Abstract
Background
Plaque hardness in carotid artery stenosis correlates with cerebral infarction. This study aimed to quantitatively compare plaque hardness with histopathological findings and identify the pathological factors involved in plaque hardness.
Methods
This study included 84 patients (89 lesions) undergoing carotid endarterectomy (CEA) at our institution. Plaque hardness was quantitatively measured immediately after excision using a hardness meter. Collagen and calcification were evaluated as the pathological factors. Collagen was stained with Elastica van Gieson stain, converted to a gray-scale image, and displayed in a 256-step histogram. The median gray-scale median (GSM) was used as the collagen content. The degree of calcification was defined by the hematoxylin–eosin stain as follows: "0:" no calcification, "1:" scattered microcalcification, or "2:" calcification greater than 1 mm or more than 2% of the total calcification. Carotid echocardiographic findings, specifically echoluminance or the brightness of the narrowest lesion of the plaque, classified as hypo-, iso-, or hyper-echoic by comparison with the intima-media complex surrounding the plaque, and clinical data were reviewed.
Results
Plaque hardness was significantly negatively correlated with GSM [Spearman's correlation coefficient: -0.7137 (p < 0.0001)]: the harder the plaque, the higher the collagen content. There were significant differences between plaque hardness and degree of calcification between "0" and "2" (p = 0.0206). For plaque hardness and echoluminance (hypo-iso-hyper), significant differences were found between hypo-iso (p = 0.0220), hypo-hyper (p = 0.0006), and iso-hyper (p = 0.0015): the harder the plaque, the higher the luminance. In single regression analysis, GSM, sex, and diabetes mellitus were significant variables, and in multiple regression analysis, only GSM was extracted as a significant variable.
Conclusions
Plaque hardness was associated with a higher amount of collagen, which is the main component of the fibrous cap. Greater plaque hardness was associated with increased plaque stability. The degree of calcification may also be associated with plaque hardness.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Carotid artery stenosis is one of the most common atherosclerotic diseases. The frequency of asymptomatic carotid artery stenosis in the general public is 0–7.5% for moderate stenosis and 0–3.1% for severe stenosis [1]. Carotid artery stenosis is caused by hypertension, diabetes, dyslipidemia, and smoking, and often occurs at the carotid bifurcation; it is defined as a plaque when its localized intimal thickening is greater than 1.1 mm [2].
The main mechanism of cerebral infarction caused by carotid artery stenosis is the reduction of cerebral blood flow due to stenosis associated with thickening of the plaque [3,4,5] and scattering of debris when fragile and soft plaques rupture [6,7,8]. Stenosis detected by cerebral angiography and carotid echocardiography are important indications for surgery for carotid artery stenosis, according to the Japanese Guidelines for the Management of Stroke 2021. However, indications for surgery are not based on plaque properties. Therefore, plaque hardness may be an important finding that strongly influences the pathogenesis, therapeutic indications, and postoperative complications of carotid artery stenosis.
Carotid endarterectomy (CEA). and carotid artery stenting (CAS) are typical surgical procedures for carotid artery stenosis. CEA involves surgically removing the plaque from the entire vascular intima [9], while CAS is an interventional therapy in which a metal stent is placed in the stenotic carotid artery to dilate it [10, 11].
In the case of carotid artery stenosis with soft unstable plaques, the risk of postoperative cerebral infarction is increased because the debris is easily dispersed by physical traction and stimulation during CEA surgery [12,13,14]. Conversely, if the plaque is hard, the tunica media may also be removed alongside the intima when the plaque is peeled off the vessel wall. Consequently, there is a risk of vessel thinning and perforation. In other words, preoperative plaque hardness provides useful information in preoperative and perioperative simulations because the precautions taken during surgical manipulation in CEA differ depending on plaque hardness.
It has been reported that during CAS, catheter manipulation can disrupt the fibrous cap causing the soft unstable plaque to disperse and embolize [15, 16]. Additionally, with hard plaques, CAS can cause plaque divergence, bradycardia due to overdilation, and restenosis due to inadequate dilation [17]. Hence, when performing CAS as well as CEA, preoperative plaque hardness is also an important finding, particularly in the determination of the type of surgery required and the device to be used, as it affects postoperative outcomes.
Several studies on plaque hardness have been reported, although most were based on subjective evaluation of hardness by the surgeon [7, 8, 18]. Although some studies have assessed plaque hardness, few have assessed quantitative measurements of the plaque, and have multiple limitations. Marher et al. performed quantitative measurements by tensile and compressive tests in 14 fresh plaques removed by CEA and compared these with echogenic findings. The results showed that calcified plaques were stiffer and hypoechoic plaques were softer in the compressive test [19]. Antonacci et al. quantitatively measured plaque hardness in mice using Brillouin microscopy, a non-contact indirect measurement. Pathological results suggested that plaque hardness was correlated with collagen abundance and inversely correlated with fat content [20]. In our previous research, we measured the hardness of various general substances, such as tofu, sliced cheese, a plastic eraser, and a stick of gum, with a hardness meter and proved the reproducibility and accuracy of the device. We also compared plaque hardness with echocardiographic findings in 44 cases, and the results showed that the harder the plaque, the higher the echo luminance [21].
Moreover, the cause and pathological basis for different plaque hardness in each case remain unknown. This study aimed to quantitatively compare plaque hardness with histopathological findings and to identify the pathological factors involved in plaque hardness.
Methods
Patient population
Eighty-nine lesions in 84 patients who underwent CEA at our institution between December 2009 and May 2018 were included in this study.
Surgical procedure
CEA in all cases was performed with the aid of a microscope, with the patient under general anesthesia. An incision was made along the anterior border of the sternocleidomastoid muscle, and the platysma was cut. The sternocleidomastoid muscle was dissected to expose the common, internal, and external carotid arteries, and superior thyroid artery. Each vessel was occluded with forceps, and a longitudinal incision was made from the common carotid artery to the internal carotid artery. After inserting a carotid shunt, the plaque was removed as a single mass along with the entire endothelium.
Quantitative measurement with hardness meter
A hardness meter (Rheometer CR-500DX-SII: SUN SCIENTIFIC CO., LTD, Tokyo, Japan) was installed in the operating room (Fig. 1a). This instrument is a commercially available industrial product for measuring the hardness of foods, resins, and pharmaceuticals. It measures the viscosity of a liquid and the elasticity of a solid. A sample was placed on the measurement table, and the upper special adaptor was moved to apply a small compressive pre- load of 0.1 N to the sample at a cross head speed of 0.1 mm/s (Fig. 1b).
The analysis software used was RheoData Analyzer VR.2.8g3 (SUN SCIENTIFIC CO. LTD, Tokyo, Japan). The unit of hardness was expressed in megapascals (MPa). Measurements were obtained within 1 h of removal.
Histopathological evaluation
Collagen is a major constituent of normal blood vessel walls; it is also present in the plaque and has high rigidity [22, 23]. Therefore, we focused on collagen as one of the pathological factors related to plaque hardness and performed Elastica van Gieson (EVG) staining of collagen and elastin. Calcification was also assessed using hematoxylin–eosin staining (HE). The resected plaques were fixed with 10% formalin immediately after hardness measurement in the operating room. Image analysis and quantitative evaluation of EVG- and HE-stained specimens were performed using the following methods:
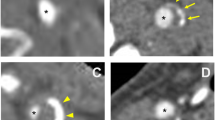
Plaque tissues were fixed for 24 to 48 h, paraffin-embedded blocks were prepared and 3-μm-thick sections were mounted on glass slides and stained with HE and EVG stains. The EVG-stained specimens were placed on a table for photography using a fixed digital camera (Nikon SLR; Nikon Corporation, Tokyo, Japan) (Fig. 2a and b). In order to eliminate errors in the image of each sample taken with a digital camera, automatic image adjustment was performed on all samples and then converted to grayscale images using image editing software (Adobe Photoshop Elements 2018, Adobe Inc. California, USA) (Fig. 2 c and d). Auto-contrast does not adjust each color channel individually, so it does not remove or create unwanted tints. Then, using image analysis software [Image J: Wayne Rasband (NIH), MD, USA], bounding rectangles were manually set up with four adjacent points, and plot profiles were constructed. Specifically, the region of interest of the image converted to grayscale was chosen using polygon selection (Fig. 2). The image was then converted for analysis to a histogram. The grayscale images of the intima and plaque were converted to a 256-step histogram from 0 to 255 and displayed. The median value of the gray-scale median (GSM) was evaluated as the collagen content (Fig. 2 e). In this grayscale image, the darkest value of 0 indicates the highest collagen content, and the lightest value of 255 indicates the lowest collagen content.
a Single-lens reflex digital camera fixed on the table for specimen photography. b Image of EVG-stained specimen taken by the camera. c Post-automatic image quality adjustment. d Grayscale converted image manually surrounded by ROIs of intima and plaque. e Grayscale image converted to a histogram and the median value determined
On HE-stained specimens, the degree of calcification was defined as follows: "0" was no calcification, "1" was scattered microcalcification, and "2" was calcification of 1 mm or more or microcalcification of 2% or more of the total.
Clinical and preoperative imaging findings
Clinical findings included age, sex, symptomatic or asymptomatic, medical history (hypertension, lipid metabolism disorders, diabetes mellitus), smoking status, and blood test results.
The preoperative stenosis rate for carotid artery stenosis was measured using the North American Symptomatic Carotid Endarterectomy Trial method on digital subtraction angiography (AREX-VC830A; Canon Medical Systems Corp., Tochigi, Japan) [24].
The XARIO SSA-660A system (Canon Medical Systems Corp., Tochigi, Japan) was used for carotid artery echocardiography, and PLT-805AT (Canon Medical Systems Corp., Tochigi, Japan) and PLT-704AT (Canon Medical Systems Corp., Tochigi, Japan) were used as transducers. Echoluminance is the brightness of the narrowest lesion of the plaque and was classified as hypo-, iso-, or hyper-echoic by comparison with the intima-media complex surrounding the plaque. Postoperative ischemic complications were assessed using diffusion-weighted images on MRI (1.5 T Magnetom Avanto, Siemens Healthcare, Erlangen, Germany) performed within 1 week after surgery.
Statistical analysis
Continuous variables are reported as mean ± standard deviation, and categorical variables are reported as counts and percentages. For comparisons between two groups, the chi-squared or Fisher's exact test was used for categorical variables, and the Student's t-test was used for categorical variables, as appropriate. Multiple comparisons using the Kruskal–Wallis test and the Steel–Dwass method were made between the three groups. Statistical significance was set at p < 0.05. Individual correlations between plaque hardness, pathological findings, and clinical data were examined using single regression analysis. In multiple regression analysis, all variables were first entered and then selected using the stepwise variable reduction method. The results are reported as partial regression coefficients, 95% confidence intervals, and p-values. IBM SPSS Statistics 26 (IBM Corp., Armonk, NY, USA) was used for data analysis.
This study was approved by the Ethics Committee of the School of Medicine, Faculty of Medicine, Toho University (approval number: A16026-25006) and the Ethics Committee of Toho University Omori Medical Center (approval number: M20250).
Results
Patient background, clinical data, and imaging findings for the 84 patients included in this study are summarized in Table 1. There were 76 men (90.5%), with a mean age of 70.6 ± 7.1 years [median 42–82], 37 symptomatic cases (42.6%), and 35 (39.3%) cases of diabetes mellitus. Echocardiographic findings were hypoechoic in 7 cases (7.9%), isoechoic in 42 cases (47.2%), and hyperechoic in 36 cases (40.4%).
The quantitatively measured plaque hardness and GSM obtained from EVG staining, and the degree of calcification on HE staining are also shown in Table 2. The mean stiffness value and GSM for all plaques was 4.0 ± 4.4 MPa and 175.7 ± 16.7 (mean ± standard deviation), respectively, and calcification was “0” in 28 cases (31.5%), “1” in 38 cases (42.7%), and “2” in 23 cases (25.8%).
Grayscale images of all plaques in this study are presented in Fig. 3.
Plaque hardness was significantly negatively correlated with GSM from EVG [Spearman's correlation coefficient: -0.7137 (p < 0.0001)], indicating that hard plaques are rich in collagen (Fig. 4).
The relationship between plaque hardness and the degree of calcification in HE showed a significant difference between "0" and "2" (p = 0.0206) (Fig. 5), and plaque containing a large amount of calcification was harder than the uncalcified plaque.
In multiple comparisons of plaque hardness and echoluminance (low-iso-high), statistically significant differences were observed in low-iso (p = 0.0220), low–high (p = 0.0006), and iso-high (p = 0.0015); that is, harder plaques were associated with higher luminance (Fig. 6).
Multiple comparisons of GSM and echoluminance showed differences in low–high (p = 0.0049) and iso-high (p = 0.0191) echoluminance, with high echoluminance showing low GSM reflecting abundant collagen (Fig. 7).
GSM, calcification, sex, age, symptoms, hypertension, dyslipidemia, diabetes mellitus, smoking status, total cholesterol, HbA1c, angiographic stenosis rate, and plaque hardness were used as objective variables to examine factors that correlate with plaque hardness and as explanatory variables for regression analysis. First, in the simple regression analysis, GSM, sex, and diabetes mellitus were significant (Table 3). In the multiple regression analysis, only GSM was extracted as a significant variable (Table 4).
Discussion
The pathogenic mechanism of cerebral infarction due to carotid artery stenosis includes hemodynamic infarction and embolism due to plaque rupture. For hemodynamic infarction, plaque stenosis rate is a clear indication for treatment; however, plaque properties are not standard indications for treatment. Soft plaques are prone causing stroke. We consider plaque hardness a standard indication for surgical treatment.
We quantitatively measured plaque hardness removed by CEA using a hardness meter and compared it with the pathological and patient background data and preoperative examination findings. Focusing on the blood vessel walls, collagen, which is a major component of plaque, and calcification as pathological factors, we quantified the hardness-determining factors of plaque. We focused on GSM as a quantification of collagen. GSM was examined to evaluate the diagnostic the echogenicity ratio using histogram analysis. Sermin et al. found that mean flexor tendons histogram analysis echogenicity/ mean median nerve histogram analysis echogenicity may useful quantitative parameters [25]. In addition, quantitative evaluation of plaque vulnerability by converting the echo brightness of the plaque into GSM has been conducted [26]. Plaque hardness quantified in this study and GSM were significantly correlated. Sex, diabetes, and echogenicity were extracted as significant findings in regression analysis performed with plaque hardness as the objective variable. GSM was the only significant factor in multiple regression analysis. Further, the hardness of the highly calcified sample was significantly higher than that of the uncalcified sample.
Our study showed that a higher echogenicity was associated with greater hardness of the plaque and that an increased amount of collagen was associated with a lower GSM. Several studies have assessed the pathological basis for the hardness of human carotid plaques. The basic pathological composition of the plaque is the fibrous cap and lipid core. The fibrous cap is mainly composed of smooth muscle cells, collagen, elastin, macrophages, foam cells, proteoglycans, and new vessels. The lipid core resides within the fibrous cap and consists of cholesterol, cell debris, foam cells, and calcium, and is severely deficient in proteoglycans and collagen [6, 27,28,29]. Collagen is a major constituent of the normal vessel wall and has a much higher stiffness than lipids, resulting in greater hardness [22, 23].
We considered collagen as a factor that determine the hardness of plaques and attempted to quantify it. Plaque collagen was EVG-stained and converted to a grayscale image, and the median value was determined by plotting the profiles in 256 steps. While some of the specimens measured were removed as whole pieces in a circumferential manner during surgery, others were removed piecemeal due to fragility. Therefore, the bounding rectangle of the plaque, including the intima, was set and measured to encompass the volume, stenosis rate, and properties of the plaque.
A stable plaque has a thick fibrous cap containing collagen, whereas an unstable plaque has a thin fibrous cap and is said to be a precursor lesion to rupture [30]. Studies on coronary arteries have also recognized unstable plaques as inflammatory lesions with thin fibrous caps that can cause acute coronary syndromes [31,32,33,34]. These findings suggest that the thickness of the fibrous cap in plaques is strongly related to their instability and tendency to rupture. Konishi et al. attempted to pathologically quantify plaque instability [35]. The authors reported that fibrous cap thickness, plaque rupture, microcalcification, and intra-plaque microvessels correlated with symptomatic plaques. Specifically, fibrous cap thickness less than 165 μm was found to be associated with unstable plaques [35]. Redgrave et al. also measured the thickness of the fibrous cap of plaques in symptomatic carotid stenosis and found that the fibrous cap in the ruptured group was thinner than that in the unruptured group. The authors also identified a cutoff value for the thickness of the fibrous cap of 200 μm [36]. In the present study, the quantitative plaque hardness was correlated with collagen content, which may indicate that the hard plaque has a thick fibrous cap with abundant collagen. In the multiple regression analysis in this study, plaque hardness was significantly correlated with GSM. Therefore, it can be said that the amount of collagen in the plaque strongly affects its hardness.
We also focused on calcification as a determinant of plaque hardness. Although pathological calcification was evaluated by HE staining, quantification of calcification was challenging in this study because it requires evaluation of the quantity of calcification and the quality of staining by shading. Therefore, the amount of calcification was evaluated by three semi-quantitative levels of "0,” "1,” and "2.” Consequently, there was a significant difference between "0,” indicating no calcification, and "2,” indicating some calcification.
Several studies have examined the relationship between plaque hardness and calcification; however, their results are controversial. For example, Mulvihill et al. used biochemical analysis by Fourier transform infrared (FTIR) spectroscopy and found that calcified specimens exhibited significantly higher stress [37]. Moreover, Barrett et al., measured composition and stiffness using FTIR analysis and micro X-ray computed tomography, which measures the calcification volume fraction relative to the total tissue content. The authors noted that the higher the lipid content, the lower the stiffness, with those with large calcification aggregates being the stiffest [38]. Conversely, a study comparing the hardness of carotid plaques using MRI and pathological findings found that calcification was not involved in plaque hardness [39]. Thus, calcification is not necessarily positively related to plaque hardness, and one reason for this may be the effect of the type of calcification. There are two types of plaque calcification: micro- and macro-calcification. Microcalcification is due to the progression of atherosclerosis and is the initial deposition of calcium in the necrotic core as a result of apoptosis of macrophages and vascular smooth muscle cells. In contrast, macrocalcification is a healing response to plaque inflammation involving grossly observable calcium deposition through the induction of osteoblast differentiation and vascular smooth muscle cell maturation. Microcalcification is more likely to be associated with plaque rupture, whereas macrocalcification is believed to lead to plaque stabilization and to be involved in plaque stiffness [40]. Macrocalcification is calcification that is visible to the naked eye, which in this semi-quantitative analysis is considered as "2.” Microcalcification is microscopic calcification and is considered as "1.” In other words, the difference in plaque hardness between "0″ and "2″ in this study indicates that macrocalcification affects plaque hardness.
In addition to GSM, sex, diabetes mellitus, and echoluminance showed significant differences in single regression analysis examining their correlation with plaque hardness. There were significant sex differences in carotid artery stenosis and most patients were male.
In the case of diabetes mellitus, there was no difference in HbA1c levels, although the presence or absence of the disease was significant, and treatment and underlying disease were not taken into account; these factors should be addressed in future studies. Our findings suggest that the echogenicity of carotid ultrasonography reflects pathological collagen content and plaque hardness.
This study also suggests that plaque hardness correlates with collagen and calcification levels. A method to preoperatively measure collagen, which shows the greatest correlation with plaque hardness, would be clinically valuable. In this study, echo findings correlated with plaque hardness during preoperative evaluation, although other preoperative evaluations such as CT and MRI were not performed. The development of a technique to quantitatively evaluate collagen and calcification based on imaging tests would be a useful preoperative evaluation for carotid artery stenosis in the future.
Limitations
This study has several limitations, which should be considered. First, the amount of collagen and extent of calcification were quantified using slices of the narrowest part for which hardness was measured. Therefore, only a slice of each plaque is used to approximate the collagen and calcification of the whole plaque. This may lead to noise in acquiring the true quantity. Second, because this is a retrospective observational study, there is a bias in the cases studied. As the subjects in this study were those with an indication for surgery, only cases with a certain degree of severity were examined. Third, over 90% of specimens were derived from male patients in this study. This is because the prevalence of carotid artery stenosis is higher in men. In a cohort study of the general public aged 50–79 years, stenosis exceeding 50% was common in men [7.9%] and found in only 1.3% of women [41]. The cause for this difference may be the effects of smoking and underlying disease.
Conclusion
Our study showed that a higher amount of collagen, which is the main component of the fibrous cap, was associated with greater plaque hardness and that increased hardness was associated with greater plaque stability. It was also shown that the amount of calcification may be related to plaque hardness. As part of preoperative evaluation, carotid echocardiography was considered a useful test because it correlates with plaque hardness.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CEA:
-
Carotid endarterectomy
- GSM:
-
Gray-scale median
- CAS:
-
Carotid artery stenting
- MPa:
-
Megapascals
- EVG:
-
Elastica van Gieson staining
- HE:
-
Hematoxylin–eosin
- FTIR:
-
Fourier transform infrared
References
de Weerd M, Greving JP, Hedblad B, Lorenz MW, Mathiesen EB, O’Leary DH, et al. Prevalence of asymptomatic carotid artery stenosis in the general population: an individual participant data meta-analysis. Stroke. 2010;41:1294–7.
Polak JF, Pencina MJ, Pencina KM, O’Donnell CJ, Wolf PA, D’Agostino RB Sr. Carotid-wall intima-media thickness and cardiovascular events. N Engl J Med. 2011;365:213–21.
Cao JJ, Thach C, Manolio TA, Psaty BM, Kuller LH, Chaves PH, et al. C-reactive protein, carotid intima–media thickness, and incidence of ischemic stroke in the elderly: the Cardiovascular Health Study. Circulation. 2003;108:166–70.
Davies MJ. The pathophysiology of acute coronary syndromes. Heart. 2000;83:361–6.
Inoue K, Matsumoto M, Shono T, Toyokawa S, Moriki A. Increased intima media thickness and atherosclerotic plaques in the carotid artery as risk factors for silent brain infarcts. J Stroke Cerebrovasc Dis. 2007;16:14–20.
Kolodgie FD, Yahagi K, Mori H, Romero ME, Trout HH, Finn AV, et al. High-risk carotid plaque: lessons learned from histopathology. Sem Vasc Surg. 2017;30:31–43.
Lee RT, Richardson SG, Loree HM, Grodzinsky AJ, Gharib SA, Schoen FJ, et al. Prediction of mechanical properties of human atherosclerotic tissue by high-frequency intravascular ultrasound imaging: an in vitro study. Arterioscler Thromb. 1992;12:1–5.
Ohayon J, Finet G, Gharib AM, Herzka DA, Tracqui P, Heroux J, et al. Necrotic core thickness and positive arterial remodeling index: Emergent biomechanical factors for evaluating the risk of plaque rupture. Am J Physiol Heart Circ Physiol. 2008;295:717–27.
Javid M, Tylon C. Neurosurgical experience with carotid endarterectomy at University Hospitals (1954–1976). Wis Med J. 1978;77:65–8.
Ederle J, Featherstone RL, Brown MM. Percutaneous transluminal angioplasty and stenting for carotid artery stenosis. Cochrane Database Syst Rev. 2007;(4):CD000515.
Diethrich EB, Ndiaye M, Reid DB. Stenting in the carotid artery. Initial experience in 110 patients. J Endvasc Surg. 1996;3:42–62.
Lennard N, Smith J, Dumville J, Abbott R, Evans DH, London NJ, et al. Prevention of postoperative thrombotic stroke after carotid endarterectomy: the role of transcranial Doppler ultrasound. J Vasc Surg. 1997;26:579–84.
Cantelmo NL, Babikian VL, Samaraweera RN, Gordon JK, Pochay VE, Winter MR. Cerebral microembolism and ischemic changes associated with carotid endarterectomy. J Vasc Surg. 1998;27:1024–31.
Mommertz G, Das M, Langer S, Koeppel TA, Krings T, Mess WH, et al. Early control of distal internal carotid artery during carotid endarterectomy: does it reduce cerebral microemboli? J Cardiovasc Surg (Torino). 2010;51:369–75.
Castaneda-Zuniga WR, Formaneck A, Tadavarthy M. The mechanism of balloon angioplasty. Radiology. 1980;135:565–71.
Crawley F, Clifton A, Buckenham T, Loosemore T, Taylor RS, Brown MM. Comparison of hemodynamic cerebral ischemia and microembolic signals detected during carotid endarterectomy and carotid angioplasty. Stroke. 1997;28:2460–4.
Coppi G, Moratto R, Veronesi J, Nicolosi E, Silingardi R. Carotid artery stent fracture identification and clinical relevance. J Vasc Surg. 2010;51:1397–405.
Williamson SD, Lam Y, Younis HF, Huang H, Patel S, Kaazempur-Mofrad MR, et al. On the sensitivity of wall stresses in diseased arteries to variable material properties. J Biomech Eng. 2003;125:147–55.
Maher E, Creane A, Sultan S, Hynes N, Lally C, Kelly DJ. Tensile and compressive properties of fresh human carotid atherosclerotic plaques. J Biomech. 2009;42:2760–7.
Antonacci G, Pedrigi RM, Kondiboyina A, Mehta MV, de Silva R, Paterson C, et al. Quantification of plaque stiffness by Brillouin microscopy in experimental thin cap fibroatheroma. J R Soc Interface. 2015;12:20150843.
Kondo K, Nemoto M, Hrada N, Fukushima D, Masuda H, Sugo N. Comparison between quantitative stiffness measurements and ultrasonographic finding of fresh carotid plaques. Ultrasound Med Biol. 2017;43:138–44.
Valentin A, Humphrey JD, Holzapfel GA. A finite element-based constrained mixture implementation for arterial growth, remodeling, and adaptation: theory and numerical verification. Int J Numer Methods Biomed Eng. 2013;29:822–49.
Holzapfel GA, Sommer G, Regitnig P. Anisotropic mechanical properties of tissue components in human atherosclerotic plaques. J Biomech Eng. 2004;126:657–65.
Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998;339:1415–25.
Sermin TU, Pinar DA. Gray scale histogram analysis of carpal tunnel syndrome with ultrasonography. Curr Med Imaging Rev. 2019;15:334–7.
Joé LK, Robbert M, Jan L, Miranda S, Job P, Jan JD, Clark JZ. Relation between B- mode gray-scale median and clinical features of carotid stenosis vulnerability. Ann Vasc Surg. 2014;28:404–11.
Kolodgie FD, Gold HK, Burke AP, Fowler DR, Kruth HS, Weber DK, et al. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med. 2003;349:2316–25.
Sluimer JC, Kolodgie FD, Bijnens AP, Maxfield K, Pacheco E, Kutys B, et al. Thin-walled microvessels in human coronary atherosclerotic plaques show incomplete endothelial junctions relevance of compromised structural integrity for intraplaque microvascular leakage. J Am Coll Cardiol. 2009;53:1517–27.
Virmani R, Joner M, Sakakura K. Recent highlights of ATVB: calcification. Arterioscler Thromb Vasc Biol. 2014;34:1329–32.
Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–75.
Carr S, Farb A, Pearce WH, Virmani R, Yao JS. Atherosclerotic plaque rupture in symptomatic carotid artery stenosis. J Vasc Surg. 1996;23:755–65 (discussion 765-6).
Carr SC, Farb A, Pearce WH, Virmani R, Yao JS. Activated inflammatory cells are associated with plaque rupture in carotid artery stenosis. Surgery. 1997;122:757–63 (discussion 763-4).
Jeziorska M, Woolley DE. Local neovascularization and cellular composition within vulnerable regions of atherosclerotic plaques of human carotid arteries. J Pathol. 1999;188:189–96.
Howard DP, van Lammeren GW, Rothwell PM, Redgrave JN, Moll FL, de Vries JP, et al. Symptomatic carotid atherosclerotic disease: Correlations between plaque composition and ipsilateral stroke risk. Stroke. 2015;46:182–9.
Konishi T, Funayama N, Yamamoto T, Morita T, Hotta D, Nomura R, et al. Pathological quantification of carotid artery plaque instability in patients undergoing carotid endarterectomy. Circ J. 2018;82:258–66.
Redgrave JN, Gallagher P, Lovett JK, Rothwell PM. Critical cap thickness and rupture in symptomatic carotid plaques: the oxford plaque study. Stroke. 2008;39:1722–9.
Mulvihill JJ, Cunnane EM, McHugh SM, Kavanagh EG, Walsh SR, Walsh MT. Mechanical, biological and structural characterization of in vitro ruptured human carotid plaque tissue. Acta Biomater. 2013;9:9027–35.
Barrett HE, Mulvihill JJ, Cunnane EM, Walsh MT. Characterising human atherosclerotic carotid plaque tissue composition and morphology using combined spectroscopic and imaging modalities. Biomed Eng Online. 2015;14(Suppl 1):S5.
Yoshida K, Narumi O, Chin M, Inoue K, Tabuchi T, Oda K, et al. Characterization of carotid atherosclerosis and detection of soft plaque with use of black-blood MR imaging. Am J Neuroradiol. 2008;29:868–74.
Shioi A, Ikari Y. Plaque Calcification During Atherosclerosis Progression and Regression. J Atheroscler Thromb. 2018;25:294–303.
Mannami T, Konishi M, Baba S, Terao A. Prevalence of asymptomatic carotid atherosclelotic lesions detected by high- resolution ultrasonography and its relation to cardiovascular risk factors in the general population of a Japanese city: the Suita study. Stroke. 1997;28:518–25.
Acknowledgements
We grateful to Chiaki Nishimura, PhD, Professor Emeritus of Toho University, for helping us with the statistical analysis. We would like to thank Editage (www.editage.com) for English language editing.
Funding
This study was not funded.
Author information
Authors and Affiliations
Contributions
DF integrated the data and completed the manuscript as a major contributor. KK and NH performed surgical procedures, measured plaque hardness, and analyzed the data. ST and KU performed part of the histopathological examinations and extracted raw data. KS performed histopathological examinations and statistical evaluation, integrated the data, and reviewed the final version of the manuscript for submission. NS conceptualized the report, integrated data, performed the statistical evaluation, revised the manuscript, and approved the manuscript. Furthermore, all authors contributed to the conceptualization, writing, reading, and approval of the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the School of Medicine, Faculty of Medicine, Toho University (approval number: A16026-25006) and the Ethics Committee of Toho University Omori Medical Center (approval number: M20250). Verbal and written consent was obtained from the participants.
Consent for publication
Participants provided oral and written permission.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fukushima, D., Kondo, K., Harada, N. et al. Quantitative comparison between carotid plaque hardness and histopathological findings: an observational study. Diagn Pathol 17, 58 (2022). https://doi.org/10.1186/s13000-022-01239-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13000-022-01239-y