Abstract
Background
Family Libellulidae is one of the largest families of suborder Anisoptera (Odonata) including 1035 species of 144 genera throughout the world. Libellulids are distributed all around the globe, while some are cosmopolitan and some are endemic. Cytogenetic data pertains to 258 libellulid species and chromosome number varies from 2n = 6–47. Majority of the species possess 2n (♂) = 25, which is the modal number of the family. The m chromosomes are considered as the fragments of autosomes and are present in 78% of studied libellulid species.
Main body
Presently, 29 libellulid species have been catalogued based on various research articles related to cytogenetic studies regarding intraspecific chromosomal variations especially due to the m chromosomes within the same or different geographical populations of the species.
Conclusions
Odonata possess holokinetic chromosomes and m chromosomes are the fragments of autosome. The break can occur at any time, at any place, which is responsible for variations in the size of m chromosomes. These variations also depend on the geographical distribution of the species which persists over generations by the action of natural selection and also play role in speciation.
Similar content being viewed by others
Background
Family Libellulidae is one of the largest families of suborder Anisoptera (Odonata) which includes 1035 species of 144 genera throughout the world and 91 species of 40 genera are present in India (Subramanian & Babu, 2017). The dragonflies of family are widely distributed from tropical to tempate regions. They commonly fly around lakes and ponds (Jarzembowski & Nel, 1996). Their size, shape and colour differ widely (Fraser, 1936). Most of the libellulids have coloured and patterned wings and anal loop is present on the hind wing. They are able to survive in low oxygen water and some species can also live in brackish water (Mitra, 2005). They act as predator in aquatic and terrestrial life. They feed on small insects like moth, chironomid midges and beetles, which act as pest on various crops. Their larvae feed on the mosquito larvae and also referred as “mosquito hawks” (Kenny & Burne, 2001).They help to control of the diseases like malaria, dengue, chikungunya, etc. Dragonflies can act as good bioindicator as they detect environmental changes and react quickly (McGeoch, 1998).They require specific habitat and their presence indicate the environmental conditions (Simaika & Samways, 2012).
Cytogenetic work plays an important role to describe the evolutionary relationships between different groups of Odonata. Cytogenetically, 258 libellulid species have been described throughout the world which also includes 45 species from India (Dasgupta, 1957; Kuznetsova & Golub, 2020; Walia & Singh, 2021). The chromosome number varies from 2n = 6–47 in the studied species. This wide range in the chromosome number is due to the chromosomal fusion/fragmentation or the presence/absence of m chromosomes in both intra and interspecific populations which is very frequent in odonates because of the holocentric chromosomes (Kuzenstova & Golub, 2020). Kiauta (1967) explained the evolution of the chromosome number in Odonates as the ancestral chromosome number is n = 9, which increased in stepwise manners as specialization increases in the order and the secondary complements in the order arise due to fusion or fragmentation of the chromosomes in different ways (Fig. 1). In the family libellulidae, 78% species possess possess 2n (♂) = 25, which is the modal number of the family, while 22% species show variations in the chromosome number (Kuzenstova & Golub, 2020, Walia & Singh, 2021). The most common sex determination mechanism in libellulid is XX (♂)/X0(♀) which is derived from primitive XX (♂)/XY (♀) sex determining mechanism in animals (Asana & Makino, 1935; Das, 1956; Oguma, 1915, 1917; Smith, 1916; White, 1954). X chromosomes shows postreductional behaviour during the meiosis and its size varies in different species. In addition to this, Neo-XY (Fig. 2) and X1X1X2X2/X1X2Y (Fig. 3) are also present in various libellulid species which are originated by the fusion of X chromosome with autosomes (Kiauta, 1968d, 1969a; Mola et al., 1999; Omura, 1955; Ray Chaudhuri & Dasgupta, 1949; Sandhu & Walia, 1994; Walia & Sandhu, 1998).
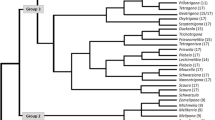
Diagrammatic representation of the karyotypic evolution of chromosome number in Odonata (Kiauta, 1967)
Representation of X1X2Y sex determining mechanism in Micrathyria ungulata given by Mola et al. (1999)
m chromosomes and their role in phylogenetic assessment of Odonata: m chromosomes are small chromosomes which possess particular meiotic behaviour as compared to both autosomes and sex chromosomes (Wilson, 1905). They were noticed for the first time in the dragonfly, Anax junius (Lefevre & McGill, 1908). They are small in size, shows negative heteropycnosis and their size varies in different species. These are smaller than or equal to half the size of immediately large chromosomes (Mola, 1992). According to Oguma (1930), m chromosomes are normal autosomes which undergo gradual diminution in volume until they eventually disappeared. This view has been accepted by Dagupta (1957) and Cumming (1964). However, Kiauta (1968a) discarded the “m chromosome theory” and described m chromsomes as the fragments of autosomes. The breaks can occur at any time, at any place in the holokinetic chromosomes which are responsible for variations in the size of m chromosomes in different and in the same specimen. These variations also depend on the geographical distribution of the species which persists over generations through the action of natural selection and also play role in speciation (Souza Bueno, 1982).
The presence/absence of m chromosomes in the species is also helpful in determining the phylogenetic relationship of species. Genus Libellula L. is mainly holoarctic in distribution and found in the temperate zones of the northern hemisphere, while it developed some species also in the transitional and subtropical zones (Kaiuta, 1968c). However, it is thought to be originated from the Eastern Hemisphere based on the penile characters. The dominancy of this genus is shifted from Eurasia in past to North America, and various centres of specialization were form during migration (Kennedy, 1922a, 1922b). Cytogenetically, same frequencies of two chromosome complements n = 12and n = 13 were found in specimens of Libellula depressa from Austria. There were total four kinds of karyotypes found in specimens—(a) complement without m chromosome, (b) cells having one bivalent had a subterminal constriction, (c) the m chromosome is clearly separated from the original bivalent, but is found lying in the prolonged longitudinal axis close to the latter and (d) complement in which the m chromosome occupies a random positions among other elements (Kiauta, 1968c). The fragmentation of the autosome in this species is important as this species is most specialized and probably represents European offshoot of the American Plathemis stock. It must have migrated to Eurasia before L. quadrimaculata entered into America. The difference between L. quadrimaculata and Platethemis is great, suggesting that it branched off much earlier (Kennedy, 1922b). Similarly, infraspeciation and radiation of Erythemis connata fusca stock is described. It is based on the distributional patterns, structural characters and the cytological evidences. The Erythemis connata fusca is supposed to be the oldest forn of connata” Artnkresis” and present in the centre of species range in the Atlantic drainage of South America, Central America and Mexico and without m chromosomes, while Erythemis connata and Erythemis minuscula present in the periphery and contain m chromosomes and thought to be originated from the central species (Kiauta & Boyes, 1972). m chromosomes are present in 78% of studied libellulid species and show variations in different pouplations of the species. Presently, intraspecific chromosomal variations based on m chromosomes in 29 libellulid species have been enlisted (Tables 1, 2 and 3).
Main text
Cytogenetically, 258 libellulid species has been studied worldwide and m chromosomes are present in 78% of libellulid species (Kuznetsova & Golub, 2020; Walia & Singh, 2021). Intraspecific karyomorphological variations in the chromosome complement due to m chromosomes have been recorded in 29 species and divided as:
-
A)
Fusion of m chromosome with an autosome pair or X chromosome (Table 1):
-
B)
Presence/absence of m chromosomes in different geographical populations of the species (Table 2):
-
C)
Two chromosome complements (with/without m chromosomes) in the same specimen of the species (Table 3):
-
A)
Fusion of m chromosome with autosome pair or X chromosome: In Odonata, fusions and fragmentations play an important role in karyotypic evolution due to holokinetic nature of chromosomes and are responsible for the increase/decrease in the chromosome number in species (Kiauta, 1968a). In the family Libellulidae, the fusion of m chromosomes with autosomal pair leads to decrease in the chromosome number from n = 13 m to n = 12 and the complement of the species is characterized by the presence of one large autosomal bivalent in Acisoma panporides, Elasmothemis cannacrioides, Erythrodiplax paraguayensis, Libellula depressa, Micrathyria hypodydima, Orthetrum brachiale, Orthetrum luzonicum (Ferreira et al., 1979; Kaur, 2016; Kiauta, 1969b, 1969c; Souza Bueno, 1982; Walia & Sandhu; 2002). Fusion of m chromosomes with X chromosome resulted in large X chromosome present in the complement with intermediate neo-XY stage in Crocothemis servilia, Orthetrum japonicum, Pontamarcha congener, Tholymis tillarga and Trithemis festiva (Higashi et al., 2001; Kaur, 2016; Omura, 1955; Sandhu & Walia, 1995a, 1995b; Walia & Sandhu, 2002).
-
B)
Presence/absence of m chromosomes in different geographical populations of the species: m chromosomes are present in one population and are absent in other populations of different geographical areas in Brachydiplax farniosa, Brechmorhoga mendax, Erythemis attala, Brachydiplax vesiculosa, Erythrodiplax basalis, Erythrodiplax fusca, Leucorrhinia hudsonica, Leucorrhinia intact, Orthemis ferruginea, Pachydiplax longipennis, Perithemis mooma, Tramea binotata, Trithemis annulata and Uracis ovipotrix (Agopian & Mola, 1988; Boyes et al., 1980; Cruden, 1968; Cumming, 1964; Dasgupta, 1957; Ferreira et al., 1979; Kaiuta and Boyes, 1972; Kiauta, 1979; Kiauta & Kiauta, 1983; Mola, 1996; Souza Bueno, 1982; Wasscher, 1985). Moreover, in Erythrodiplax fusca, size of m chromosomes varies in comparison to X chromosome as it is of the same size of X chromosome in Brazalian population (Ferreira et al., 1979), larger than X chromosome in Chile and Brazalian population (Kaiuta and Boyes, 1972; Souza Bueno, 1982) and is half the size of X chromosome in Argentina population (Mola, 1996). The variations in the size of m chromosomes are because of the site of breakage in the holokinetic chromosomes. Chromosomal breaks and fusions can occur at any times in the holokinetic chromosomes and are retained because of their abilities to attach the spindle apparatus during divison. The kinetic activity of holokinetic chromsomes do not depends on specific nucleotide sequences while it is epigentically controlled (Guerra et al., 2010).
Environment plays an important role for the survival of species in the particular area. Geographical populations are related to the surrounding environment conditions which act as the stimulant for the chromosomal rearrangements. In Erythrodiplax berenice, shows the venational “anomalies” as increase in the number of marginal cells in the Northern populations (Shortness, 1930). Two populations of species have been studied from North Carolina, USA and North Dakota, USA. The chromosome number was 13 and 14 m (Cruden, 1968; Hung, 1971). The populations close to the northernmost limit of species increase the recombination index by increase in the chromosome number, thus promoting the ability of species to vary and adapt to changing environmental conditions, morphologically and genetically (Kiauta & Boyes, 1972). Similarly, Erythrodiplax basalis, geographical variation in the male addominal and wing colouration is resulted due to the change in chromosome complement of Brazil, Bolivia and Surinam populations (Cumming, 1964; Ferriera et al.1979; Kiauta, 1979).
-
C)
Two chromosome complement (with/without m chromosomes) in the same speciemen of species: The numerical variations in the chromosome complement (with m/without m chromosomes) have been observed in the cells of same individual of Diplocodes haematodes, Elasmothemis williamsoni, Erythrodiplax berenice, Leucorrhinia frigida, Ologoclada laetitia, Orthetrum coerulescens and Tholymis tillarga (Cruden, 1968; Hung, 1971; Kaur, 2016; Kiauta, 1969b, 1971, 1979; Souza Bueno, 1982). The presence of two complements indicates the new adaptive level of the recombinations by the species which suits their evolutionary requirements. It is not a sudden event, the successful arrangement becomes stable in a population and others are eliminated (Kiauta, 1983). As in case of Crocothemis servilia, a large autosomal bivalent originated due to fusion of m chromosome with autosome pair is present only in the population of Japan and is absent in the other populations of species from China, Philippines, Taiwan and India. It represents the retention of suitable rearrangements in the population. Kiauta (1983) erected new genus Crocothemis servilia marrinae based on this cytological character as these rearrangements have been occurring from the generations to generation and play an important role in speciations.
Conclusions
In Odonata, chromosomes are holokinetic in nature and large numbers of genetic rearrangements are possible in these chromosomes. The m chromosomes are the fragments of autosomes and variations in the size of m chromosomes, their presence/absence in the complement, fusion of m chromosome with autosome pair and two different chromosome complement (with/without m chromosome) in the same specimen have been catalogued for 29 libellilid species.
Availability of data and material
Not applicable.
References
Agopian, S. S., & Mola, L. M. (1988). Intra and interspecific karyotype variability in five species of Libellulidae (Odonata, Anisoptera). Caryologia, 41(1), 69–78.
Asana, J. J., & Makino, S. (1935). A comparative study of the chromosomes in the Indian dragonflies. Journal of the Faculty of Science, Hokkaido University Series 6, Zoology, 4(2), 67–86.
Boyes, J. W., Van Brink, J. M., & Kiauta, B. (1980). Sixteen dragonfly karyotypes from the republic of South Africa and Swaziland, with evidence on the possible hybrid nature of Orthetrum julia falsum Longfeild (Anisoptera: Libellulidae). Odonatologica, 9, 131–145.
Carnoy, B. J. (1885). La cytodierese chez les arthropods. IV. Pseudo-Nevropteres. La Cellule, 1, 279–282.
Cruden, R. W. (1968). Chromosome numbers of some North American dragonflies (Odonata). Canadian Journal of Genetics and Cytology, 10, 200–214. https://doi.org/10.1139/g68-029
Cumming, R. B. (1964). Cytogenetic studies in the order Odonata. In PhD thesis. Austin: University of Texas.
Das, C. (1956). Studies on the association between Non-Homologus chromosomes during meiosis in four species of the Indian dragonflies (Odonata). Journal of Zoological Society of India, 8, 129–132.
Dasgupta, J. (1957). Cytological studies of some Indian dragonflies. II: A study of the chromosomes during meiosis in thirty species of Indian Odonata (Insecta). Proceedings of the Zoological Society, Calcutta, 10, 1–65.
Ferreira, A., Kiauta, B., & Zaha, A. (1979). Male germ cell chromosomes of thirty-two Brazilian dragonflies. Odonatologica, 8, 5–22.
Francovic, M., & Jurecic, R. (1986). Prilog citogenetickim i citotaksonomskim istrazivanjima vrste Libellula depressa L. (Odonata, Libellulidae). In Plenarni Referati VII Kongres Biologa Jugoslavije, Budva, (pp. 341).
Francovic, M., & Jurecic, R. (1989). Comparative cytogenetic analysis of karyotype morphology and organization in males of species Libellula depressa L. and L. fulva Müll. (Insecta: Odonata). Periodicum Biologorum, 91(1), 32–33.
Fraser, F. C. (1936). The fauna of British India, including Ceylon and Burma. Odonata., III, 1–418.
Guerra, M., Cabral, G., Cuacos, M., González-García, M., González-Sánchez, M., Vega, J., & Puertas, M. J. (2010). Neocentrics and holokinetics (holocentrics): Chromosomes out of the centromeric rules. Cytogenetic and Genome Research, 129(1–3), 82–96. https://doi.org/10.1159/000314289
Handa, S. M., & Batra, H. N. (1980). Cytology of ten species of dragonflies (Anisoptera: Odonata). In proceedings of the 67th Indian science congress, Part III, Calcutta. (pp. 103).
Handa, S. M., Mittal, O. P., & Batra, H. N. (1984). Chromosomes in ten species of dragonflies (Anisoptera: Odonata). Research Bulletin of the Panjab University, 35, 65–75.
Higashi, K., & Kayano, H. (1993). The distribution of distinct karyomorphs of Crocothemis servilia Drury (Anisoptera, Libellulidae) in Kyushu and the south-western islands of Japan. Japanese Journal of Entomology, 61, 1–10.
Higashi, K., Lee, C. E., Kayano, H., & Kayano, A. (2001). Korea strait delimiting distribution of distinct karyomorphs of Crocothemis servilia (Drury) (Anisoptera: Libellulidae). Odonatologica, 30(3), 265–270.
Hogben, L. (1921). Studies on synapsis, III. The nuclear organisation of the germ cells in Libellula depressa. Philosophical Transactions of the Royal Society of London Series B, 92, 60–80.
Hung, A. C. F. (1971). Cytological studies of five dragonflies (Odonata: Anisoptera). Entomological News, 82, 103–106.
Jarzembowski, E. A., & Nel, A. (1996). New fossil dragonflies from the early cretaceous of SE England and the phylogeny of the superfamily Libelluloidea (Insecta: Odonata). Cretaceous Research, 17, 67–85.
Katatani, N. (1987). On the chromosomes of dragonflies, 1. Synopsis on the studies in some Japanese dragonflies. Aeschna, 20, 21–31.
Kaur, J. (2016). Cytogenetic and molecular studies on some species of family Libellulidae (Odonata:Anisoptera). In Ph.D. thesis. Patiala: Punjabi University.
Kennedy, C. H. (1922a). The phylogeny and the geographical distribution or genus Libellula (Odonata). Ent. News, 33, 65–71.
Kennedy, C. H. (1922b). The phylogeny and the geographical distribution or genus Libellula (Odonata). Ent. News, 33, 105–111.
Kenny, L. P. & Burne, M. R. (2001). A Field Guide to the Animals of Vernal Pools. Natural Heritage and Endangered Species Program (p. 77). Westborough: MA.
Kiauta, B. (1967). Considerations on the evolution of the chromosome complement in Odonata. Genetica, 38(4), 430–446.
Kiauta, B. (1968a). Evolution of the chromosome complement in Odonata. Entomologische Berichten, Amsterdam, 28(5), 97–100.
Kiauta, B. (1968b). The chromosome numbers of eight old world dragonflies (Odonata). Chromosome Information Service, Tokyo, 9, 3–4.
Kiauta, B. (1968c). Autosomal fragmentations and fusions in Odonata and their evolutionary implications. Genetica, 40(2), 158–180. https://doi.org/10.1007/BF01787347
Kiauta, B. (1968d). Morphology and kinetic behaviour of the Odonata sex chromosomes, with a review of the distribution of sex determination mechanisms in the order. Genen Phaenen, 12(2), 21–24.
Kiauta, B. (1969a). Sex chromosomes and sex determining mechanisms in Odonata, with a review of the cytological conditions in the family Gomphidae, and reference to the karyotypic evolution in the order. Genetica, 40(2), 127–157. https://doi.org/10.1007/BF01787346
Kiauta, B. (1969b). The chromosomes of eight dragonfly species from continental Africa and Madagascar (Odonata). Arnoldia (rhodesia), 4(15), 1–8.
Kiauta, B. (1971). Studies on the germ cell chromosome cytology of some cytotaxonomically interesting or hitherto not studied Odonata from the autonomous region Friuli-Venezia Giulia (northern Italy). Atti Del Museo Civico Di Storia Naturale Di Trieste, 27, 65–127.
Kiauta, B. (1973). Notes on new or little known dragonfly karyotypes. IV. Spermatocyte chromosomes of Calopteryx splendens splendens Harris (Zygoptera: Calopterygidae), Gomphus pulchellus Selys, Libellula depressa Linnaeus (Anisoptera: Gomphidae, Libellulidae) from northern France. Genen En Phaenen, 16(2), 55–60.
Kiauta, B. (1975). Cytotaxonomy of dragonflies, with special reference to the Nepalese fauna. Lectures delivered at the Tribhuvan University, Kathmandu (Vol. 2, p. 78). Nepal Research Center.
Kiauta, B. (1979). The karyotypes of some Anisoptera from Surinam. Odonatologica, 2, 267–283.
Kiauta, B. (1983). The status of the Japanese Crocothemis servilia (Drury) as revealed by karyotypic morphology (Anisoptera: Libellulidae). Odonatologica, 12, 381–388.
Kiauta, B., & Boyes, J. W. (1972). Cytology of ten South American Libellulidae, with cytophylogenetic consideration of the genera Orthemis Hagen and Erythrodipax Brauer (Odonata, Anisoptera). Genetica, 43(3), 407–421.
Kiauta, B., & Kiauta, M. (1980). On a small collection of dragonfly karyotypes from the Philippines. Odonatologica, 9(3), 237–245.
Kiauta, B., & Kiauta, M. (1982). The chromosome numbers of sixteen dragonfly species from the Arun Valley, Eastern Nepal. Notulae Odonatologicae, 9(1), 143–146.
Kiauta, B., & Kiauta, M. (1983). The chromosome numbers of some Odonata from Thailand. Notulae Odonatologicae, 2(2), 17–32.
Kiauta, B., & Ochssée, B. V. (1979). Some dragonfly karyotypes from the Voltiac Republic (Haute Volta), West Africa. Odonatologica, 8, 47–54.
Kichijo, H. (1942). Insect chromosomes. III. Order of dragonflies, Pt. 1. Nagasaki Medical Journal, 20(7), 1084–1092. [In Japanese].
Kuznetova, V. G., & Golub, N. V. (2020). A checklist of chromosWome numbers and review of karyotype variation in Odonata of the World. Comp Cytogenet., 14(4), 501–540.
Kuznetsova, V. G., Maryańska-Nadachowska, A., Shapoval, N. A., Anokhin, B. A., & Shapoval, A. P. (2018). Cytogenetic characterization of eight Odonata species originating from the Curonian Spit (the Baltic Sea, Russia) using C-banding and FISH with 18S rDNA and telomeric (TTAGG)n n probes. Cytogenetic and Genome Research, 153, 147–157.
Lefevre, G., & McGill, C. C. (1908). The chromosomes of Anasa tristis and Anax junius. American Journal of ASNT, 7, 467–487.
Makino, S. A. (1935). Comparative study of the chromosomes in the Indian dragonflies. Japanese Journal of Genetics, 11, 234–235. https://doi.org/10.1266/jjg.11.234
McGeoch, M. A. (1998). The selection, testing and application of terrestrial insects as bioindicators. Biological Review, 73, 181–201
Mitra, T. R. (2005). Evolutionary adaptations in morphology and ecology of Tholymis Tillarga (Fabricius) and Bradinopyga geminata (Rambur) (Insecta: Odonata). Records of Zoological Survey of India, 104(1-2), 101–104.
Mola, L. M. (1992) Estudios cromosomics en libelulas (Order Odonata). Thesis de Doctorado, Fac. CS. Exactas y Naturales, Universidad de Buenos Aires, Argentina.
Mola, L. M. (1996). Meiotic studies in nine species of Erythrodiplax (Libellulidae, Odonata). Neo-XY sex chromosome system in Erythrodiplax media. Cytologia, 61, 349–357.
Mola, L. M., Papeschi, A. G., & Carrillo, T. (1999). Cytogenetics of seven species of dragonflies. A novel sex chromosome determining system in Micrathyria ungulata. Hereditas, 131, 147–153.
Oguma, K. (1917). Entomology and cytology. Nawa-kinen-rombonshu, 105–114
Oguma, K. (1915). A study of the chromosomes of dragonflies. Zoological Magazine, Tokyo., 27, 241–250.
Oguma, K. (1930). A comparative study of the spermatocyte chromosome in allied species of the dragonfly. Journal of Faculty of Sciences, Hokkaido University., VI, 1–32.
Omura, T. (1955). A comparative study of the spermatogenesis in the Japanese dragonflies. I. Family Libellulidae. Biological Journal of Okayama University, 2(2–3), 95–135.
Perepelov, E. A., Bugrov, A. G., & Warchałowska-Śliwa, E. (1998). C banded karyotypes of some dragonfly species from Russia. Folia Biologica (kraków), 46, 137–142.
Prasad, K., & Thomas, K. I. (1992). C-band pattern homogeneity in dragonflies (Odonata). Caryologia, 45, 57–68.
Ray Chaudhuri, S. P., & Dasgupta, J. (1949). Cytological studies on the Indian dragonflies I. Structure and behaviour of chromosomes in six species of dragonflies (Odonata). Proceedings of the Zoological Society of Bengal, 2, 81–93.
Sandhu, R., & Walia, G. K. (1994). Chromosomal studies of three species of Libellulids (Anisoptera: Odonata). La Kromosoma II, 75–76, 2599–2604.
Sandhu, R., & Walia, G. K. (1995a). A note on the karyotype of Potamarcha conger (Anisoptera: Libellulidae). Chromosome Information Service, 58, 24–25.
Sandhu, R., & Walia, G. K. (1995b). A note on the karyotype of Potamarcha congener (Anisoptera: Libellulidae). Chromosome Information Service, 58, 24–25.
Shortness, S. I. (1930). Variation in wing reticulation and size of Erythrodiplax berenice, with regard to geographical distribution (Odonata: Libellulidae). Transactions of the American Entomological Society, 55(936), 415–423.
Simaika, J. P. & Samways, M. J. (2012). Using dragonflies to monitor and prioritize lotic systems: a South African perspective. Organism Diversity Evolution, 12, 251–259.
Smith, E. A. (1916). Spermatogensis of the dragonfly Sympetrum semicinctum with remarks upon Libellula basalis. Biological Bulletin, 31, 269–290.
Souza Bueno, A. M. (1982). Estudos cromossomicos na ordem Odonata (p. 140). Universidad Estatal Paulista.
Subramanian, K. A. (2017). A checklist of Odonata (Insecta) of India. In Zoological Survey of India, Kolkata. Version 3.0. www.zsi.gov.in.
Thomas, K. I., & Prasad, R. (1981). The chromosomes of five Indian dragonflies (Odonata). Perspectives in Cytology and Genetics, 3, 629–632.
Tyagi, B. K. (1982). Cytotaxonomy of Indian dragonflies. Indian Review of Life Sciences, 2, 149–161.
Walia, G. K. (2008). Comparative cytological data on twenty six species of family Libellulidae (Anisoptera: Odonata). Fraseria, 7, 77–82.
Walia, G. K., Kaur, H., & Kaur, J. (2010). Cytogenetical studies on five species of the family Libellulidae (Anisoptera: Odonata). Hislopia Journal, 3(2), 149–157.
Walia, G. K., & Sandhu, R. (1998). Female karyotypic study of four species of family Libellulidae (Anisoptera: Odonata). Fraseria, 5, 63–67.
Walia, G. K., & Sandhu, R. (2002). Chromosomal data on seven species of genus Orthetrum (Libellulidae: Anisoptera: Odonata). Bionature, 22, 7–12.
Walia, G. K., & Singh, H. (2021). First cytogenetic report of four species of family Libellulidae (Odonata: Anisoptera) from India. International Journal of Entomology Research, 2(6), 223–227.
Wasscher, M. (1985). The karyotypes of some dragonflies from Kenya and Sudan. Notulae Odonatologicae, 2(6), 105–106.
White, M. J. D. (1954). Animal cytology and evolution (2nd ed.). Cambridge University Press.
Wilson, E. B. (1905). The chromosomes in relation to the determination of sex in insects. Science, 22, 500–502.
Yadav, J. S. (1979). A note on the karyotypic variability in Crocothemis erythraea Brulle and Crocothemis servilia Drury (Anisoptera: Libellulidae). Notulae Odonatologica, 1(4), 53–84.
Acknowledgements
We are thankful to the Department of Zoology and Environmental Sciences, Punjabi University, Patiala, for providing all the laboratory facilities and the Council of Scientific and Industrial Research, New Delhi, India, for financial support under the scheme CSIR-UGC NET (JRF) with a grant number (09/140(0169)/2018-EMR-I).
Funding
This work has been supported by the Council of Scientific and Industrial Research, New Delhi, India, under the scheme CSIR-UGC NET (JRF) with a grant number (09/140(0169)/2018-EMR-I).
Author information
Authors and Affiliations
Contributions
HS carried out the literature survey and prepared the mensucript. GKW checked and reframed the manuscript for publication. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Competing interests
We have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Walia, G.K., Singh, H. A review on intraspecific karyomorphological variations of m chromosomes in family Libellulidae (Anisoptera: Odonata). JoBAZ 83, 47 (2022). https://doi.org/10.1186/s41936-022-00310-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41936-022-00310-w