Abstract
Background
Breast cancer is one of the most common diseases worldwide that affects women of reproductive age. miR-221 and miR-222 are two highly homogeneous microRNAs that play pivotal roles in many cellular processes and regulate the Wnt/β-catenin signaling pathway. Curcumin (CUR), a yellow polyphenolic compound, targets numerous signaling pathways relevant to cancer therapy. The main aim of this study was to compare the ability of chitosan curcumin nanoparticle (CC-CUR) formulation with the curcumin in modulating miR-221 and miR-222 expression through Wnt/β-catenin signaling pathway in MCF-7, MDA-MB-231 and SK-BR-3 breast cancer cell lines.
Method
Chitosan-cyclodextrin-tripolyphosphate containing curcumin nanoparticles (CC-CUR) were prepared. Cytotoxicity of the CUR and CC-CUR was evaluated. Experimental groups including CC-CUR, CUR and negative control were designed. The expression of miR-221 and miR-222 and Wnt/β-catenin pathway genes was measured.
Results
The level of miR-221 and miR-222 and β-catenin genes decreased in MCF-7 and MDA-MB-231 cells and WIF1 gene increased in all cells in CC-CUR group. However, the results in SK-BR-3 cell line were unexpected; since miRs and WIF1 gene expressions were increased following CC-CUR administration and β-catenin decreased by administration of CUR.
Conclusion
Although the composite form of curcumin decreased the expression of miR-221 and miR-222 in MCF-7 and MDA cells, with significant decreasing of β-catenin and increasing of WIF1 gene in almost all three cell lines, we can conclude than this formulation exerts its effect mainly through the Wnt/β-catenin pathway. These preliminary findings may pave the way for the use of curcumin nanoparticles in the treatment of some known cancers.
Similar content being viewed by others
Introduction
Breast cancer is the most common malignancy in women and the second leading cause of cancer-related deaths worldwide. Global statistics show an increase in the incidence of breast cancer mostly in developing countries [1]. It has pathophysiological appearance and its high prevalence and differences in response patterns to various treatment modalities as well as differences in clinical outcomes provide a strong rationale for identifying natural and synthetic treatment agents [2]. Breast cancer is divided into 3 types based on the presence or absence of different proteins in the cancerous cells. 70% of breast cancer cases are hormone receptor–positive type which has either estrogen receptor (ER) or progesterone receptor (PR) protein in the cancer cells; 15–20% of cases are ERBB2-positive (formerly known as human epidermal growth factor receptor 2 (HER2)-positive) which has high levels of ERBB2 protein on the cancer cells; and the remaining 15% of cases are triple-negative (TNBC) type that does not have ER, PR, or ERBB2 protein in the cancer cells [3, 4]. Among these types of breast cancers, TNBC is the most aggressive tumor with poor prognosis in young women. Because estrogen and progesterone receptors are not expressed in this type, TNBC do not benefit from hormone therapy and although they respond to chemotherapies, prognosis remains poor [5, 6]. The lack of appropriate treatment has prompted researchers to identify the molecular pathways involved in the proliferation and growth of these tumors, which can be a new hope for TNBC tumor treatment [7, 8]. Micro RNAs are a group of small non-coding RNAs with a length of about 18–24 nucleotides. These molecules control gene expression by inhibiting the translation of target mRNAs [9]. Studies have shown that Mic RNAs regulate the expression of a large number of genes involved in various biological processes e.g., proliferation, differentiation, survival, migration, apoptosis, and cell death. Mic RNAs are classified into oncogenic and tumor suppressor groups [9]. The miR-221 and miR-222 are two highly homogeneous mic RNAs that act as a gene cluster (miR-221/222) in cellular regulation [10]. The expression levels of miR-221 and miR-222 varies in different types of breast cancers [11, 12]. MiR-221/222 regulate the Wnt/β-catenin signaling pathway, which plays an important role in fetal growth, proliferation, polarity, and cell migration [13]. Wnt protein contains a large family of signaling molecules that affect a large number of biological processes and cell growth. Under normal conditions, the β-catenin gene is rapidly phosphorylated by the action of a multiprotein compound, marked and decomposed by ubiquitination [4]. miR-221/222 are positive regulators at different levels of β-catenin gene expression. β-catenin is a key regulator in the Wnt signaling cascade and appears to be involved in the transcription of genes involved in estrogen-independent growth in cancer cells [13]. Wnt-inhibitory factor-1 (WIF1) is a secreted protein in Wnt/β-catenin signaling pathway that binds to Wnt proteins and inhibits their activities [4].
Medicinal plants have long been used to treat various diseases including cancers [14], but the way to deliver the drug to these cells and as a result the effectiveness of the drug is one of the important factors that should be taken into consideration. Curcumin (diferuloylmethane), an orange-yellow polyphenolic compound of the curcuminoid family is a natural product isolated from the rhizome of Curcuma longa. For centuries, it has been used in medicinal preparations and also as a food colorant. In recent years, extensive in vitro and in vivo studies have suggested that curcumin possesses activity against cancer, viral infection, arthritis, amyloid aggregation, oxidation and inflammation [15]. Curcumin exerts anticancer effects primarily by activating apoptotic pathways in cancer cells and inhibiting pro-cancer processes, including inflammation, angiogenesis and metastasis [16]. Curcumin targets numerous signaling pathways relevant to cancer therapy, including those mediated by p53, Ras, phosphatidylinositol-3-kinase, protein kinase B, Wnt/β-catenin and the mammalian target of rapamycin [15]. At the molecular level, curcumin increased Tp53 gene expression [17, 18]. Curcumin may inhibit the ability of camptothecin, mechlorethamine and doxorubicin to induce apoptosis by up to 70% in MCF-7, MDA-MB-231 and BT-474 human breast cancer cells [15]. On the other hand, its bioavailability is low, due to its low solubility [19]. Therefore, drug delivery systems based on nanoparticles can increase its stability and solubility in water [20]. There are several ways to formulate curcumin, including nanocrystals, micelles, and other conjugates that increase its permeability. Chitosan is a cellulose-dependent mucopolysaccharide obtained by deactivating chitin. Chitosan is a non-toxic, biodegradable, biocompatible and safe molecule. Chitosan nanoparticles showed a better physicochemical, antibacterial, and biological properties than the other vectors due to their small size and surface-to-volume ratio [21, 22]. Therefore, the aim of this study was to formulate the curcumin in chitosan nanoparticles and then evaluation and comparing the effect of parent curcumin and its nano form (composite) on the miR-221 and miR-222 and Wnt/β-catenin signaling pathway in three different kinds of breast cancer cells, MCF-7, MDA-MB-231 and SK-BR-3.
Materials and methods
Chitosan (low molecular weight, viscosity 20–300 cP, 1% wt in 1% acetic acid), sodium tripolyphosphate (STPP), Dulbecco’s modified eagle’s medium (DMEM), penicillin–streptomycin (100 μg/ml), phosphate-buffered saline (PBS), 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT reagent), Tween 80 and Span 80 were all purchased from Sigma-Aldrich (Sigma-Aldrich company. Mo, USA). β- Cyclodextrin (CD) with molecular weight of 1135 g/mol and curcumin were obtained from Merck Company (Darmstadt, Germany).
Manufacture of chitosan-cyclodextrin-tripolyphosphate containing curcumin nanoparticles (CS/CD/TPP/CUR) (CC-CUR)
Nanoparticles were prepared by ionotropic gelling. To achieve a homogeneous solution of curcumin, first, a solution of tween in water and curcumin in span was made, and then the two solutions were mixed and homogenized well by ultrasonication. Chitosan noncomplex was prepared according to our previous study [21]. Briefly, Chitosan aqueous solution (CS) was mixed with a cyclodextrin (CD) aqueous solution (with the cross linker TPP) using a magnetic stirrer. In order to enter the curcumin into the nanoparticle, 1 mg/ml of curcumin solution was directly placed in tripolyphosphate and added to the chitosan mixture and stirred to allow the complete formation of the system for 30 min. The final product was used for further experiments.
Evaluation of physicochemical characteristics of produced nanoparticles
The particle size and zeta potential of CC-CUR nanoparticles were performed using Zetasizer (Malvern Instrument, USA) device. Fourier-transform infrared spectroscopy (FT-IR) was used to identify chemical properties of CC-CUR. Scanning electron microscope (SEM) was used to measure CC-CUR nanoparticles’ size, morphology and topography. X-Ray diffraction analysis (XRD) was used to determine the crystallographic structure of CC-CUR nanoparticles. Energy dispersive X-ray (EDX) microanalysis was used to identify the elemental composition of CC-CUR nanoparticles. Differential scanning calorimetry (DSC) as a thermo-analytical technique was used to determine the thermal properties of CC-CUR by using a differential scanning calorimeter.
Curcumin release profile
Phosphate-buffered saline (PBS, pH 5.8) was used to evaluate the release of curcumin from nanoparticles at different time intervals. After dialyzing the samples by dialysis membrane (MWCO 12,000 Da) against PBS, free curcumin was quantified in the supernatant by Optizen 3220UV (South Korea) instrument at 430 nm absorbance.
Cell culture and cytotoxicity evaluation
Cytotoxicity of CC-CUR and CUR was assessed by MTT assay according to the manufacturer’s protocol. MCF-7 cells were seeded in 96-well plates with a density of 1 × 104 cells/well in three replicates and incubated for 24 h in a 37 ○C humidified incubator with 5% CO2 in air. Twenty μl of different concentrations (0.05, 0.5 and 5 mg/ml) of CUR and CC-CUR were mixed with 80 μl serum free medium and kept at room temperature for 20 min. The mixtures were transferred into each well containing different cells and incubated for 48 h. 10 μl of MTT solution was added to each well and incubated for four hours. The supernatant was replaced by 100 μl of DMSO. The absorbance was read at 570–630 nm by an ELISA reader (Bio-tech Instruments, USA).
Transfection of cells
DMEM-F12 supplemented with 10% FBS was used and the cells were maintained in a humidified incubator with 5% CO2 at 37 °C. One day before transfection, cells at a density of 4˟105 were seeded in the fresh serum-free medium (2% FBS), in 6 cm dishes until they reached 60–70% confluence. After 24 h of incubation, the medium was changed with 100 μl of CUR and CC-CUR nanocomposite in serum-free medium without antibiotics. RNA extraction was done according to a published protocol [23].
RNA extraction and gene expression
Cancerous cells were lysed by adding 1 ml of RiboEx™ (Cat No., 301–001, GeneAll, Portugal) reagent following the manufacturer’s instructions. Phenol/chloroform was used to remove proteins to obtain an A260:A280 ratio of 1.81 ± 0.06, using a UV spectrophotometer (Thermo Scientific™ Nanodrop 2000). cDNA was synthesized using the Parstous cDNA synthesis kit (Cat No., A101161, Parstous Biotechnology company, Mashhad, Iran) according to the manufacturer’s recommendations. 0.1 μg of total RNA was used for every first strand cDNA synthesis as follow: primer annealing at 25 ºC for 10 min, denaturation at 47 ºC for 60 min and heat inactivation at 85 ºC for 5 min.
Real time PCR
Real-time PCR was performed using the Sina SYBR blue reaction mix without low ROX (Sina Clone) in a magnetic induction cycler (mic) Real-time PCR system, using 1.5 μl of each cDNA sample, and 10 pmol of each primer. The reaction program was 95 ºC for 7 min, followed by 40 cycles of 95 ºC for 15s, 65 ºC for 20s, and 72 ºC for 35s. The CT (threshold cycle) values were analyzed using 2−∆∆CT methods. β-actin and GAPDH were used as the endogenous reference genes for normalizing the fold change in gene expression. The intrinsic expression of the control group was normalized by the reference genes and set to one. The sequence of primers is shown Table 1.
Statistical analysis
All experiments were performed in triplicate and in three independent assays. Data were expressed as mean ± SD. Microsoft Excel 2019 was used to manage data and draw graphs. Data were statistically analyzed by using SPSS software version 16. p < 0.05 was considered statistically significant.
Results
Evaluation of physicochemical characteristics of nanoparticles
The X-ray diffraction patterns taken for chitosan nano-complex are presented in Fig. 1. Chitosan exhibited a crystalline peak at 2θ = 20.20°, with a slight shift to a higher diffraction angle, indicating better crystalline nature of the chitosan complex. The lower intensity exhibited by the diffraction peaks of CS/CD/TPP nano-complex revealed that they are amorphous in nature. The absence of other diffraction peaks related to impurities in the XRD patterns of CS/CD/TPP nano-complex confirmed their high purity. The ionic interaction between TPP and –NH3+ of chitosan molecules has resulted in the formation of CS/CD/TPP nano-complex. In case of CS/CD/TPP nano-complex, the intensity of diffraction peaks was increased as a consequence of transforming amorphous chitosan into crystallized form after reaction with TPP. The diffraction peak of pure chitosan which was usually observed at 20.20° has slightly shifted to a lower value (19.85°), this can be attributed to the reaction of CS/CD/TPP nano-complex with TPP and the crystallized structure of CS/CD/TPP nano-complex.
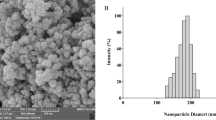
The SEM images of CS/CD/TPP nano-complex samples are shown in Fig. 2. The SEM image of CS/CD/TPP nano-complex sample showed presence of diverse shapes of particles with sizes from 50 to 150 nm. In addition, its lower magnification image revealed that chitosan contained irregular sheet-like particles. Moreover, the surface of the chitosan particles was smooth in the absence of voids.
DSC curve of CS/CD/TPP nano-complex showed both endothermic and exothermic peaks. An endothermic peak at 100 °C is probably due to water loss from the hydrophilic groups; whilst an exothermic peak at 306.0 °C indicates degradation due to dehydration and de polymerization (Fig. 3).
Curcumin release profile
Data of the release pattern of curcumin showed that 0, 13, 19, 25, 88 and 93% of the curcumin was released in 0, 1, 2, 3, 16 and 24 h, respectively (Fig. 4).
Cytotoxicity
MCF7 cells were treated in different concentrations for 48 h and the viability of the cells were measured by MTT assay. Results showed that difference between the cell viability of CUR and CC-CUR groups was much clearer at concentrations of 0.5 and 0.05 mg/ml (p < 0.05). It should be noted that CC-CUR nanocomposite was more toxic than CUR at lower concentrations (0.05 mg/ml) (Fig. 5).
Gene expression analysis
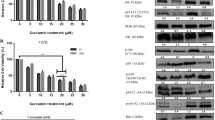
Comparing the expression of different genes in MCF-7 cells indicated that the level of miR-221 gene in the CC-CUR group was significantly decreased (P < 0.01). CUR also decreased the expression level of this gene, although it did not reach a significant level. MiR-222 level did not show any significant difference between groups although its level in CC-CUR group was lower than in the other groups. Expression of β-catenin gene decreased significantly in CC-CUR and CUR groups in comparison to the control group, although this decrease was higher in the CC-CUR group (P < 0.0001). The level of WIF1 gene expression in the CC-CUR group was significantly increased compared to both CUR and control groups (P < 0.0001). CUR also non-significantly increased the level of WIF1 gene expression in comparison to the control group (Fig. 6A).
In MDA-MB-231 cells the level of miR-221 and miR-222 genes expression in CUR group was significantly higher than in the control and CC-CUR groups while there was no significant difference between the CC-CUR and control groups. β-catenin level significantly decreased in both CC-CUR and CUR groups in comparison to the control group (P < 0.0001), a significant difference between CC-CUR and CUR groups was also detected. The level of WIF1 gene significantly increased in CC-CUR group compared to the control and CUR groups (P value < 0.001). WIF1 expression level in the CUR group also non-significantly increased compared to the control group (Fig. 6B).
The level of miR-221 and miR-222 genes in SKBR3 cells significantly increased in CC-CUR group compared to the CUR and control groups (P < 0.0001), However, the expression level of these genes in CUR group was almost similar to the control group. CUR significantly decreased the expression level of β-catenin gene compared to both CC-CUR and control groups. WIF1 expression level in the CC-CUR group was significantly higher than that in the CUR and control groups (P < 0.0001) (Fig. 6C).
Discussion
miR-221 and miR-222 are two oncomiRs that are consistently up-regulated in breast cancer tissues [24]. Activation of β-catenin by miR-221 and miR-222 promotes estrogen-independent growth of cancer cells, whereas Wnt-inhibitory factor-1 (WIF1) is suppressed by these miRNAs [25]. WIF1 acts as a tumor suppressor gene in Wnt/β-catenin signaling pathway [26]. Curcumin inhibits breast cancer cell proliferation by altering expression of signaling proteins, including Ras, phosphatidylinositol-3-kinase (PI3K), protein kinase B (Akt), mammalian target of rapamycin (mTOR) and Wnt/β-catenin pathway [15]. Therefore, targeting oncogenes and tumor suppressor genes is one of the most effective methods in the treatment of cancers. The present study investigated the possible anti-cancer effects of nano curcumin (CC-CUR) against different types of breast cancer cell lines by analyzing the expression level of miR-221, miR-222 and β-catenin as oncogene and WIF1 as a tumor suppressor gene. The results of cell viability assay which showed more toxicity of CC-CUR nanocomposite at lower concentrations than CUR reveals that in lower doses, CC-CUR has higher toxicity than CUR on breast cancer cells and to achieve more toxicity, higher doses of parent curcumin is needed which can be explained by the fact that the nanocurcumin is more soluble in addition to higher cellular uptake.
MCF-7 cell line is a Luminal A subtype expressing estrogen receptor (ER) and progesterone receptor (PR). This cell line is considered non-invasive and non-tumorigenic in vivo unless supplemented with estrogen, meaning they rely on estrogens for growth [27]. MDA-MB-231 is a basal type hormone-independent TNBC that is thought to be more aggressive and invasive than other breast cancer subtypes [28]. Also, SKBR3 cells represent basal-like BC that are estrogen-independent (her2+, ER/PR−) [29].
The results of previous studies showed that the expression of miR-221 and miR-222 was increased in breast cancer tissues compared with normal breast tissues [10, 30]. In addition, the level of miR-221 and miR-222 were associated with the advanced clinical stage and different tumor types [31]. miR-221 and miR-222 represses multiple negative regulators of the Wnt/β-catenin signaling pathway, including WIF1, SFRP2, DKK2, and AXIN2, to activate the pathway. Notably, the patient survival is inversely correlated with miR-221 and miR-222 expression level whereas it is positively correlated with that of WIF1, DKK2, SFRP2, and AXIN2 genes [13].
Our results indicated that not only the level of miR-221 and miR-222 genes are different in different types of intact breast cancers, but also the response rate of these genes to treatment is different. In our study the expression level of miR-221 and miR-222 reached to the lowest level in MCF7 cells compared to MDA-MB-231 and SKBR3 cells after treating with curcumin. Therefore, we can conclude that the effects of curcumin on the expression of miR-221 and miR-222 is more prominent in hormone dependent cancerous cells and it may lead to the hypothesis that these cells are more sensitive with better responding to certain treatments.
Many researches indicated that curcumin could acts as an anti-cancer agent in breast cancer cell line due to the regulation of different pathways such as miR-221 and miR-222 and Wnt/β-catenin [32, 33]. In this study we observed different effects of curcumin on different cell lines. Evaluating the effects of parent curcumin (CUR) and its nanocomposite form (CC-CUR) on three different cells lines showed that CC-CUR down-regulated the expression of miR-221 and miR-222 in MCF-7 cells, although its effect on the Wnt/β-catenin pathway was more prominent. CUR also modulated these pathways but the effect of the CC-CUR was more significant.
Liu et al., reported that Wnt/β-catenin signaling is hyperactivated in TNBC, promoting the invasive potential of TNBC. Inhibiting miR-221/222 expression in a TNBC cell line (MDA-MB-231) suppressed its proliferation, viability, epithelial-to-mesenchymal transition, and migration. Whereas expression of miR-221/222 in a non-TNBC line (MCF-7) promotes all the aforementioned cancer characteristics [13]. However, the effects of our treatment on Wnt/β-catenin pathway on both MDA-MB-231 and MCF-7 cells was similar and CC-CUR effect was significantly better than CUR. While the expression of miR-221 and miR-222 was not satisfactory in MDA-MB-231 cells, especially in the CUR group, as the level of these genes increased compared to other groups. The CC-CUR also could not decrease the expression level of miR genes in MDA-MB-231 cells and its level was equal to the control group.
The results in SKBR3 cells were quite unexpected as the expression level of miR genes in the CC-CUR group increased considerably. Treatment of SKBR3 cells with CC-CUR significantly increased the level of WIF1 gene expression without decreasing the level of β-catenin. On the other hand, CUR did not change the level of miR genes compared to the control group, while it significantly decreased β-catenin expression. Kim et al. claimed that the expression levels of miR-221 and miR-222 were associated with the aggressive characteristics of breast cancer subtypes and their expression level vary in breast cancers according to tumor characteristics [34]. Generally, the gene expression values in our study indicated that curcumin in its parent or composite forms affects cancerous cell death through the Wnt/β-catenin pathway and the significant point is that this effect is not basically by modulating the expression of miR-221 and miR-222 genes and shows the effect of other paths involved in this process.
WIF1 has been shown to be down-regulated in various human cancers, including breast cancer, and has been regarded as a tumor suppressor gene [26]. Rubin et al. demonstrated that loss of WIF1 could trigger Wnt/β-catenin signaling and thereby contributes to tumor invasion and metastasis [35]. Therefore, up-regulation of this gene can be a promising strategy in controlling tumor proliferation and metastasis and our results indicated that in all three cell lines the level of WIF1 significantly increased after using CC-CUR.
Previous studies demonstrated that up-regulated miR-221/222 may simultaneously suppress multiple inhibitors of Wnt/β-catenin signaling pathway such as WIF1, supporting the idea that miRNA represents a potent activator of the pathway [13]. While our results indicating the importance of other genes in activation of Wnt/β-catenin pathways. Therefore, it remains to be clarified whether miR-221 and miR-222 regulate other signaling pathways to promote oncogenesis or other genes regulating Wnt/β-catenin pathway.
Conclusion
We conclude that since different genes are involved in cancers, investigating different signaling pathways contributed in these diseases is very important. MCF-7 cells are more sensitive to curcumin treatment especially its nanocomposite form than MDA-MB-231 and SKBR3 cells due to the expression of miR-221 and miR-222 genes. Furthermore, curcumin decoration with chitosan nanoparticles strongly affected Wnt/β-catenin pathway by reducing β-catenin and increasing WIF1 gene in almost all three breast cancer cell lines, the findings that need to be further investigated in different cancers as well as in vivo conditions.
Data availability
No datasets were generated or analysed during the current study.
References
Sharma GN, Dave R, Sanadya J, Sharma P, Sharma K. Various types and management of breast cancer: an overview. J Adv Pharm Tech Res. 2010;1(2):109.
Abdullah ML, Hafez MM, Al-Hoshani A, Al-Shabanah O. Anti-metastatic and anti-proliferative activity of eugenol against triple negative and HER2 positive breast cancer cells. BMC Complement Altern Med. 2018;18(1):1–11.
Iqbal BM, Buch A. Hormone receptor (ER, PR, HER2/neu) status and proliferation index marker (Ki-67) in breast cancers: their onco-pathological correlation, shortcomings and future trends. Med J Dr DY Patil Univ. 2016;9(6):674–9.
Hsieh J-C, Kodjabachian L, Rebbert ML, Rattner A, Smallwood PM, Samos CH, et al. A new secreted protein that binds to wnt proteins and inhibits their activites. Nature. 1999;398(6726):431–6.
Singh PG, Basalingappa KM, Gopenath T, Navya Raj M, Prathibha Rajashekara S, Sushma B. Awareness of breast cancer and current perspectives: an overview. Int J Pharm Sci. 2021;12(2):b54–9.
Lee KK, Chng WJ, Jha S. Prognostic biomarkers for breast cancer metastasis. Cancer Metastasis: IntechOpen; 2018.
Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17(11):1359–70.
Feng Y, Spezia M, Huang S, Yuan C, Zeng Z, Zhang L, et al. Breast cancer development and progression: risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018;5(2):77–106.
Skaftnesmo KO, Prestegarden L, Micklem DR, Lorens JB. MicroRNAs in tumorigenesis. Curr Pharm Biotechnol. 2007;8(6):320–5.
Zong Y, Zhang Y, Sun X, Xu T, Cheng X, Qin Y. miR-221/222 promote tumor growth and suppress apoptosis by targeting lncRNA GAS5 in breast cancer. Biosci Rep. 2019;39(1).
Liang Y-K, Lin H-Y, Dou X-W, Chen M, Wei X-L, Zhang Y-Q, et al. MiR-221/222 promote epithelial-mesenchymal transition by targeting Notch3 in breast cancer cell lines. NPJ Breast cancer. 2018;4(1):20.
Mobini K, Eskandari F, Tamaddon G, Mohammadi-Bardbori A. Expression of miR-221/222 is affected by Triclosan in MCF-7 cells. Trends Pharm Sci. 2019;5(3):145–52.
Liu S, Wang Z, Liu Z, Shi S, Zhang Z, Zhang J, et al. miR-221/222 activate the Wnt/β-catenin signaling to promote triple-negative breast cancer. J Mol Cell Biol. 2018;10(4):302–15.
Roy A, Ahuja S, Bharadvaja N. A review on medicinal plants against cancer. J Plant Sci Agricultural Res. 2017;2(1):008.
Song X, Zhang M, Dai E, Luo Y. Molecular targets of curcumin in breast cancer. Mol Med Rep. 2019;19(1):23–9.
Joshi P, Joshi S, Semwal D, Bisht A, Paliwal S, Dwivedi J, et al. Curcumin: an insight into molecular pathways involved in anticancer activity. Mini Rev Med Chem. 2021;21(17):2420–57.
Shishodia S. Molecular mechanisms of curcumin action: gene expression. BioFactors. 2013;39(1):37–55.
Ramachandran C, You W. Differential sensitivity of human mammary epithelial and breast carcinoma cell lines to curcumin. Breast Cancer Res Treat. 1999;54:269–78.
Mohanty C, Das M, Sahoo SK. Emerging role of nanocarriers to increase the solubility and bioavailability of curcumin. Expert Opin Drug Deliv. 2012;9(11):1347–64.
Mirzaie Z, Barati M, Tokmedash MA. Anticancer drug delivery systems based on curcumin nanostructures: a review. Pharm Chem J. 2020;54:353–60.
Eslaminejad T, Nematollahi-Mahani SN, Ansari M. Cationic β-cyclodextrin–chitosan conjugates as potential carrier for pmCherry-C1 gene delivery. Mol Biotechnol. 2016;58:287–98.
Assa F, Jafarizadeh-Malmiri H, Ajamein H, Vaghari H, Anarjan N, Ahmadi O, et al. Chitosan magnetic nanoparticles for drug delivery systems. Crit Rev Biotechnol. 2017;37(4):492–509.
Mirzaie V, Eslaminejad T, Babaei H, Nematollahi-Mahani SN. Evaluation of the butyrylcholinesterase expression and activity in CHO, HEK-293 and vero cell lines transformed by dual promoter expression vector. J Cell Biotechnol. 2022;8(1):23–35.
Zong Y, Zhang Y, Sun X, Xu T, Cheng X, Qin Y. miR-221/222 promote tumor growth and suppress apoptosis by targeting lncRNA GAS5 in breast cancer. Biosci Rep. 2019;39(1):BSR20181859.
Rao X, Di Leva G, Li M, Fang F, Devlin C, Hartman-Frey C, et al. MicroRNA-221/222 confers breast cancer fulvestrant resistance by regulating multiple signaling pathways. Oncogene. 2011;30(9):1082–97.
Zhang J, Zhou B, Liu Y, Chen K, Bao P, Wang Y, et al. Wnt inhibitory factor-1 functions as a tumor suppressor through modulating Wnt/β-catenin signaling in neuroblastoma. Cancer Lett. 2014;348(1–2):12–9.
Qiao G, Wang W, Duan W, Zheng F, Sinclair AJ, Chatwin CR. Bioimpedance analysis for the characterization of breast cancer cells in suspension. IEEE Trans Biomed Eng. 2012;59(8):2321–9.
Moon H-r, Ospina-Muñoz N, Noe-Kim V, Yang Y, Elzey BD, Konieczny SF, et al. Subtype-specific characterization of breast cancer invasion using a microfluidic tumor platform. PLoS ONE. 2020;15(6):e0234012.
Chen J-Q, Russo J. ERα-negative and triple negative breast cancer: molecular features and potential therapeutic approaches. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer. 2009;1796(2):162–75.
Falkenberg N, Anastasov N, Rappl K, Braselmann H, Auer G, Walch A, et al. MiR-221/-222 differentiate prognostic groups in advanced breast cancers and influence cell invasion. Br J Cancer. 2013;109(10):2714–23.
Shah MY, Calin GA. MicroRNAs miR-221 and miR-222: a new level of regulation in aggressive breast cancer. Genome Med. 2011;3:1–4.
Kaboli PJ, Rahmat A, Ismail P, Ling K-H. MicroRNA-based therapy and breast cancer: a comprehensive review of novel therapeutic strategies from diagnosis to treatment. Pharmacol Res. 2015;97:104–21.
Farooqi AA, Pinheiro M, Granja A, Farabegoli F, Reis S, Attar R, et al. EGCG mediated targeting of deregulated signaling pathways and non-coding RNAs in different cancers: focus on JAK/STAT, Wnt/β-Catenin, TGF/SMAD, NOTCH, SHH/GLI, and TRAIL mediated signaling pathways. Cancers. 2020;12(4):951.
Kim J-Y, Jung EJ, Kim J-M, Son Y, Lee HS, Kwag S-J, et al. MiR–221 and miR–222 regulate cell cycle progression and affect chemosensitivity in breast cancer by targeting ANXA3. Experimental Therapeutic Med. 2023;25(3):1–13.
Rubin EM, Guo Y, Tu K, Xie J, Zi X, Hoang BH. Wnt inhibitory factor 1 decreases tumorigenesis and metastasis in osteosarcomawif-1 modulates tumor progression in osteosarcoma. Mol Cancer Ther. 2010;9(3):731–41.
Funding
Kerman University of Medical Science vice chancellor for research provided the financial support for this study.
Author information
Authors and Affiliations
Contributions
Conceptualization of the study: VM, TE. Study design: TE, ML, VM. Performed the experiments: TE, ML, VM. Contributed reagents/materials/analytical tools: VM, TE, MA, SNNM and ML. Statistical analysis: VM and TE. Wrote the first draft: VM and TE, Edited the manuscript: VM and SNNM. Approved the submission for publication: TE, ML, MA, SNNM and VM.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
The study was approved by ethics board committee for the care and use of animals at Kerman University of Medical Science, Kerman, Iran.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Eslaminejad, T., Nematollahi-Mahani, S.N., Sargazi, M.L. et al. Evaluating the effects of curcumin nano-chitosan on miR-221 and miR-222 expression and Wnt/β-catenin pathways in MCF-7, MDA-MB-231 and SKBR3 cell lines. Diagn Pathol 19, 35 (2024). https://doi.org/10.1186/s13000-024-01468-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13000-024-01468-3