Abstract
Curcumin (CUR) a phenolic compound originally derived from the turmeric plant is known as a promising therapeutic agent for several human diseases including malignancies. Despite remarkable anti-cancer effects, disadvantages including short half-life time and low bioavailability limit its usage for efficient cancer therapy. Curcumin was first encapsulated into PLGA-PEG nanoparticles. Then, using DLS, FE-SEM, and FTIR assays, the synthesized NPs were characterized. Furthermore, MCF-7 cells were exposed to different concentrations of free CUR and NP-CUR, and then the cell survival rates and gene expression profile were followed utilizing the MTT and qRT-PCR techniques, respectively. The obtained results illustrated that CUR was efficiently encapsulated into PLGA-PEG NPs. Also, MTT assay indicated that NP-curcumin more effectively inhibited MCF-7 cell viability than free curcumin treatment. Besides, qRT-PCR results evidenced that exposure of cells to CUR and NP-CUR led to upregulation of P53 and miR-132, and subsequent downregulation of hTRET and Cyclin D1 genes expression. However, changes in the expression profiles of these genes were remarkably higher in NP-CUR group. Taken together, the findings of this study suggested that encapsulation of curcumin into PLGA-PEG could increase its anti-cancer effects on breast cancer cells by modulating P53, Cyclin D1, hTRET, and MicroRNA-132 axis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer, one of the most severe threat to human health, remains a major challenge and steadily increases the mortality rate worldwide. Breast cancer is considered the most prevalent malignancy, with an estimated 2.3 million new cases (11.7%) in 2020 and a leading health challenge among females. According to some estimates in recent years, it is responsible for 6.9% of mortality cases in female breast cancers [1, 2]. Despite the significant progression in therapeutic strategies for breast cancer, many patients suffer from relapse and metastasis. The etiology of insufficient therapies can be related to ambiguous insight into molecular mechanisms involved in breast cancer initiation, progression, and metastasis. Various genomic and epigenetic events have long been considered hallmarks for the incidence of cancer formation and development of the disease [3]. According to studies, 90% of cancer cells have activated telomerase which Inhibition of the telomerase function disrupts cell proliferation and leads to shortening of telomere and apoptosis [4].
As a reverse transcriptase enzyme, telomerase ribonucleoprotein maintains the length of repetitive sequences of telomeres via modeling its ribonucleic acid portion. Telomeres, or nucleoprotein compounds, are complexes located at the ends of chromosomes in eukaryotic with several main functions, including protecting the ends of chromosomes, regulating some gene expression, and the standard of cellular living time. Due to the terminal replication problem, telomere lengths continuously decrease during each cell division, causing the cell cycle to stop. Therefore, telomeres, as the internal control clock, determine the number of cell divisions [5]. Cells with oncogenic modifications maintain their proliferative immortality via activating or upregulating the telomerase coding gene, Human telomerase reverse transcriptase (hTERT). hTERT is usually not expressed in somatic cells and is involved in aberrant cell differentiation, stemness, and most tumor metastasis [6]. Although the molecular mechanisms that regulate the activation and function of hTERT remain unclear, hTERT-based therapy approaches such as gene therapy and immunotherapy have indicated that Inhibiting hTERT may lead to the eradication of cancer cells through telomere shortening [7, 8].
MicroRNAs, endogenic short non-coding RNAs with 20 to 24 nucleotides, negatively regulate gene expression protein translation through interaction with target messenger RNAs (mRNAs) [9]. Dysregulation of miRNAs has been identified in many types of tumors. Various cellular and molecular processes in the initiation and development of breast cancer, including aberrant cell division, self-renewal capacity, apoptotic response, metastatic spread, the poor response to chemotherapy, and relapse of the tumor, are mediated via either the downregulation of tumor suppressor miRNAs (tsmiRs) or upregulation of oncogenic miRNAs (oncomiRs) [10]. Among these miRNAs, downregulated miR-132 has been mentioned in the tissues of patients with breast cancer, especially high-grade tumors, also in other cancers as diverse as colorectal, prostate, non-small cell lung, and hepatocellular carcinomas [11]. Furthermore, miR-132 can be considered a prominent tumor marker for many types of cancers, including breast cancer. miR-132 functions as tumor suppressor and its exogenous overexpression restrains the ability of breast cancer cell to proliferate and form colonies through targeting FOXA1 [11]. It was shown that, miR-132 reduces the invasion and migration in breast cancer cells by downregulating LAPTM4B and subsequently modulating signaling pathways involved in epithelial-mesenchymal transition [12]. The potential effects of miR-132 expression regulation and associated signaling pathways can relieve symptoms and reduce the disease’s severity.
Cyclin-dependent kinases (cdks) are known as key regulatory factors in the cell cycle. Therefore, it plays an important role in maintaining cell cycle [13]. Cyclin D1 has been reported to be associated with cancer aggressiveness when overexpressed [14]. It has been widely reported in several reports on human breast cancer that overexpression of the cyclin D1 gene is present in 50% of breast cancer cases [15, 16]. Therefore, ablation of cyclin D1 appears to be a strong target for future research in breast cancer therapy.
According to recent studies, curcumin, an herbal compound isolated from the plant Curcuma longa, alone or combined with other agents can have anti-cancer therapeutic effects in clinical oncology. There is evidence of curcumin biological activities including antioxidant, antimicrobial, antiviral, and anti-cancer in various diseases [17, 18], introducing curcumin as a medicinal agent in clinical studies. Especially, curcumin has been reported to diminish breast cancer cell proliferation, induce apoptosis and decrease in vivo and in vitro angiogenesis, invasion and metastasis [19]. Also, accumulating studies indicated that curcumin inhibits tumor initiation via interfering with different molecular mechanisms, especially telomerase and microRNAs [20, 21]. In fact, curcumin causes changes in expression profiles of multiple miRNAs such as miR-21, miR-19 and miR-181a in various human cancer cells, including breast cancer cells [22]. For instance, exposure to curcumin leads to Bcl-2 suppression through miR-15a and miR-16 expression resulting in apoptosis induction in MCF-7 breast cancer cells [23]. Despite curcumin application in clinical medicine, including cancer therapy, its inadequate bioavailability, low-aqueous solubility, and poor absorption are considered as disadvantages in oral administration [24]. To overcome these obstacles and enhance its anticancer efficiency, the use of a nanotechnology-based strategy, as an alternative strategy, is suggested to improve the chemopreventive and chemotherapeutic effects of curcumin [25, 26]. More importantly, due to their capacity in slow, constant, and controlled release of therapeutic agents, using nanoparticles (NPs), including polyethylene glycol–poly lactic acid-co-glycolic acid (PEG–PLGA) copolymers have been illustrated to be an effective way to overwhelm these drawbacks. The presence of PLGA-PEG copolymer prevents fat cells from approaching curcumin nanoparticles, and thus curcumins easily reach cancer tissues and destroy them by connecting with cancer cells [27, 28].
Subsequently, in the current study, we aimed to encapsulate curcumin compound into PEG–PLGA NPs and investigate the improvement of its therapeutic outcomes in MCF-7 breast cancer cells through modulating the expression of miR-132 and hTRET, as the important molecules involved in tumorigenesis.
Methods and Materials
PLGA/PEG Copolymer Synthesis
Ring open polymerization of glycolide and DL-lactide in a 3:1 molar ratio was used to synthesis PLGA-PEG copolymers. PEG and PLGA preparation as the initiators, were done through a melt polymerization method under nitrogen, in the presence of Sn(Oct)2 catalyst. Subsequently, glycolide (0.57 g), PEG4000 (1.54 g), and DL-lactide (2.882 g) in a bottleneck flask, under nitrogen gas atmosphere, were warmed to 140 °C for complete melting. Afterward, Sn(Oct)2 (0.05% w/w) was inserted and reaction heat was set at 180 °C for 4 h to polymerization be completed. Finally, the recovery of synthesized copolymers was done by means of dissolving in Dichloromethane (DCM) and subsequent precipitation in chilled diethyl ether.
Nanoparticle Formulation
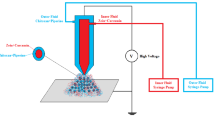
To prepare curcumin-loaded PLGA-PEG NPs, the Water-in-Oil-in-Water (W/O/W) double emulsions technique was employed. For this aim, curcumin in amount of 20 mg and PLGA-PEG in amount of 200 mg were suspended in DCM and 0.5% PVD, respectively, in a weight ratio of 1:10. Then, PLGA-PEG solution was homogenized for 20 min while, drop by drop, curcumin solution was added gradually to generate W/O emulsion. In the next step, the remainder of solvents in the emulsion was extracted by 15 min evaporation using a Heidolph Instruments rotary evaporator (Hei-VAP series, Germany) under a low vacuum. Finally, the formed NPs were collected using Amicon® Ultra Centrifugal filters by centrifugation at 14,000 g for 40 min. The gathered emulsion was moved to micro vials, then lyophilized and deposited at − 20 °C.
The Characterization of Designed Nanoparticle
To characterize the surface charge (zeta potential), hydrodynamic size of particle [29] and polydispersity index (PDI) of designed NPs, we used a DLS (dynamic light scattering) system (Zetasizer Nano ZS; Malvern Instrument) possessing a helium–neon laser beam at 633 nm wavelength. Furthermore, to determine the shape and surface morphology of designed NPs, they were coated with gold and then subjected to Hitachi S-4800 field emission scanning electron microscopy (FE-SEM) system (Japan). Also, to further evaluate curcumin’s successful loading on designed NPs, free curcumin, free NPs, and NP-curcumin first were prepared in KBr disks in the region of 4000–400 cm−1 and then by means of Perkin-Elmer Spectrum One model FT-IR, their IR spectra were determined. Moreover, the HPLC technique was employed to assess the drug loading efficiency (DLE) and drug loading capacity (DLC) of designed NPs, as described previously [30].
Drug Release Assessment
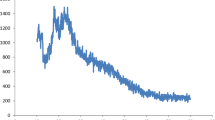
To investigate in vitro release of curcumin from PLGA-PEG NPs, the dialysis technique was employed. For this aim, NP-curcumin in amount of 25 mg was suspended in PBS buffer (5 ml) (Ph 7.4 and pH 4.4) and then transferred into dialysis membrane tubing which was placed in vials containing 25 ml PBS on a heat block stirrer set at 37 °C and 125 rpm. At the certain time periods, vial solution containing released curcumin was switched with 25 ml of new PBS and then subjected to PerkinElmer Visible–UV spectrophotometer (Lambda 950, CA, USA) to measure the concentration of curcumin according to a calibration curve at its maximum absorbance (427 nm wavelength). The function of time was regarded as the accumulative ratios of the released curcumin.
Cell Culture
MCF-7 (breast cancer cell line) and HEK 293 (normal cell lines) were attained from the Pasteur Institute’s National Cell Bank (Tehran, Iran) and cultured in Gibco Roswell Park Memorial Institute (RPMI) 1640 medium containing 2 g glucose, pH indicator, salts, amino acids, and vitamins supplement with 10% FBS (, USA) and antibiotics, including streptomycin (100 μg/mL and penicillin (100 IU/mL). The cultivation condition was 37 °C temperature, 5% CO2, and 80% humidity. As they reached 80–90% confluence, the cells were harvested using Trypsin–EDTA (0.25%, Gibco) and sub-cultured.
MTT Assay
To evaluate the effect of synthesized NPs in increasing the cytotoxicity of curcumin on MCF-7 cells while HEK 293 normal cell line was used for evaluating the toxicity, MTT assay was carried out. Consequently, MCF-7 cells, at a density of 10 × 103 cells per well, were seeded into 96-well culture plates and after incubation for 24 h to become confluent, they were treated with various concentrations of curcumin and NP-curcumin (5 μM–50 μM) and further incubated for 24, 48, and 72 h. Then, the medium was aspirated and MTT solution (5 mg/ml suspended in PBS, Sigma Aldrich) in volume of 50 μL was added to each well. Following the 4 h incubation at 37 °C, to solve formed formazan crystals, MTT solution was substituted with 100 μL dimethyl sulfoxide and the plate was incubated for 30 min. Finally, using the Thermo Fisher Scientific’s Varioskan Flash microplate reader, the wells’ absorbance at the wavelength of 570 nm was calculated to estimate the inhibitory concentrations, including IC50, according to the following equation:
RNA Extraction and qRT-PCR
To further evaluate mechanisms underlying curcumin cytotoxicity on MCF-7 cells, qRT-PCR was operated to follow changes in P53, Cyclin D1, hTRET and miR-132 expression levels through different treatments. At the density of 20 × 104 cells per well, MCF-7 cells were seeded into 6-well plates and cultivated for 48 h. Then, the cells reached to confluency were treated with curcumin and NP-curcumin in various concentrations, including 2, 5, and 10 μM and further cultivated for 48 h. Hereinafter, total RNA in treatment groups was extracted by means of Trizol RNA extraction kit (GeneAll, Korea) regarding supplied protocols. Then, considering the optical densities at 260 nm and 280 nm wavelengths, the concentration and quality of RNA were evaluated by the Thermo Fisher Scientific’s NanoDrop spectrophotometer. Additionally, total RNA was electrophoresed on agarose gel (1% in TAE buffer) to investigate its integrity. The qualified RNA samples were subjected to complementary DNA (cDNA) synthesis; to evaluate miRNA and mRNA expression levels, 1000 nanogram of RNA was initially consumed to synthesize cDNA using the Universal cDNA Synthesis miRCURY LNATM kit and BioFACT cDNA synthesis kit (Korea), respectively. Then, real-time PCR was carried out in n the StepOnePlus Real-Time PCR instrument (Applied Biosystems) using a Real-Time PCR Master Mix (BioFACT). To normalize miR-132 and hTRET expression, U6 and beta-actin were used as internal controls. The reactions were carried out in triplicates and the obtained data were analyzed according the comparative 2−ΔΔCT (Livak) method. Table 1 represents the primer sequences that are used though the current study.
Statistical Analysis
To perform all statistical analysis and design the graphs, we used GraphPad 8 Prism software (CA, USA). All experiments in the current research were repeated three times and all data were stated as mean values ± standard error (SE). The comparison between two groups and more than two groups was done using t-test and two-way analysis of variance (ANOVA), respectively. Statistical significance and multi-group comparisons of data were analyzed using two-way ANOVA followed by Tukey's post hoc test. A p-value of < 0.05 was considered significant between groups.
Results and Discussion
The Properties of Designed NPs
Due to their remarkable advantages, including being bioavailable, stability in blood, less toxic effects, better encapsulation, and controlled release of drugs, biodegradable polymeric nanomaterials have been increasingly subjected to innovate novel drug delivery systems in applications aiming to improve cancer therapy [30,31,32]. More importantly, as the well-known biocompatible biomaterials, PLGA-PEG copolymers, are widely used in nano-capsulation of anti-cancer therapies and their delivery, which have been illustrated the effects of therapies [33, 34]. Subsequently, considering the therapeutic significance of curcumin through the treatment of human malignancies, including breast adenocarcinoma [35], to improve its cytotoxic effects and better follow underlying mechanisms, curcumin was loaded on PLGA-PEG copolymers.
In this study, encapsulation of curcumin in PLGA-PEG NPs was performed using the emulsion solvent evaporation method. Our results showed that curcumin was efficiently loaded on PLGA-PEG NPs, regarding the high encapsulation efficiency equal to 80.22% and remarkable loading capacity around 13.5 ± 3.5. Subsequently, the hydrophobic nature of curcumin was suggested to be the main reason for its efficient encapsulation [36]. Furthermore, the results obtained from DLS analysis (Fig. 1B) illustrated that the average hydrodynamic diameter of PLGA-PEG NPs was approximately 180 nm with uniform dispersion. Also, it was estimated that in designed NPs loaded by curcumin, the average hydrodynamic diameter was approximately 205 nm with a particle size distribution between 100 and 300 nm. Besides, as represented in Fig. 1A, FE-SEM analysis showed that curcumin loaded-PLGA-PEG NPs had a spherical shape and uniform distributions with an averaged diameter of 250 ± 11.43 nm. Considering that NPs should have diameters less than 400 nm can internalize to cancer cells as well as they should be large enough to avoid quick and uncontrolled release into the blood capillaries and evade from macrophages [37], the designed NPs through this study could be considered as an effective delivery system for curcumin.
To more approve the loading of curcumin on designed NPs, we employed FTIR spectroscopy to characterize the structures of PLGA-PEG and curcumin-loaded PLGA-PEG NPs. The major peaks for PLGA-PEG NPs were observed at 2840–3000 cm−1,1750–1765 cm−1 and 1090–1300 cm−1 and 1085–1150 cm−1 that were indicators for stretching vibration of C–H bond, carbonyl ester bond, C–C/C–O, and polyethylene glycol ether bond, respectively. Furthermore, FTIR spectroscopy of NP-curcumin showed absorption at 2944 cm−1 related to stretching vibration of C–H bond, at 1756 cm−1 representing carbonyl ester bond, at 1095 cm−1 C–C/C–O bonds, and at 1385 cm−1 related to polyethylene glycol ether bond. Also, absorption peaks were detected at 950, 1189, and 1634 cm−1, which were in accordance with the IR absorption spectrum of curcumin (Fig. 2); further illustrating successful encapsulation of curcumin in designed NPs [38].
The Profile of in Vitro Release of Curcumin
Controlled/sustained release of drugs, especially chemotherapy agents, is an important factor for improving drug bioavailability and effectiveness through reducing side effects, inhibiting premature degradation, and maintaining drug concentration within the therapeutic range [39,40,41]. Consequently, in this study, the kinetics of in vitro release of curcumin from designed NPs was investigated for 7 days at pH 7.4 as the simulator of body fluid condition and pH 4.4 as the simulator of lysosome condition. The results (Fig. 3) evidenced that nearly 20% of curcumin was increasingly released from copolymeric NPs during the first 3 h, at pH 7.4. After that, during 5 days approximately 75% of curcumin was released from NPs in a moderately slower release pattern (steady controlled release phase) despite the initial quick release. Furthermore, curcumin was shown to exhibit a quicker release rate at pH 4.4 compared to its release at pH 7.4. In fact, 80% of curcumin was released from NPs only during 2 days. Considering that, due to the instability of the bonds between curcumin and NPs at lower pH, it may be released at the tumor site efficiently where pH is acidic.
The Synthesized NPs Increased the Cytotoxicity of Curcumin on MCF-7 Cells
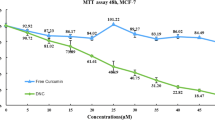
For evaluating the effectiveness of designed curcumin loaded NPs on inhibition of MCF-7 cell proliferation, MTT assay was carried out. As illustrated in Fig. 4 and presented in Table 2, the obtained results indicated that curcumin in a dose-dependent manner was capable to decrease MCF-7 cell viability compared to the control. However, encapsulation of curcumin using PLGA-PEG NPs remarkably increased the curcumin cytotoxic effects on these cells. As seen in Table 2, the IC50 of curcumin was reduced from 50.5 ± 1.11 μM in free curcumin-treated cells to 23.7 ± 1.06 μM in cells treated with NP-curcumin during 24 h incubation. Besides, despite that free curcumin showed no significant time-dependent effect on MCF-7 cells, the IC50 of NP-curcumin was dramatically decreased from 23.7 ± 1.06 μM at 24 h after incubation to 7.3 ± 1.15 μM during 72 h incubation. Overall, these results established that designed NPs improved the cytotoxic effect of curcumin on MCF-7 cells.
In consistent with our results, Fatemeh Tavakoli and colleagues previously reported that encapsulation of curcumin into PLGA-PEG NPs also improved its inhibitory power on melanoma tumor growth and progression through upregulation of tissue inhibitors of metalloproteinase (TIMPs) and subsequent downregulation of MMP-2 and MMP-9 [37]. Furthermore, the same effect was observed by Javid Lotfi-Attari and colleagues on colorectal cancer cells. They encapsulated curcumin into PEGylated PLGA NPs and illustrated its elevated anti-proliferation effects through downregulation of hTRET in Caco-2 colorectal cancer cells [38].
Curcumin and NP-Curcumin Inhibits MCF-7 Cell Proliferation Through Modulating P53, Cyclin D1, hTRET, and miR-132 Expression
In recent years, accumulating studies have illustrated that curcumin exerts its suppressive effects on tumorigenesis via modulating multiple genes and miRNAs involved in various cellular pathway mechanisms [21]. Accordingly, curcumin was illustrated to downregulate miR-21 expression as a promising oncomiR in MCF-7 cells, which in turn lead to upregulation of PTEN and deactivation of Akt signaling; resulting in the inhibition of cell viability and apoptosis induction through cleavage of caspase-3 and caspase-9 [42]. Furthermore, it was shown that curcumin also could upregulate p53 expression and signaling, which in turn induces apoptosis through regulating Bax activity in breast cancer cells [43].
Notably, curcumin was revealed to participate in inhibiting tumor development through regulating hTRET expression [37]. TERT, as an important catalytic subunit of telomerase responsible, was established to be overexpressed in human malignancies and participate in tumorigenesis through modulating cell proliferation, metastasis, and stemness [44]. Interestingly, it was reported that curcumin suppresses telomerase activity through downregulating hTRET expression in breast and cervical cancer cells [37, 45,46,47]. Besides, miR-132 which is known as a tumor suppressor miRNA was also shown to be involved in curcumin-mediated anti-cancer effects on malignant cells [48]. miR-132 was previously reported to be downregulated through breast tumorigenesis [49] and its elevated expression could inhibit cell proliferation and metastasis by directly targeting HN1 and FOXA1 genes [11, 50]. Consistently, curcumin was reported to synergistically with Chrysin decrease cell viability and induce cell cycle arrest at G2/M phases as well as apoptosis through miR-132 upregulation and consequent downregulation of HN1, as its direct target, in MDA-MB-231 breast cancer cells [30]. Consequently, in the current study, to evaluate whether curcumin is involved in the inhibition of MCF-7 cells via regulating hTRET and miR-132 expression, qRT-PCR was employed. As presented in Fig. 5, our results illustrated that increasing curcumin contractions lead to dose depended downregulation hTRET mRNA expression levels compared to the control. Moreover, NP-curcumin significantly increased the suppressive effect of curcumin on hTRET expression. Conversely, treatment of cells with free curcumin and NP-curcumin upregulated miR-132 expression in a dose-dependent manner in comparison with control cells; suggesting the negative regulatory effect of miR-132 on hTRET expression. Furthermore, it was shown that miR-132 expression levels were higher in NP-curcumin treated cells than that of free curcumin-treated cells. Collectively, these results indicated curcumin may affect MCF-7 in vitro cell proliferation through modulating the miR-132/hTRET axis and this effect was improved through the loading of curcumin into PLGA-PEG NPs.
Conclusion
Taken together, the results of this study further confirmed the anti-proliferative effects of curcumin on breast cancer cells. Also, it was shown that curcumin may exert its cytotoxic effects on MCF-7 cells through modulating P53, Cyclin D1, miR-132, and hTRET expression, revealing a new promising therapeutic axis that is affected by curcumin. Furthermore, our results showed that encapsulation of curcumin in PLGA-PEG NPs remarkably improved its inhibitory effects on breast cancer cells, which may be regarded as a promising and potential drug delivery system for better management of breast cancer.
References
E. Adlravan, K. Nejati, M. A. Karimi, H. Mousazadeh, A. Abbasi, and M. Dadashpour (2021). Potential activity of free and PLGA/PEG nanoencapsulated nasturtium officinale extract in inducing cytotoxicity and apoptosis in human lung carcinoma A549 cells. Journal of Drug Delivery Science and Technology 61, 102256.
S. Samadzadeh, H. Mousazadeh, S. Ghareghomi, M. Dadashpour, M. Babazadeh, and N. Zarghami (2021). In vitro anticancer efficacy of Metformin-loaded PLGA nanofibers towards the post-surgical therapy of lung cancer. Journal of Drug Delivery Science and Technology 61, 102318.
A. I. Riggio, K. E. Varley, and A. L. Welm (2021). The lingering mysteries of metastatic recurrence in breast cancer. British Journal of Cancer 124 (1), 13–26.
M. A. Jafri, S. A. Ansari, M. H. Alqahtani, and J. W. Shay (2016). Roles of telomeres and telomerase in cancer, and advances in telomerase-targeted therapies. Genome Medicine 8 (1), 69.
D. E. Gomez, R. G. Armando, H. G. Farina, P. L. Menna, C. S. Cerrudo, P. D. Ghiringhelli, et al. (2012). Telomere structure and telomerase in health and disease (review). International Journal of Oncology 41 (5), 1561–1569.
R. Leão, J. D. Apolónio, D. Lee, A. Figueiredo, U. Tabori, and P. Castelo-Branco (2018). Mechanisms of human telomerase reverse transcriptase (hTERT) regulation: clinical impacts in cancer. Journal of Biomedical Science 25 (1), 22.
K. Jäger, M. Walter (2016). Therapeutic Targeting of Telomerase. Genes(Basel) 7(7), 39.
N. Hassani, D. Jafari-Gharabaghlou, M. Dadashpour, N. Zarghami (2022). The effect of dual bioactive compounds artemisinin and metformin co-loaded in PLGA-PEG nano-particles on breast cancer cell lines: potential apoptotic and anti-proliferative action. Applied Biochemistry and Biotechnology 194 (10), 4930–4945.
F. Jeddi, S. Alipour, N. Najafzadeh, M. Dadashpour, F. Pouremamali, M. R. Sadeghi, et al. (2019). Reduced levels of miR–28 and miR–200a act as predictor biomarkers of aggressive clinicopathological characteristics in gastric cancer patients. Galen Medical Journal 8, e1329.
M. V. Iorio and C. M. Croce (2012). MicroRNA dysregulation in cancer: diagnostics, monitoring and therapeutics. A comprehensive Review. EMBO Molecular Medicine 4 (3), 143–159.
D. Wang, J. Ren, H. Ren, J.-l Fu, and D. Yu (2018). MicroRNA-132 suppresses cell proliferation in human breast cancer by directly targeting FOXA1. Acta Pharmacologica Sinica 39 (1), 124–131.
S. Li, J. J. Xu, and Q. Y. Zhang (2019). MicroRNA-132-3p inhibits tumor malignant progression by regulating lysosomal-associated protein transmembrane 4 beta in breast cancer. Cancer Science 110 (10), 3098–3109.
L. Ding, J. Cao, W. Lin, H. Chen, X. Xiong, H. Ao, et al. (2020). The roles of cyclin-dependent kinases in cell-cycle progression and therapeutic strategies in human breast cancer. International Journal of Molecular Sciences 21 (6), 1960.
F. I. Montalto and F. De Amicis (2020). Cyclin D1 in cancer: a molecular connection for cell cycle control, adhesion and invasion in tumor and stroma. Cells 9 (12), 2648.
M. Chatran, Y. Pilehvar-Soltanahmadi, M. Dadashpour, L. Faramarzi, S. Rasouli, D. Jafari-Gharabaghlou, et al. (2018). Synergistic anti-proliferative effects of metformin and silibinin combination on T47D breast cancer cells via hTERT and cyclin D1 inhibition. Drug Research 68 (12), 710–716.
S. Rasouli and N. Zarghami (2018). Synergistic growth inhibitory effects of chrysin and metformin combination on breast cancer cells through hTERT and cyclin D1 suppression. Asian Pacific journal of cancer prevention: APJCP. 19 (4), 977.
M. Rai, R. Pandit, S. Gaikwad, A. Yadav, and A. Gade (2015). Potential applications of curcumin and curcumin nanoparticles: from traditional therapeutics to modern nanomedicine. Nanotechnology Reviews 4 (2), 161–172.
H. Sadeghzadeh, Y. Pilehvar-Soltanahmadi, A. Akbarzadeh, H. Dariushnejad, F. Sanjarian, and N. Zarghami (2017). The effects of nanoencapsulated curcumin-Fe3O4 on proliferation and hTERT gene expression in lung cancer cells. Anti-Cancer Agents in Medicinal Chemistry Formerly Current Medicinal Chemistry-Anti-Cancer Agents 17 (10), 1363–1373.
A. S. Ombredane, V. R. Silva, L. R. Andrade, W. O. Pinheiro, M. Simonelly, J. V. Oliveira, et al. (2021). In vivo efficacy and toxicity of curcumin nanoparticles in breast cancer treatment: a systematic review. Frontiers in Oncology 11, 612903.
K. A. Lewis, T. O. Tollefsbol (2016). Regulation of the telomerase reverse transcriptase subunit through epigenetic mechanisms. Frontiers in Genetics 7, 83.
S. Zhou, S. Zhang, H. Shen, W. Chen, H. Xu, X. Chen, et al. (2017). Curcumin inhibits cancer progression through regulating expression of microRNAs. Tumor Biology 39 (2), 1010428317691680.
Y. Liu, H. Sun, B. Makabel, Q. Cui, J. Li, C. Su, et al. (2019). The targeting of non-coding RNAs by curcumin: Facts and hopes for cancer therapy (Review). Oncol Rep 42 (1), 20–34.
J. Yang, Y. Cao, J. Sun, and Y. Zhang (2010). Curcumin reduces the expression of Bcl-2 by upregulating miR-15a and miR-16 in MCF-7 cells. Medical oncology (Northwood, London, England). 27 (4), 1114–1118.
R. Tabanelli, S. Brogi, and V. Calderone (2021). Improving Curcumin Bioavailability: Current Strategies and Future Perspectives. Pharmaceutics 13 (10), 1715.
G. P. Nagaraju, S. Aliya, S. F. Zafar, R. Basha, R. Diaz, and B. F. El-Rayes (2012). The impact of curcumin on breast cancer. Integrative Biology 4 (9), 996–1007.
S. Fathi Karkan, M. Mohammadhosseini, Y. Panahi, M. Milani, N. Zarghami, A. Akbarzadeh, et al. (2017). Magnetic nanoparticles in cancer diagnosis and treatment: a review. Artificial cells, Nanomedicine, and Biotechnology 45 (1), 1–5.
K. Zhang, X. Tang, J. Zhang, W. Lu, X. Lin, Y. Zhang, et al. (2014). PEG–PLGA copolymers: their structure and structure-influenced drug delivery applications. Journal of Controlled Release 183, 77–86.
A. Eatemadi, H. Daraee, H. T. Aiyelabegan, B. Negahdari, B. Rajeian, and N. Zarghami (2016). Synthesis and characterization of chrysin-loaded PCL-PEG-PCL nanoparticle and its effect on breast cancer cell line. Biomedicine & Pharmacotherapy. 84, 1915–1922.
M. Wu, H. Xu, J. Liu, X. Tan, S. Wan, M. Guo, et al. (2021). Metformin and fibrosis: a review of existing evidence and mechanisms. Journal of Diabetes Research 2021, 6673525.
N. Javan, M. H. Khadem Ansari, M. Dadashpour, M. Khojastehfard, M. Bastami, M. Rahmati-Yamchi, et al. (2019). Synergistic antiproliferative effects of co-nanoencapsulated curcumin and chrysin on mda-mb-231 breast cancer cells through upregulating mir-132 and mir-502c. Nutrition and Cancer 71 (7), 1201–1213.
M. Dadashpour, M. Ganjibakhsh, H. Mousazadeh, K. Nejati (2022). Increased pro-apoptotic and anti-proliferative activities of simvastatin encapsulated PCL-PEG nanoparticles on human breast cancer adenocarcinoma cells. Journal of Cluster Science 33(1), 1–12.
K. Nejati, M. Rastegar, F. Fathi, M. Dadashpour, A. Arabzadeh (2022). Nanoparticle-based drug delivery systems to overcome gastric cancer drug resistance. Journal of Drug Delivery Science and Technology 70, 103231.
A. Pourgholi, M. Dadashpour, A. Mousapour, A. F. Amandi, and N. Zarghami (2021). Anticancer Potential of Silibinin Loaded Polymeric Nanoparticles against Breast Cancer Cells: Insight into the Apoptotic Genes Targets. Asian Pacific Journal of Cancer Prevention: APJCP 22 (8), 2587.
E. S. Javan, F. Lotfi, D. Jafari-Gharabaghlou, H. Mousazadeh, M. Dadashpour, and N. Zarghami (2022). Development of a magnetic nanostructure for co-delivery of metformin and silibinin on growth of lung cancer cells: Possible action through leptin gene and its receptor regulation. Asian Pacific Journal of Cancer Prevention: APJCP 23 (2), 519.
A. Giordano and G. Tommonaro (2019). Curcumin and cancer. Nutrients 11 (10), 2376.
L. Youssouf, A. Bhaw-Luximon, N. Diotel, A. Catan, P. Giraud, F. Gimié, et al. (2019). Enhanced effects of curcumin encapsulated in polycaprolactone-grafted oligocarrageenan nanomicelles, a novel nanoparticle drug delivery system. Carbohydrate Polymers 217, 35–45.
F. Tavakoli, R. Jahanban-Esfahlan, K. Seidi, M. Jabbari, R. Behzadi, Y. Pilehvar-Soltanahmadi, et al. (2018). Effects of nano-encapsulated curcumin-chrysin on telomerase, MMPs and TIMPs gene expression in mouse B16F10 melanoma tumour model. Artificial cells, Nanomedicine, and Biotechnology 46 (sup2), 75–86.
J. Lotfi-Attari, Y. Pilehvar-Soltanahmadi, M. Dadashpour, S. Alipour, R. Farajzadeh, S. Javidfar, et al. (2017). Co-delivery of curcumin and chrysin by polymeric nanoparticles inhibit synergistically growth and hTERT gene expression in human colorectal cancer cells. Nutrition and Cancer 69 (8), 1290–1299.
R.-V. Kalaydina, K. Bajwa, B. Qorri, A. Decarlo, and M. R. Szewczuk (2018). Recent advances in “smart” delivery systems for extended drug release in cancer therapy. International Journal of Nanomedicine 13, 4727.
G. Nys and M. Fillet (2018). Microfluidics contribution to pharmaceutical sciences: From drug discovery to post marketing product management. Journal of Pharmaceutical and Biomedical Analysis 159, 348–362.
L. Khoshravan Azar, M. Dadashpour, M. Hashemi, and N. Zarghami (2022). Design and Development of Nanostructured Co Delivery of Artemisinin and Chrysin for Targeting hTERT Gene Expression in Breast Cancer Cell Line: Possible Clinical Application in Cancer Treatment. Asian Pacific Journal of Cancer Prevention 23 (3), 919–927.
X. Wang, Y. Hang, J. Liu, Y. Hou, N. Wang, and M. Wang (2017). Anticancer effect of curcumin inhibits cell growth through miR-21/PTEN/Akt pathway in breast cancer cell. Oncology Letters 13 (6), 4825–4831.
T. Choudhuri, S. Pal, M. L. Agwarwal, T. Das, and G. Sa (2002). Curcumin induces apoptosis in human breast cancer cells through p53-dependent Bax induction. FEBS Letters 512 (1–3), 334–340.
M.-H. Lü, Z.-L. Liao, X.-Y. Zhao, Y.-H. Fan, X.-L. Lin, D.-C. Fang, et al. (2012). hTERT-based therapy: a universal anticancer approach. Oncology Reports 28 (6), 1945–1952.
M. Singh and N. Singh (2009). Molecular mechanism of curcumin induced cytotoxicity in human cervical carcinoma cells. Molecular and Cellular Biochemistry 325 (1), 107–119.
S. Ghasemali, K. Nejati-Koshki, A. Akbarzadeh, E. Tafsiri, N. Zarghami, M. Rahmati-Yamchi, et al. (2013). Inhibitory effects of β-cyclodextrin-helenalin complexes on H-TERT gene expression in the T47D breast cancer cell line-results of real time quantitative PCR. Asian Pacific Journal of Cancer Prevention 14 (11), 6949–6953.
E. Salmani Javan, F. Lotfi, D. Jafari-Gharabaghlou, H. Mousazadeh, M. Dadashpour, and N. Zarghami (2022). Development of a magnetic nanostructure for co-delivery of metformin and silibinin on growth of lung cancer cells: Possible action through leptin gene and its receptor regulation. Asian Pacific Journal of Cancer Prevention 23 (2), 519–527.
Y.-B. Zheng, H.-P. Luo, Q. Shi, Z.-N. Hao, Y. Ding, Q.-S. Wang, et al. (2014). miR-132 inhibits colorectal cancer invasion and metastasis via directly targeting ZEB2. World Journal of Gastroenterology: WJG 20 (21), 6515.
Z. Damavandi, S. Torkashvand, M. Vasei, B. M. Soltani, M. Tavallaei, and S. J. Mowla (2016). Aberrant expression of breast development-related microRNAs, miR-22, miR-132, and miR-212, in breast tumor tissues. Journal of Breast Cancer 19 (2), 148.
Z.-G. Zhang, W.-X. Chen, Y.-H. Wu, H.-F. Liang, and B.-X. Zhang (2014). MiR-132 prohibits proliferation, invasion, migration, and metastasis in breast cancer by targeting HN1. Biochemical and Biophysical Research Communications 454 (1), 109–114.
Acknowledgements
All authors participated in the current research would like to appreciate the supports from the Stem Cell Research Center.
Funding
This study was financially supported by grant No: 4000166 of the National Institute for Medical Research Development.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The existence of no conflict of interests was declared by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Amirsaadat, S., Jafari-Gharabaghlou, D., Dadashpour, M. et al. Potential Anti-Proliferative Effect of Nano-formulated Curcumin Through Modulating Micro RNA- 132, Cyclin D1, and hTERT Genes Expression in Breast Cancer Cell Lines. J Clust Sci 34, 2537–2546 (2023). https://doi.org/10.1007/s10876-023-02404-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-023-02404-z