitis C patients. Med J Viral Hepat 5(2):9–15
Sanai FM, Sobki S, Bzeizi KI, Shaikh SA, Alswat K, Al-Hamoudi W et al (2010) Assessment of alpha-fetoprotein in the diagnosis of hepatocellular carcinoma in Middle Eastern patients. Digest Dis Sci 55(12):3568–75
Sherman M (2001) Alphafetoprotein: an obituary. J Hepatol 34(4):603–5
Tsochatzis E, Papatheodoridis GV, Koliaraki V, Hadziyannis E, Kafiri G, Manesis EK et al (2010) Serum hepcidin levels are related to the severity of liver histological lesions in chronic hepatitis C. J Viral Hepat 17(11):800–6
Wang J, Dong A, Liu G, Anderson GJ, Hu TY, Shi J et al (2016) Correlation of serum hepcidin levels with disease progression in hepatitis B virus-related disease assessed by nanopore film based assay. Sci Rep 6:34252
Detivaud L, Elizabeta nemeth, Karim Boudjema, Olvier Lore al, et al (2005) Hepcidin levels in humans are correlated with hepatic iron stores, haemoglobin levels, and hepatic function. Blood 106:746–8
Marzouk HA, Zayed NA, Al-Ansary M et al (2013) Hepcidin levels in Egyptian patients with chronic hepatitis C and the effect of anti-viral therapy. World Appl Sci J 22(8):1140–1145
Rosário C, Zandman-Goddard G, Meyron-Holtz EG, D’Cruz DP, Shoenfeld Y (2013) The hyperferritinemic syndrome: macrophage activation syndrome, Still’s disease, septic shock, and catastrophic antiphospholipid syndrome. BMC Med 11:185
Mohamed S, Morsy A , Mohamed N, Mohamed A (2019) Estimation of serum hepcidin and ferritin in patients with chronic liver disease. Egypt J Hosp Med 74(8):1817–1825. Article 18
Toyoda H, Kumada T, Osaki Y, Oka H, Kudo M (2007) Role of tumor markers in assessment of tumor progression and prediction of outcomes in patients with hepatocellular carcinoma. Hepatol Res 37(Suppl 2):166–71
Acknowledgements
The authors are thankful to all the patients who took part in this study.
Funding
The authors declare that no funding, grants, or other forms of support were received.
Author information
Authors and Affiliations
Contributions
MA was in charge of the practical section, data analysis, and manuscript preparation. All authors provided critical feedback and helped shape and revise the research, the analysis, and the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This research was conducted in line with ethical principles. Before beginning the research, the Faculty of Medicine, Alexandria University Ethical Committee, granted clearance on April 15, 2021, and the study protocol adheres to the ethical principles outlined in the 1975 Declaration of Helsinki. Each participant’s consent was acquired in advance. The committee’s serial number is 0106755, and the reference number is FWA no.: 00018699.
Consent for publication
Both patients and the control group provided written informed consent. Patients participating in this research consent to data publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohiedeen, K.M., Tahoon, M.M., Hanna, C.S.S. et al. Serum level of hepcidin in cirrhotic patients as a marker for hepatocellular carcinoma. Egypt Liver Journal 14, 10 (2024). https://doi.org/10.1186/s43066-023-00307-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43066-023-00307-2